Background: Hyperphosphorylated Tau is a component of neurofibrillary tangles, the pathological hallmark in brains with tauopathies.
Results: Pin1 binds phospho-Tau and stimulates its dephosphorylation at Cdk5-mediated phosphorylation sites.
Conclusion: Efficient Tau dephosphorylation at Alzheimer-related sites requires Pin1 activity, thereby preventing Tau hyperphosphorylation.
Significance: Disruption of Pin1-dependent facilitation of Tau dephosphorylation may be a critical mechanism underlying the etiology of tauopathies.
Keywords: Alzheimer Disease, CDK (Cyclin-dependent Kinase), Phosphorylation, Prolyl Isomerase, Tau, Biacore, Cdk5, FTDP-17, Dephosphorylation
Abstract
Neurodegenerative diseases associated with the pathological aggregation of microtubule-associated protein Tau are classified as tauopathies. Alzheimer disease, the most common tauopathy, is characterized by neurofibrillary tangles that are mainly composed of abnormally phosphorylated Tau. Similar hyperphosphorylated Tau lesions are found in patients with frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) that is induced by mutations within the tau gene. To further understand the etiology of tauopathies, it will be important to elucidate the mechanism underlying Tau hyperphosphorylation. Tau phosphorylation occurs mainly at proline-directed Ser/Thr sites, which are targeted by protein kinases such as GSK3β and Cdk5. We reported previously that dephosphorylation of Tau at Cdk5-mediated sites was enhanced by Pin1, a peptidyl-prolyl isomerase that stimulates dephosphorylation at proline-directed sites by protein phosphatase 2A. Pin1 deficiency is suggested to cause Tau hyperphosphorylation in Alzheimer disease. Up to the present, Pin1 binding was only shown for two Tau phosphorylation sites (Thr-212 and Thr-231) despite the presence of many more hyperphosphorylated sites. Here, we analyzed the interaction of Pin1 with Tau phosphorylated by Cdk5-p25 using a GST pulldown assay and Biacore approach. We found that Pin1 binds and stimulates dephosphorylation of Tau at all Cdk5-mediated sites (Ser-202, Thr-205, Ser-235, and Ser-404). Furthermore, FTDP-17 mutant Tau (P301L or R406W) showed slightly weaker Pin1 binding than non-mutated Tau, suggesting that FTDP-17 mutations induce hyperphosphorylation by reducing the interaction between Pin1 and Tau. Together, these results indicate that Pin1 is generally involved in the regulation of Tau hyperphosphorylation and hence the etiology of tauopathies.
Introduction
The neuropathological hallmarks of Alzheimer disease (AD)2 include neurofibrillary tangles, which are composed mainly of abnormally phosphorylated microtubule-associated protein Tau (1). Aggregates of hyperphosphorylated Tau are also found in other neurodegenerative diseases that are collectively called tauopathies including Pick disease, progressive supranuclear palsy, corticobasal degeneration, and frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP-17) (2). FTDP-17 is an inherited form of tauopathy that is caused by mutations within the tau gene and is characterized by lesions containing hyperphosphorylated Tau (3–5). Genetically modified mice featuring the tau mutations of FTDP-17 developed similar aggregates of hyperphosphorylated Tau and showed dementia-like memory impairments, indicating a causative role of the tau mutations (2, 6, 7). However, it is not yet known why these Tau mutations induce Tau aggregation and neurodegeneration. Understanding the molecular mechanisms that induce Tau hyperphosphorylation and aggregation in AD and FTDP-17 may be critical to unravel the processes underlying the etiology of tauopathies.
Tau in neurofibrillary tangles is phosphorylated at more than 30 sites with most of them being located in the flanking regions of the microtubule-binding repeats (8–10). Many protein kinases have been implicated in Tau phosphorylation. Proline-directed protein kinases (PDPKs) such as glycogen synthase kinase 3β (GSK3β) and cyclin-dependent kinase 5 (Cdk5) have been thought to be critically involved in abnormal Tau phosphorylation because many proline-directed sites are hyperphosphorylated in Tau (2, 8, 10–12).
Cdk5, originally purified as Tau kinase II (13), is a serine/threonine kinase with pleiotropic functions in postmitotic neurons (14, 15). Cdk5 requires binding of the activation subunit, p35, for activation. The active holoenzyme Cdk5-p35 is localized to the cell membrane via the myristoylation of p35 (16–18). Membrane-associated Cdk5-p35 exhibits moderate kinase activity due to a short half-life of p35, which is degraded by the proteasome (19). Alternatively, p35 can be cleaved to p25 by calpain, and the Cdk5-p25 holoenzyme can subsequently relocalize to the cytoplasm and/or nucleus (16, 20, 21). The Cdk5 activator, p25, has a long half-life (16, 21) and induces aberrant Cdk5 activity toward Tau (22, 23). Consistently, silencing of Cdk5 reduced the phosphorylation of Tau in primary neuronal cultures and in brain and decreased the number of neurofibrillary tangles in the hippocampi of transgenic Alzheimer disease mice (24). However, it is not clear how Cdk5-p25 causes Tau hyperphosphorylation and aggregation.
In FTDP-17 patients and transgenic mouse models, Tau is hyperphosphorylated (2, 8, 10, 11, 25). In contrast, FTDP-17 mutant Tau is less phosphorylated than wild-type (WT) Tau in vitro or in cell cultures (26–29). These studies suggest that disruption of dephosphorylation rather than increased phosphorylation contributes to the hyperphosphorylated state of Tau. Accordingly, protein phosphatase 2A (PP2A) activity is decreased in AD brains (30–32), and highly phosphorylated Tau in paired helical filament is relatively resistant to dephosphorylation by PP2A (33). Furthermore, PP2A preferentially dephosphorylated phospho-(Ser/Thr)-Pro motifs in trans conformation when synthetic phospho-Thr-231 Tau peptide was used as a substrate (34, 35). Peptidyl-prolyl cis/trans isomerase, NIMA-interacting 1 (Pin1) is a peptidylprolyl isomerase composed of two functional domains, the N-terminal WW domain, which binds to phosphorylated Ser or Thr at proline-directed sites, and the C-terminal cis/trans isomerase domain (36, 37). Pin1 is found in neurofibrillary tangles, and Tau hyperphosphorylation is reported in Pin1-deficient mice (38). Hence, Pin1 could be a critical regulator of Tau dephosphorylation to (i) restore physiological Tau function such as microtubule binding and (ii) suppress neurofibrillary tangle formation by enhancing dephosphorylation by PP2A. We reported recently that Pin1 stimulates dephosphorylation of Tau phosphorylated by Cdk5-p25, suggesting that there are more Pin1 binding motifs in Tau (39). The Pin1 binding sites in Tau were shown to be phospho-Thr-231 (34, 40) and phospho-Thr-212 (41). However, these two Pin1 binding sites alone cannot prevent abnormal Tau phosphorylation at all the other hyperphosphorylation sites. Therefore, we wanted to assess Pin1 binding at additional Tau phosphorylation sites.
Here we analyzed interaction of Pin1 with Cdk5-mediated Tau phosphorylation sites using a GST pulldown assay and Biacore technique. We observed that Pin1 binds to Tau and stimulates its dephosphorylation at all Cdk5 phosphorylation sites including Ser-202, Thr-205, Ser-235, and Ser-404. Furthermore, Tau carrying the FTDP-17 mutation P301L or R406W showed slightly weaker binding to Pin1 than WT Tau, suggesting that FTDP-17 mutations induce Tau hyperphosphorylation by reducing its interaction with Pin1.
EXPERIMENTAL PROCEDURES
Antibodies and Chemicals
Anti-Pin1 and anti-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), anti-human Tau (A0024) was obtained from Dako Denmark (Glostrup, Denmark), anti-Tau Ab-2 (Tau5) was purchased from Thermo Fisher Scientific Anatomical Pathology (Fremont, CA), Tau-C was prepared as described previously (42), anti-phospho-Tau (Ser(P)-202, Thr(P)-205, and Ser(P)-404) was obtained from Invitrogen, and anti-phospho-Tau (Ser(P)-235) was from Abcam (Cambridge, UK). Goat anti-GST antibody was purchased from GE Healthcare. Phos-tag acrylamide and 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride (Pefabloc) were purchased from Wako Chemicals (Osaka, Japan). Leupeptin was obtained from the Peptide Institute (Osaka, Japan). 5-Hydroxy-1,4-naphthoquinone (juglone) and Dulbecco's modified Eagle's medium (DMEM) were from Sigma-Aldrich. HilyMax transfection reagent was from Dojindo Laboratories (Kumamoto, Japan).
Plasmid Construction of Mutant Tau
The 412-amino acid isoform (1N4R; containing one N-terminal insertion and four microtubule-binding repeats) of human Tau was used (29). Alanine mutants of Tau at Ser-202 (S202A), Thr-205 (T205A), Thr-212 (T212A), Thr-231 (T231A), or Ser-235 (S235A) were generated using 1N4R human tau as a template with the QuikChange site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The primers used were as follows: 5′-GGGAGTGCCTGGGGCGCCGGGGCTGCT-3′ and 5′-AGCAGCCCCGGCGCCCCAGGCACTCCC-3′ for S202A, 5′-GCTCCCCAGGCGCTCCCGGCAGCCGCT-3′ and 5′-AGCGGCTGCCGGGAGCGCCTGGGGAGC-3′ for T205A, 5′-CAGCCGCTCCCGCGCCCCGTCCCTTCC-3′ and 5′-GGAAGGGACGGGGCGCGGGAGCGGCTG-3′ for T212A, 5′-GGCAGTGGTCCGTGCTCCACCCAAGTC-3′ and 5′-GACTTGGGTGGAGCACGGACCACTGCC-3′ for T231A, and 5′-TACTCCACCCAAGGCGCCGTCTTCCGC-3′ and 5′-GCGGAAGACGGCGCCTTGGGTGGAGTA-3′ for S235A. Ser-202, Thr-205, Ser-235, and Ser-404 were mutated to Ala in Tau-4A by PCR using the primers described above. Tau-3A mutants with a single phosphorylation site were constructed by adding back one of Ser-202, Thr-205, Ser-235, or Ser-404 to Tau-4A.
