Background: Tppp1 regulates microtubule dynamics and cell growth.
Results: Tppp1-mediated inhibition of the G1/S-phase and the mitosis to G1-phase transitions are relieved by Rock and Cdk1 phosphorylation.
Conclusion: Cell cycle-dependent Tppp1 phosphorylation regulates cell proliferation.
Significance: The newly discovered role of Tppp1 and its regulatory pathways in cell growth might have important implications in hyper-proliferative diseases.
Keywords: Cell Cycle, Cell Proliferation, Microtubules, Phosphorylation, Signal Transduction, Cyclin-dependent Kinase, Rho Kinase, TPPP1/p25
Abstract
Tubulin polymerization promoting protein 1 (Tppp1) regulates microtubule (MT) dynamics via promoting MT polymerization and inhibiting histone deacetylase 6 (Hdac6) activity to increase MT acetylation. Our results reveal that as a consequence, Tppp1 inhibits cell proliferation by delaying the G1/S-phase and the mitosis to G1-phase transitions. We show that phosphorylation of Tppp1 by Rho-associated coiled-coil kinase (Rock) prevents its Hdac6 inhibitory activity to enable cells to enter S-phase. Whereas, our analysis of the role of Tppp1 during mitosis revealed that inhibition of its MT polymerizing and Hdac6 regulatory activities were necessary for cells to re-enter the G1-phase. During this investigation, we also discovered that Tppp1 is a novel Cyclin B/Cdk1 (cyclin-dependent kinase) substrate and that Cdk phosphorylation of Tppp1 inhibits its MT polymerizing activity. Overall, our results show that dual Rock and Cdk phosphorylation of Tppp1 inhibits its regulation of the cell cycle to increase cell proliferation.
Introduction
Cell proliferation is a complex physiological process, which involves the concerted activation of multiple signaling pathways in response to intra- and extracellular cues. During cell proliferation, the microtubule (MT)3 network undergoes a dynamic reorganization, such as assembly of the mitotic spindle that provides the structural framework for the morphological changes necessary for cells to progress through the cell-cycle phases, to generate daughter cells.
A large number of proteins are dedicated to the regulation of the MT network. Tubulin polymerization promoting protein 1 (Tppp1) is a unique regulatory protein that modulates MT dynamics in two ways. First, it binds to tubulin heterodimers and promotes their incorporation into the growing microtubule filaments (1). Second, it binds to histone deacetylase 6 (Hdac6), a major MT deacetylase, and inhibits its activity leading to increased MT acetylation, a polymer stabilizing modification (2, 3). Our recent study established that Rock-mediated phosphorylation of Tppp1 inhibits its interaction with Hdac6 resulting in decreased MT acetylation in cells without altering Tppp1-mediated MT polymerization (4). Tppp1 is highly phosphorylated in cells on residues distinct from the Rock-mediated sites raising the possibility that its MT polymerizing activity is regulated by other kinases and signaling pathways (5–10).
Hdac6 is a class IIb atypical deacetylase that cleaves the acetyl groups of lysine 40 (Lys-40) within α-tubulin. Recent reports demonstrated that MT acetylation is important for the regulation of cell proliferation. Hdac6-null mouse embryonic fibroblasts exhibit high MT acetylation that promotes their resistance to oncogenic Ras and ErbB2 transformation (11). Additionally, knockdown of Hdac6 in a number of cancer cell lines inhibits their anchorage-independent proliferation (11). Furthermore, overexpression of the tumor suppressor gene cylindromatosis, which inhibits Hdac6 activity, causes delays in the cell cycle (12). Conversely, overexpression of Hdac6 promotes anchorage-independent cell proliferation (11). Because Tppp1 is a regulator of Hdac6 activity, these previous studies imply its potential role in cell proliferation.
Other important regulators of cell proliferation are the cyclin-dependent kinases (Cdks). They are key cell cycle regulatory molecules that are activated transiently through binding to their complementary cyclins. Mitogenic stimulation during G1-phase leads to increased Cyclin D levels, which then interact with Cdk4 or Cdk6 to promote their activation (13, 14). Cyclin D/Cdk4/6-mediated phosphorylation of the retinoblastoma protein (Rb) results in its dissociation from Hdac and alleviates its inhibitory effect on the E2F transcription factor. This partially activates E2F-mediated transcriptional up-regulation of genes including cyclin E, cyclin A, and Cdk1. Increased cyclin E levels in cells promotes the formation and activation of the Cyclin E/Cdk2 complex at late G1-phase to drive cells into S-phase (15). Subsequent Cyclin A/Cdk2 and then Cyclin A/Cdk1 complex formation promotes the progression of cells through S- and G2-phases, respectively (16, 17). Finally, Cyclin B/Cdk1 complex formation is essential for progression through mitosis (18).
In this study, we investigated the role of Tppp1 in the regulation of cell proliferation. We hypothesized that Tppp1 regulates the transition of cells through the G1/S-phase and mitosis via its inhibition of Hdac6 activity as well as its modulation of MT polymerization. Furthermore, we explored possible mechanisms for Tppp1 regulation that have important consequences in cell proliferation. We show here that Rock- and Cdk-mediated phosphorylation of Tppp1 control cell cycle progression and cell proliferation.
EXPERIMENTAL PROCEDURES
Plasmid Constructs
pGEX4T1-GST-Tppp1, pBABE-FLAG-Tppp1 (2), pBABE-Flag-Tppp13Ala, and pBABE-FLAG-Tppp13Glu (4) constructs were generated as previously described. Single pGEX4T1-GST-Tppp1 T14A, S18A, S45A, and S160A mutants were generated by site-directed mutagenesis (4) using the following primers: T14A, 5′-GCCAACAGGGCGCCCCCCAAG-3′; S18A, 5′-CGCCCCCCAAGGCCCCGGGGGAC-3′; S45A, 5′-GCAGCCGCAGCCCCTGAGCTC-3′; S160A, 5′-AAGCCATCTCGGCGCCCACAGTG-3′ and antisense 5′-TGGCAGCTTTGGCAGGCTTG-3′. cDNA containing the quadruple Tppp1 mutations as well as the dual Rock/Cdk phospho-site mutations were synthesized by Geneart (Invitrogen) and cloned into the BamHI and SalI restriction sites of pGEX4T1-GST-Tppp1 and/or pBABE-FLAG-Tppp1.
Tissue Culture
Stable U2OS cell lines were generated by viral transduction and selection in DMEM supplemented with 10% FBS and puromycin (4 μg/ml) as previously described (4). Cells were treated with Y-27632 (10 μm, water solvent; Calbiochem), roscovitine (10 μm, dimethyl sulfoxide solvent; Sigma), or their solvents for 16 h prior to analyses. For synchronization, cells were arrested in G0/G1-phase by incubation in DMEM supplemented with 0.2% FBS for 72 h; in S-phase by double thymidine block (2 mm; Sigma) for two 18-h incubations performed before and after a 9-h recovery; and G2/M-phase by a 16-h incubation with nocodazole (200 ng/ml; Sigma).
Cell Proliferation
Cell proliferation assays were conducted in a 6-well plate format. Cells were seeded at a density of 2 × 105 cells/well 24 h prior to trypsinization and resuspension in DMEM supplemented with 10% FBS. Total cell counts were performed with a hemacytometer and a light microscope. Three fields of each sample were counted. Proliferation results were analyzed by two-way analysis of variance, with a Bonferroni's post-test.
Propidium Iodide Staining
Cells were trypsinized and fixed in 70% (v/v) ethanol overnight at 4 °C. They were washed three times in PBS and incubated in the dark with propidium iodide (PI) staining buffer (10 mm Tris-HCl, pH 7.5, 5 mm MgCl2, 20 μg/ml of RNase A, and 5 μg/ml of PI) for 30 min at 37 °C. For experiments analyzing the recovery of cells from a G2/M-phase arrest, cells were treated with nocodazole (200 ng/ml) for 16 h. After treatment, mitotic cells were harvested by shaking the flask (mitotic shake-off) and re-plated for the indicated time points followed by collection of the suspension and adherent cells for analysis.
