Background: Proteins generally require the YidC and SecYEG machineries for membrane insertion.
Results: The specific features of membrane protein sequences determine YidC/SecYEG requirements for insertion.
Conclusion: The charge composition of membrane proteins determines the YidC and Sec translocase requirements in E. coli.
Significance: Translocase requirements for membrane insertion are determined by the charge composition of membrane proteins.
Keywords: Membrane, Membrane Biogenesis, Membrane Proteins, Protein Export, Protein Translocation, SecYEG, YidC, Hydrophobic Force, Membrane Protein Insertion, Proton Motive Force
Abstract
We have investigated the features of single-span model membrane proteins based upon leader peptidase that determines whether the proteins insert by a YidC/Sec-independent, YidC-only, or YidC/Sec mechanism. We find that a protein with a highly hydrophobic transmembrane segment that inserts into the membrane by a YidC/Sec-independent mechanism becomes YidC-dependent if negatively charged residues are inserted into the translocated periplasmic domain or if the hydrophobicity of the transmembrane segment is reduced by substituting polar residues for nonpolar ones. This suggests that charged residues in the translocated domain and the hydrophobicity within the transmembrane segment are important determinants of the insertion pathway. Strikingly, the addition of a positively charged residue to either the translocated region or the transmembrane region can switch the insertion requirements such that insertion requires both YidC and SecYEG. To test conclusions from the model protein studies, we confirmed that a positively charged residue is a SecYEG determinant for the endogenous proteins ATP synthase subunits a and b and the TatC subunit of the Tat translocase. These findings provide deeper insights into how pathways are selected for the insertion of proteins into the Escherichia coli inner membrane.
Introduction
In Escherichia coli, inner membrane proteins generally require the YidC and SecYEG machineries to insert into the inner membrane (1), although some proteins can insert independently of YidC and the Sec machinery via an unknown insertion mechanism. For example, KdpD does not require YidC or SecYEG for insertion into the inner membrane (2), and a variant of the Pf3 coat protein with a very hydrophobic TM2 segment can insert into protein-free lipid vesicles (3). SecYEG and YidC can work independently or in concert to catalyze insertion. A fundamental question addressed here is what features of the primary sequence of a membrane protein determine its insertion pathway. Although others (3–6) have addressed this question in various systems, there has not been a systematic study, especially of single-span membrane proteins. We have used a set of model single-span membrane proteins engineered from leader peptidase (Lep) to examine systematically how the charge compositions of transmembrane and translocated segments affect selection of the insertion pathway.
The Sec translocase is composed of the translocation channel SecYEG and several accessory components: SecA, SecDFYajC, and YidC (1, 7). The SecA ATPase catalyzes protein translocation and membrane insertion using a ratchet mechanism to move hydrophilic domains across the membrane (8). SecDF facilitates protein export (9) and membrane protein insertion (10), most likely as a chaperone, by binding to the emerging translocating domain of the inserting protein (11, 12). In this fashion, SecDF promotes the forward movement of the translocated region and prevents its backward movement. YidC can catalyze the insertion of proteins independently and in conjunction with the Sec machinery. The Sec-dependent F0a (subunit a of the F1F0-ATP synthase) (13–15), CyoA (16–18), and NuoK (4) are strictly dependent on YidC for membrane insertion. YidC also acts as a chaperone in the folding of the Sec-dependent LacY (20) and MalF proteins (21).
Some proteins require only YidC for insertion into the inner membrane. In this pathway, YidC acts as an insertase independently from the Sec translocase. Although a portion of YidC is associated with the Sec translocase (12, 22), much of it is free from the Sec machinery, because it is much more abundant than the SecYEG translocation channel (23). The YidC that is free of the Sec apparatus can mediate independent membrane protein insertion. Known substrates of YidC that are Sec-independent include subunit c of the F1F0-ATP synthase (13, 14, 24, 25), MscL (26), the M13 procoat protein (27, 28), and Pf3 coat protein (29). Whereas MscL is targeted to the membrane in a signal recognition particle (SRP)-dependent manner (26), subunit c (for a different view, see Ref. 24), M13 procoat, and Pf3 coat do not require SRP (14, 25, 30, 31).
These observations raise important questions about the features of membrane proteins that determine whether a protein inserts by a YidC/Sec-independent mechanism, by YidC only, or by both the YidC and the Sec pathway. Are translocase requirements connected to the overall hydrophobicity of TM helices of a substrate, or are the requirements related to charged residues within TM anchors or translocated domains, as observed in some cases (10)? The membrane-protein substrate features that determine whether a protein requires YidC for insertion have been controversial. One possibility is that YidC is required for the insertion of proteins when negatively charged residues are present in the TM segments but not when positively charged residues are present (4). Another possibility is that YidC is required for insertion of membrane proteins that have an unfavorable distribution of positively charged residues around the TM segments (5). A third possibility is that YidC is required for insertion of proteins with a weakly hydrophobic TM segment, because increasing the hydrophobicity of the TM segment of the YidC-dependent Pf3 coat protein allows this protein to insert by the autonomous pathway (3).
To expand our understanding of the determinants of insertion pathway selection, we systematically examine in this paper the features of model single-span membrane proteins that determine which translocation pathway is required for membrane insertion. Although Price and Driessen (4) found that negative charges in two TM segments of NuoK determined the YidC dependence of NuoK, we show that the addition of a negative charge to a TM segment or periplasmic segment requires YidC, whereas the addition of a positive charge imposes a requirement for both YidC and SecYEG. In addition, although Gray et al. (5) observed that YidC is required for proteins that have a topology with an unfavorable positive inside rule, we find that charged residues within proteins that have a favorable positive inside rule are also YidC determinants. Looking beyond the model single-span proteins, we found that positively charged residues can also function as Sec determinants for the endogenous proteins subunits a and b of the ATP synthase and the TatC subunit of the twin-arginine translocase.
EXPERIMENTAL PROCEDURES
Materials
Phenylmethylsulfonyl fluoride, sodium azide, carbonyl cyanide m-chlorophenylhydrazone (CCCP), and lysozyme were all purchased from Sigma. Proteinase K (protK) was purchased from Qiagen. IPTG was from Research Products International Corp. Trans-[35S]-label, a mixture of 85% [35S]methionione and 15% [35S]cysteine, 1000 Ci/mmol, was from PerkinElmer. Antisera to leader peptidase (anti-Lep) and outer membrane protein A (anti-OmpA) were from our own laboratory collection. Antiserum to the T7 tag was from Invitrogen.
Site-directed Mutagenesis, Molecular Biology Techniques, and Plasmids
The techniques described previously (32) were used for DNA manipulations. Site-directed mutations were made by the QuikChange method. DNA sequencing of the entire gene verified all mutations. Pf3-LepTM, subunit a-LepTM, Pf3-aTM, and a-aTM were cloned into the IPTG-inducible pMS119 vector. pMS119 was used to express these proteins in the JS7131 strain. The pLZ1 vector containing the RSF origin, T7/lacUV5 promoter, and kanamycin resistance was constructed in the previous study (33) and used to express various protein derivatives in CM124. F0a and TatC genes were amplified from E. coli (K-12) genomic DNA and fused at the 3′-end of the DNA with a DNA fragment encoding the LepP2 domain (residues 223–323) of leader peptidase. The coding regions of F0a-LepP2 and TatC-LepP2 were subcloned into the expression vector pLZ1 using the restriction sites for the NcoI and EcoRI enzymes.
Strains and Growth Conditions
JS7131, the E. coli YidC depletion strain, and MC1060 are from our collection. CM124, the SecE depletion strain, was received from Beth Traxler and has been described (34). yidC and secE genes in the JS7131 and CM124 strains are under the control of the araBAD promoter.
JS7131 cells are cultured at 37 °C for 3 h in LB medium with 0.2% arabinose (YidC expression conditions) or 0.2% glucose (YidC depletion conditions) (27). The SecE depletion strain CM124 was cultured in M9 medium supplemented with 0.4% glucose (SecE depletion conditions) or 0.2% arabinose plus 0.4% glucose (SecE expression conditions) (34). Prior to induction of the plasmid-encoded proteins in JS7131 and CM124, the medium was exchanged into M9 medium (35) containing 0.5% fructose and 50 μg/ml of each amino acid except methionine and shaken for 30 min at 37 °C.
Protease Accessibility Studies
Expression of the constructs was induced by 1 mm IPTG (final concentration) for 3 min. Cells were labeled with [35S]methionine for 1 min and converted to spheroplasts (36). Briefly, the pulse-labeled cells were collected by centrifugation and resuspended with spheroplast buffer (33 mm Tris·HCl, pH 8.0, 40% (m/v) sucrose). The resuspended cells were treated with 1 mm EDTA (pH 8.0) and 5 μg/ml Lysozyme on ice for 15 min. An aliquot was then incubated with protK (0.75 mg/ml) for 1 h on ice, and then the protease reaction was quenched by the addition of 5 mm PMSF for 5 min. An equal volume of 20% (m/v) TCA buffer was added to the solution and incubated on ice for another 1 h. The total protein was then spun down at 14,000 × g for 10 min and washed with 1 ml of ice-cold acetone. The protein pellet was then solubilized with Tris-SDS buffer (10 mm Tris·HCl, pH 8.0, 2% (m/v) SDS). The samples were immunoprecipitated with antiserum to leader peptidase, which precipitates the Lep derivatives, or precipitated with antiserum to OmpA. For the SecA-dependent studies, cells were treated with 3 mm (final concentration) sodium azide for 5 min prior to labeling. For the proton motive force (pmf)-dependent studies, the cells were pretreated with 50 μm (final concentration) CCCP for 45 s prior to labeling of the cells. The samples were analyzed by SDS-PAGE and phosphorimaging.
Western Blotting
E. coli strains Wam121, JS7131, and CM124 were grown for various times (Wam121 for 3 h, JS7131 for 3 h, and CM124 for 8 h) in the presence of arabinose or glucose at 37 °C. The cells were then collected by centrifugation and washed with ice-cold PBS buffer (137 mm NaCl, 2.7 mm KCl, 10 mm Na2HPO4, 2 mm KH2PO4 (pH 7.4)). After normalizing the cells to A600 of 1.2, the cell samples (1 ml) were pelleted, washed, and dissolved in 100 μl of SDS gel loading buffer, and 2 μl of the protein samples were loaded on a 15% SDS-polyacrylamide gel. Ffh and YidC were analyzed by a Western blot using the total protein. To analyze the protein expression of SecY, membrane vesicles were prepared from the 1 ml of cells grown in arabinose and glucose conditions, adjusted to A600 ∼1.2, respectively. The membrane vesicles were then solubilized in DDM buffer (20 mm Tris-HCl (pH 8.0) and 1% n-Dodecyl β-d-maltoside) and the proteins resolved on the 15% SDS-polyacrylamide gel. Antisera to Ffh, YidC, and SecY (each diluted 1:5000) and secondary antibody (goat-to-rabbit IgG horseradish peroxidase, 1:10,000) were used for the Western blot.