Expression and Purification of Recombinant Human Tau
Expression of recombinant human Tau (either the WT or Ala mutants) and FTDP-17 mutant Tau (P301L and R406W) was performed in Escherichia coli BL21-CodonPlus (DE3)-RP as described previously (29). Tau proteins were purified from heat-treated extracts using a Mono S column with an ÄKTA purifier (GE Healthcare). The amount of Tau protein was estimated by Coomassie Brilliant Blue (CBB) staining of gels using bovine serum albumin (BSA) as a standard.
Cloning, Mutant Construction, and Expression of Pin1
Pin1 cDNA was amplified from an adult mouse brain cDNA library by PCR using oligonucleotides 5′-GAATTCATGGCGGACGAGGAGAAG-3′ and 5′-CTCGAGTCATTCTGTGCGCAGGAT-3′ as the forward and reverse primers, respectively. GST-Pin1 was generated by inserting Pin1 cDNA into EcoRI/Xho1 sites of pGEX4T-1 (GE Healthcare). Asp mutants of Pin1 at Tyr-23 (Y23D) and Trp-34 (W34D) were generated using mouse Pin1 as a template with the QuikChange site-directed mutagenesis kit. The primers used were as follows: 5′-CAGGCCGGGTGGACTACTTCAATCACA-3′ and 5′-TGTGATTGAAGTAGTCCACCCGGCCTG-3′ for Y23D and 5′-CACCAACGCCAGCCAGGACGAGCGGCCCAGCGGCG-3′ and 5′-CGCCGCTGGGCCGCTCGTCCTGGCTGGCGTTGGTG-3′ for W34D. GST-Pin1 or its mutants were expressed in the BL21 E. coli strain (DE3) and purified from the cell extract using GSH beads (GE Healthcare).
Preparation of Extracts from COS-7 Cells or Mouse or Human Brain
COS-7 cells were maintained in DMEM supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 0.1 mg/ml streptomycin. COS-7 cells were transfected with p25, Cdk5, tau, and/or Pin1 expression plasmids by HilyMax transfection reagent (Dojindo Laboratories). Twenty-four hours after transfection, the cells were lysed in 20 mm HEPES, pH 7.4, 1 mm MgCl2, 100 mm NaCl, 0.5% Nonidet P-40, 0.4 mm Pefabloc, 10 μg/ml leupeptin, and 1 mm dithiothreitol (DTT) on ice for 10 min. After centrifugation at 15,000 × g for 15 min, the supernatant was collected as a COS-7 cell extract. Four-week-old C57BL/6J mice were obtained from Sankyo Labo Service (Tokyo, Japan). Brain cortex was homogenized in 20 mm HEPES, pH 7.4, 100 mm NaCl, 0.1% Nonidet P-40, 2 mm MgCl2, 1 mm EGTA, 0.4 mm Pefabloc, 10 μg/ml leupeptin, and 1 mm DTT. After centrifugation at 15,000 × g for 20 min, the supernatant was used as a brain extract. The extract was incubated with or without 1 mm ATP and/or 20 μm roscovitine (Calbiochem) at 35 °C for 1 h to induce phosphorylation of Tau. The human brain tissues were kindly provided by the University College London Queen Square Brain Bank (London, UK). Lysates were prepared from snap frozen human brain tissues from a male FTDP-17 patient and three male non-AD controls. Small frozen tissue samples were homogenized in ice-cold P2 buffer containing protease and phosphatase inhibitors as described previously (12).
Preparation of Cdk5-p25 from Sf9 Cells and Phosphorylation of Tau
Cdk5-p25 was purified from Sf9 cells infected by baculovirus encoding Cdk5 and p25 as described previously (43). Tau at 0.1 mg/ml was phosphorylated by Cdk5-p25 at 35 °C for 1.5 h in 10 mm MOPS, pH 6.8, 2 mm MgCl2, 0.1 mm EDTA, 0.1 mm EGTA, 0.1% Nonidet P-40, and 1 mm ATP or 0.1 mm [γ-32P]ATP. Phosphorylation was detected by autoradiography after SDS-PAGE on a 10% polyacrylamide gel, and the extent of Tau phosphorylation was quantified using a FLA7000 bioimage analyzer (Fujifilm, Tokyo, Japan). Two-dimensional phosphopeptide map analysis of phospho-Tau was performed as described previously (29).
GST Pulldown Assay
GST or GST-Pin1 was incubated with GSH-Sepharose 4B (GE Healthcare) at 4 °C for 1 h and then incubated with mouse brain extract or E. coli extract containing Tau at 4 °C for 2 h. The beads were washed five times with HEPES buffer (20 mm HEPES, pH 7.4, 100 mm NaCl, 2 mm MgCl2, 0.5% Nonidet P-40, 1 mm EGTA, 10 mm NaF, 10 mm β-glycerophosphate, 1 mm Na3VO4, 10 μg/ml leupeptin, 0.2 mm Pefabloc, and 1 mm DTT), and the proteins bound to beads were eluted by boiling in SDS sample buffer.
Surface Plasmon Resonance Analysis of the Binding between Pin1 and Phosphorylated Tau
The affinity of phosphorylated Tau binding to Pin1 was measured with a Biacore 2000 surface plasmon resonance spectroscope (GE Healthcare) as described previously (44). Briefly, anti-GST antibody was immobilized on a CM5 sensor chip using an amine coupling kit (GE Healthcare) according to the manufacturer's protocol. All measurements were performed using 10 mm HEPES, pH 7.0, 100 mm NaCl, 1.5 mm EDTA, and 0.01% Nonidet P-40. Purified Tau was injected at a rate of 5 μl/min at 25 °C. For the kinetic analysis, various concentrations of Tau were loaded onto the sensor surface to equilibrate the Tau-Pin1 interaction, and the amount of the complex (the response at equilibrium (Req)) was measured in resonance units. The correlation between Req, the concentration of ligand (C) passed over the sensor surface, and the total binding capacity (Rmax) of the immobilized protein was calculated as: Req/C = KaRmax − KaReq. The association constant (Ka) was determined from the plot of Req/C versus Req estimated at different Tau concentrations by Scatchard plot analysis. Statistical analysis was performed using a linear regression method.
SDS-PAGE, Phos-tag SDS-PAGE, and Immunoblotting
SDS-PAGE was performed according to the method of Laemmli (45) using a 12.5% polyacrylamide gel. Phos-tag SDS-PAGE was performed with 7.5% polyacrylamide gels containing 50 μm Phos-tag acrylamide and 150 μm MnCl2 (46, 47). Proteins were transferred to a PVDF membrane using a submerged blotting system. The reaction was detected using an enhanced chemiluminescence detection kit (GE Healthcare).
RESULTS
Cdk5 Phosphorylation Is a Prerequisite for Pin1 Binding to Tau
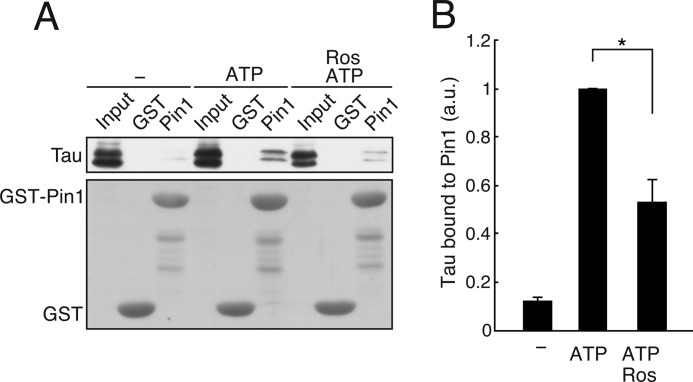
We have reported previously that recombinant Tau phosphorylated by Cdk5-p35 is dephosphorylated by PP2A faster in WT mouse brain extract than in Pin1-deficient mouse brain extract (39). This result suggests that Pin1 recognizes Cdk5-phosphorylated Tau to stimulate its dephosphorylation. However, there has been no report describing Pin1 binding to Cdk5-mediated Tau phosphorylation sites. Therefore, we wanted to know whether endogenous Tau in mouse brain binds to Pin1. Tau was phosphorylated by incubation with ATP at 35 °C for 1 h in the presence or absence of the Cdk5 inhibitor roscovitine, and the binding of Tau to Pin1 was assessed using a GST-Pin1 pulldown assay. Incubation with ATP increased the amount of Tau bound to Pin1 more than 7-fold. In contrast, application of roscovitine decreased the amount of Tau bound to Pin1 by more than 30% (Fig. 1). These results suggest that Cdk5-mediated phosphorylation facilitates the binding of Tau to Pin1. Other Tau kinases (e.g. GSK3β) that phosphorylate Tau at similar sites may also increase the interaction between Tau and Pin1.
FIGURE 1.
Endogenous Tau phosphorylated by Cdk5 in mouse brain extracts binds to Pin1. Mouse brain extract was incubated with ATP in the presence or absence of 20 μm roscovitine (Ros) at 35 °C for 1 h. The incubated extracts were subjected to a pulldown assay using GST-Pin1 (Pin1) or GST. A, the binding of Tau was detected by immunoblotting with anti-Tau antibody (Tau5) (upper panel). CBB staining of GST-Pin1 and GST is shown in the lower panel. B, quantification of Tau bound to GST-Pin1. The results are expressed as mean ± S.E. (error bars) (n = 3; *, p < 0.05). a.u., arbitrary units.