BrdU Labeling
Cells plated at 1 × 106 cells/10-cm dish for 24 h were incubated with 1 μg/ml of 5-bromo-2′-deoxyuridine (BrdU) for 3 h, trypsinized, and fixed in 70% ethanol overnight at 4 °C. Cell pellets were washed in PBS and incubated with ice-cold 0.1% Triton X-100, 0.1 n HCl on ice for 1 min followed by resuspension in DNA denaturing buffer (150 μm NaCl, 15 μm trisodium citrate dihydrate) at 90 °C for 5 min and on ice for 5 min. Cells were resuspended in BrdU staining buffer (PBS, 0.1% Triton X-100, 1% (w/v) BSA) and incubated with 2 μg/ml of FITC-conjugated mouse anti-BrdU or anti-IgGκ isotype control, 20 μg/ml of RNase A, and 5 μg/ml of PI for 30 min at room temperature.
Flow Cytometry
The cells prepared above were analyzed with the FACSCalibur Flow Cytometer (BD Bioscience) using the CellQuest Pro software. Channel compensation was performed using unlabeled and single-labeled samples. Population gates and numerical analyses were performed with the FlowJo (version 8.8.6) software.
Immunoblotting
Immunoblotting was performed as previously described (4). The following antibodies were used in this study: anti-acetyl-α-tubulin (1:5000) (Sigma), anti-c-Myc (1:1000) (Invitrogen), anti-FLAG 9H1 clone (1:3000) (WEHI monoclonal Ab facility, Melbourne, Australia), anti-GAPDH-HRP (1:3000) (Cell Signaling), anti-GST (1:5000) (Merck), anti-Hdac6 (1:1000) (Sigma), anti-phospho-histone 3 (1:500) (Roche Diagnostics), anti-phospho-Mlc Ser-18/Thr-19 (1:1000) (Cell Signaling), anti-Tppp1 monclonal and polyclonal Abs (1:1000, 1:500) (2), and anti-α-tubulin (1:5000) (Sigma).
In Vitro Kinase Assays
In vitro kinase assays were performed as described previously (4). Tppp1 phosphorylation levels following cyclin/Cdk phosphorylation were calculated by compensating for fold-differences in complex activity, which were obtained by analysis of Rb protein phosphorylation.
Metabolic Labeling
Log-phase HEK293T cells plated at a density of 2 × 106 cells/10-cm dish were transfected with the appropriate DNA constructs 24 h prior to incubation with Roswell Park Memorial Institute (RPMI) 1640 media without phosphate and l-glutamine for 16 h. 10 μm Y-27632 or vehicle were added 1 h prior to the addition of 0.1 mCi/ml of [32P]orthophosphate for 6 h. Cell cycle-dependent phosphorylation was evaluated by synchronizing the stable U2OS-FLAG-Tppp1 cell line in G0/G1-phase, S-phase, or G2/M-phase as described. Synchronized cells were incubated with 0.1 mCi/ml of [32P]orthophosphate 6 h prior to the conclusion of the treatment periods. Labeled cells were washed twice in cold PBS, harvested in metabolic labeling buffer (50 mm Tris-HCl, pH 7.4, 150 mm NaCl, and 0.1% (v/v) Triton X-100), and lysed by centrifugation at 16,000 × g for 10 min at 4 °C.
Microtubule Polymerization and Immunofluorescence Microscopy
Briefly, tubulin polymerization assays were performed using a Tubulin polymerization assay kit (catalogue number BK006P, Cytoskeleton). For immunofluorescence microscopy cells were fixed in 100% methanol and blocked in 10% FBS followed by incubations with primary and secondary antibodies as previously described (4).
RESULTS
Tppp1 Inhibits Cell Proliferation
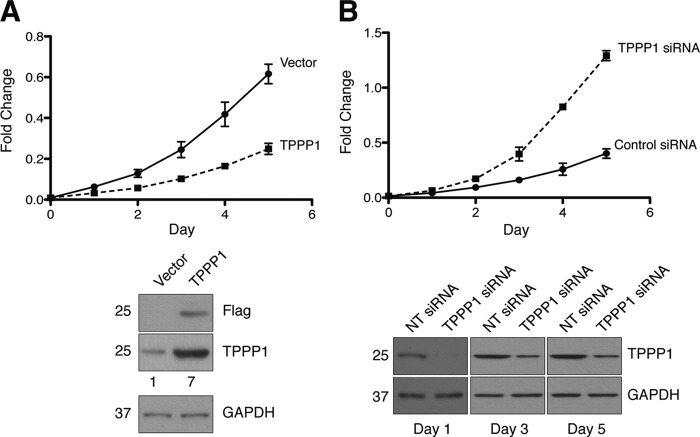
Dynamic rearrangement of the microtubule network is imperative for the transition of cells through the cell cycle phases and ultimately for cell proliferation. We hypothesized that Tppp1, as a modulator of MT dynamics, regulates cell proliferation. Our studies revealed that overexpression of FLAG-Tppp1 in U2OS cells, resulting in a 7-fold increase in Tppp1 expression, significantly reduced the rate of cell proliferation (Fig. 1A). In contrast, RNAi-mediated knockdown of Tppp1 significantly increased cell proliferation compared with non-targeting siRNA-transfected cells (Fig. 1B).
FIGURE 1.
TPPP1 inhibits cell proliferation. TPPP1 overexpression reduces cell proliferation. A, the proliferation of U2OS cells stably expressing FLAG-TPPP1 (TPPP1) or vector were analyzed. Assays were performed at daily intervals for a 5-day period as described under “Experimental Procedures” (p < 0.0001). Immunoblot analysis of U2OS cells stably expressing F-TPPP1 or vector that were probed for FLAG, TPPP1, and GAPDH (loading control) showed a 7-fold increase in TPPP1 expression compared with vector. B, TPPP1 knockdown increases cell proliferation. Proliferation assays were performed for 5 days as described under “Experimental Procedures” (p < 0.0001). Immunoblot analysis of TPPP1 knockdown probed for TPPP1 and GAPDH (loading control). Data in A and B were analyzed by two-way analysis of variance with Bonferroni's post-test.
Tppp1-mediated Delay of the G1/S-phase Transition Is Inhibited by Rock Signaling
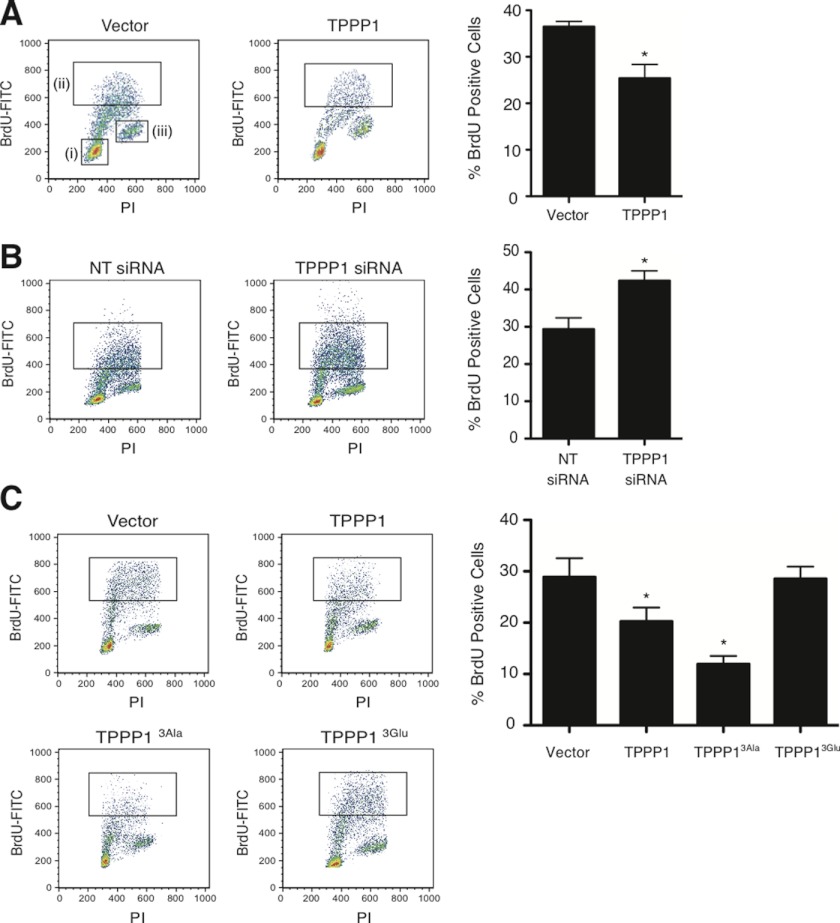
Cell cycle checkpoints are important transitional stages whereby the ability of cells to commit to S-phase and mitosis is controlled. We therefore investigated whether the observed Tppp1-mediated regulation of cell proliferation is through its modulation of the G1/S-phase transition. Analysis of U2OS cells in S-phase by measurement of the incorporation of BrdU (5-bromo-2′-deoxyuridine) revealed that overexpression and knockdown of Tppp1 decreased (25.4 ± 3.8%) and increased (42.4 ± 4.2%) BrdU incorporation, respectively, compared with the control cells (36.5 ± 1.8% and 29.4 ± 4.7%) (Fig. 2, A and B), suggesting that Tppp1 regulates the entry and/or transition of cells through S-phase.
