Members of the heat shock protein 70 family bind to the macrophage mannose receptor, potentially contributing to appropriate localization and trafficking.
Keywords: receptor, binding proteins, cytoplasmic tail
Abstract
The macrophage MR has been the subject of investigation for over 20 years, and several important physiological functions have been described. However, the molecular mechanisms that regulate MR signaling and trafficking during these processes still remain elusive. The focus of the current paper was to identify potential cellular MR-interacting proteins. An initial screen of binding proteins in MR-expressing cells was performed using coimmunoprecipitation, followed by identification of matching peptide sequences using proteomics and MS. The major class of binding proteins identified belonged to the heat shock family of proteins. The specific interaction of the MR with HSP70 family members was validated by Western blot analysis, ligand binding assays, and intracellular colocalization using confocal microscopy. Additional studies indicated that inhibition of the HSP BiP by treatment of cells with EGCG reduced BiP interaction with and surface expression of the MR. Studies of possible motifs within the cytoplasmic tail of the receptor suggested that a juxtamembrane dibasic sequence may contribute to the interaction with BiP. These findings suggest that the molecular association of the MR with HSP70 family members via the receptor cytoplasmic tail may contribute to MR trafficking in macrophages.
Introduction
The macrophage MR is a key receptor involved in uptake of extracellular glycoproteins such as hydrolases and peroxidases [1] and in ingestion of a variety of pathogens, including Pseudomonas, Pneumocystis, and Candida [2]. Studies from our laboratory and others have provided compelling evidence that the MR plays a central role in resolution of inflammation by clearance of potentially harmful extracellular enzymes [3], as well as a key role in innate host defense [4]. The MR is a prototypic recycling receptor that appears to constitutively enter cells, traffic through the endosomal compartment, and return to the cell surface. The receptor is a 175-kDa type I transmembrane protein expressed predominantly on the cell surface of macrophages. Binding of extracellular ligands occurs through eight conserved carbohydrate recognition domains in the N-terminal portion of the molecule [5]. The cytoplasmic tail, which contains only 45 aa, is presumed to mediate binding of cytosolic cellular proteins, although very little information is available as to the specific motifs involved and which cellular proteins might interact with the MR. Early work from several laboratories suggested that the tyrosine residue at position 1429 was involved in endocytosis and phagocytosis, although other domains within the tail region also appear to participate [6, 7]. Motifs have been identified in other recycling receptors that mediate interaction with proteins of the endocytic machinery, including a tyrosine (Y) residue and a dileucine (SDXXXL/V/M) motif [8, 9]. The MR contains both of these motifs, and both have been implicated as required sequences for endocytosis [10]. The focus in the current study was to identify cellular proteins that interact with the MR and to determine the specific residues in the MR tail region that are involved in this binding.
As an initial screen to identify potential cellular proteins that bind to the MR, an immunoprecipitation/proteomics approach was used. Several proteins that might be predicted to interact with endocytic receptors were detected, including IQGAP1, which is involved in formation of the phagocytic cup [11], and eEF1α2, which plays a role in macrophage deactivation [12]. A surprising finding was the high level of binding of several members of the HSP family members. HSPs belong to a family of proteins that participate in a wide variety of functions to maintain cellular homeostasis, including protein folding, substrate stabilization, and assembly of macromolecular structures [13]. In support of a broader role in cellular function, recent reports have suggested that HSPs may be involved in several aspects of immunological and physiological homeostasis [14]. Specifically, a number of receptors involved in uptake and/or clearance of cellular proteins and nutrients have been shown to be regulated by interaction of HSPs with intracellular domains of these receptors [15, 16]. In the current report, the interaction of the HSP70 family of proteins with the MR was studied to begin to understand the role that HSPs play in regulation of MR-mediated uptake and clearance of glycosylated proteins and particles.
MATERIALS AND METHODS
Plasmids and antibodies
Studies on MR trafficking have been hampered by the lack of an appropriately expressed full-length MR. It has been suggested that the MR cDNA may contain “poison” or toxic sequences that preclude successful cloning and expression in mammalian cells. To avoid these sequences, an opti-MR cDNA lacking the putative poison sequences but still encoding a full-length functional MR protein was obtained from GeneArt AG (Regensberg, Germany; unpublished results). Analysis of the resulting cDNA indicated that it is free of negative cis-acting motifs, including internal TATA boxes, Chi sites, and ribosomal entry sites; AT- or GC-rich sequence stretches; repeat sequences and RNA secondary structures; (cryptic) splice donor and acceptor sites; and branch points that may negatively influence expression [17]. Additionally, the GC content was increased to 60% in an effort to prolong mRNA half-life. The optimized cDNA was subcloned into pcDNA3.1. Transient transfection of HeLa or 293T cells with this vector resulted in high levels of MR expression, as detected by FACS, confocal, and Western analyses.
mAb against the human MR used for confocal assays was obtained from BD Biosciences (San Jose, CA, USA). MR mAb (15-2) and polyclonal antibodies (C-20) used for immunoprecipitation and Western blot analysis were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Antibodies against the HSP70 family of proteins were obtained from the following sources: rabbit and goat polyclonal antibody against BiP/Grp78 were obtained from Assay Designs (Ann Arbor, MI, USA) and Santa Cruz Biotechnology, respectively; Hsc70 mAb and polyclonal antibodies were obtained from Santa Cruz Biotechnology; and Grp75 (Mortalin) mAb was obtained from Assay Designs. Anti-GST mAb was obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies conjugated to Alexa dyes (488 and 568) for use in confocal studies were obtained from Molecular Probes (Eugene, OR, USA).