Pin1 Binds to Tau When Phosphorylated by Cdk5-p25 and GSK3β but Not by the Catalytic Subunit of cAMP-dependent Protein Kinase (PKA)
To demonstrate more conclusively that Pin1 can bind to Tau phosphorylated by Cdk5, recombinant Tau was phosphorylated by Cdk5-p25 in vitro, and its binding to Pin1 was examined. Tau was also phosphorylated by GSK3β, another PDPK, and the PKA, a non-PDPK, and their binding to Pin1 was examined as a positive and negative control, respectively. Tau phosphorylated by Cdk5-p25 or GSK3β, but not by PKA, bound to GST-Pin1 (Fig. 2A). No binding was observed with control GST beads. These results indicate that Pin1 binds to Tau at proline-directed Ser/Thr phosphorylation sites that are modified by Cdk5 and GSK3β.
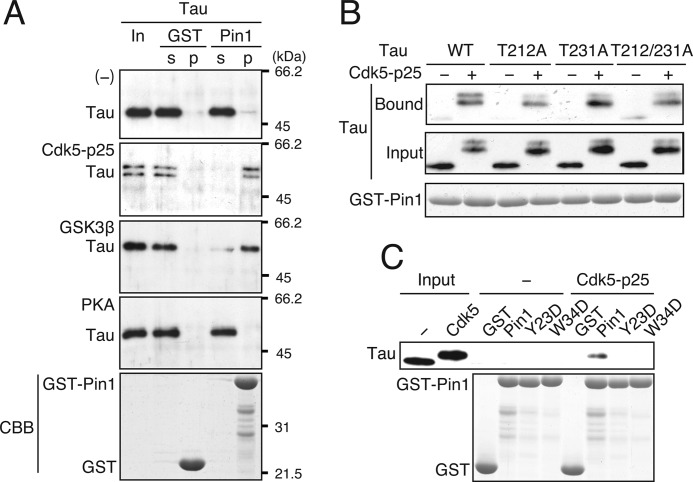
FIGURE 2.
Recombinant Tau phosphorylated in vitro by Cdk5-p25 or GSK3β, but not by PKA, binds to Pin1. A, recombinant Tau phosphorylated by Cdk5-p25, GSK3β, or PKA was subjected to a GST-Pin1 pulldown assay. s, supernatant; p, pellet. Unphosphorylated Tau is shown in the first panel ([−]). Tau bound to GST-Pin1 was detected by immunoblotting with Tau5 (first to fourth panels). The input is shown in the lanes labeled “In.” GST-Pin1 and GST are shown by CBB staining (lower panel). B, Tau binds to Pin1 at phosphorylation sites other than Thr-212 and Thr-231. Tau-T212A, Tau-T231A, or Tau-T212A/T231A was phosphorylated by Cdk5-p25 and subjected to the GST pulldown assay. Tau bound to Pin1 (upper panel) and in the input (second panel) was detected by immunoblotting with Tau5. CBB staining shows GST-Pin1 (lower panel). C, the WW domain-dependent binding of Pin1 to Cdk5-phosphorylated Tau. Unphosphorylated Tau (−) or Cdk5-phosphorylated Tau (Cdk5-p25) was subjected to the GST pulldown assay using an Asp mutant of Pin1 at Tyr-23 (Y23D) or Trp-34 (W34D) in the WW domain. Input shows unphosphorylated (−) and Cdk5-phosphorylated (Cdk5) Tau. CBB staining shows GST-Pin1 and GST (lower panel).
There are six isoforms of Tau in the human brain (48) that differ in the N-terminal insertion of exons 2 and/or 3 and in the C terminus of exon 10. The latter splicing produces Tau isoforms with three (3R) or four (4R) microtubule-binding repeats. The ratio of Tau 3R to 4R in neurofibrillary tangles differs between tauopathies (2, 49). We next examined whether individual Tau isoforms have distinct binding affinities for Pin1. All Tau isoforms bound to Pin1 after phosphorylation by Cdk5, and the binding did not differ between isoforms (supplemental Fig. 1). Therefore, we decided to use the Tau 1N4R isoform in the following experiments.
Pin1 Binds to Tau at Phosphorylation Sites Other than Thr-212 and Thr-231
It has been reported that Thr-212 and Thr-231 in Thr-Pro sequences are the Pin1-binding sites (40, 41). Thr-212 and Thr-231 are not major Cdk5-mediated phosphorylation sites (29); nevertheless, it is possible that these sites can be phosphorylated by Cdk5 in vitro and hence explain the increased Pin1 binding after Cdk5-mediated phosphorylation. To test this possibility, we generated mutant Tau in which the threonine residues at position 212 and/or 231 were replaced with non-phosphorylatable alanine (T212A, T231A, and T212A/T231A), and we examined the binding of the mutant Tau to GST-Pin1 after Cdk5 phosphorylation (Fig. 2B). The upward shift of Tau-T212A, Tau-T231A, and Tau-T212A/T231A was similar to that of WT Tau, suggesting that these sites are not efficiently phosphorylated by Cdk5. Consistently, an in vitro kinase assay revealed that the Tau mutants (T212A, T231A, and T212A/T231A) exhibited phosphorylation levels comparable with those of WT Tau (supplemental Fig. 2). All of these Ala mutants phosphorylated by Cdk5-p25 bound to Pin1 (Fig. 2B). The results suggest that Pin1 can bind to sites other than Thr-212 and Thr-231 in Cdk5-phosphorylated Tau. Pin1 binds to the phospho-(Ser/Thr)-Pro sequences via its N-terminal WW domain (36, 37). To confirm that Cdk5-phosphorylated Tau binds to Pin1 via the WW domain, we generated Asp or Trp mutants of Pin1 at the critical Tyr-23 residue (Pin1-Y23D) or Trp-34 residue (Pin1-W34D) in the WW domain, and we examined their binding to Cdk5-phosphorylated Tau. These Pin1 mutants did not bind to phosphorylated Tau (Fig. 2C), indicating that Pin1 binding to Cdk5-phosphorylated Tau is dependent on WW domain-mediated interaction.
The Major Cdk5 Phosphorylation Sites in Tau Are Ser-202, Ser-235, Ser-404, and Thr-205
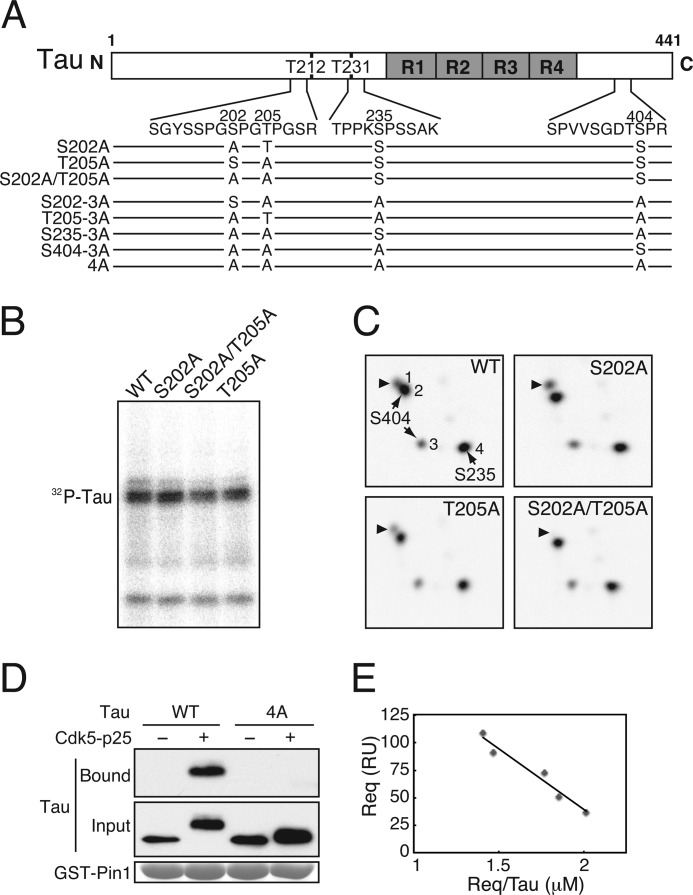
The Cdk5 phosphorylation sites in Tau reported so far include Thr-153, Thr-181, Thr-199, Ser-202, Thr-205, Thr-212, Ser-214, Thr-231, Ser-235, Ser-396, and Ser-404 (8, 11, 29, 39, 50). Some of these sites may be minor sites that were identified with phosphorylation-specific antibodies. To identify Pin1-binding Cdk5 phosphorylation sites, we first needed to identify all major Cdk5 phosphorylation sites. We have previously identified the major Cdk5 phosphorylation sites in Tau as Ser-202, Ser-235, and Ser-404 biochemically using two-dimensional phosphopeptide mapping (29, 39, 51). However, there was ambiguity about Thr-205, which is included in the same tryptic peptide as Ser-202 (Fig. 2A, amino acid sequences shown between the domain structure of Tau and mutants). We then determined whether Thr-205 could also be phosphorylated by Cdk5-p25. Ala mutants of Tau at Ser-202, Thr-205, or both were phosphorylated by Cdk5-p25 (Fig. 3B) and then subjected to two-dimensional phosphopeptide map analysis (Fig. 3C). WT Tau phosphorylated by Cdk5-p25 is shown in Fig. 3A, which shows four major phosphorylation spots that are identical to our previous results (29, 39). We previously assigned each spot to its corresponding phosphorylation site, Ser-202 for spot 1, Ser-404 for spots 2 and 3, and Ser-235 for spot 4. To determine whether phospho-Thr-205 is included in the same peptide as phospho-Ser-202, the two-dimensional phosphopeptide pattern of Tau-S202A (Fig. 3B) was compared with that of WT Tau. Spot 1 was still detected (Fig. 3C, S202A). To identify the remaining phosphorylation site in spot 1, we generated a Tau-S202A/T205A mutant and subjected it to two-dimensional phosphopeptide map analysis after phosphorylation by Cdk5-p25. Spot 1 disappeared in the double mutant S202A/T205A (Fig. 3C). Detection of spot 1 in T205A (Fig. 3C) confirmed the phosphorylation of Ser-202. These results indicate that spot 1 is the phosphopeptide containing either phospho-Ser-202 or phospho-Thr-205. Thus, Thr-205 in addition to Ser-202, Ser-235, and Ser-404 can be phosphorylated by Cdk5-p25 in in vitro.