FIGURE 2.
TPPP1 and the ROCK-TPPP1 signaling pathway regulate the G1/S-phase cell cycle transition. Altered TPPP1 levels modulate the G1/S-phase transition. A, U2OS cell lines stably expressing FLAG-TPPP1 or vector were incubated with 1 μg/ml of 5-bromo-2-deoxyuridine (BrdU) for 3 h followed by fixation in 70% ethanol. Fixed cells stained with mouse anti-BrdU-FITC or mouse anti-IgGκ FITC (isotype control) antibodies and PI were analyzed by flow cytometry. Inset i represents the low PI positive, G1-phase cell population; inset ii is the BrdU and PI positive population, that represents cells that were in S-phase during the incubation period; and inset iii represents the high PI, G2/M-phase population. Graphical representation of the (ii) gated population (*, p = 0.0252) (right panel). B, representative dot plots of U2OS cells transiently transfected with TPPP1 or non-targeting (NT) siRNA that were analyzed as in A as a graphical representation of the data (*, p = 0.0322) (right panel). C, ROCK phosphorylation of TPPP1 relieves its inhibition of the G1/S-phase transition. Representative dot plots of stable U2OS cells expressing wild-type TPPP1, TPPP13Ala, TPPP13Glu, or vector. Graphical representation of experiments as described in A of the (ii) gated cell population (*, p = 0.0476; **, p = 0.0134). Data are expressed as mean ± S.E. of three independent experiments and analyzed by two-tailed unpaired t tests.
We previously established that Rock-mediated phosphorylation of Tppp1 prevents its interaction with Hdac6 to alleviate its deacetylase inhibitory activity (4). As a major tubulin deacetylase, Hdac6 regulates the level of MT acetylation, which controls MT dynamics and ultimately determine cellular quiescence (11, 12). Therefore, we investigated if the molecular mechanism responsible for Tppp1-mediated modulation of G1/S-phase is dependent on its Hdac6 regulatory activity. We analyzed the efficiency of U2OS cells that were stably expressing wild-type Tppp1, Rock phosphoinhibitory Tppp13Ala, Rock phosphomimetic Tppp13Glu, or vector to transit through the G1/S-phase boundary. These studies revealed that expression of the Tppp13Ala protein retards the entry of cells into S-phase, with 12 ± 2.5% of BrdU positive cells compared with 28.8 ± 6.4% of BrdU positive cells transfected with empty vector (Fig. 2C). Conversely, expression of the phosphomimetic Tppp13Glu, abolished the Tppp1-mediated decreases in the G1/S-phase transition of cells, resulting in 28.6 ± 4.1% of BrdU positive cells (Fig. 2C). These results strongly suggest that Tppp1-mediated regulation of the G1/S-phase transition is dependent on its Hdac6 regulatory activity.
Tppp1 Expression Inhibits the Transition of Cells through Mitosis
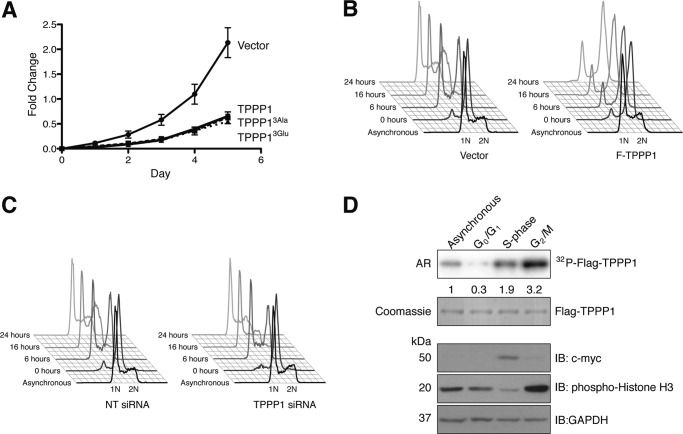
We establish here that Tppp1 overexpression reduces cell proliferation, whereas its down-regulation enhanced proliferation (Fig. 1, A and B). Furthermore, we show that Tppp1-mediated inhibition of the G1/S-phase transition is dependent on its phosphorylation by Rock. Therefore, to investigate if Rock-Tppp1 signaling is sufficient to inhibit Tppp1-mediated reductions in cell proliferation we performed proliferation assays with U2OS cells stably expressing wild-type Tppp1, its Rock phospho-mutants, or vector control. We demonstrated that expression of all Tppp1 proteins reduced cell proliferation compared with the vector control (Fig. 3A). These results indicate that Rock phosphorylation of Tppp1 is not sufficient to inhibit its regulation of cell proliferation, thereby suggesting that its MT polymerization activity, which is retained when phosphorylated by Rock, is also important for its regulation of cell proliferation.
FIGURE 3.
TPPP1 inhibits the re-entry of cells into the G1-phase from mitosis and its phosphorylation is cell cycle-dependent. A, ROCK-TPPP1 signaling does not affect TPPP1-mediated inhibition of cell proliferation. U2OS cells stably expressing wild-type F-TPPP1, TPPP13Ala, TPPP13Glu, or vector were subjected to proliferation assays that were performed at daily intervals for a 5-day period as described under “Experimental Procedures.” Data are expressed as mean ± S.E. of three independent experiments (p < 0.0001). B, TPPP1 regulates the re-entry of cells into G1-phase from mitosis. Stable U2OS cells expressing TPPP1 or vector were synchronized in the G2/M-phase by nocodazole treatment (200 ng/ml) and mitotic cells were collected by shake-off. After washout of nocodazole and re-plating, cells were harvested at the indicated time points and fixed in 70% ethanol. Cells were stained with PI and analyzed by flow cytometry. 1N represents the G1-phase cell population, with only one copy of the genome, whereas 2N is the G2/M-phase population with two copies of the genome. C, U2OS cells transiently transfected with TPPP1 or non-targeting (NT) siRNA were analyzed as described in B. D, TPPP1 phosphorylation is cell cycle dependent. Stable U2OS cells expressing Flag-TPPP1 (TPPP1) were synchronized in G0/G1- (lane 2), S- (lane 3), or G2/M-phase (lane 4) as described under “Experimental Procedures.” Asynchronous and synchronized cells, in the presence of the arrest inducing reagents, were labeled with [32P]orthophosphate. The phosphorylation of the immunoprecipitated F-TPPP1 was analyzed by immunoblotting (IB) and autoradiography (AR). Total lysates were probed with anti-histone H3 (mitotic marker), anti-c-myc (S-phase marker), and anti-GAPDH (loading control) antibodies. The numbers below the top panel represent the level of TPPP1 phosphorylation relative to the asynchronous cells.
Mitosis is dependent on dynamic morphological changes of the cell afforded by the MT network. Therefore, we hypothesized that Tppp1 might also be involved in the regulation of cell proliferation through modulation of mitotic progression. To study the role of Tppp1 in completion of mitosis we analyzed the G1-phase entry of cells that were arrested in mitosis by nocodazole (200 ng/ml) treatment. Overexpression of Tppp1 delayed the progression of cells from mitosis into the G1-phase compared with vector control cells (Fig. 3B), whereas knockdown of Tppp1 increased the rate of progression through mitosis (Fig. 3C). Therefore, we concluded that Tppp1 regulates cell proliferation via modulation of the G1/S-phase transition as well as the mitosis to G1-phase transition.
Because Tppp1 activity is regulated by Rock signaling and it is phosphorylated on residues distinct from the Rock phosphorylation sites (5, 7, 19), we tested if other kinase signaling pathways contribute to its role in the progression of cells through mitosis. We first investigated the level of Tppp1 phosphorylation during the cell cycle by analysis of cells metabolically labeled with [32P]orthophosphate that were arrested in G0/G1-, S-, or G2/M-phase. These experiments revealed that Tppp1 phosphorylation was lowest at the G0/G1-phase, whereas it increased during S-phase and peaked at the G2/M-phase compared with an asynchronous cell population (Fig. 3D). Previous studies demonstrated that Rock activity was necessary for the transition of cells from G1- into S-phase (20), which is likely to reflect the increase in Tppp1 phosphorylation at this stage. However, further increases in Tppp1 phosphorylation during the G2/M-phase suggest that it is subject to further kinase regulation.