Supplemental Fig. S1 examines the YidC and Sec dependence of key mutants in which the H2 and H3 segments of the Lep domain were deleted. Supplemental Fig. S2 shows the SecA and SRP dependence of key mutants.
RESULTS
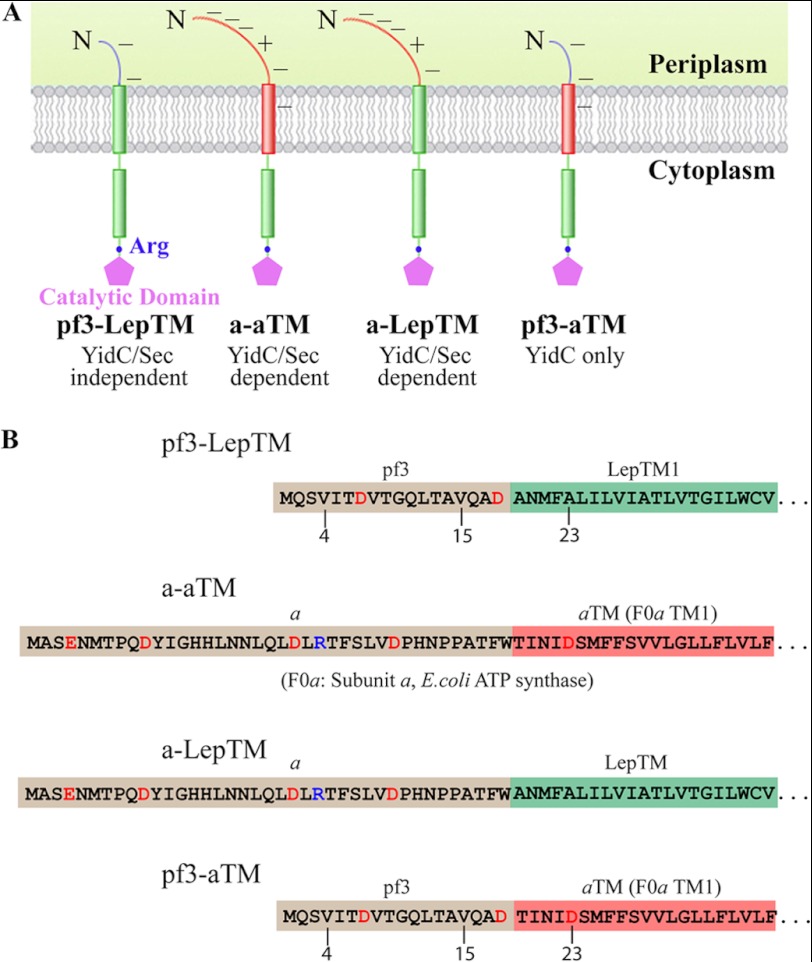
To examine the features of a membrane protein that determine which translocase is required, we studied four single-span model membrane proteins (Fig. 1A) based upon E. coli Lep. The model proteins differ only in the translocated N-terminal region (N-tail) or the TM segment. The constructs have two-part names; the first part represents the N-tail region, and the second represents the TM segment (Fig. 1B). The Pf3-LepTM construct contains the Pf3 coat periplasmic N-tail fused to Lep. To prevent translocation of Lep TM2, we introduced a single arginine after TM2, which has previously been demonstrated to prevent it from functioning as a TM segment (37). The Pf3-aTM and a-aTM constructs both contain TM1 of subunit a of the F1F0-ATP synthase (referred to here as F0a) but different N-tails; Pf3-aTM has the Pf3 N-tail fused to F0a TM1, and a-aTM has both F0a N-tail and the F0a TM1 domain. All of the constructs contain Lep residues 23–323 plus the Arg at the C terminus of TM2. These constructs simplify topology assays using protK, because only the N-tails are translocated. To assay the insertion pathways of the constructs, we used YidC- and SecE-depleted E. coli strains.
FIGURE 1.
Topology and amino acid sequences of the N-tail and TM segments of the model proteins. A, the constructs are named with the N-tail region designated first followed by the TM segment name (i.e. the Pf3-Lep model protein contains the Pf3 N-tail and the LepTM segment). The rectangles correspond to the TM segment of a membrane protein. The TM segment of each construct is followed by residues 23–323 of Lep, where an arginine is present at position 79 of Lep following TM2. B, the amino acid sequences of the N-tail and TM segments of the model proteins Pf3-LepTM, a-aTM, a-LepTM, and Pf3-aTM.
YidC dependence of membrane insertion was assayed using the YidC depletion strain JS7131, which has the yidc gene under the control of the araBAD promoter (27). Membrane insertion was studied under YidC depletion conditions by growing the bacteria in growth medium supplemented with glucose. JS7131 cells expressing different protein constructs were labeled with [35S]methionine for 1 min under YidC expression (0.2% arabinose) and YidC depletion conditions (0.2% glucose). They were then analyzed by the protease accessibility assay (38). JS7131 cells were converted to spheroplasts to allow access to the inner membrane and then treated with protK for 60 min. To determine the Sec dependence of the various constructs, we used CM124, the SecE depletion strain (34). It is well established that proteins requiring the SecYEG machinery are strongly inhibited in this strain when SecE is depleted, whereas Sec-independent proteins are unaffected. CM124 cells expressing the model proteins were labeled with [35S]methionine for 1 min under SecE depletion conditions (SecE−), and protease mapping was carried out as described for JS7131.
For all experiments reported here, we used the protease degradation of OmpA as a positive control for efficiency of spheroplast formation because OmpA is not digestible in intact cells. It can, however, be digested from the periplasmic side of the membrane in properly prepared spheroplasts. OmpA was completely digested in all the protK+ studies, showing that the cells were converted to spheroplasts (Fig. 2A, right). These positive controls are included for all data presented in Fig. 2.
FIGURE 2.

Ffh, YidC, and Sec dependence of the model membrane proteins. A, E. coli Wam121 (the Ffh depletion strain) cells bearing different plasmids were grown for 3 h under Ffh expression (0.2% arabinose) or Ffh depletion conditions (0.2% glucose) and analyzed by protease mapping (see “Experimental Procedures”). The plasmid pMS119 expressed the proteins Pf3-LepTM, a-aTM, a-LepTM, and Pf3-aTM. B, E. coli JS7131 (the YidC depletion strain) cells expressing the different model proteins were grown for 3 h under YidC expression (0.2% arabinose) or YidC depletion conditions (0.2% glucose) and subjected to protease-mapping analysis, as described under “Experimental Procedures.” C, E. coli CM124 transformed with pLZ1 expressing the model proteins or Procoat-Lep were grown under SecE expression (0.2% arabinose) or SecE depletion conditions (0.4% glucose) and analyzed by a protease-mapping procedure, as described under “Experimental Procedures.” D, Western blot of YidC, SecY, and Ffh proteins under the Ffh-, YidC-, and SecE-expressed and -depleted conditions. For details, see “Experimental Procedures.”
All of the Model Membrane Proteins Are Targeted via the SRP Pathway
Because of the possibility that the targeting pathway might differ among the model proteins, we first established that all were targeted via the SRP pathway. In bacteria, SRP is composed of the protein component Ffh and a 4.5 S RNA. To test whether SRP is required for targeting, we used Wam121, the SRP depletion strain. Wam121 contains Ffh under the control of the araBAD promoter similar to the depletion strains described earlier. As can be seen, Pf3-LepTM, a-aTM, a-LepTM, and Pf3-aTM insert efficiently under Ffh+ conditions (Fig. 2A). In contrast, insertion is inefficient under Ffh− conditions, indicating that SRP is required for membrane targeting and insertion of all model proteins. As a positive control, we confirmed that the SRP-dependent construct a-P2 (composed of F0a with residues 223–323 of Lep attached) (14) is strongly inhibited under Ffh depletion conditions. As a negative control, we confirm that Ffh depletion does not affect the chemical amounts of YidC and SecY, both of which require Ffh for membrane targeting (Fig. 2D, left). These results show that the different translocase requirements described below were not due to different targeting requirements; all are targeted using the SRP pathway.
The N-tail and TM Segment Are Determinants of Translocase Requirements
We first examined the insertion pathways of Pf3-LepTM and a-aTM. The 35S-labeled Pf3-LepTM inserted efficiently under YidC depletion conditions (39), whereas a-aTM was blocked (Fig. 2B). Only a small amount of the shifted band was observed for a-aTM, with mainly the full-length protein detected upon the addition of protK, whereas only a shifted band due to cleavage of the N-tail was seen with Pf3-LepTM (Fig. 2B). The difference in the YidC dependence of Pf3-LepTM and a-aTM suggests that YidC determinants are present in the N-tail or TM segment of the proteins. To examine this idea further, we determined the YidC dependence of a-LepTM, in which the F0a N-tail replaces the Pf3 N-tail preceding Lep TM1. Remarkably, a-LepTM requires YidC for translocation of the N-tail domain (Fig. 2B) because the protein is resistant to protK digestion under YidC depletion conditions but not under YidC-expression conditions. This indicated that the N-tail domain of F0a contains a YidC pathway determinant. We also tested the membrane insertion requirements of Pf3-aTM with the Pf3 coat tail attached to the F0a TM segment. Translocation of the N terminus of Pf3-aTM also required YidC (Fig. 2B), showing that the F0a TM1 segment contains a YidC pathway determinant as well. As a negative control, we confirmed by Western blotting that YidC depletion did not affect the level of Ffh or the amount of SecY (Fig. 2D, middle).