FIGURE 3.
Tau lacking four Cdk5 phosphorylation sites does not bind to Pin1. A, domain structure and major Cdk5 phosphorylation sites in the longest human Tau comprising 441 amino acids. R1–R4 are microtubule-binding repeats, and Thr-212 (T212) and Thr-231 (T231) are the reported Pin1-binding sites. Amino acid sequences of tryptic peptides containing the major Cdk5 phosphorylation sites are indicated below. Tau mutants with Ala replacement at the Cdk5 phosphorylation sites used in this study are also shown. B, an autoradiograph of WT Tau and Ala mutants at Ser-202 and/or Thr-205 phosphorylated by Cdk5-p25. C, two-dimensional phosphopeptide map of WT Tau and Ala mutants at Ser-202 and/or Thr-205 phosphorylated by Cdk5-p25. Four spots, 1–4, were detected with Tau WT. The phosphorylation spots 4 and 2/3 correspond to Ser-235 and Ser-404, respectively (29, 39). Arrowheads indicate the spot including phospho-Ser-202 or phospho-Thr-205. D, Tau-4A does not bind to Pin1. Tau WT or Tau-4A was phosphorylated by Cdk5-p25, and their binding to Pin1 was examined by a GST-Pin1 pulldown assay (Bound). Input is shown in the middle panel, and GST-Pin1 is shown in the lower panel by CBB staining. E, affinity of Cdk5-phosphorylated Tau binding to Pin1. WT Tau phosphorylated by Cdk5-p25 was applied to a Pin1-bound sensor tip in a Biacore instrument as described under “Experimental Procedures.” The Scatchard plot was obtained by plotting the resonance units (RU) at equilibrium (Req)/Tau concentration (μm) against resonance units. A straight line was drawn using the least squares method. The linear regression equation between Req and Req/Tau (μm) was: Req = Rmax − Req/Tau × Kd (95% confidence interval for the regression coefficient, −1.53 × 10−4 to −0.67 × 10−4, and the coefficient of determination was r2 = 0.96). The calculated Kd value was 1.11 ± 0.14 × 10−4 m (±S.D.).
Pin1 Binds to Tau at Any of the Four Cdk5 Phosphorylation Sites
To see whether Pin1 binds to Cdk5 phosphorylation sites in Tau, we generated a Tau-4A mutant in which all four major Cdk5 phosphorylation sites at Ser-202, Thr-205, Ser-235, and Ser-404 were mutated to alanine. Although a slight upward shift remained, the Cdk5-dependent upward shift was largely abolished in Tau-4A (Fig. 3D, lane 4, Input), confirming that these four residues are the major Cdk5 phosphorylation sites. Cdk5-phosphorylated Tau-4A abolished the binding of Pin1 to Tau (Fig. 3D), demonstrating that Pin1 binds to Tau at Cdk5-mediated sites. Next, we determined the affinity of WT Tau phosphorylated by Cdk5-p25 binding to Pin1 using Biacore technology. The amount of Tau bound to a constant amount of GST-Pin1 in the sensor tip was measured as resonance units at five different concentrations of Tau (Fig. 3E). These experiments were performed in the presence of 0.1 m NaCl, the same concentration used for the GST binding assay. Under this condition, we did not observe the binding of unphosphorylated Tau to Pin1 (data not shown). In contrast, phosphorylated Tau did bind to Pin1, and the amount of bound Tau increased with rising Tau concentrations with a dissociation constant (Kd) of 1.11 ± 0.14 × 10−4 m (Fig. 3E).
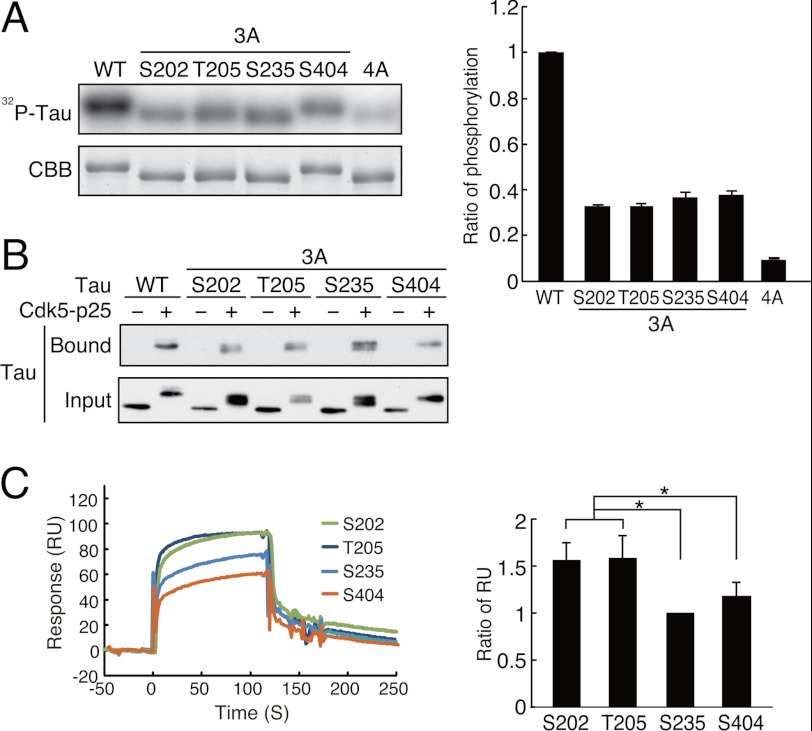
To identify the Pin1-binding sites conclusively, we constructed four triple alanine (3A) mutants of Tau by adding back Ser-202 (Tau-S202–3A), Thr-205 (Tau-T205–3A), Ser-235 (Tau-S235–3A), or Ser-404 (Tau-S404–3A) to Tau-4A (Fig. 3A, lower panel). Consistent with the idea that Cdk5 phosphorylates Tau mainly at Ser-202, Thr-205, Ser-235, and Ser-404, all of the 3A mutants were phosphorylated by Cdk5 to a similar extent, exhibiting about one-third of WT Tau phosphorylation (Fig. 4A). Moreover, Tau that was mutated at all four sites (Tau-4A) acted as a poor substrate for Cdk5. All four 3A mutants bound to Pin1 in a phosphorylation-dependent manner (Fig. 4B, Bound), indicating that Pin1 can recognize and bind to any of the major Cdk5-mediated sites including Ser-202, Thr-205, Ser-235, and Ser-404.
FIGURE 4.
Tau with a single phosphorylation at any site of Ser-202, Thr-205, Ser-235, or Ser-404 binds to Pin1 after phosphorylation by Cdk5-p25. A, phosphorylation of Tau-3A mutants together with Tau WT and Tau-4A by Cdk5-p25. Tau WT and mutants at 0.1 mg/ml were phosphorylated by Cdk5-p25 in the presence of 0.1 mm [γ-32P]ATP for 2 h at 35 °C. The extent of phosphorylation (right panel) was measured with an image analyzer (left panel, 32P-Tau) followed by normalization with Tau protein (left panel, CBB). B, the binding of phospho-Tau-3A mutants to Pin1. After phosphorylation with (+) or without (−) Cdk5-p25, the WT Tau or Tau-3A mutants were subjected to a GST-Pin1 pulldown assay. Tau was detected by immunoblotting with Tau5. C, binding of Cdk5-phosphorylated Tau-3A to Pin1 was measured on a Biacore instrument. Tau-3A mutants were phosphorylated by Cdk5-p25 followed by measurement of the amount bound to Pin1 measured on a Biacore instrument. The binding profiles are shown as resonance units (RU) in the left panel, and the relative ratio of each Tau-3A bound to Pin1 is shown in the right panel (mean ± S.E. (error bars); n = 4; *, p < 0.05).
We wondered whether there might be differences between the binding strength of the individual mutants, and hence we compared their site-specific binding to Pin1 using Biacore technology. The protein concentration and phosphorylation were adjusted carefully in the Tau-3A mutants. When the extent of phosphorylation was the same, Tau-Ser202–3A and Tau-Thr205–3A showed slightly greater binding than did Tau-Ser235–3A and Tau-Ser404–3A (Fig. 4C). We observed similar results with three different preparations of Tau but could not derive reliable Kd values at the respective sites probably because of the relatively weak binding affinity.