Tppp1 Is a Cyclin/Cdk1/2 Substrate in Vitro and in Cells
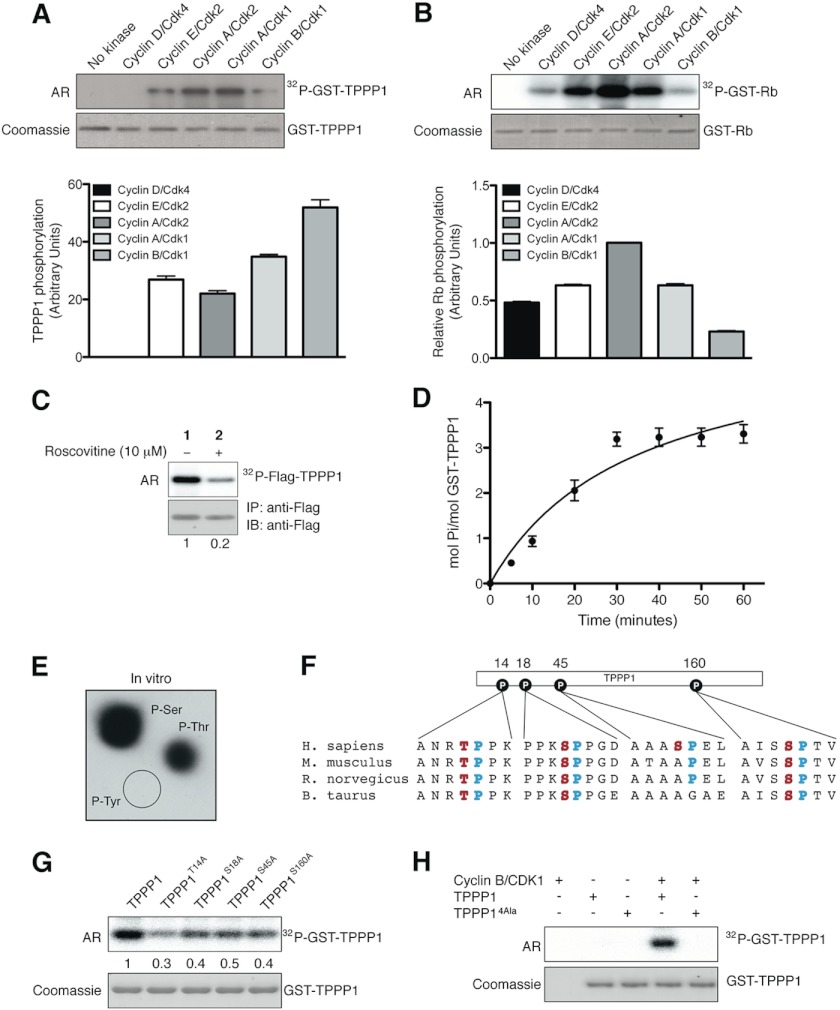
Cyclin/Cdks are the classical cell cycle regulatory complexes that phosphorylate a vast array of substrates including MT regulatory proteins (21–24); therefore, we tested the hypothesis that Tppp1 is a cyclin/Cdk substrate. We first analyzed the ability of the Cyclin/Cdk complexes to phosphorylate Tppp1 in vitro. In vitro kinase assays with Cyclin D/Cdk4 (G1-phase), Cyclin E/Cdk2 (late G1-phase), Cyclin A/Cdk2 (S-phase), Cyclin A/Cdk1 (early G2-phase), and Cyclin B/Cdk1 (mitosis) showed that TPPP1 is a cyclin/Cdk1/2 substrate in vitro (Fig. 4A). Moreover, quantification of the level of Tppp1 phosphorylation relative to Cyclin/Cdk complex activities, which was obtained through analysis of their phosphorylation of the Rb (Fig. 4B), a known Cdk substrate, revealed that the highest level of Tppp1 phosphorylation was achieved in the presence of Cyclin B/Cdk1 (Fig. 4A, lower panel). We next tested if Tppp1 is also phosphorylated by Cdks in cells. Treatment of cells with the Cdk inhibitor roscovitine (10 μm) showed that inhibition of Cdk activity significantly reduced Tppp1 phosphorylation compared with vehicle-treated cells (Fig. 4C). Therefore, we establish that Tppp1 is a Cyclin/Cdk substrate in vitro and in cells.
FIGURE 4.
TPPP1 is a Cyclin/Cdk substrate in vitro and in cells. A and B, TPPP1 is a Cdk substrate in vitro. In vitro kinase assays were performed in the presence of the bacterially expressed and purified TPPP1 (A) or Rb (B) proteins and the cyclin D/Cdk4, cyclin E/Cdk2, cyclin A/Cdk2, cyclin A/Cdk1, and cyclin B/Cdk1 complexes as described under “Experimental Procedures.” TPPP1 phosphorylation levels (A) were normalized according to cyclin/Cdk complex activities based on their level of Rb phosphorylation (B). Data are expressed as mean ± S.E. of three independent experiments. C, TPPP1 is an in vivo Cdk substrate. U2OS cells transiently transfected with FLAG-TPPP1 were treated with roscovitine (10 μm) or vehicle (dimethyl sulfoxide) prior to incubation with [32P]orthophosphate (0.1 mCi/ml) for 6 h. TPPP1 phosphorylation in cells was determined by immunoprecipitation (IP) of the F-TPPP1 protein followed by autoradiography (AR) and immunoblotting (IB). D, TPPP1 is phosphorylated on 3–4 serine/threonine residues by Cdk1. Michaelis-Menten kinetics assays performed as described under “Experimental Procedures” revealed that Cdk phosphorylates TPPP1 on 3–4 residues. E, phosphoamino acid analysis of in vitro phosphorylated TPPP1 showed that TPPP1 is phosphorylated by Cdk on serine and threonine residues. F, amino acid sequence alignment of four mammalian TPPP1 species showing their evolutionary conservation, potential Cdk phosphorylation sites: TPPP1 Thr-14, Ser-18, Ser-45, and Ser-160 (red) and their minimal Cdk motif proline residue (+1; blue). G, alanine substitution mutations of TPPP1 Thr-14, Ser-18, Ser-45, or Ser-160 residues decreased their phosphorylation by cyclin B/Cdk1. In vitro kinase assays with cyclin B/Cdk1 and wild-type TPPP1, TPPP1-T14A, TPPP1-S18A, TPPP1-S45A, or TPPP1-S160A were performed as described under “Experimental Procedures.” H, the quadruple T14A/S18A/S45A/S160A (TPPP14Ala) mutations abolish TPPP1 phosphorylation by cyclin B/Cdk1 in in vitro kinase assays. The numbers below the panels in C and G represent their level of phosphorylation relative to the wild-type TPPP1 protein. Data in A, B, and D are expressed as mean ± S.E. of three and two independent experiments, respectively.
Phosphoamino acid analysis as well as phosphorylation kinetics experiments revealed that Cyclin B/Cdk1 phosphorylates Tppp1 on four serine/threonine residues in vitro (Fig. 4, D and E). A literature search of the Tppp1 amino acid sequence for residues that are part of minimal cyclin/Cdk consensus motifs (S/T-P) (25–27) and that are phosphorylated in cells, led to the identification of Thr-14, Ser-18, Ser-45, and Ser-160 as candidate phosphorylation sites (Fig. 4F). We therefore generated single Tppp1 alanine substitution mutants and in vitro kinase assays showed that mutation of each of these sites reduced Tppp1 phosphorylation by Cyclin B/Cdk1 (Fig. 4G). Subsequent analysis of a quadruple Tppp1 alanine mutant (Tppp14Ala) confirmed that mutation of these sites abolished Tppp1 phosphorylation by Cyclin B/Cdk1 (Fig. 4H).