How do the differences between the constructs affect SecYEG pathway dependence? We found that the YidC-independent construct Pf3-LepTM was also independent of SecYEG because insertion was unaffected by SecE depletion (Fig. 2C); protK digestion completely converted the radiolabeled Pf3-LepTM protein to a shifted band under SecE depletion conditions. As controls, we show that the Sec-independent Procoat-Lep is unaffected by SecE depletion, and the Sec-dependent pro-OmpA accumulated in the precursor form in the cytoplasm under SecE-depleted conditions and as a result was protK-resistant (Fig. 2C, right). Note also that a Western blot study shows that SecE depletion does not affect the amount of Ffh or levels of the Sec-dependent YidC protein (Fig. 2D, right). Similarly, the YidC-dependent construct Pf3-aTM also inserted by a Sec-independent process (Fig. 2C). On the other hand, a-aTM and a-LepTM were inhibited when SecE was depleted, suggesting that these constructs require the Sec translocase to be inserted efficiently (Fig. 2C). We concluded that YidC acting alone cannot translocate the F0a N-tail; SecYEG is required for translocation, suggesting that F0a N-tail possesses a Sec substrate determinant.
The Charge Composition of Periplasmic Tails Can Change the Insertion Requirement from YidC-independent to YidC-dependent
The N-tail of a-LepTM contains one positively and four negatively charged residues, whereas the N-tail of Pf3-LepTM contains only two negatively charged and no positively charged residues (Fig. 1, A and B). The former is YidC-dependent, and the latter is YidC-independent. Could the differences in N-tail charge composition account for the differing YidC requirements?
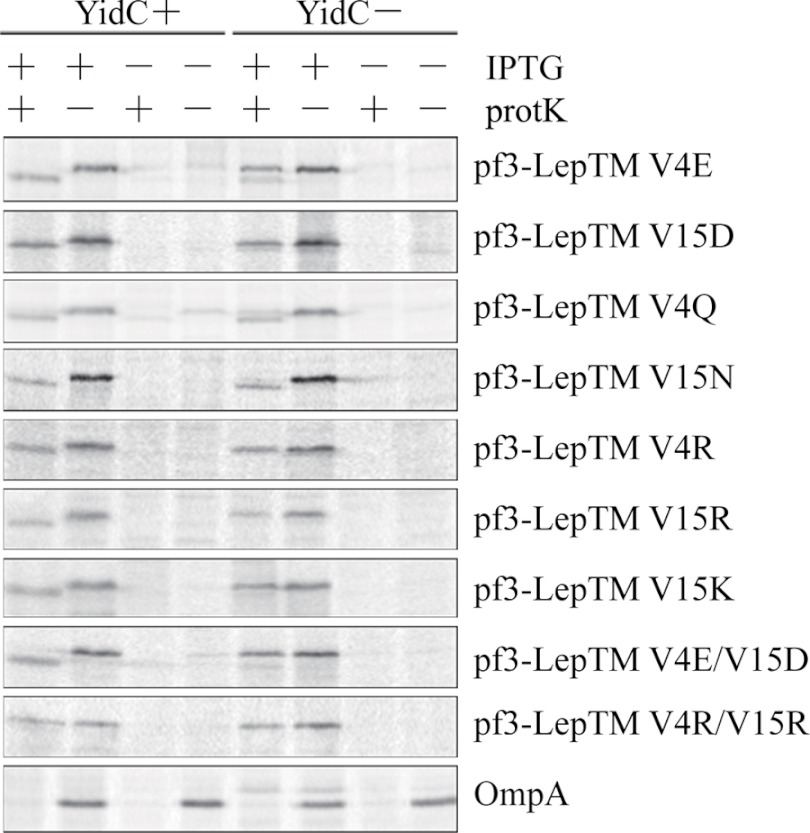
To test whether negatively charged residues in an N-tail can function as a YidC substrate determinant, we added additional negative charges to the Pf3-LepTM N-tail by substituting negatively charged residues for non-polar ones. When JS7131 cells expressing the Pf3-LepTM(V4E) or Pf3-LepTM(V15D) were grown under YidC depletion conditions, membrane insertion of the proteins was blocked (Fig. 3). As can be seen, protK digestion of the N-tail is inhibited under YidC depletion conditions but not under YidC expression conditions, indicating that the addition of one negatively charged residue to the N-tail caused Pf3-Lep insertion to become YidC-dependent. The double mutant Pf3-Lep(V4E/V15D) was also YidC-dependent for membrane insertion (Fig. 3). Were the results due to charge polarity (net negative) or to the total amount of charge? To answer this question, we replaced the non-polar valines at positions 4 and 15 with the positively charged residues Arg and Lys. As observed for the negative charge mutants, Pf3-LepTM(V4R) and Pf3-LepTM(V15R) became YidC-dependent (Fig. 3). Fig. 3 also shows that Pf3-LepTM(V4K) and Pf3-LepTM(V15K) also caused Pf3-Lep to become YidC-dependent for insertion. However, the double positively charged mutant Pf3-LepTM(V4R/V15R) (Fig. 3) was blocked in membrane insertion under YidC-expression conditions, perhaps as a result of positive inside rule (40–43).
FIGURE 3.
Both negatively and positively charged residues in the periplasmic region can function as YidC determinants. E. coli JS7131 cells bearing different plasmids were grown under YidC expression or YidC depletion conditions, labeled, and analyzed for translocation of the N-tail using the protease accessibility assay as described in the legend to Fig. 2. The plasmids encoded the proteins Pf3-LepTM V4E, V15D, V4Q, V15N, V4R, V15R, V15K, V4E/V15D, and V4R/V15R. A representative OmpA immunoprecipitation data under YidC+ or YidC− conditions is shown at the bottom. PK, proteinase K.
Are these charge substitution effects due to the charge itself, or are they due simply to replacement of non-polar residues with polar ones? The charge itself is apparently important because we found that Pf3-LepTM(V4Q) and Pf3-LepTM(V15N) remained YidC-independent, as indicated by efficient insertion under YidC depletion conditions (Fig. 3). We conclude that the charge composition of the N-tails of our single-span model membrane proteins can affect their YidC dependence. Another difference between the F0a and Pf3 N-tails is their length (Fig. 1B). Whether length plays a role requires further study, as does the distribution of the charges along the N-tails.
The Sec Translocase Is Required to Translocate the N-tail of a Protein When Positively Charged Residues Are Introduced
The Pf3-LepTM constructs with a positively charged residue introduced into the N-tail became YidC-dependent. Intriguingly, these same constructs require, in addition, the Sec machinery for translocation, whereas the constructs with a negatively charged residue introduced do not (Fig. 4). Pf3-LepTM(V4R) and Pf3-LepTM(V15R) are strongly inhibited in membrane insertion under SecE depletion conditions. A similar result was obtained for Pf3-LepTM(V15K). However, there is a limit to the number of positively charged residues that can be translocated because the double-arginine mutant Pf3-LepTM(V4R/V15R) was blocked in membrane insertion even under SecE expression conditions (Fig. 4). Because this same construct was also blocked under YidC expression conditions, the double arginine is a powerful inhibitor of insertion in general, possibly because of the positive inside rule.
FIGURE 4.
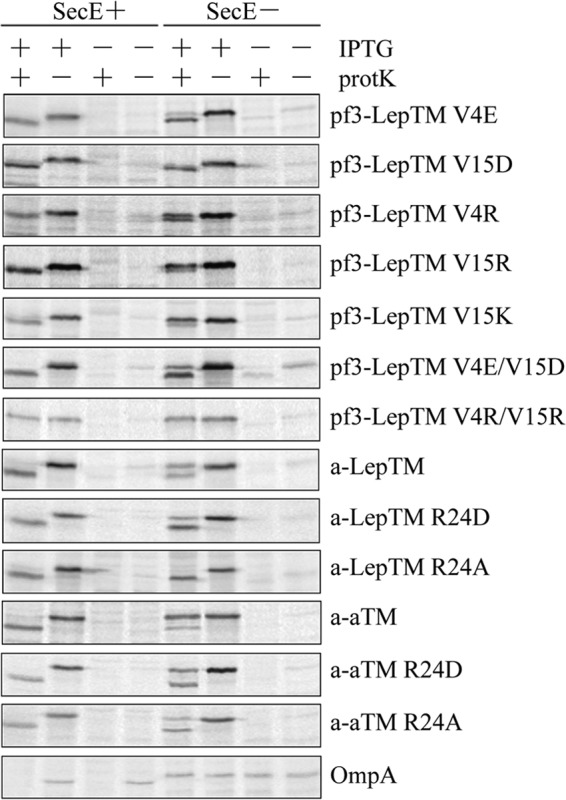
The Sec translocase is required to translocate the Pf3 tail or the F0a N-tail with a positively charged residue. E. coli CM124 cells bearing different plasmids were radiolabeled and analyzed by the protease accessibility assay, as described in the legend to Fig. 2. The plasmids expressed Pf3-LepTM V4E, V15D, V4R, V15R, V15K, V4E/V15D, or V4R/V15R; a-LepTM, a-LepTM R24D or R24A; or a-aTM, a-aTM R24D or R24A. Representative OmpA data for the protease-mapping study is shown at the bottom.
In contrast to these positive residue results, the negatively charged mutants Pf3-LepTM(V4E), Pf3-LepTM(V15D), and Pf3-LepTM (V4E/V15D) did not require the Sec machinery for efficient insertion (Fig. 4). These results may explain why the constructs a-aTM and a-LepTM require the Sec translocase for efficient insertion (Fig. 2C); the F0a N-tail region has one positively charged residue, Arg-24, in addition to four negatively charged ones (Fig. 1A). To test whether the arginine at position 24 makes these constructs Sec-dependent, we mutated the arginine to an alanine or aspartic acid. Fig. 4 shows that a-aTM(R24A) inserts very efficiently under SecE depletion condition, whereas the a-aTM(R24D) mutant is less Sec-dependent than the wild-type protein. Similar results were found with the a-LepTM constructs. The a-LepTM(R24A) mutant is not Sec-dependent (Fig. 4), showing that the arginine within the N-tail makes protein insertion Sec-dependent. Insertion of a-LepTM(R24D) (Fig. 4) is slightly Sec-dependent. Overall, these results suggest that the Sec translocase is required for efficient insertion when a positively charged residue is introduced into the N-terminal tail.
YidC Is Required for the Insertion of TM Segments with Marginal Hydrophobicity
Having established the importance of N-tail charge composition for translocase requirements, we next asked how the composition of the TM segments affects the YidC requirement for insertion. Unlike LepTM, the F0a TM segment contains an Asp (position 23; Fig. 1B). To test the effect of this Asp as a YidC determinant, we substituted an Ala for the Asp. Fig. 5A shows that the Asp residue within the TM segment is indeed important for YidC dependence because insertion of Pf3-aTM(D23A) is independent of YidC. As can be seen, the addition of protK leads to a Pf3-aTM(D23A)-shifted band under YidC depletion conditions. A similar result is seen with the Pf3-aTM(D23I) protein (Fig. 5A). These results suggest that the presence of the Asp in the F0a TM segment signaled YidC dependence.