Effects of Pin1 on Dephosphorylation at Cdk5-mediated Tau Phosphorylation Sites
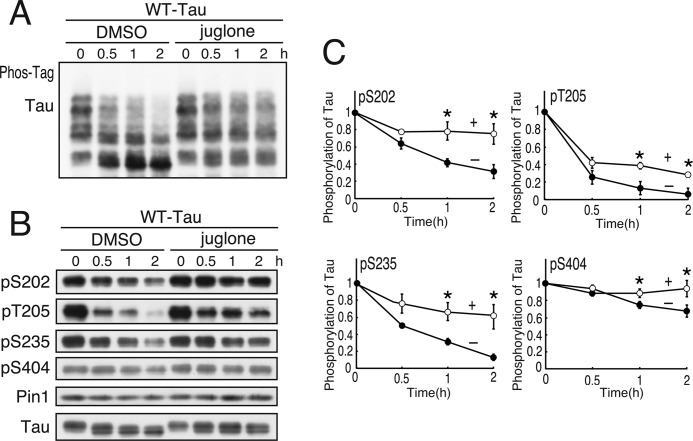
Pin1 increases dephosphorylation of Tau at Thr-231 (34, 38, 40). Although we have reported the increased dephosphorylation of Cdk5-phosphorylated Tau in rat brain extract (39), we did not examine the sites. We next tried to identify the sites for which dephosphorylation can be facilitated by Pin1. Tau was phosphorylated in COS-7 cells by co-expression of Cdk5 and p25 and subsequently dephosphorylated in cell extracts in the presence or absence of the Pin1 inhibitor juglone (52). To detect the complete phosphorylation state of Tau at a glance, we used Phos-tag SDS-PAGE in which the phosphorylated proteins are separated according to their phosphorylation states. WT Tau was separated into four major bands and many minor bands, indicating varied phosphorylation states in COS-7 cells. Incubation of the cell extract increased the electrophoretic mobility of Tau due to its dephosphorylation. Juglone delayed the mobility shift of Tau, indicating a decreased rate of Tau dephosphorylation (Fig. 5A).
FIGURE 5.
Effects of Pin1 on dephosphorylation of Tau at Cdk5 phosphorylation sites. A, the extracts of COS-7 cells expressing Tau and Cdk5-p25 were incubated with (+) or without (−) 5 μm juglone at 35 °C for the times indicated. Phosphorylation of Tau was examined by immunoblotting with anti-human Tau antibody after Phos-tag SDS-PAGE (A) or with phosphospecific antibodies against Ser-202 (pS202), Thr-205 (pT205), Ser-235 (pS235), or Ser-404 (pS404) after Laemmli SDS-PAGE (B). C, the rate of dephosphorylation at each Cdk5 phosphorylation site is shown as a comparison between the immunoblotting before (0 h) and after incubation (0.5, 1, or 2 h) in B. The results are expressed as mean ± S.E. (error bars) (n = 3; *, p < 0.05).
We then tried to identify which sites were affected by juglone by immunoblotting with phosphospecific antibodies to Ser-202, Thr-205, Ser-235, or Ser-404 (Fig. 5B). Juglone decreased the dephosphorylation rate of Tau at any of the four sites (Fig. 5C). Consistent with our previous results (39), the dephosphorylation rate was slowest at Ser-404, although the rate was additionally reduced by juglone. Addition of Pin1 increased the rate of downward shift and Tau dephosphorylation (supplemental Fig. 3), although this effect was minor compared with Pin1 inhibition by juglone. To rule out the possibility that juglone inhibited protein phosphatase directly, we examined the effect of juglone on PKA-dependent Tau phosphorylation. Contrary to Cdk5 phosphorylation of Tau that was inhibited by juglone, PKA phosphorylation of Tau was not affected by juglone (supplemental Fig. 4). These results suggest that Pin1 stimulates the dephosphorylation of Tau at the Cdk5-mediated sites Ser-202, Thr-205, Ser-235, and Ser-404.
Binding of Cdk5-phosphorylated FTDP-17 Mutant Tau to Pin1
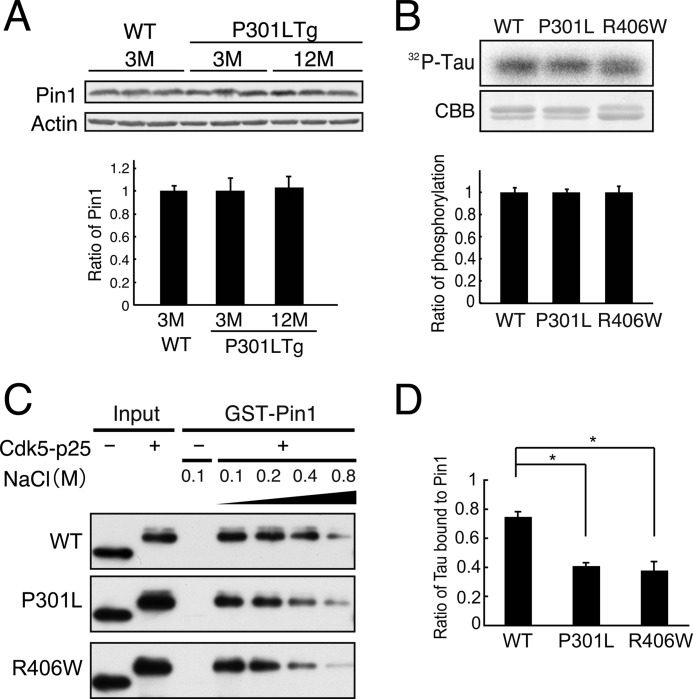
FTDP-17 Tau mutants are abnormally phosphorylated in the brains of FTDP-17 patients and in the brains of FTDP-17 mouse models (2–5, 25). However, it is not known how the Tau hyperphosphorylation is induced in FTDP-17. It is reported that Pin1 is reduced in AD brains (40). We examined Pin1 expression in one FTDP-17 patient and control brains but could not detect a change in Pin1 level (supplemental Fig. 5). Then we compared Pin1 in P301L transgenic mouse brains before and after appearance of pathological lesions. There was no difference between WT and P301L mouse brains at 3 months and in P301L mouse brains between 3 and 12 months (Fig. 6A). These results suggest that changes in Pin1 expression are not the underlying cause for Tau hyperphosphorylation in FTDP-17.
FIGURE 6.
Binding of Cdk5-phosphorylated FTDP-17 mutants to Pin1. A, levels of Pin1 in P301L transgenic mouse brains. Pin1 was probed in whole brain lysates of wild-type mouse at 3 months and P301L transgenic (Tg) mouse at 3 and 12 months by immunoblotting. Actin is the loading control. Quantification is shown below. B, WT Tau and FTDP-17 Tau mutants P301L and R406W were phosphorylated by Cdk5-p25 in the presence of 0.1 mm [γ-32P]ATP for 1.5 h at 35 °C. Phosphorylation of Tau was detected by autoradiography after SDS-PAGE (32P-Tau) and quantified with an image analyzer (lower panel). The relative ratio is expressed against Tau WT after normalization with Tau protein (mean ± S.E. (error bars), n = 3). C, the binding of phospho-Tau P301L or R406W to Pin1. Tau-P301L or Tau-R406W was phosphorylated by Cdk5-p25 (+) and then subjected to a GST pulldown assay in the presence of increasing concentrations of NaCl from 0.1 to 0.8 m. Input is shown in the left two lanes. D, Tau bound to Pin1 in the presence of 0.4 m NaCl was expressed as the percent ratio against the binding in the presence of 0.1 m NaCl. The results are expressed as mean ± S.E. (error bars) (n = 3; *, p < 0.05).
Tau-P301L and -R406W mutants are more resistant to Pin1-dependent dephosphorylation by PP2A compared with WT Tau (39). We suspected that the binding ability of Cdk5-phosphorylated Tau-P301L or -R406W to Pin1 might be weaker than that of WT Tau. Tau-P301L and -R406W were phosphorylated in vitro by Cdk5-p25 and subjected to a Pin1 binding assay. Both P301L and R406W Tau bound to Pin1 to the same extent as did WT Tau in the presence of 0.1 m NaCl when the phosphorylation levels were adjusted carefully (Fig. 6B). We then increased the concentration of NaCl in the binding buffer to reduce the binding (53). The amount of Tau bound to Pin1 decreased with increasing NaCl concentration (Fig. 6C). The ratio of Tau bound to Pin1 at 0.4 m NaCl was expressed relative to the amount of Tau bound to Pin1 in the presence of 0.1 m NaCl (Fig. 6D). The ratio was 41 ± 2.1% for P301L and 38 ± 6.2% for R406W, which were much lower than the 75 ± 3.4% for WT Tau. These results suggest that the affinity of Pin 1 binding to Tau carrying the FTDP-17 mutation P301L or R406W is slightly weaker than that to WT Tau.
DISCUSSION
In this study, we investigated the interaction of Pin1 with Cdk5-phosphorylated Tau and the effect on dephosphorylation at Cdk5 phosphorylation sites. Pin1 binds to Tau phosphorylated by Cdk5-p25 at any of its major phosphorylation sites, Ser-202, Thr-205, Ser-235, and Ser-404. The binding was slightly stronger to phospho-Ser-202 and -Thr-205 than to phospho-Ser-235 and -Ser-404. Pin1 facilitated the dephosphorylation of Tau at any of these sites. These are all abnormal phosphorylation sites found in AD (1, 2, 49). The FTDP-17 mutant Tau, P301L or R406W, showed slightly weaker binding to Pin1 than did WT Tau. These results support the idea that reduced Pin1-dependent dephosphorylation may underlie Tau hyperphosphorylation in tauopathies.
Phospho-Thr-212 and -Thr-231 were the only sites that had been previously mapped as Pin1-interacting sites in Tau (38, 40, 41, 54). Phospho-Thr-231 was first identified by an ELISA as a site among many synthetic phospho-Tau peptides (40). Phospho-Thr-212 was subsequently found to be another Pin1-binding site among GSK3β phosphorylation sites (41). Although the latter authors suggested that Thr-212 and Thr-231 are not unique as Pin1-binding sites based on the observation that Tau mutants at Thr-212 and Thr-231 still bound to Pin1 after phosphorylation in COS-7 cells (41), no other sites have been reported. We show here that there are at least four additional Pin1-binding phosphorylation sites in Tau at Ser-202, Thr-205, Ser-235, and Ser-404.