Cdk-Tppp1 Signaling Inhibits Tppp1-mediated MT Polymerization without Altering Its Hdac6 Binding
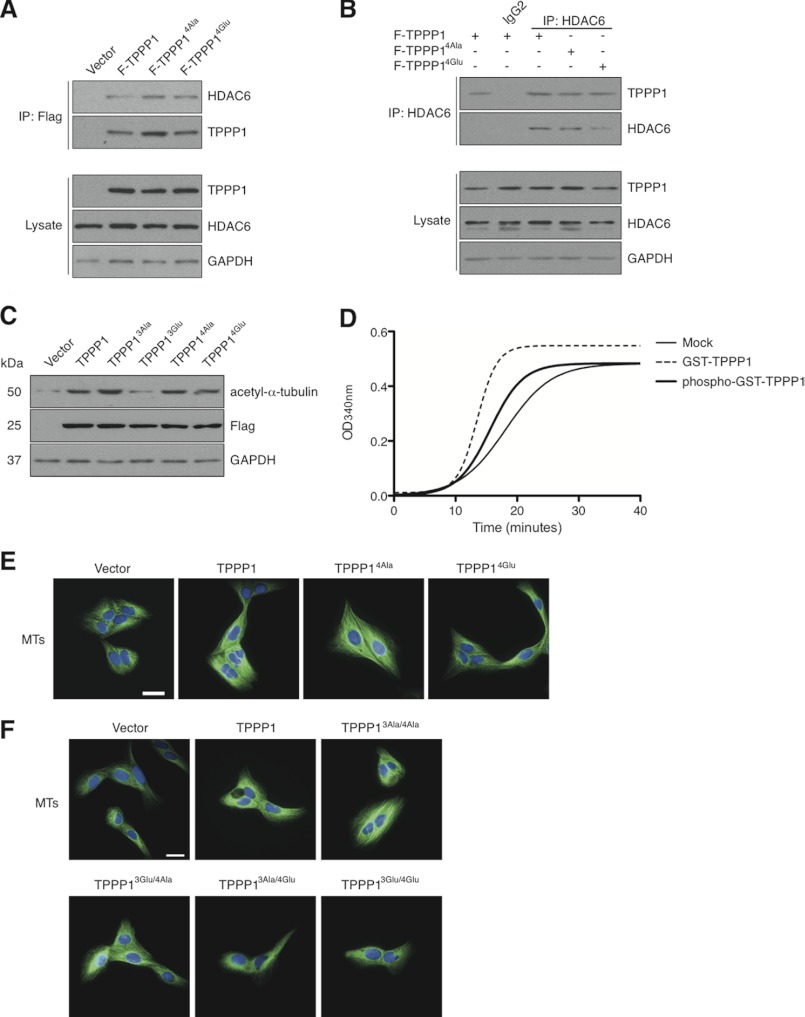
Our initial investigation into the functional implications of the Cdk-Tppp1 signaling pathway revealed that Tppp1 phosphorylation by Cdk did not alter its interaction with Hdac6 or the level of acetylated MT in cells (Fig. 5, A–C). To examine the impact of Tppp1 phosphorylation by Cdk in vitro we performed tubulin polymerization assays (Fig. 5D) and visualized the amount of polymerized MTs in cells expressing wild-type Tppp1, Cdk phosphoinhibitory Tppp14Ala, Cdk phosphomimetic Tppp14Glu, or vector (Fig. 5E). These studies demonstrated that Tppp1 phosphorylation by Cdk inhibits its MT polymerization in vitro and reduces the level of MTs in cells. To further confirm that only Cdk phosphorylation of Tppp1 inhibits its MT polymerizing activity, we generated stable U2OS cell lines expressing the dual Rock and Cdk Tppp1 phosphoinhibitory and phosphomimetic proteins (Table 1). Analysis of MT levels in these cells by immunofluorescence microscopy revealed that phosphorylation of Tppp1 by Cdk, irrelevant of its phosphorylation by Rock inhibited Tppp1-mediated increases in MT levels in cells (Fig. 5F). Therefore, we confirm that Cdk-Tppp1 signaling inhibits Tppp1-mediated MT polymerizing activity in vitro and in cells.
FIGURE 5.
Cdk-TPPP1 signaling inhibits its MT polymerizing activity. A, U2OS cells expressing F-TPPP1, F-TPPP14Ala, F-TPPP14Glu, or vector were immunoprecipitated (IP) and analyzed by immunoblotting together with cell lysates that were probed for FLAG-TPPP1, HDAC6, and GAPDH (loading control). B, U2OS cell extracts from A were immunoprecipitated with an anti-HDAC6 antibody and their interaction with F-TPPP1 proteins and total cell lysates were analyzed as described in A. C, stable U2OS cell line extracts expressing the indicated TPPP1 proteins or vector were immunoblotted for acetyl-α-tubulin, FLAG, and GAPDH (loading control) levels. D and E, TPPP1 phosphorylation by cyclin B/Cdk1 inhibits its MT polymerizing activity in vitro and in cells. D, in vitro MT polymerization assays in the presence of GST- or GST-TPPP1 phosphorylated in vitro by cyclin B/Cdk1 as described under “Experimental Procedures.” E, phosphorylation of TPPP1 by Cdk inhibits its microtubule polymerizing activity in cells. U2OS cells stably expressing wild-type TPPP1, TPPP14Ala, TPPP14Glu, or vector were stained for MTs (green) and nuclei (blue). Immunofluorescence microscopy revealed that expression of Cdk-mediated phosphomimetic TPPP1 (TPPP14Glu) reduces the level of MT staining compared with cells expressing wild-type and phosphoinhibitory TPPP1 (TPPP14Ala). F, only TPPP1 phosphorylation by Cdk inhibits its MT polymerizing activity. U2OS cells stably expressing wild-type TPPP1, TPPP13Ala/4Ala, TPPP13Glu//4Ala, TPPP13Ala/4Glu, TPPP13Glu/4Glu, or vector were analyzed as described in E. Scale bar is 50 μm.
TABLE 1.
Dual ROCK and Cdk phosphoinhibitory and phosphomimetic TPPP1 mutants
| Construct | Mutant sites | Mutant type |
|---|---|---|
| Tppp13Ala/4Ala | S32A/S107A/S159A-T14A/S18A/S45A/S160A | Dual phosphoinhibitory |
| Tppp13Ala/4Glu | S32A/S107A/S159A-T14E/S18E/S45E/S160E | Rock phosphoinhibitory/Cdk phosphomimetic |
| Tppp13Glu/4Ala | S32E/S107E/S159E-T14A/S18A/S45A/S160A | Rock phosphomimetic/Cdk phosphoinhibitory |
| Tppp13Glu/4Glu | S32E/S107E/S159E-T14E/S18E/S45E/S160E | Dual phosphomimetic |
Tppp1-mediated MT Polymerization Does Not Contribute to Its Inhibition of the G1/S-phase Transition
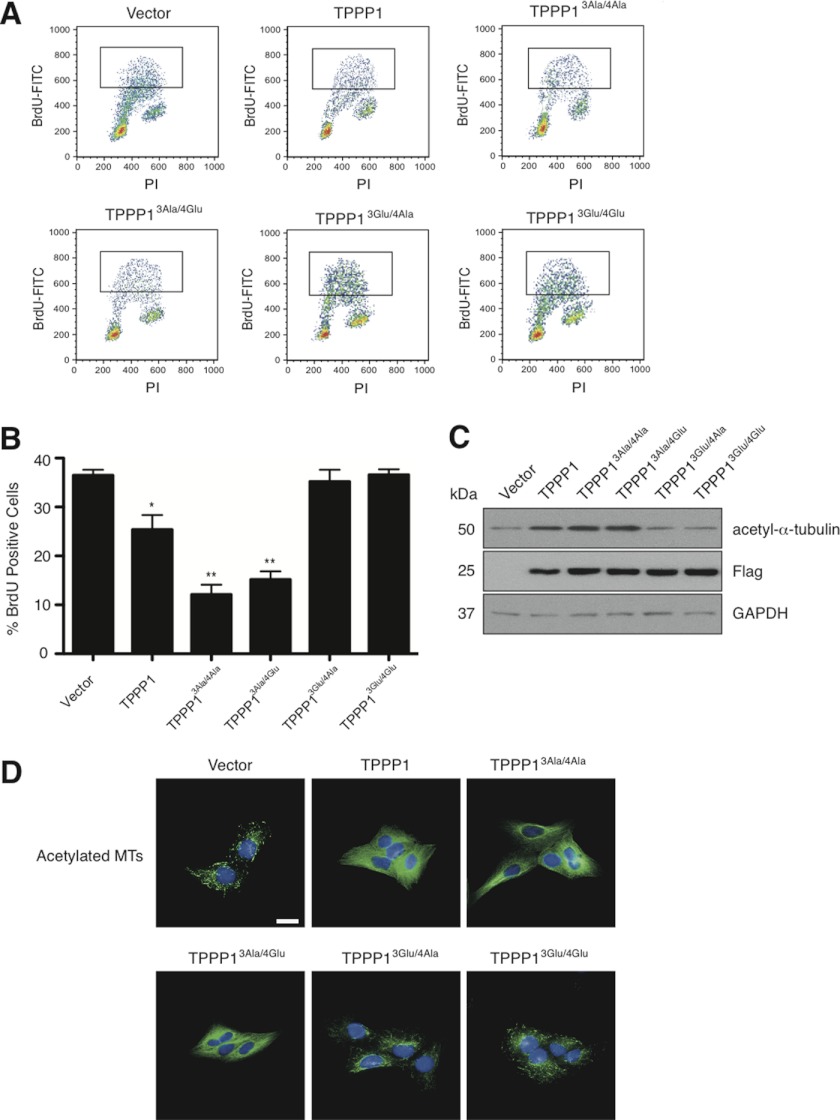
Our results thus far suggest that Rock-mediated phosphorylation of Tppp1 relieves its inhibitory effect on G1/S-phase progression (Fig. 2). To rule out the possibility that Tppp1-mediated MT polymerization is also contributing to its inhibition of the G1/S-phase transition, we analyzed the level of BrdU in cells expressing the dual Rock and Cdk phospho-site Tppp1 mutants displayed in Table 1. Analysis of BrdU incorporation in cells revealed that expression of wild-type Tppp1 (25.4 ± 2.71%), Tppp13Ala/4Ala (12.1 ± 2.12%), or Tppp13Ala/4Glu (15.2 ± 1.24%) significantly decreased the level of cellular BrdU compared with the vector expressing cells (36.5 ± 1.06%) (Fig. 6, A and B). In contrast, expression of Tppp13Glu/4Ala (35.2 ± 2.36%) or Tppp13Glu/4Glu (36.6 ± 1.36%) did not alter BrdU levels. Therefore, we conclude that Tppp1 retards the G1/S-phase transition only via its Hdac6 inhibitory activity.