FIGURE 5.
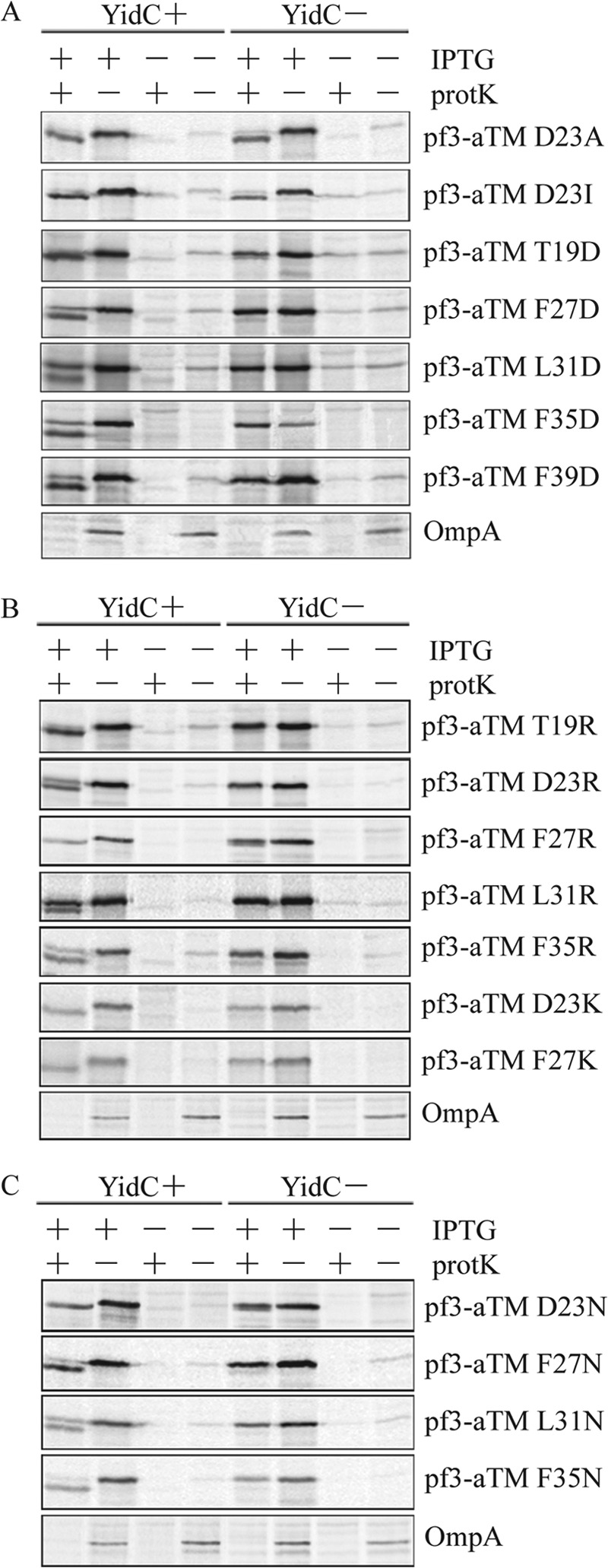
Aspartic acid, arginine, and asparagine scanning of the TM segment of Pf3-aTM. JS7131 expressing different plasmids was analyzed for N-tail translocation using the protease mapping assay described in the legend to Fig. 2. A, for the aspartic acid scanning study, the plasmids encoded the protein Pf3-aTM D23A, D23I, T19D, F27D, L31D, F35D, or F39D. The negatively charged mutants were made using Pf3-aTM D23A as a template. B, for the arginine scanning study, JS7131 expressing different plasmids was grown under YidC plus or YidC minus conditions, labeled with [35S]methionine, and analyzed using the protease-mapping assay, as described under “Experimental Procedures.” The plasmids expressed the proteins Pf3-aTM T19R, D23R, F27R, L31R, F35R, D23K, and F27K. Except for the D23R and D23K constructs, these mutants were made in the D23A background. C, for the asparagine scanning study, JS7131 expressing different plasmids was analyzed by protease mapping, as described in the legend to Fig. 2. The plasmids expressed the proteins Pf3-aTM D23N, F27N, L31N, and F35N. Except for the D23N construct, the mutants were made in the D23A background. Representative OmpA data are shown at the bottom of A–C.
We next investigated whether the position of a negatively charged residue in the TM segment is important for YidC dependence. Starting with the Pf3-aTM(D23A) construct, we introduced an Asp at position 19, 27, 31, 35, or 39 within the TM segment (Fig. 5A). In all cases, membrane insertion was YidC-dependent (Fig. 5A; compare YidC− and YidC+ conditions). None of the constructs with negative charges were digested by protK under YidC depletion conditions, indicating that the position of the negatively charged amino acid is not a YidC determinant.
Possible explanations for the effect of Asp in the TM segment are that YidC dependence is determined by the sign of the charge or by the overall hydrophobicity of the segment. To test these possibilities, we performed arginine-scanning (Fig. 5B) and asparagine-scanning mutagenesis (Fig. 5C) within the TM segment of F0a. Fig. 5B shows that the Pf3-aTM mutants with arginine at position 19, 23, 27, 31, or 35 within the TM segment are YidC-dependent for insertion. These constructs are strongly resistant to protK digestion, demonstrating that YidC is strictly required for N-tail translocation when a positively charged residue is introduced into the TM segment. We also tested whether lysine substituted in the TM segment would also cause a change in YidC dependence. As can be seen, both Pf3-aTM(D23K) and Pf3-aTM(F27K) are YidC-dependent for insertion (Fig. 5B). Therefore, the nature of the positive charge does not seem to be important for determining YidC dependence. Similar results are found for the asparagine positional scanning mutants (Fig. 5C). No shifted band is observed upon the addition of protK to the 35S-labeled cells under YidC− conditions (Fig. 5C), but the asparagine mutants are inserted efficiently when YidC is expressed. Taken together, the results suggest that YidC is needed to insert a membrane protein when the polarity of the TM segment is increased with charged or very polar residues. This conclusion is generally supported by the hydrophobicity values of the TM segments shown in Table 1 that were computed using the translocon-based apparent free energy (ΔGapp) scale (44). Except for the T19R, the ΔGapp values are more positive (less hydrophobic) relative to the D23A mutant.
TABLE 1.
The ΔGapp (kcal mol−1) of the TM domain of the Pf3-aTM constructs
The TM domain hydrophobicity ΔGapp (kcal mol−1) of Pf3-aTM constructs were calculated using the D23A mutant as a basis (ΔGapp of *D23A mutant = −1.17). ΔGapp is the computed free energy of a TM segment from a Sec61 translocon to a dog pancreas microsomal membrane as described (44, 55) (reviewed in Ref. 19). The free energy values of individual amino acids depend upon their positions in the TM helix. Arg, Lys, Asp, and Glu are strongly position-dependent; they affect insertion marginally near helix ends but have a big effect near helix centers. The position dependence is apparent in the values listed (e.g. compare F35D and F39D). The values in the table were computed with the totalizer module of MPEx (51) using the translocon hydrophobicity values (44).
| Asp mutants | ΔGapp | Arg mutants | ΔGapp | Lys mutants | ΔGapp | Asn mutants | ΔGapp |
|---|---|---|---|---|---|---|---|
| T19D | −0.87 | T19R | −1.17 | ||||
| D23Ia | −1.42 | D23R | −0.93 | D23K | −0.46 | D23N | −0.43 |
| F27D | +0.81 | F27R | +0.48 | F27K | +0.84 | F27N | +0.37 |
| L31D | +1.28 | L31R | +0.91 | L31N | +0.79 | ||
| F35D | +0.52 | F35R | −0.36 | F35N | +0.42 | ||
| F39D | −0.29 |
a Insertion independent of YidC. All other TM segments require YidC.
The Addition of a Positively Charged Residue to the TM Segment Switches Insertion from YidC/Sec-independent to YidC/Sec-dependent
Because both the Sec translocase and YidC are required to translocate an N-tail region with a positive charge, we next tested whether the Sec machinery is also required for membrane insertion when a positively charged residue is added to the TM segment. Fig. 6A shows that membrane insertion of Pf3-LepTM(A23R) with arginine substituted for aspartate at position 23 is strongly inhibited when SecE is depleted, whereas Pf3-LepTM(A23I) and Pf3-LepTM(A23D) insert efficiently, suggesting that the Sec machinery is only needed for insertion of the LepTM when a positive charge is present. To confirm this idea, we examined further the effect of a negatively charged residue on Sec dependence. Insertion of Pf3-aTM with a negatively charged residue at position 19 (T19D) or 23 (D23) used a Sec-independent mechanism (Fig. 6B). However, the F27D, L31D, and F35D variants were slightly Sec-dependent (Fig. 6B). As a control, we confirmed that the Sec-dependent OmpA is blocked under SecE-depleted conditions but fully translocated in SecE expression conditions. The combined study shows that the Sec translocase is strictly required for insertion of TM segments possessing a positively charge residue but generally is not needed for efficient insertion of TM segments with negatively charged residues.
FIGURE 6.