The binding of Pin1 to Tau at the Alzheimer-related phosphorylation site AT8, whose epitope is generated by phosphorylation of Tau at sites including Ser-202 and Thr-205 (55, 56), has been reported recently in rat cortical neurons (57); this finding is consistent with our present results. However, we do not know why these sites were not detected as Pin1-binding sites in previous work. It might be simply that no study has focused on Cdk5 phosphorylation sites, but we think there might be some mismatching between the protein kinases used and the phosphorylation sites examined. There are 16 (Ser/Thr)-Pro sequences in the longest isoform of Tau (2, 8, 10), many of which are phosphorylated distinctly and with an overlap by different PDPKs and are not necessarily phosphorylated stoichiometrically. Some of them are minor phosphorylation sites that can be detected only with highly sensitive phosphospecific antibodies. In the present study, we used Cdk5-p25, which has a substrate specificity similar to that of Cdk1 toward Tau (11, 50, 51). Having determined all major Cdk5 phosphorylation sites by a combination of phosphopeptide mapping and site-specific mutagenesis, we have shown that all major Cdk5 phosphorylation sites are also newly identified Pin1-binding sites. Extending this approach to other (Ser/Thr)-Pro sites could identify more Pin1-binding sites.
The dissociation constant Kd of Pin1 to Cdk5-phosphorylated full-length Tau was measured as 1.11 ± 0.14 × 10−4 m using Biacore technology. Two different Kd values have been reported for the binding between Pin1 and phosphorylated Tau or a phosphorylated Tau peptide: ∼40 nm with the phospho-Thr-231 peptide when measured by an ELISA (40) and 3.8 ± 1.0 × 10−4 m at phospho-Thr-231 or 1.0 ± 0.3 × 10−4 m at phospho-Thr-212 by NMR (41). Our Kd value is close to the values measured by NMR. Furthermore, a similar Kd value, ∼1 × 10−4 m, was reported for Csc25, a well known Pin1-binding protein (58). This Kd is relatively weak compared with many other protein-protein interactions. Nevertheless, GST pulldown assay worked well for both Cdc25 and Tau (59). The affinity of phospho-(Ser/Thr)-Pro sequences for Pin1 appears to be affected by various experimental conditions including the methods of measurement, the ionic strength of the solution, the number of phosphorylation sites, the use of phosphopeptides versus phosphorylated full-length proteins, and the WW domain of Pin1 versus full-length Pin1. We used phosphorylated full-length Tau instead of phosphopeptides, which may explain the low binding affinity. However, the weak affinity measured in vitro does not necessarily mean that the binding does not occur in cells. Tau is highly concentrated in the vicinity of microtubules. Furthermore, in vivo Tau, particularly in brains with tauopathy, is phosphorylated at multiple (Ser/Thr)-Pro sites by multiple PDPKs, which may increase the binding affinity for Pin1. When highly phosphorylated, Tau may become a better target for Pin1, which may act on highly phosphorylated Tau in predisease conditions to prevent further phosphorylation. In other words, decreased Pin1 activity could be a consequence of Tau hyperphosphorylation. It has been reported that Pin1 is reduced in AD brains (40). We also examined the Pin1 level in an FTDP-17 brain and P301L transgenic mouse brains but could not detect differences between normal and patient or transgenic mouse brains (Fig. 6A and supplemental Fig. 5). We think that there must be other unknown factors that reduce the Pin1 activity in FTDP-17 brains.
The binding affinity also depends on the phospho-(Ser/Thr)-Pro sequences. For example, Smad2/3 has four Pin1-binding (Ser/Thr)-Pro sequences in the linker region, which can be phosphorylated by Cdk and MAPK family members but with strong binding at only one site (60, 61). In the case of Tau, phospho-Thr-212 has a lower Kd than phospho-Thr-231 (41, 54). We also observed here that the Cdk5 phosphorylation sites phospho-Ser-202 and -Thr-205 showed slightly stronger binding to Pin1 than did phospho-Ser-235 and -Ser-404. However, the correlation between Pin1 binding and dephosphorylation is not clear. The phosphorylation site grasped by the WW domain can be attacked by peptidylprolyl isomerase activity in the same Pin1 molecule (36, 37). Ser-202 with strong binding to Pin1 was dephosphorylated faster than was the weaker Ser-404 binding site. However, Ser-235, the weakest binding site, was dephosphorylated as fast as the stronger binding site Ser-202. By contrast, Pin1-binding sites are not always the sites whose dephosphorylation is stimulated by Pin1. It has been reported that peptidylprolyl isomerase catalyzes a different phosphorylation site close to the WW domain-binding site when the target protein is phosphorylated at multiple sites (53, 54). Tau is a multiphosphorylated protein with many clusters of (Ser/Thr)-Pro sequences. Previous research has also identified hierarchical phosphorylation by which GSK3β phosphorylation is facilitated by priming the phosphorylation by Cdk5 or PKA (13, 42, 62, 63). For example, Ser-404 phosphorylation is thought to be a priming site for Ser-400 and Ser-396 phosphorylation by GSK3β. The binding of Pin1 to phospho-Ser-404 may stimulate isomerization and then dephosphorylation at the Ser-396 GSK3β phosphorylation site. A similar relationship has been observed between the Ser-235 Cdk5 site and the Thr-231 GSK3β site (64).
FTDP-17 mutant Tau is hyperphosphorylated in brains of patients, but it is not known why. The phosphorylation levels of several FTDP-17 Tau mutants are similar to those of WT Tau when examined in cultured cells or in vitro (26–29, 65). R406W Tau shows even less phosphorylation by Cdk5 (29) probably because of mutation of Arg-406 to Trp, which changes the site to an unfavorable Cdk5 target sequence, S404PWH from S404PRH. We showed previously that P301L and R406W mutants do not show Pin1 dependence in dephosphorylation at the Cdk5 phosphorylation sites (39). We thought that Pin1 might not recognize the P301L or R406W Tau mutant phosphorylated by Cdk5, but this turned out not to be the case. At a physiological salt concentration, Pin1 bound to Cdk5-phosphorylated P301L and R406W Tau to the same extent as did WT Tau. By contrast, the protein stability of P301L Tau, but not WT Tau, was decreased in Pin1 knockdown or knock-out neurons, suggesting that the P301L mutation affects the interaction of Tau with Pin1 (66). The binding of Pin1 to both P301L and R406W Tau was slightly weaker than that to WT Tau when the binding was measured in the presence of a higher salt concentration. The slight difference in the binding affinity might be increased in the cellular condition by an unidentified factor. Further detailed biochemical studies are required to understand the Pin1-dependent regulation of Tau phosphorylation.
We found that Pin1 binds to and stimulates dephosphorylation of Tau at Cdk5-mediated sites Ser-202, Thr-205, Ser-235, and Ser-404. All of these sites conform at least in part to well known Tau hyperphosphorylation epitopes in AD (Ser-202 and Thr-205 for AT8, Ser-235 for AT180, and Ser-404 for PHF1). An involvement of Pin1 in the Tau hyperphosphorylation in AD has been suggested (38), but the two Pin1-binding sites identified so far could not explain the many other abnormally phosphorylated Tau sites. Our results identify novel Pin1-binding phosphorylation sites in Tau and characterize them as Cdk5-specific sites. Therefore, thorough analysis of the remaining (Ser/Thr)-Pro sites phosphorylated by other PDPKs may indeed reveal to what extent Pin1 can contribute to abnormal Tau phosphorylation in tauopathies.
Acknowledgments
We thank Junichiro Suzuki and Yutaka Sato for statistical data analysis.
This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to S. H.).

This article contains supplemental Figs. 1–5.
- AD
- Alzheimer disease
- Cdk
- cyclin-dependent kinase
- FTDP-17
- frontotemporal dementia with parkinsonism linked to chromosome 17
- Pin1
- peptidyl-prolyl cis/trans isomerase, NIMA-interacting 1
- GSK3β
- glycogen synthase kinase 3β
- PDPK
- proline-directed protein kinase
- PP2A
- protein phosphatase 2A
- Req
- response at equilibrium
- juglone
- 5-hydroxy-1,4-naphthoquinone
- CBB
- Coomassie Brilliant Blue.