FIGURE 6.
ROCK-mediated TPPP1 phosphorylation inhibits its delay in the G1/S-phase transition. A, U2OS cells stably expressing wild-type TPPP1, TPPP13Ala/4Ala, TPPP13Glu//4Ala, TPPP13Ala/4Glu, TPPP13Glu/4Glu, or vector were incubated with 1 μg/ml of 5-bromo-2-deoxyuridine (BrdU) for 3 h followed by fixation in 70% ethanol. Fixed cells stained with mouse anti-BrdU-FITC or mouse anti-IgGκ FITC (isotype control) antibodies and PI were analyzed by flow cytometry. Representative dot plots of flow cytometry analyzed cells. Insets represent the BrdU-labeled cell population showing the number of cells that have transitioned through S-phase during the incubation period. B, graphical representation of the data in A (*, p = 0.025; **, p = 0.0005). Data are expressed as mean ± S.E. of three independent experiments. C, TPPP1-mediated MT polymerization does not affect its inhibition of HDAC6 activity or regulation of MT acetylation. U2OS cell lysates from cell lines described in A were analyzed by immunoblotting. Total lysates were probed with anti-FLAG, anti-acetyl-α-tubulin, and anti-GAPDH (loading control) antibodies. D, immunofluorescence microscopy of cells described in A that were stained for acetylated tubulin (green) and nuclei (blue). Scale bar is 50 μm.
To confirm that Tppp1-mediated inhibition of Hdac6 activity and its inhibition of the G1/S-phase transition is the result of increased microtubule acetylation, we analyzed the level of MT acetylation in these cell lines by immunoblotting (Fig. 6C) and immunofluorescence microscopy (Fig. 6D). The results show that increases and decreases in the number of cells in S-phase correlates with increased and decreased Tppp1 phosphorylation by Rock and is dependent on its regulation of MT acetylation. Therefore, our results clearly show that Tppp1-mediated inhibition of Hdac6 activity and the resulting increase in MT acetylation is the only Tppp1 function necessary for the inhibition of the G1/S-phase transition.
Phosphorylation of Tppp1 by Rock and Cdk Is Necessary to Inhibit Tppp1 Activity and Its Regulation of Cell Proliferation
To investigate the impact of Rock- and Cdk-mediated Tppp1 phosphorylation on its regulation of the mitosis to G1-phase transition, we analyzed the recovery of cells arrested in the G2/M-phase with nocodazole treatment. Our results revealed that inhibition of Tppp1 phosphorylation by Rock and/or Cdk delayed the transition of cells into the G1-phase compared with vector control cells (Fig. 7A). Interestingly, Tppp1 phosphorylation by Rock or Cdk increases the rate of entry of cells into the G1-phase compared with wild-type Tppp1 expressing cells, suggesting that loss of its Hdac6 regulatory or MT polymerizing function partially inhibits its modulation of mitosis to G1-phase transition. In contrast, expression of the dual Rock/Cdk phosphomimetic Tppp1 mutant (Tppp13Glu/4Glu) showed no difference in the transition of cells into G1-phase compared with the vector control. Therefore, we concluded that Tppp1 phosphorylation by both Rock and Cdk is necessary to inhibit its Hdac6 regulatory and MT polymerizing activities, respectively, to enable cells to re-enter the G1-phase.
FIGURE 7.
Dual TPPP1 phosphorylation by Cdk and ROCK inhibits its regulation of cell proliferation. A, U2OS cells stably expressing wild-type TPPP1, TPPP13Ala/4Ala, TPPP13Ala/4Glu, TPPP13Glu/4Ala, TPPP13Glu/4Glu, or vector were synchronized in the G2/M-phase by nocodazole treatment (200 ng/ml) and the mitotic population was collected by shake-off. After nocodazole washout and re-plating, cells were harvested at the indicated time points fixed in 70% ethanol, stained with PI, and analyzed by flow cytometry. 1N represents the G1-phase cell population, with only one copy of the genome, whereas 2N is the G2/M-phase population with two copies of the genome. B, proliferation assays of the cells described in A were performed as described under “Experimental Procedures.”
To confirm that Tppp1 phosphorylation by Rock and Cdk suppresses its cell proliferation effect, we performed proliferation assays with the cell lines described above. These results confirmed that neither Rock (Tppp13Glu/4Ala) nor Cdk phosphorylation (Tppp13Ala/4Glu) of Tppp1 alone was sufficient to alleviate its inhibition of cell proliferation. However, the proliferation of cells expressing the dual Tppp1 phosphomimetic mutant (Tppp13Glu/4Glu) was similar to that of the vector expressing cells (Fig. 7B), therefore confirming that Tppp1 phosphorylation by Rock and Cdk is required to modulate the progression of cells through the cell cycle.
DISCUSSION
Our study establishes that Tppp1 reduces cell proliferation through inhibition of the G1/S-phase transition and the progression of cells into G1 from G2/M-phase. We demonstrate here that Rock-mediated Tppp1 phosphorylation inhibits its regulation of the G1/S-phase transition, whereas Rock- and Cdk-mediated phosphorylation are necessary to inhibit its mitotic regulatory activity. Therefore, our findings reveal that temporal cell cycle-dependent phosphorylation of Tppp1, which inhibits its activity, is necessary for cells to progress through the cell cycle and has important consequences in cell proliferation.
We establish that Tppp1 overexpression reduces, whereas its knockdown increases the proliferation of U2OS cells. These results are supported by reports that oligodendrocytes show similar defects in replication upon prolonged Tppp1 expression (28) and increased cell proliferation when Tppp1 expression is reduced using the microRNA-206 (miR-206) (29). Our findings also provide further insight into the mechanism by which Tppp1 temporally regulates cell proliferation, whereby it modulates the transition of cells from G1 into S-phase as well as from mitosis into G1-phase.
Herein, we focused on the signaling pathways that regulate Tppp1 activity and their impact on its regulation of cell proliferation. This initially involved the evaluation of our recently established Rock-Tppp1 signaling pathway (4). We show that Rock-mediated phosphorylation of Tppp1, which inhibits TPPP1 binding to Hdac6 and results in reduced MT acetylation, promotes the transition of cells through G1/S-phase. These results suggest that down-regulation of acetyl-MT levels is a critical event at the onset of the cell cycle. This is supported by our studies demonstrating that knockdown of Tppp1 promotes the G1/S-phase transition and those of others showing that increased MT acetylation delays cell cycle entry and promotes cell senescence (12, 30, 31). Moreover, Rock signaling has previously been implicated in the regulation of the G1/S-phase transition, with the observation that Rock activation enhances the entry of cells into S-phase (20), whereas inhibition of its activity leads to a marked decrease in DNA synthesis (32) and blocked G1/S-phase cell cycle progression (33–35). Therefore, our studies now show that Rock signaling regulates the G1/S-phase transition, at least in part via inhibition of the Tppp1/Hdac6 interaction.