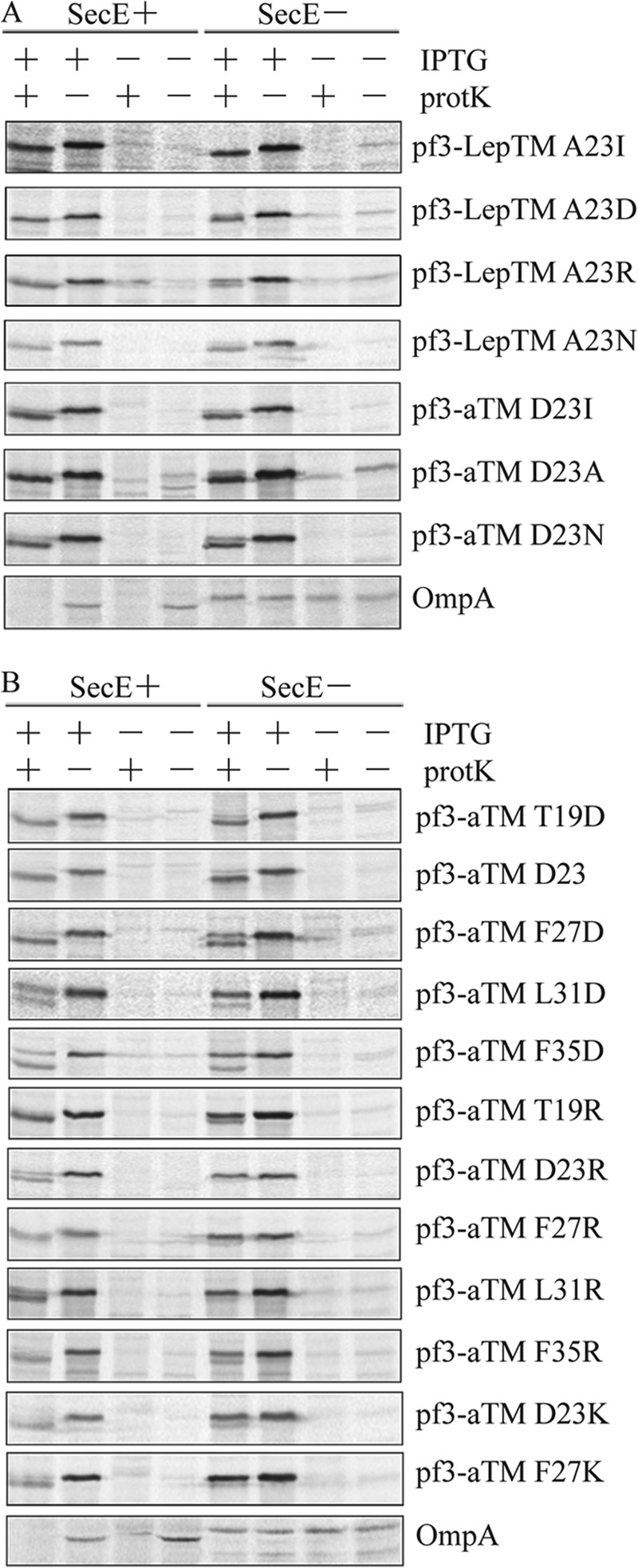
Sec-dependent insertion with an arginine in the TM segment. A, E. coli CM124 cells bearing different plasmids were analyzed for translocation of the N-tail as described in the legend to Fig. 2. The plasmids encoded the protein Pf3-LepTM A23I, A23D, A23R, or A23N or Pf3-aTM D23I, D23A, or D23N. B, for the positional scanning study, E. coli CM124 cells bearing different plasmids were analyzed as described in the legend to Fig. 2. The plasmids expressed Pf3-aTM T19D, D23, F27D, L31D, F35D, T19R, D23R, F27R, L31R, F35R, D23K, and F27K. The negatively and positively charged mutants were made using Pf3-aTM D23A as a template. Representative OmpA data are shown at the bottom of A and B.
Similar Sec-independent insertion results were found with Pf3-aTM when the aspartic acid at position 23 within the aTM domain was replaced with a hydrophobic or neutral polar residue as shown in Fig. 6A for Pf3-aTM(D23I), Pf3-aTM(D23A), and Pf3-aTM(D23N). As a control, we demonstrated that OmpA is translocated under SecE+ conditions while blocked under SecE− conditions. Taken together with the YidC− data (Fig. 5), these results show that Pf3-aTM inserts in a YidC/Sec-independent manner when the TM segment is more hydrophobic, as with D23I or D23A substitution, whereas YidC alone is required with a neutral polar residue as with D23N substitution.
Strikingly, in every case, the Sec translocase is required to insert Pf3-aTM with a positively charged residue introduced within the TM segment. Insertion of the positively charged mutants (T19R, D23R, F27R, L31R, and F35R) was strongly inhibited under SecE depletion conditions (Fig. 6B), showing that the effect of the positively charged residue on Sec dependence is independent of its position in the TM segment. Consistent with this result, we found that insertion of Pf3-aTM(D23K) and Pf3-aTM(F27K) was Sec-dependent (Fig. 6B). Thus, the insertion of TM segments with either a lysine or arginine requires both the Sec machinery and the YidC insertase.
Proton Motive Force Is Generally Required for the YidC-dependent Proteins with Negatively Charged Residues in the Tail
The inner membrane pmf has been shown to be an important driving force for membrane protein translocation for some but not all proteins. For example, the inner membrane pmf is required for the translocation of the N-tail of YidC-dependent Pf3 coat (45) and F0a (14) but is not required for translocation of the amino terminus of the YidC-independent Pf3-Lep (39). To test the pmf requirements of our model single-span proteins, cells expressing various constructs were induced with IPTG for 3 min and then treated with a 50 μm concentration of the proton uncoupler CCCP for 45 s to dissipate the pmf prior to labeling the protein for 1 min. Fig. 7 shows that the YidC-dependent constructs a-aTM, a-LepTM, Pf3-aTM, Pf3-LepTM(V4E), Pf3-LepTM(V15D), and Pf3-LepTM(V4E/V15D), all with acidic tails, are pmf-dependent for insertion. However, pmf-independent insertion was observed with YidC-dependent Pf3-LepTM, which also contains an acidic tail (Fig. 7A).
FIGURE 7.
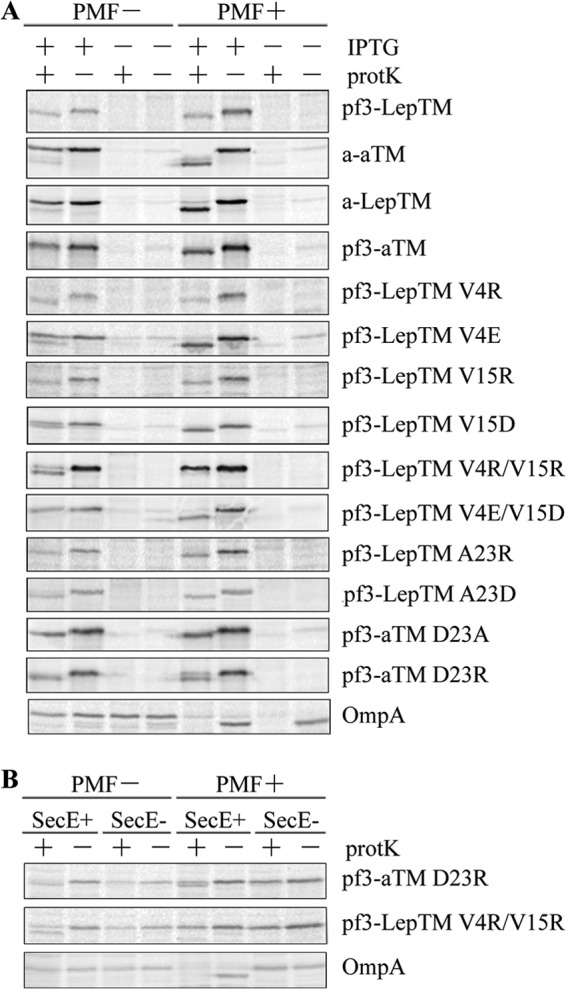
The pmf is required for insertion of YidC-dependent constructs that contain negative charges in the tail. A, cells in the exponential growth phase were labeled with [35S]methionine for 1 min and analyzed by protease mapping, as described under “Experimental Procedures.” Where indicated, samples were treated with CCCP (50 μm final concentration) for 45 s prior to adding [35S]methionine and analyzed by protease mapping as before. The plasmids expressed the YidC-dependent protein a-aTM, a-LepTM, Pf3-aTM, Pf3-LepTM V4R, Pf3-LepTM V4E, Pf3-LepTM V15R, Pf3-LepTM V15D, Pf3-LepTM V4R/V15R, Pf3-LepTM V4E/V15D, Pf3-LepTM A23R, Pf3-LepTM A23D, or Pf3-aTM D23R, or the plasmids expressed the YidC-independent protein Pf3-LepTM or Pf3-aTM D23A. B, E. coli CM124 cells containing the vector encoding Pf3-aTM D23R or Pf3-LepTM V4R/V15R were analyzed by the protease accessibility assay. CM124 cells grown under SecE+ and SecE− conditions were treated with CCCP (50 μm final concentration) for 45 s prior to pulse labeling to dissipate the pmf. Protease accessibility was analyzed as described in the legend to Fig. 2. The Sec-dependent protein OmpA is shown as a control.
In contrast, the pmf is not required for insertion for the YidC-dependent Pf3-LepTM(V4R) and Pf3-LepTM(V15R) that have a positively charged residue added to the N-tail region. Strikingly, the pmf can even impede the translocation of positively charged residues; the double arginine mutant Pf3-Lep(V4R/V15R), which is not inserted in the presence of the pmf, can insert when the pmf is collapsed (Fig. 7A). This result reinforces the idea that the pmf impedes the translocation of positively charged residues, as was demonstrated with M13 procoat mutants (46). To test whether the pmf inhibits or is not required for insertion of positive charges in the TM segment, we investigated Pf3-LepTM(A23R) and Pf3-aTM(D23R). Whereas the Pf3-aTM(D23R) mutant inserted better in the absence of the pmf, the YidC-dependent protein substrate Pf3-LepTM(A23R) inserted into the membrane independent of the pmf. These results show that the YidC dependence is not directly linked to the pmf dependence. In all cases, we found that the pmf-dependent OmpA is blocked in membrane insertion when the pmf is dissipated; pro-OmpA accumulates and is not digested by protK. The YidC/Sec-independent constructs Pf3-LepTM and Pf3-aTM(D23A) with “hydrophobic” TM segments insert independent of the pmf. The data of Fig. 7A suggest that the pmf can facilitate the translocation of a negatively charged residue and can inhibit the translocation of a positive residue.
Another explanation for the Sec dependence of positively charged mutant translocation is that the pmf inhibits insertion of the positively charged mutants by the YidC pathway and therefore requires the SecYEG channel for insertion. If this were the case, it is possible that insertion of the positively charged mutant would occur independent of the Sec translocase if the pmf were abolished. To test this hypothesis, we examined the Sec dependence of membrane insertion of Pf3-aTM(D23R) and Pf3-LepTM(V4R/V15R) when the pmf was abolished. As can be seen from Fig. 7B, the D23R mutant is still Sec-dependent when the pmf is dissipated. The Pf3-aTM(D23R), with a positively charged residue in the TM segment, inserts more efficiently under SecE+/pmf− conditions compared with the SecE+/pmf+ conditions, as seen in Fig. 7B. However, even in the absence of the pmf, insertion is Sec-dependent. For Pf3-Lep(V4R/V15R) that has positively charged residues at the periplasmic tail, insertion cannot occur by a Sec-independent process when the pmf is abolished (Fig. 7B). Whereas insertion occurs under SecE+/pmf− conditions (Fig. 7B), insertion is blocked under SecE−/pmf− conditions, showing that translocation of the positively charged residues cannot occur by the YidC-only pathway and that the pmf inhibits Sec-dependent translocation. These results rule out the possibility that the membrane potential (Δψ) could inhibit the translocation of positively charged residues by the YidC-only pathway and therefore make the protein Sec dependent for insertion.