REFERENCES
- 1. Ballatore C., Lee V. M., Trojanowski J. Q. (2007) Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nat. Rev. Neurosci. 8, 663–672 [DOI] [PubMed] [Google Scholar]
- 2. Goedert M., Jakes R. (2005) Mutations causing neurodegenerative tauopathies. Biochim. Biophys. Acta 1739, 240–250 [DOI] [PubMed] [Google Scholar]
- 3. Hutton M., Lendon C. L., Rizzu P., Baker M., Froelich S., Houlden H., Pickering-Brown S., Chakraverty S., Isaacs A., Grover A., Hackett J., Adamson J., Lincoln S., Dickson D., Davies P., Petersen R. C., Stevens M., de Graaff E., Wauters E., van Baren J., Hillebrand M., Joosse M., Kwon J. M., Nowotny P., Che L. K., Norton J., Morris J. C., Reed L. A., Trojanowski J., Basun H., Lannfelt L., Neystat M., Fahn S., Dark F., Tannenberg T., Dodd P. R., Hayward N., Kwok J. B., Schofield P. R., Andreadis A., Snowden J., Craufurd D., Neary D., Owen F., Oostra B. A., Hardy J., Goate A., van Swieten J., Mann D., Lynch T., Heutink P. (1998) Association of missense and 5′-splice-site mutations in tau with the inherited dementia FTDP-17. Nature 393, 702–705 [DOI] [PubMed] [Google Scholar]
- 4. Poorkaj P., Bird T. D., Wijsman E., Nemens E., Garruto R. M., Anderson L., Andreadis A., Wiederholt W. C., Raskind M., Schellenberg G. D. (1998) Tau is a candidate gene for chromosome 17 frontotemporal dementia. Ann. Neurol. 43, 815–825 [DOI] [PubMed] [Google Scholar]
- 5. Clark L. N., Poorkaj P., Wszolek Z., Geschwind D. H., Nasreddine Z. S., Miller B., Li D., Payami H., Awert F., Markopoulou K., Andreadis A., D'Souza I., Lee V. M., Reed L., Trojanowski J. Q., Zhukareva V., Bird T., Schellenberg G., Wilhelmsen K. C. (1998) Pathogenic implications of mutations in the tau gene in pallido-ponto-nigral degeneration and related neurodegenerative disorders linked to chromosome 17. Proc. Natl. Acad. Sci. U.S.A. 95, 13103–13107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Allen B., Ingram E., Takao M., Smith M. J., Jakes R., Virdee K., Yoshida H., Holzer M., Craxton M., Emson P. C., Atzori C., Migheli A., Crowther R. A., Ghetti B., Spillantini M. G., Goedert M. (2002) Abundant tau filaments and nonapoptotic neurodegeneration in transgenic mice expressing human P301S tau protein. J. Neurosci. 22, 9340–9351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ikeda M., Shoji M., Kawarai T., Kawarabayashi T., Matsubara E., Murakami T., Sasaki A., Tomidokoro Y., Ikarashi Y., Kuribara H., Ishiguro K., Hasegawa M., Yen S. H., Chishti M. A., Harigaya Y., Abe K., Okamoto K., St George-Hyslop P., Westaway D. (2005) Accumulation of filamentous tau in the cerebral cortex of human tau R406W transgenic mice. Am. J. Pathol. 166, 521–531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hanger D. P., Anderton B. H., Noble W. (2009) Tau phosphorylation: the therapeutic challenge for neurodegenerative disease. Trends Mol. Med. 15, 112–119 [DOI] [PubMed] [Google Scholar]
- 9. Morishima-Kawashima M., Hasegawa M., Takio K., Suzuki M., Yoshida H., Watanabe A., Titani K., Ihara Y. (1995) Hyperphosphorylation of tau in PHF. Neurobiol. Aging 16, 365–371 [DOI] [PubMed] [Google Scholar]
- 10. Johnson G. V., Stoothoff W. H. (2004) Tau phosphorylation in neuronal cell function and dysfunction. J. Cell Sci. 117, 5721–5729 [DOI] [PubMed] [Google Scholar]
- 11. Imahori K., Uchida T. (1997) Physiology and pathology of tau protein kinases in relation to Alzheimer's disease. J. Biochem. 121, 179–188 [PubMed] [Google Scholar]
- 12. Plattner F., Angelo M., Giese K. P. (2006) The roles of cyclin-dependent kinase 5 and glycogen synthase kinase 3 in tau hyperphosphorylation. J. Biol. Chem. 281, 25457–25465 [DOI] [PubMed] [Google Scholar]
- 13. Ishiguro K., Takamatsu M., Tomizawa K., Omori A., Takahashi M., Arioka M., Uchida T. (1992) Tau protein kinase I converts normal tau protein into A68-like component of paired helical filaments. J. Biol. Chem. 267, 10897–10901 [PubMed] [Google Scholar]
- 14. Dhavan R., Tsai L. H. (2001) A decade of CDK5. Nat. Rev. Mol. Cell Biol. 2, 749–759 [DOI] [PubMed] [Google Scholar]
- 15. Hisanaga S., Endo R. (2010) Regulation and role of cyclin-dependent kinase activity in neuronal survival and death. J. Neurochem. 115, 1309–1321 [DOI] [PubMed] [Google Scholar]
- 16. Patrick G. N., Zukerberg L., Nikolic M., de la Monte S., Dikkes P., Tsai L. H. (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402, 615–622 [DOI] [PubMed] [Google Scholar]
- 17. Asada A., Yamamoto N., Gohda M., Saito T., Hayashi N., Hisanaga S. (2008) Myristoylation of p39 and p35 is a determinant of cytoplasmic or nuclear localization of active cyclin-dependent kinase 5 complexes. J. Neurochem. 106, 1325–1336 [DOI] [PubMed] [Google Scholar]
- 18. Asada A., Saito T., Hisanaga S. (2012) Phosphorylation of p35 and p39 by Cdk5 determines the subcellular location of the holokinase in a phosphorylation-site-specific manner. J. Cell Sci. 125, 3421–3429 [DOI] [PubMed] [Google Scholar]
- 19. Minegishi S., Asada A., Miyauchi S., Fuchigami T., Saito T., Hisanaga S. (2010) Membrane association facilitates degradation and cleavage of the cyclin-dependent kinase 5 activators p35 and p39. Biochemistry 49, 5482–5493 [DOI] [PubMed] [Google Scholar]
- 20. Kusakawa G., Saito T., Onuki R., Ishiguro K., Kishimoto T., Hisanaga S. (2000) Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J. Biol. Chem. 275, 17166–17172 [DOI] [PubMed] [Google Scholar]
- 21. Lee M. S., Kwon Y. T., Li M., Peng J., Friedlander R. M., Tsai L. H. (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405, 360–364 [DOI] [PubMed] [Google Scholar]
- 22. Cruz J. C., Tseng H. C., Goldman J. A., Shih H., Tsai L. H. (2003) Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 40, 471–483 [DOI] [PubMed] [Google Scholar]
- 23. Noble W., Olm V., Takata K., Casey E., Mary O., Meyerson J., Gaynor K., LaFrancois J., Wang L., Kondo T., Davies P., Burns M., Veeranna, Nixon R., Dickson D., Matsuoka Y., Ahlijanian M., Lau L. F., Duff K. (2003) Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron 38, 555–565 [DOI] [PubMed] [Google Scholar]
- 24. Piedrahita D., Hernández I., López-Tobón A., Fedorov D., Obara B., Manjunath B. S., Boudreau R. L., Davidson B., Laferla F., Gallego-Gómez J. C., Kosik K. S., Cardona-Gómez G. P. (2010) Silencing of CDK5 reduces neurofibrillary tangles in transgenic Alzheimer's mice. J. Neurosci. 30, 13966–13976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lambourne S. L., Sellers L. A., Bush T. G., Choudhury S. K., Emson P. C., Suh Y. H., Wilkinson L. S. (2005) Increased tau phosphorylation on mitogen-activated protein kinase consensus sites and cognitive decline in transgenic models for Alzheimer's disease and FTDP-17: evidence for distinct molecular processes underlying tau abnormalities. Mol. Cell. Biol. 25, 278–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Matsumura N., Yamazaki T., Ihara Y. (1999) Stable expression in Chinese hamster ovary cells of mutated tau genes causing frontotemporal dementia and parkinsonism linked to chromosome 17 (FTDP-17). Am. J. Pathol. 154, 1649–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pérez M., Lim F., Arrasate M., Avila J. (2000) The FTDP-17-linked mutation R406W abolishes the interaction of phosphorylated tau with microtubules. J. Neurochem. 74, 2583–2589 [DOI] [PubMed] [Google Scholar]
- 28. Connell J. W., Gibb G. M., Betts J. C., Blackstock W. P., Gallo J., Lovestone S., Hutton M., Anderton B. H. (2001) Effects of FTDP-17 mutations on the in vitro phosphorylation of tau by glycogen synthase kinase 3β identified by mass spectrometry demonstrate certain mutations exert long-range conformational changes. FEBS Lett. 493, 40–44 [DOI] [PubMed] [Google Scholar]
- 29. Sakaue F., Saito T., Sato Y., Asada A., Ishiguro K., Hasegawa M., Hisanaga S. (2005) Phosphorylation of FTDP-17 mutant tau by cyclin-dependent kinase 5 complexed with p35, p25, or p39. J. Biol. Chem. 280, 31522–31529 [DOI] [PubMed] [Google Scholar]
- 30. Wang J. Z., Gong C. X, Zaidi T., Grundke-Iqbal I., Iqbal K. (1995) Dephosphorylation of Alzheimer paired helical filaments by protein phosphatase-2A and -2B. J. Biol. Chem. 270, 4854–4860 [DOI] [PubMed] [Google Scholar]
- 31. Sontag E., Nunbhakdi-Craig V., Lee G., Bloom G. S., Mumby M. C. (1996) Regulation of the phosphorylation state and microtubule-binding activity of Tau by protein phosphatase 2A. Neuron 17, 1201–1207 [DOI] [PubMed] [Google Scholar]
- 32. Liang Z., Liu F., Iqbal K., Grundke-Iqbal I., Wegiel J., Gong C. X. (2008) Decrease of protein phosphatase 2A and its association with accumulation and hyperphosphorylation of tau in Down syndrome. J. Alzheimers Dis. 13, 295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yamamoto H., Hasegawa M., Ono T., Tashima K., Ihara Y., Miyamoto E. (1995) Dephosphorylation of fetal-tau and paired helical filaments-tau by protein phosphatases 1 and 2A and calcineurin. J. Biochem. 118, 1224–1231 [DOI] [PubMed] [Google Scholar]