Our investigation into the role of Tppp1 in the regulation of the progression through mitosis into G1-phase demonstrates that Rock-mediated phosphorylation of Tppp1 alone does not inhibit this activity. However, further analysis of the Tppp1 regulatory pathways identified that it is a novel Cyclin B/Cdk1 substrate in vitro and a Cdk substrate in cells. The data presented here clearly show that Cdk-mediated Tppp1 phosphorylation inhibits its MT polymerizing activity in vitro and in cells, without affecting its Hdac6 regulatory activity. Finally, an integrated analysis of the role of Tppp1 phosphorylation by Rock and Cdk in the regulation of the transition of cells from mitosis into G1-phase shows that dual Tppp1 phosphorylation is necessary to completely inhibit Tppp1 activity and its regulation of mitosis.
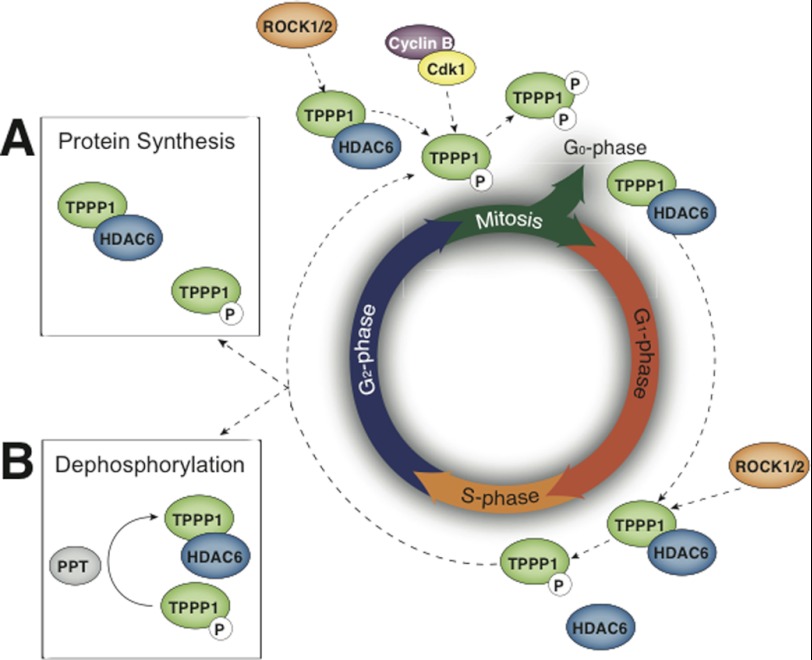
Although it appears that Tppp1 activity is regulated during the cell cycle via its sequential phosphorylation at late G1 by Rock to enable cells to transit into S-phase followed by its further phosphorylation at the G2/M-phase by cyclin B/Cdk1 enabling cells to re-enter G1-phase, the regulation of Tppp1 during the cell cycle is likely to be more complex. The results presented here and those of others demonstrate that the mitotic apparatus is enriched in acetylated MTs, suggesting that an increase in MT acetyltransferase activity or a reduction in deacetylase activity is crucial for the regulation of mitosis. Furthermore, Rock activity is required for the completion of mitosis (36, 37) and Tppp1 regulation by Rock and Cdk is not interdependent, since we show that bacterially expressed Tppp1 is an in vitro substrate of both kinases. Therefore, we propose a model whereby the cellular Tppp1 pool is phosphorylated or partially phosphorylated at late G1-phase by Rock to permit cells to enter S-phase, followed by synthesis of the new Tppp1 protein or its de-phosphorylation during G2-phase to re-establish its Hdac6 inhibitory activity and increase MT acetylation, enabling the formation of the stable mitotic apparatus. Finally, subsequent phosphorylation of Tppp1 during mitosis by Rock and cyclin B/Cdk1 completely inhibits its activity and enables cells to re-enter the G1-phase (Fig. 8).
FIGURE 8.
A proposed model of the dynamic phosphorylation of TPPP1 and its activation/deactivation during the cell cycle. TPPP1 phosphorylation by ROCK during late G1-phase inhibits its HDAC6 regulatory activity to decrease MT acetylation and enable cells to enter and/or progress through S-phase. TPPP1 phosphorylation by ROCK and Cdk, which inhibits its HDAC6 regulatory and MT polymerizing activity, respectively, is necessary for cells to exit mitosis and re-enter the G1-phase. It was previously suggested that an increase in MT acetylation is required for mitosis. Therefore, we propose that TPPP1-mediated inhibition of HDAC6 activity, resulting in increased MT acetylation, occurs during the G2-phase. We suggest two alternative models whereby the TPPP1 pool is phosphorylated or partially phosphorylated at late G1-phase by ROCK to permit cells transition into S-phase. This is followed by the synthesis of new TPPP1 protein (A) or its dephosphorylation by a phosphatase (PPT) (B) during the G2-phase to re-establish its inhibition of HDAC6 activity and increase MT acetylation to form a stable mitotic apparatus.
In summary, we establish a role for Tppp1 in the regulation of cell proliferation via inhibition of the entry of cells into S-phase and re-entry into G1-phase from mitosis, respectively. Tppp1 reduces the G1/S-phase transition through inhibition of Hdac6 activity, which modulates MT acetylation levels. Moreover, we discovered a novel Cdk-Tppp1 signaling pathway that inhibits Tppp1-mediated MT polymerization. Finally, we demonstrate that dual phosphorylation of Tppp1 by Rock and Cdk during mitosis is necessary to inhibit its activity and enable cells to re-enter the G1-phase.
This work was supported in part by grants from the National Health and Medical Research Council (NHMRC), the Australian Research Council (ARC), the Cancer Council of Victoria, and the Victorian Government's Operational Infrastructure Support Program.
- MT
- microtubule
- Tppp1
- tubulin polymerization promoting protein 1
- Hdac6
- histone deacetylase 6
- Cdk
- cyclin-dependent kinase
- Rb
- retinoblastoma
- PI
- propidium iodide.