Positively Charged Residues in the Translocated Domains of Endogenous Proteins Can Function as Sec Determinants
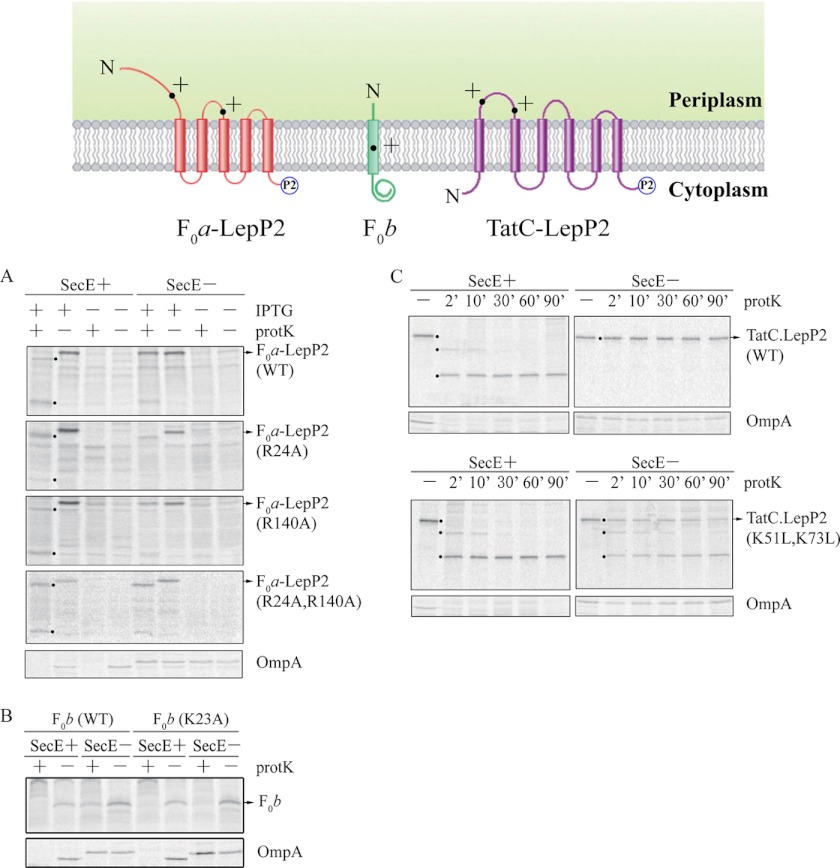
Do the rules observed for our single-span model membrane proteins apply to endogenous proteins? Previously, Yi et al. studied extensively the insertase requirements of subunits a (F0a) and b (F0b) of E. coli ATP synthase (13, 14). They found that both proteins required YidC and the Sec translocase for insertion. F0a spans the membrane five times with its N-tail periplasmic and its C terminus cytoplasmic (14, 47); F0b spans the membrane once with its N-tail periplasmic (48). The periplasmic N-tail and periplasmic loop P2 of F0a each contain a single Arg, which allowed us to test the hypothesis that positive charge is a determinant for translocation by SecYEG. Subunit b has a single TM helix at the N terminus with a single Lys residue that is predicted to be within the TM segment. Subunit b thus provides an opportunity to test the hypothesis that the Sec machinery is only needed for insertion of a TM segment when it contains a positive charge. In addition, the TatC protein (49, 50) with six TM segments (N and C termini in the cytoplasm) also provides an opportunity of testing the rules for pathway selection because of positively charged residues in periplasmic loop P1.
We made several mutants of F0a, which has one arginine (Arg-24) located in the 39-residue N-tail and one (Arg-140) in the 10-residue P2 loop. Translocation of the N-tail and the P2 loop can be monitored by protK digestion, whereas translocation of the P3 loop cannot because it is protease-inaccessible (14). A tag LepP2 consisting of 103 residues of the P2 domain of leader peptidase was added at the C terminus of the protein so that the full-length protein and digested C-terminal fragments could be detected by immunoprecipitation. To determine whether the Arg residues were Sec determinants, we examined the translocation of the N-tail and the second periplasmic loop of F0a-LepP2 under SecE+ and SecE− conditions. ProtK digestion of the F0a-LepP2 wild type under SecE+ conditions gave rise to two shifted bands. The higher shifted fragment corresponds to digestion of the tail region, whereas the lower shifted fragment corresponds to digestion of the periplasmic loop 2 (see Fig. 8A, top) (14). Under SecE− conditions, the amounts of shifted bands were markedly decreased, and the full-length protein was protected from protK digestion, confirming that translocation of the N-tail and the periplasmic loop 2 is Sec-dependent.
FIGURE 8.
Positively charged residues can function as Sec determinants for endogenous membrane protein. The top shows the membrane topology of F0a-LepP2, F0b, and TatC-LepP2. A, E. coli CM124 cells bearing F0a-LepP2 WT, R24A, R140A, or the double R24A/R140A mutant were analyzed for insertion using the protease accessibility assay as described in the legend to Fig. 2. Samples were immunoprecipitated with Lep or OmpA antiserum and analyzed by SDS-PAGE and phosphorimaging. The higher shifted fragment results from protK digestion within the N-tail, whereas the lower shifted fragment corresponds to protK digestion within the periplasmic 2 loop (see Ref. 14). B, E. coli CM124 cells bearing the subunit b derivative (F0b, with a t7 tag at the N terminus of F0b) of F1F0-ATPase were examined by protease accessibility assay as described in the legend to Fig. 2. The protein samples were immunoprecipitated with T7 monoclonal antibody (purchased from Invitrogen) or OmpA antiserum and then analyzed by Tricine-SDS-PAGE or SDS-PAGE, respectively. The band corresponds to the full-length F0b protein. The translocated T7 tag of F0b will be digested by protK and therefore it will not be immunoprecipitated by the T7 monoclonal antibody. C, E. coli CM124 cells bearing TatC-LepP2 proteins (WT or K51L/K73L) were analyzed for insertion by protK digestion for various time periods (min), as described in the legend to Fig. 2. Samples were immunoprecipitated with Lep or OmpA antiserum and analyzed by SDS-PAGE and phosphorimaging. The higher shifted fragment results from the cleavage by protK at the first periplasmic loop of TatC-LepP2, whereas the lower shifted fragment is due to the cleavage by protK within the second periplasmic loop of TatC-LepP2. However, the third periplasmic loop is not proteinase-accessible.
To test whether Arg-24 is a Sec determinant for N-tail translocation, we mutated it to Ala. If positive charges are Sec determinants, then the N-tail of the R24A construct should insert even under SecE− conditions. Fig. 8A shows that the full-length F0a-LepP2(R24A) is completely digested under SecE depletion conditions, consistent with the loss of Sec dependence of N-tail translocation. The amount of the higher shifted band is similar to that produced under SecE+ conditions, whereas very little of the lower shifted band is seen, because the periplasmic loop 2 presumably remains Sec-dependent. The dependence of periplasmic loop 2 on Sec was confirmed using a R140A mutant. In this case, the lower molecular weight band was detected, whereas the larger molecular weight fragment band was not. Finally, the F0a-LepP2(R24A/R140A) double mutant was found to be completely Sec-independent; bands with shifted fragments are identical for SecE+ and SecE− (Fig. 8A). The results show that positive charges in the translocated regions of a protein with five TM segments are Sec substrate determinants, as predicted by the results from the single-span model proteins.
We then used F0b to test the hypothesis that a positive charge in a TM segment is a Sec substrate determinant. Previously, Yi et al. (14) showed that F0b with a T7 tag (MASMTGGQQMG) at the N terminus requires YidC for insertion and that SecDF is needed for efficient insertion, suggesting that F0b inserts by the YidC/Sec mechanism. Excluding the T7 tag, MPEx (51) using the translocon scale predicts residues Ala-5 through Trp-26 of the N-terminal hydrophobic stretch to be a TM helix containing a Lys at position 23. To determine if Lys-23 is a Sec substrate determinant, we used a F0b(K23A) mutant. Translocation of the N-terminal T7 was assayed using protK digestion and immunoprecipitation with T7 antiserum. Fig. 8B shows that the WT protein with the T7 tag is Sec-dependent for insertion, because membrane insertion is inhibited in the SecE depletion strain, whereas it inserts efficiently under SecE expression conditions. In contrast, the N-tail of F0b(K23A) translocates across the membrane even under SecE depletion conditions, consistent with Lys-23 being a Sec substrate determinant.
Finally, we examined the Sec substrate determinants for TatC, which contains two positively charge residues (Lys-51 and Lys-73) in periplasmic loop 1. For determination of Sec dependence, we used TatC-LepP2 to allow precipitation of the protein using leader peptidase antiserum. Periplasmic loops 1 and 2 are sensitive to protK digestion, whereas loop P3 is protease-resistant (33). Digestion only at the P1 loop leads to the production of a higher molecular weight fragment, whereas digestion at the P2 loop leads to the smaller protease-resistant fragment (Fig. 8C, top left, see 2 min protK time point). Fig. 8C shows that TatC-LepP2 (WT) is strongly inhibited under SecE depletion conditions but not under SecE expression conditions. The TatC-LepP2 (WT) is fully digested by protK after 10 min or longer times. Remarkably, although the wild-type TatC-LepP2 and TatC-LepP2(K51L/K73L) are fully inserted under SecE expression conditions, the mutant inserts more efficiently than the wild-type protein under SecE depletion conditions. In addition to the major fragment (digested by protK at periplasmic loop 2), the larger fragment (digested by protK at periplasmic loop 1) can also be seen at the 2 min, 10 min, and 30 min time points in both SecE+ and SecE− conditions. These results show that both periplasmic loop 1 and loop 2 of TatC-LepP2(K51L/K73L) are translocated efficiently under SecE− conditions, consistent with the F0a and F0b results. We conclude that positively charged residues can function as Sec determinants, even for endogenous membrane proteins.