- 34. Zhou X. Z., Kops O., Werner A., Lu P. J., Shen M., Stoller G., Küllertz G., Stark M., Fischer G., Lu K. P. (2000) Pin1-dependent prolyl isomerization regulates dephosphorylation of Cdc25C and tau proteins. Mol. Cell 6, 873–883 [DOI] [PubMed] [Google Scholar]
- 35. Nakamura K., Greenwood A., Binder L., Bigio E. H., Denial S., Nicholson L., Zhou X. Z., Lu K. P. (2012) Proline isomer-specific antibodies reveal the early pathogenic tau conformation in Alzheimer's disease. Cell 149, 232–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lippens G., Landrieu I., Smet C. (2007) Molecular mechanisms of the phospho-dependent prolyl cis/trans isomerase Pin1. FEBS J. 274, 5211–5222 [DOI] [PubMed] [Google Scholar]
- 37. Liou Y. C., Zhou X. Z., Lu K. P. (2011) Prolyl isomerase Pin1 as a molecular switch to determine the fate of phosphoproteins. Trends Biochem. Sci. 36, 501–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liou Y. C., Sun A., Ryo A., Zhou X. Z., Yu Z. X., Huang H. K., Uchida T., Bronson R., Bing G., Li X., Hunter T., Lu K. P. (2003) Role of the prolyl isomerase Pin1 in protecting against age-dependent neurodegeneration. Nature 424, 556–561 [DOI] [PubMed] [Google Scholar]
- 39. Yotsumoto K., Saito T., Asada A., Oikawa T., Kimura T., Uchida C., Ishiguro K., Uchida T., Hasegawa M., Hisanaga S. (2009) Effect of Pin1 or microtubule binding on dephosphorylation of FTDP-17 mutant tau. J. Biol. Chem. 284, 16840–16847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lu P. J., Wulf G., Zhou X. Z., Davies P., Lu K. P. (1999) The prolyl isomerase Pin1 restores the function of Alzheimer-associated phosphorylated tau protein. Nature 399, 784–788 [DOI] [PubMed] [Google Scholar]
- 41. Smet C., Sambo A. V., Wieruszeski J. M., Leroy A., Landrieu I., Buée L., Lippens G. (2004) The peptidyl prolyl cis/trans-isomerase Pin1 recognizes the phospho-Thr212-Pro213 site on Tau. Biochemistry 43, 2032–2040 [DOI] [PubMed] [Google Scholar]
- 42. Ishiguro K., Sato K., Takamatsu M., Park J., Uchida T., Imahori K. (1995) Analysis of phosphorylation of tau with antibodies specific for phosphorylation sites. Neurosci. Lett. 202, 81–84 [DOI] [PubMed] [Google Scholar]
- 43. Yamada M., Saito T., Sato Y., Kawai Y., Sekigawa A., Hamazumi Y., Asada A., Wada M., Doi H., Hisanaga S. (2007) Cdk5-p39 is a labile complex with the similar substrate specificity to Cdk5-p35. J. Neurochem. 102, 1477–1487 [DOI] [PubMed] [Google Scholar]
- 44. Taoka M., Ichimura T., Wakamiya-Tsuruta A., Kubota Y., Araki T., Obinata T., Isobe T. (2003) V-1, a protein expressed transiently during murine cerebellar development, regulates actin polymerization via interaction with capping protein. J. Biol. Chem. 278, 5864–5870 [DOI] [PubMed] [Google Scholar]
- 45. Laemmli U. K. (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685 [DOI] [PubMed] [Google Scholar]
- 46. Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T. (2006) Phosphate-binding tag, a new tool to visualize phosphorylated proteins. Mol. Cell. Proteomics. 5, 49–57 [DOI] [PubMed] [Google Scholar]
- 47. Hosokawa T., Saito T., Asada A., Fukunaga K., Hisanaga S. (2010) Quantitative measurement of in vivo phosphorylation states of Cdk5 activator p35 by Phos-tag SDS-PAGE. Mol. Cell. Proteomics. 9, 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Goedert M., Spillantini M. G., Potier M. C., Ulrich J., Crowther R. A. (1989) Cloning and sequencing of the cDNA encoding an isoform of microtubule-associated protein tau containing four tandem repeats: differential expression of tau protein mRNAs in human brain. EMBO J. 8, 393–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lee V. M., Goedert M., Trojanowski J. Q. (2001) Neurodegenerative tauopathies. Annu. Rev. Neurosci. 24, 1121–1159 [DOI] [PubMed] [Google Scholar]
- 50. Illenberger S., Zheng-Fischhöfer Q., Preuss U., Stamer K., Baumann K., Trinczek B., Biernat J., Godemann R., Mandelkow E. M., Mandelkow E. (1998) The endogenous and cell cycle-dependent phosphorylation of tau protein in living cells: implications for Alzheimer's disease. Mol. Biol. Cell 9, 1495–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wada Y., Ishiguro K., Itoh T. J., Uchida T., Hotani H., Saito T., Kishimoto T., Hisanaga S. (1998) Microtubule-stimulated phosphorylation of tau at Ser202 and Thr205 by cdk5 decreases its microtubule nucleation activity. J. Biochem. 124, 738–746 [DOI] [PubMed] [Google Scholar]
- 52. Hennig L., Christner C., Kipping M., Schelbert B., Rücknagel K. P., Grabley S., Küllertz G., Fischer G. (1998) Selective inactivation of parvulin-like peptidyl-prolyl cis/trans isomerases by juglone. Biochemistry 37, 5953–5960 [DOI] [PubMed] [Google Scholar]
- 53. Daum S., Fanghänel J., Wildemann D., Schiene-Fischer C. (2006) Thermodynamics of phosphopeptide binding to the human peptidyl prolyl cis/trans isomerase Pin1. Biochemistry 45, 12125–12135 [DOI] [PubMed] [Google Scholar]
- 54. Smet C., Duckert J. F., Wieruszeski J. M., Landrieu I., Buée L., Lippens G., Déprez B. (2005) Control of protein-protein interactions: structure-based discovery of low molecular weight inhibitors of the interactions between Pin1 WW domain and phosphopeptides. J. Med. Chem. 48, 4815–4823 [DOI] [PubMed] [Google Scholar]
- 55. Goedert M., Jakes R., Vanmechelen E., (1995) Monoclonal antibody AT8 recognises tau protein phosphorylated at both serine 202 and threonine 205. Neurosci. Lett. 189, 167–169 [DOI] [PubMed] [Google Scholar]
- 56. Shahpasand K., Uemura I., Saito T., Asano T., Hata K., Shibata K., Toyoshima Y., Hasegawa M., Hisanaga S. (2012) Regulation of mitochondrial transport and inter-microtubule spacing by tau phosphorylation at the sites hyperphosphorylated in Alzheimer's disease. J. Neurosci. 32, 2430–2441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Nykänen N. P., Kysenius K., Sakha P., Tammela P., Huttunen H. J. (2012) γ-Aminobutyric acid type A (GABAA) receptor activation modulates tau phosphorylation. J. Biol. Chem. 287, 6743–6752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Wintjens R., Wieruszeski J. M., Drobecq H., Rousselot-Pailley P., Buée L., Lippens G., Landrieu I. (2001) 1H NMR study on the binding of Pin1 Trp-Trp domain with phosphothreonine peptides. J. Biol. Chem. 276, 25150–25156 [DOI] [PubMed] [Google Scholar]
- 59. Crenshaw D. G., Yang J., Means A. R., Kornbluth S. (1998) The mitotic peptidyl-prolyl isomerase, Pin1, interacts with Cdc25 and Plx1. EMBO J. 17, 1315–1327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Nakano A., Koinuma D., Miyazawa K., Uchida T., Saitoh M., Kawabata M., Hanai J., Akiyama H., Abe M., Miyazono K., Matsumoto T., Imamura T. (2009) Pin1 down-regulates transforming growth factor-β (TGF-β) signaling by inducing degradation of Smad proteins. J. Biol. Chem. 284, 6109–6115 [DOI] [PubMed] [Google Scholar]
- 61. Matsuura I., Chiang K. N., Lai C. Y., He D., Wang G., Ramkumar R., Uchida T., Ryo A., Lu K., Liu F. (2010) Pin1 promotes transforming growth factor-β-induced migration and invasion. J. Biol. Chem. 285, 1754–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Pei J. J., Grundke-Iqbal I., Iqbal K., Bogdanovic N., Winblad B., Cowburn R. F. (1998) Accumulation of cyclin-dependent kinase 5 (cdk5) in neurons with early stages of Alzheimer's disease neurofibrillary degeneration. Brain Res. 797, 267–277 [DOI] [PubMed] [Google Scholar]
- 63. Michel G., Mercken M., Murayama M., Noguchi K., Ishiguro K., Imahori K., Takashima A. (1998) Characterization of tau phosphorylation in glycogen synthase kinase-3β and cyclin dependent kinase-5 activator (p23) transfected cells. Biochim Biophys. Acta 1380, 177–182 [DOI] [PubMed] [Google Scholar]
- 64. Ishiguro K., Kobayashi S., Omori A., Takamatsu M., Yonekura S., Anzai K., Imahori K., Uchida T. (1994) Identification of the 23 kDa subunit of tau protein kinase II as a putative activator of cdk5 in bovine brain. FEBS Lett. 342, 203–208 [DOI] [PubMed] [Google Scholar]
- 65. DeTure M., Ko L. W., Easson C., Yen S. H. (2002) Tau assembly in inducible transfectants expressing wild-type or FTDP-17 tau. Am. J. Pathol. 161, 1711–1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lim J., Balastik M., Lee T. H., Nakamura K., Liou Y. C., Sun A., Finn G., Pastorino L., Lee V. M., Lu K. P. (2008) Pin1 has opposite effects on wild-type and P301L tau stability and tauopathy. J. Clin. Investig. 118, 1877–1889 [DOI] [PMC free article] [PubMed] [Google Scholar]