REFERENCES
- 1. Hlavanda E., Kovács J., Oláh J., Orosz F., Medzihradszky K. F., Ovádi J. (2002) Brain-specific p25 protein binds to tubulin and microtubules and induces aberrant microtubule assemblies at substoichiometric concentrations. Biochemistry 41, 8657–8664 [DOI] [PubMed] [Google Scholar]
- 2. Acevedo K., Li R., Soo P., Suryadinata R., Sarcevic B., Valova V. A., Graham M. E., Robinson P. J., Bernard O. (2007) The phosphorylation of p25/TPPP by LIM kinase 1 inhibits its ability to assemble microtubules. Exp. Cell Res. 313, 4091–4106 [DOI] [PubMed] [Google Scholar]
- 3. Tokési N., Lehotzky A., Horváth I., Szabó B., Oláh J., Lau P., Ovádi J. (2010) TPPP/p25 promotes tubulin acetylation by inhibiting histone deacetylase 6. J. Biol. Chem. 285, 17896–17906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Schofield A. V., Steel R., Bernard O. (2012) Rho-associated coiled-coil kinase (ROCK) protein controls microtubule dynamics in a novel signaling pathway that regulates cell migration. J. Biol. Chem. 287, 43620–43629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hlavanda E., Klement E., Kókai E., Kovács J., Vincze O., Tökési N., Orosz F., Medzihradszky K. F., Dombrádi V., Ovádi J. (2007) Phosphorylation blocks the activity of tubulin polymerization-promoting protein (TPPP). Identification of sites targeted by different kinases. J. Biol. Chem. 282, 29531–29539 [DOI] [PubMed] [Google Scholar]
- 6. Trinidad J. C., Specht C. G., Thalhammer A., Schoepfer R., Burlingame A. L. (2006) Comprehensive identification of phosphorylation sites in postsynaptic density preparations. Mol. Cell Proteomics 5, 914–922 [DOI] [PubMed] [Google Scholar]
- 7. Dephoure N., Zhou C., Villén J., Beausoleil S. A., Bakalarski C. E., Elledge S. J., Gygi S. P. (2008) A quantitative atlas of mitotic phosphorylation. Proc. Natl. Acad. Sci. U.S.A. 105, 10762–10767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Skorobogatko Y. V., Deuso J., Adolf-Bryfogle J., Adolf-Bergfoyle J., Nowak M. G., Gong Y., Lippa C. F., Vosseller K. (2011) Human Alzheimer's disease synaptic O-GlcNAc site mapping and iTRAQ expression proteomics with ion trap mass spectrometry. Amino Acids 40, 765–779 [DOI] [PubMed] [Google Scholar]
- 9. Wiśniewski J. R., Nagaraj N., Zougman A., Gnad F., Mann M. (2010) Brain phosphoproteome obtained by a FASP-based method reveals plasma membrane protein topology. J. Proteome Res. 9, 3280–3289 [DOI] [PubMed] [Google Scholar]
- 10. Tweedie-Cullen R. Y., Reck J. M., Mansuy I. M. (2009) Comprehensive mapping of post-translational modifications on synaptic, nuclear, and histone proteins in the adult mouse brain. J. Proteome Res. 8, 4966–4982 [DOI] [PubMed] [Google Scholar]
- 11. Lee Y. S., Lim K. H., Guo X., Kawaguchi Y., Gao Y., Barrientos T., Ordentlich P., Wang X. F., Counter C. M., Yao T. P. (2008) The cytoplasmic deacetylase HDAC6 is required for efficient oncogenic tumorigenesis. Cancer Res. 68, 7561–7569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wickström S. A., Masoumi K. C., Khochbin S., Fässler R., Massoumi R. (2010) CYLD negatively regulates cell-cycle progression by inactivating HDAC6 and increasing the levels of acetylated tubulin. EMBO J. 29, 131–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. (1992) Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell 71, 323–334 [DOI] [PubMed] [Google Scholar]
- 14. Meyerson M., Harlow E. (1994) Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell Biol. 14, 2077–2086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ohtsubo M., Theodoras A. M., Schumacher J., Roberts J. M., Pagano M. (1995) Human cyclin E, a nuclear protein essential for the G1 to S phase transition. Mol. Cell. Biol. 15, 2612–2624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pagano M., Draetta G., Jansen-Dürr P. (1992) Association of cdk2 kinase with the transcription factor E2F during S phase. Science 255, 1144–1147 [DOI] [PubMed] [Google Scholar]
- 17. Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. (1992) Cyclin A is required at two points in the human cell cycle. EMBO J. 11, 961–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Draetta G., Beach D. (1988) Activation of cdc2 protein kinase during mitosis in human cells. Cell cycle-dependent phosphorylation and subunit rearrangement. Cell 54, 17–26 [DOI] [PubMed] [Google Scholar]
- 19. Tirián L., Hlavanda E., Oláh J., Horváth I., Orosz F., Szabó B., Kovács J., Szabad J., Ovádi J. (2003) TPPP/p25 promotes tubulin assemblies and blocks mitotic spindle formation. Proc. Natl. Acad. Sci. U.S.A. 100, 13976–13981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Croft D. R., Olson M. F. (2006) The Rho GTPase effector ROCK regulates cyclin A, cyclin D1, and p27Kip1 levels by distinct mechanisms. Mol. Cell. Biol. 26, 4612–4627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cassimeris L. (2002) The oncoprotein 18/stathmin family of microtubule destabilizers. Curr. Opin. Cell Biol. 14, 18–24 [DOI] [PubMed] [Google Scholar]
- 22. Ookata K., Hisanaga S., Sugita M., Okuyama A., Murofushi H., Kitazawa H., Chari S., Bulinski J. C., Kishimoto T. (1997) MAP4 is the in vivo substrate for CDC2 kinase in HeLa cells. Identification of an M-phase specific and a cell cycle-independent phosphorylation site in MAP4. Biochemistry 36, 15873–15883 [DOI] [PubMed] [Google Scholar]
- 23. Masson D., Kreis T. E. (1995) Binding of E-MAP-115 to microtubules is regulated by cell cycle-dependent phosphorylation. J. Cell Biol. 131, 1015–1024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Charrasse S., Lorca T., Dorée M., Larroque C. (2000) The Xenopus XMAP215 and its human homologue TOG proteins interact with cyclin B1 to target p34cdc2 to microtubules during mitosis. Exp. Cell Res. 254, 249–256 [DOI] [PubMed] [Google Scholar]
- 25. Songyang Z., Blechner S., Hoagland N., Hoekstra M. F., Piwnica-Worms H., Cantley L. C. (1994) Use of an oriented peptide library to determine the optimal substrates of protein kinases. Curr. Biol. 4, 973–982 [DOI] [PubMed] [Google Scholar]
- 26. Srinivasan J., Koszelak M., Mendelow M., Kwon Y. G., Lawrence D. S. (1995) The design of peptide-based substrates for the cdc2 protein kinase. Biochem. J. 309, 927–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Songyang Z., Lu K. P., Kwon Y. T., Tsai L. H., Filhol O., Cochet C., Brickey D. A., Soderling T. R., Bartleson C., Graves D. J., DeMaggio A. J., Hoekstra M. F., Blenis J., Hunter T., Cantley L. C. (1996) A structural basis for substrate specificities of protein Ser/Thr kinases. Primary sequence preference of casein kinases I and II, NIMA, phosphorylase kinase, calmodulin-dependent kinase II, CDK5, and Erk1. Mol. Cell Biol. 16, 6486–6493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lehotzky A., Tirián L., Tökési N., Lénárt P., Szabó B., Kovács J., Ovádi J. (2004) Dynamic targeting of microtubules by TPPP/p25 affects cell survival. J. Cell Sci. 117, 6249–6259 [DOI] [PubMed] [Google Scholar]
- 29. Lehotzky A., Lau P., Tokési N., Muja N., Hudson L. D., Ovádi J. (2010) Tubulin polymerization-promoting protein (TPPP/p25) is critical for oligodendrocyte differentiation. Glia 58, 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li A., Saito M., Chuang J. Z., Tseng Y. Y., Dedesma C., Tomizawa K., Kaitsuka T., Sung C. H. (2011) Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat. Cell Biol. 13, 402–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim S., Zaghloul N. A., Bubenshchikova E., Oh E. C., Rankin S., Katsanis N., Obara T., Tsiokas L. (2011) NdeI-mediated inhibition of ciliogenesis affects cell cycle re-entry. Nat. Cell Biol. 13, 351–360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Harvey S. A., Anderson S. C., SundarRaj N. (2004) Downstream effects of ROCK signaling in cultured human corneal stromal cells. Microarray analysis of gene expression. Invest. Ophthalmol. Vis. Sci. 45, 2168–2176 [DOI] [PubMed] [Google Scholar]
- 33. Chen J., Guerriero E., Lathrop K., SundarRaj N. (2008) Rho/ROCK signaling in regulation of corneal epithelial cell cycle progression. Invest. Ophthalmol. Vis. Sci. 49, 175–183 [DOI] [PubMed] [Google Scholar]
- 34. Zhang S., Tang Q., Xu F., Xue Y., Zhen Z., Deng Y., Liu M., Chen J., Liu S., Qiu M., Liao Z., Li Z., Luo D., Shi F., Zheng Y., Bi F. (2009) RhoA regulates G1-S progression of gastric cancer cells by modulation of multiple INK4 family tumor suppressors. Mol Cancer Res. 7, 570–580 [DOI] [PubMed] [Google Scholar]
- 35. Ishizaki T., Uehata M., Tamechika I., Keel J., Nonomura K., Maekawa M., Narumiya S. (2000) Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol. Pharmacol. 57, 976–983 [PubMed] [Google Scholar]
- 36. Kosako H., Yoshida T., Matsumura F., Ishizaki T., Narumiya S., Inagaki M. (2000) Rho-kinase/ROCK is involved in cytokinesis through the phosphorylation of myosin light chain and not ezrin/radixin/moesin proteins at the cleavage furrow. Oncogene 19, 6059–6064 [DOI] [PubMed] [Google Scholar]
- 37. Lowery D. M., Clauser K. R., Hjerrild M., Lim D., Alexander J., Kishi K., Ong S. E., Gammeltoft S., Carr S. A., Yaffe M. B. (2007) Proteomic screen defines the Polo-box domain interactome and identifies Rock2 as a Plk1 substrate. EMBO J. 26, 2262–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]