DISCUSSION
We studied the translocase requirements of four single-span model membrane proteins (Fig. 1A) based upon N-terminal fusions of a periplasmic N-tail and a TM domain to the N terminus of the cytoplasmic loop preceding TM2 helix 2 of E. coli leader peptidase (called TM2-Lep). An Arg residue at the C terminus of TM2 prevented insertion of TM2 into the inner membrane and consequently translocation of the leader peptidase catalytic domain (see Refs. 36 and 37). We eliminated the possibility that the presence of the Lep TM2 in the models created a “frustrated” topology problem (52) that alters the translocase requirements of model proteins with charged residues by repeating key experiments using constructs lacking TM2 (supplemental Fig. S1). To examine sequence features that determine which translocase pathway the proteins used for insertion, we made mutations within the N-tail or the TM domain of each model protein.
We made two important observations about the role of charged residues. First, a positively or negatively charged residue added to the N-tail region of a model membrane protein can cause membrane insertion to be YidC-dependent. Second, a positively charged residue added to the N-tail or to the TM segment leads to insertion via by the YidC/Sec pathway, indicating that positively charged residues can act as a YidC/Sec determinant. This conclusion differs from that of Price and Driessen (4), who showed that only negatively charged residues in TM segments of multispanning membrane proteins are YidC determinants. We found in every case, with no exceptions, that the model single-span proteins become YidC-dependent when a negative charge or a positive charge is added to either a translocated region or a TM segment. Gray et al. (5) observed that YidC is required for proteins that have a topology with an unfavorable positive inside rule. In contrast, for membrane proteins with a favorable positive inside rule, our results show that the YidC requirement is due to an added positive or negative charge.
Our results reinforce the important role of hydrophobicity in insertion pathway selection. Previously, Ernst et al. (3) showed that insertion of two hydrophobic residues into the TM segment allows the Pf3 coat protein to insert into the membrane in a YidC-independent manner, suggesting that YidC is required to insert a TM segment with low hydrophobicity. By making substitutions within a TM segment of fixed length by scanning mutagenesis, using aspartic acid, arginine, and asparagine, we found that the YidC requirement correlates with a decreased hydrophobicity of a TM segment, which strengthens the previous findings (3).
Neugebauer et al. (6) showed that adding an increasing number of negatively charged residues to the periplasmic loop of MscL, which spans the membrane twice with N and C termini in the cytoplasm, caused the protein to become progressively more dependent on SecYEG for membrane insertion. All of our single-span model proteins have negatively charged N termini located in the periplasm, yet their translocase requirements are quite variable (Fig. 1A). Our results show that only when a positively charged residue is added to the N-tail or TM segment does the protein become Sec-dependent Figs. 4 and 6. The Sec dependence of MscL with added positive charges was not investigated.
The observation that the positively charged residues in the N-tails (Fig. 4) were an important determinant of translocation requirements led us to examine more closely the role of positively charged residues in the TM segments. We found that SecYEG is strictly required along with YidC for the insertion of proteins with a positively charged residue in the TM segment but is not required with negatively charged residues (Fig. 6). However, YidC alone suffices for insertion with negatively charged residues. It is possible that YidC is sufficient to translocate negative charges, because the pmf also contributes to the translocation process for negative charges. However, YidC by itself is insufficient to translocate positive charges, because the pmf impedes translocation for positive charges (Fig. 7). This led us to test if translocation of positive charges could occur in a Sec-independent process when the pmf is abolished. Experiments using CCCP to discharge the pmf revealed that, even in the absence of a pmf, the insertion of a positively charged TM segment is SecYEG-dependent. Therefore, translocation of positively charged residues cannot occur by a YidC-only pathway even in the absence of the pmf.
Because of the possibility that the targeting pathway might be different among the four model single-span proteins and the possibility that the different translocase requirements of the positively and negatively charged constructs might have to do with the nature of membrane targeting, we confirmed that the four model proteins (Fig. 2A) and key charged constructs (supplemental Fig. S2B) are SRP-dependent for insertion. This shows that all of the proteins are delivered by the same targeting mechanism. Moreover, we confirmed that SecA (supplemental Fig. S2A) facilitates membrane insertion to a small extent only for the SecE-dependent constructs.
Our studies of model membrane proteins clarified the translocase requirements of single-span membrane proteins. An important question was whether natural endogenous proteins follow the same rules. To examine this question, we used site-directed mutagenesis to determine the effects of positively charged residues on Sec dependence using subunit a and subunit b of ATP synthase (F0a and F0b), and TatC. F0a spans the membrane five times with the N terminus periplasmic and the C terminus cytoplasmic (14, 47); F0b spans the membrane once with N-tail periplasmic (48), and TatC spans the membrane six times with N and C termini in the cytoplasm (49, 50). For the F0a protein, we found that the arginines in the N-tail and periplasmic loop 2, respectively, make F0a require SecYEG for insertion (Fig. 8A). In the case of F0b, the Sec substrate determinant involves the positively charged residue in the TM segment, because removal of the positively charged residue results in Sec-independent insertion. Finally, the lysines within the periplasmic loop1 of TatC are Sec-dependent substrate determinants. These results indicate that positive charges are Sec determinants for endogenous proteins that span the membrane once or multiple times.
Recently it was shown by in vitro experiments that multispan membrane proteins can insert either by the YidC-only pathway or by the SecYEG pathway (53). The authors suggested that the SecYEG-associated function of YidC is not essential for membrane insertion of TatC and mannitol permease. Our data showing that both YidC and the Sec translocase are strictly required for insertion of the positively charged mutants (Figs. 4 and 6) clearly show that a Sec/YidC pathway exists in bacteria.
We hypothesize that a membrane protein with a highly hydrophobic TM segment has the capability to insert by an autonomous (Sec-independent/YidC-independent) pathway because the hydrophobic force of TM1 is sufficient to drive membrane translocation across the membrane. Consistent with this hypothesis, Ulmschneider et al. (54) have shown by microsecond scale molecular dynamics simulations that polyleucine transmembrane segments with eight or more leucines can insert spontaneously. Another possibility with the highly hydrophobic TM segment is that the protein can use either the YidC-only or the Sec pathway. This would explain why, when YidC is depleted, insertion is not affected because it can go by the SecYEG pathway or, when SecE is depleted, the protein is inserted by the YidC insertase. The only way to distinguish between these possibilities would be to make the YidC/SecYEG double depletion strain, which would be extremely challenging.
We have shown for the first time that positively charged residues are Sec determinants for endogenous proteins as well as single-span membrane proteins (Figs. 4, 6, and 8). Evidently, both YidC and SecYEG must be surveying the elongating chain and acting cooperatively as the chain passes across the membrane. However, why both YidC and SecYEG are required for insertion of the positively charged mutants and YidC on its own can insert the negatively charged mutants is not clear. This is difficult to answer because we do not know how YidC and SecYEG talk with each other, and we do not have a YidC structure.
This work was supported, in whole or in part, by National Institutes of Health Grant GM074637 (to S. H. W.). This work was also supported by National Science Foundation Grant MCB-1052033 (to R. E. D.).

This article contains supplemental Figs. S1 and S2.
- TM
- transmembrane
- pmf
- proton motive force
- CCCP
- carbonyl cyanide m-chlorophenylhydrazone
- protK
- proteinase K
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- Lep
- leader peptidase
- OmpA
- outer membrane protein A
- SRP
- signal recognition particle
- Tricine
- N-[2-hydroxy-1,1-bis(hydroxymethyl)ethyl]glycine.
REFERENCES
- 1. Dalbey R. E., Wang P., Kuhn A. (2011) Assembly of bacterial inner membrane proteins. Annu. Rev. Biochem. 80, 161–187 [DOI] [PubMed] [Google Scholar]
- 2. Facey S. J., Kuhn A. (2003) The sensor protein KdpD inserts into the Escherichia coli membrane independent of the Sec translocase and YidC. Eur. J. Biochem. 270, 1724–1734 [DOI] [PubMed] [Google Scholar]
- 3. Ernst S., Schönbauer A. K., Bär G., Börsch M., Kuhn A. (2011) YidC-driven membrane insertion of single fluorescent Pf3 coat proteins. J. Mol. Biol. 412, 165–175 [DOI] [PubMed] [Google Scholar]
- 4. Price C. E., Driessen A. J. (2010) Conserved negative charges in the transmembrane segments of subunit K of the NADH:ubiquinone oxidoreductase determine its dependence on YidC for membrane insertion. J. Biol. Chem. 285, 3575–3581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gray A. N., Henderson-Frost J. M., Boyd D., Shirafi S., Niki H., Goldberg M. B. (2011) Unbalanced charge distribution as a determinant for dependence of a subset of Escherichia coli membrane proteins on the membrane insertase YidC. MBio 2, e00238–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Neugebauer S. A., Baulig A., Kuhn A., Facey S. J. (2012) Membrane protein insertion of variant MscL proteins occurs at YidC and SecYEG of Escherichia coli. J. Mol. Biol. 417, 375–386 [DOI] [PubMed] [Google Scholar]
- 7. Driessen A. J., Nouwen N. (2008) Protein translocation across the bacterial cytoplasmic membrane. Annu. Rev. Biochem. 77, 643–667 [DOI] [PubMed] [Google Scholar]
- 8. Papanikou E., Karamanou S., Economou A. (2007) Bacterial protein secretion through the translocase nanomachine. Nat. Rev. Microbiol. 5, 839–851 [DOI] [PubMed] [Google Scholar]
- 9. Pogliano J. A., Beckwith J. (1994) SecD and SecF facilitate protein export in Escherichia coli. EMBO J. 13, 554–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen M., Xie K., Yuan J., Yi L., Facey S. J., Pradel N., Wu L. F., Kuhn A., Dalbey R. E. (2005) Involvement of SecDF and YidC in the membrane insertion of M13 procoat mutants. Biochemistry 44, 10741–10749 [DOI] [PubMed] [Google Scholar]
- 11. Tsukazaki T., Mori H., Echizen Y., Ishitani R., Fukai S., Tanaka T., Perederina A., Vassylyev D. G., Kohno T., Maturana A. D., Ito K., Nureki O. (2011) Structure and function of a membrane component SecDF that enhances protein export. Nature 474, 235–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nouwen N., Driessen A. J. (2002) SecDFyajC forms a heterotetrameric complex with YidC. Mol. Microbiol. 44, 1397–1405 [DOI] [PubMed] [Google Scholar]
- 13. Yi L., Jiang F., Chen M., Cain B., Bolhuis A., Dalbey R. E. (2003) YidC is strictly required for membrane insertion of subunits a and c of the F1F0-ATP synthase and SecE of the SecYEG translocase. Biochemistry 42, 10537–10544 [DOI] [PubMed] [Google Scholar]
- 14. Yi L., Celebi N., Chen M., Dalbey R. E. (2004) Sec/SRP requirements and energetics of membrane insertion of subunits a, b, and c of the Escherichia coli F1F0 ATP synthase. J. Biol. Chem. 279, 39260–39267 [DOI] [PubMed] [Google Scholar]
- 15. Kol S., Majczak W., Heerlien R., van der Berg J. P., Nouwen N., Driessen A. J. (2009) Subunit a of the F1F0 ATP synthase requires YidC and SecYEG for membrane insertion. J. Mol. Biol. 390, 893–901 [DOI] [PubMed] [Google Scholar]
- 16. Celebi N., Yi L., Facey S. J., Kuhn A., Dalbey R. E. (2006) Membrane biogenesis of subunit II of cytochrome bo oxidase. Contrasting requirements for insertion of N-terminal and C-terminal domains. J. Mol. Biol. 357, 1428–1436 [DOI] [PubMed] [Google Scholar]
- 17. van Bloois E., Haan G. J., de Gier J. W., Oudega B., Luirink J. (2006) Distinct requirements for translocation of the N-tail and C-tail of the Escherichia coli inner membrane protein CyoA. J. Biol. Chem. 281, 10002–10009 [DOI] [PubMed] [Google Scholar]
- 18. du Plessis D. J., Nouwen N., Driessen A. J. (2006) Subunit a of cytochrome o oxidase requires both YidC and SecYEG for membrane insertion. J. Biol. Chem. 281, 12248–12252 [DOI] [PubMed] [Google Scholar]
- 19. White S. H., von Heijne G. (2008) How translocons select transmembrane helices. Annu. Rev. Biophys. 37, 23–42 [DOI] [PubMed] [Google Scholar]
- 20. Nagamori S., Smirnova I. N., Kaback H. R. (2004) Role of YidC in folding of polytopic membrane proteins. J. Cell Biol. 165, 53–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wagner S., Pop O. I., Haan G. J., Baars L., Koningstein G., Klepsch M. M., Genevaux P., Luirink J., de Gier J. W. (2008) Biogenesis of MalF and the MalFGK2 maltose transport complex in Escherichia coli requires YidC. J. Biol. Chem. 283, 17881–17890 [DOI] [PubMed] [Google Scholar]
- 22. Scotti P. A., Urbanus M. L., Brunner J., de Gier J. W., von Heijne G., van der Does C., Driessen A. J., Oudega B., Luirink J. (2000) YidC, the Escherichia coli homologue of mitochondrial Oxa1p, is a component of the Sec translocase. EMBO J. 19, 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Urbanus M. L., Fröderberg L., Drew D., Björk P., de Gier J. W., Brunner J., Oudega B., Luirink J. (2002) Targeting, insertion, and localization of Escherichia coli YidC. J. Biol. Chem. 277, 12718–12723 [DOI] [PubMed] [Google Scholar]
- 24. van Bloois E., Jan Haan G., de Gier J. W., Oudega B., Luirink J. (2004) F1F0 ATP synthase subunit c is targeted by the SRP to YidC in the E. coli inner membrane. FEBS Lett. 576, 97–100 [DOI] [PubMed] [Google Scholar]
- 25. van der Laan M., Bechtluft P., Kol S., Nouwen N., Driessen A. J. (2004) F1F0 ATP synthase subunit c is a substrate of the novel YidC pathway for membrane protein biogenesis. J. Cell Biol. 165, 213–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Facey S. J., Neugebauer S. A., Krauss S., Kuhn A. (2007) The mechanosensitive channel protein MscL is targeted by the SRP to the novel YidC membrane insertion pathway of Escherichia coli. J. Mol. Biol. 365, 995–1004 [DOI] [PubMed] [Google Scholar]
- 27. Samuelson J. C., Chen M., Jiang F., Möller I., Wiedmann M., Kuhn A., Phillips G. J., Dalbey R. E. (2000) YidC mediates membrane protein insertion in bacteria. Nature 406, 637–641 [DOI] [PubMed] [Google Scholar]
- 28. Samuelson J. C., Jiang F., Yi L., Chen M., de Gier J. W. (2001) Function of YidC for the insertion of M13 procoat protein in E. coli. Translocation of mutants that show differences in their membrane potential dependence and Sec requirement. J. Biol. Chem. 276, 34847–34852 [DOI] [PubMed] [Google Scholar]
- 29. Chen M., Samuelson J. C., Jiang F., Muller M., Kuhn A., Dalbey R. E. (2002) Direct interaction of YidC with the Sec-independent Pf3 coat protein during its membrane protein insertion. J. Biol. Chem. 277, 7670–7675 [DOI] [PubMed] [Google Scholar]
- 30. Gallusser A., Kuhn A. (1990) Initial steps in protein membrane insertion. Bacteriophage M13 procoat protein binds to the membrane surface by electrostatic interaction. EMBO J. 9, 2723–2729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Serek J., Bauer-Manz G., Struhalla G., van den Berg L., Kiefer D. (2004) Escherichia coli YidC is a membrane insertase for Sec-independent proteins. EMBO J. 23, 294–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maniatis T., Fritsch E. F., Sambrook J. (1982) Molecular Cloning: A Laboratory Manual, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 33. Zhu L., Klenner C., Kuhn A., Dalbey R. E. (2012) Both YidC and SecYEG are required for translocation of the periplasmic loops 1 and 2 of the multispanning membrane protein TatC. J. Mol. Biol. 424, 354–367 [DOI] [PubMed] [Google Scholar]
- 34. Traxler B., Murphy C. (1996) Insertion of the polytopic membrane protein MalF is dependent on the bacterial secretion machinery. J. Biol. Chem. 271, 12394–12400 [DOI] [PubMed] [Google Scholar]
- 35. Miller J. H. (1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 36. Delgado-Partin V. M., Dalbey R. E. (1998) The proton motive force, acting on acidic residues, promotes translocation of amino-terminal domains of membrane proteins when the hydrophobicity of the translocation signal is low. J. Biol. Chem. 273, 9927–9934 [DOI] [PubMed] [Google Scholar]
- 37. Cao G., Dalbey R. E. (1994) Translocation of N-terminal tails across the plasma membrane. EMBO J. 13, 4662–4669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dalbey R. E., Wickner W. (1986) The role of the polar, carboxyl-terminal domain of Escherichia coli leader peptidase in its translocation across the plasma membrane. J. Biol. Chem. 261, 13844–13849 [PubMed] [Google Scholar]
- 39. Lee J. I., Kuhn A., Dalbey R. E. (1992) Distinct domains of an oligotopic membrane protein are Sec-dependent and Sec-independent for membrane insertion. J. Biol. Chem. 267, 938–943 [PubMed] [Google Scholar]
- 40. Heijne G. (1986) The distribution of positively charged residues in bacterial inner membrane proteins correlates with the trans-membrane topology. EMBO J. 5, 3021–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. von Heijne G. (1989) Control of topology and mode of assembly of a polytopic membrane protein by positively charged residues. Nature 341, 456–458 [DOI] [PubMed] [Google Scholar]
- 42. Boyd D., Beckwith J. (1989) Positively charged amino acid residues can act as topogenic determinants in membrane proteins. Proc. Natl. Acad. Sci. U.S.A. 86, 9446–9450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Laws J. K., Dalbey R. E. (1989) Positive charges in the cytoplasmic domain of Escherichia coli leader peptidase prevent an apolar domain from functioning as a signal. EMBO J. 8, 2095–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hessa T., Meindl-Beinker N. M., Bernsel A., Kim H., Sato Y., Lerch-Bader M., Nilsson I., White S. H., von Heijne G. (2007) Molecular code for transmembrane-helix recognition by the Sec61 translocon. Nature 450, 1026–1030 [DOI] [PubMed] [Google Scholar]
- 45. Rohrer J., Kuhn A. (1990) The function of a leader peptide in translocating charged amino acyl residues across a membrane. Science 250, 1418–1421 [DOI] [PubMed] [Google Scholar]
- 46. Schuenemann T. A., Delgado-Nixon V. M., Dalbey R. E. (1999) Direct evidence that the proton motive force inhibits membrane translocation of positively charged residues within membrane proteins. J. Biol. Chem. 274, 6855–6864 [DOI] [PubMed] [Google Scholar]
- 47. Fillingame R. H., Jiang W., Dmitriev O. Y. (2000) Coupling H+ transport to rotary catalysis in F-type ATP synthases. Structure and organization of the transmembrane rotary motor. J. Exp. Biol. 203, 9–17 [DOI] [PubMed] [Google Scholar]
- 48. Steffens K., Schneider E., Deckers-Hebestreit G., Altendorf K. (1987) F0 portion of Escherichia coli ATP synthase. Further resolution of trypsin-generated fragments from subunit b. J. Biol. Chem. 262, 5866–5869 [PubMed] [Google Scholar]
- 49. Punginelli C., Maldonado B., Grahl S., Jack R., Alami M., Schröder J., Berks B. C., Palmer T. (2007) Cysteine scanning mutagenesis and topological mapping of the Escherichia coli twin-arginine translocase TatC component. J. Bacteriol. 189, 5482–5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Behrendt J., Standar K., Lindenstrauss U., Brüser T. (2004) Topological studies on the twin-arginine translocase component TatC. FEMS Microbiol. Lett. 234, 303–308 [DOI] [PubMed] [Google Scholar]
- 51. Snider C., Jayasinghe S., Hristova K., White S. H. (2009) MPEx. A tool for exploring membrane proteins. Protein Sci. 18, 2624–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gafvelin G., von Heijne G. (1994) Topological “frustration” in multispanning E. coli inner membrane proteins. Cell 77, 401–412 [DOI] [PubMed] [Google Scholar]
- 53. Welte T., Kudva R., Kuhn P., Sturm L., Braig D., Müller M., Warscheid B., Drepper F., Koch H. G. (2012) Promiscuous targeting of polytopic membrane proteins to SecYEG or YidC by the Escherichia coli signal recognition particle. Mol. Biol. Cell 23, 464–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ulmschneider J. P., Smith J. C., White S. H., Ulmschneider M. B. (2011) In silico partitioning and transmembrane insertion of hydrophobic peptides under equilibrium conditions. J. Am. Chem. Soc. 133, 15487–15495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hessa T., Kim H., Bihlmaier K., Lundin C., Boekel J. (2005) Recognition of transmembrane helices by the endoplasmic reticulum translocon. Nature 433, 377–381 [DOI] [PubMed] [Google Scholar]