Cells
For studies involving macrophages, a novel macrophage hybridoma cell line (43 cells), provided by Kirk Sperber (Mt. Sinai Medical School, New York, NY, USA), was used [18]. The cells were maintained in RPMI-1640 medium with 10% FBS, 100 U/ml penicillin and streptomycin, and 100 ng/ml gentamycin. A subset of cells expressing the MR was selected by FACS and maintained as a stable cell line (43MR). These cells exhibited a number of properties of mature macrophages [19]. The epithelial cell line 293T and the continuous HeLa cell line (obtained from the American Type Culture Collection, Manassas, VA, USA) were used for studies involving transient expression of the MR, and cells were maintained in RPMI-1640 medium with 10% FBS, 100 U/ml penicillin and streptomycin, and 100 ng/ml gentamycin. For studies using primary macrophages, human MDM lysates were obtained from Dr. Larry Schlesinger (The Ohio State University, Columbus, OH, USA), prepared in their laboratory as described previously [20].
Transfection of cells
For Western blot analysis and GST and ligand pull-down experiments, HeLa and 293T cells were transfected using Polyfect transfection reagent (Qiagen, Valencia, CA, USA), according to the manufacturer's directions. Briefly, cells were cultured at a concentration of 2 × 106 cells/P-100 dish overnight. Plasmid DNA (10 μg) was transfected into cells using 80 μl Polyfect reagent, and cells were incubated overnight at 37°C in media plus serum and antibiotics. Transfection of cells for confocal experiments was performed in 35-mm glass-bottom dishes (Mattek, Ashland, MA, USA). Briefly, 5 × 105 cells were seeded into each dish and incubated overnight. Plasmid DNA (2 μg) was transfected into cells using 20 μl Polyfect reagent. Following overnight incubation, the cells were washed, and fresh RPMI with 10% FBS was added. Cells were collected 24 h and 48 h post-transfection.
Confocal analysis
HeLa cells were plated at a density of 103/well in Mattek dishes and maintained in DMEM plus 10% FBS. For staining of MR and interacting proteins, cells were washed with PBS after 36 h of transfection, fixed with 4% paraformaldehyde, and permeabilized with 0.2% Triton X-100. Following fixation and permeabilization, cells were stained in a two-step process with primary anti-MR or anti-HSP antibodies, followed by goat anti-mouse Alexa-488 or Alexa-568 (Invitrogen, Carlsbad, CA, USA). Nuclei were stained for visualization with TO-PRO-3 (Invitrogen). Cells were mounted in Vectashield mounting media (Vector Laboratories, Burlingame, CA, USA) between coverslips and examined by confocal imaging using a Zeiss LSM 510 confocal microscope in the VUMC Cell Imaging Shared Resource (Nashville, TN, USA). Images were collected using a 63× oil-immersion objective. Image processing was performed using Adobe Photoshop CS imaging software.
Immunoprecipitation and Western blot analysis
Prior to transfection, cells were plated at a density of 5 × 105 cells/well in six-well dishes or 1 × 106/P-100 tissue-culture dish and incubated overnight in complete RPMI media. Following overnight incubation, fresh medium was added, and transfection was performed. Following treatment, the cells were lysed in lysis buffer containing 0.5% NP-40. Total cellular protein was determined by BCA protein assay (Pierce, Rockford, IL, USA), and 10 μg total protein was resolved by electrophoresis on a 7.5% SDS-PAGE gel at 150 V constant at 4°C. Proteins were transferred to nitrocellulose at 100 V, followed by blocking in TBS containing 0.1% Tween and 5% powdered milk. Proteins were visualized on radiographic film using primary antibodies to the desired protein, followed by incubation with HRP-conjugated secondary antibodies and chemiluminescence reagent. For immunoprecipitation, cells were seeded into six-well dishes at 5 × 105 cells/well and treated accordingly. Cells were lysed in 0.5% NP-40 lysis buffer, and lysate was precleared with protein A-Sepharose overnight at 4°C, followed by immunoprecipitation using rabbit polyclonal antibody against MR. The immunoprecipitated receptor was detected by immunoblot analysis after electrophoretic separation on 7.5% SDS-PAGE gels. Bands were visualized by chemiluminescence and exposure of Kodak BioMax film. The density of the bands was quantified using the UN-SCAN IT densitometry software from Silk Scientific (Orem, UT, USA).
Flow cytometry
Flow cytometry was performed using a FACSCaliber (BD Biosciences) bench-top analyzer in the Vanderbilt University Flow Cytometry Core Facility. Cells were stained with PE-conjugated antibodies as follows: cells were collected by centrifugation, followed by suspension in staining buffer (1% BSA, 0.1% sodium azide, 0.5% normal goat serum in PBS). Normal goat serum was included in the stain buffer to saturate the FcR and minimize autofluorescence. The appropriate concentrations of PE-conjugated antibodies were diluted in staining buffer and added to the cells. In addition, an isotype-matched control was performed to account for nonspecific fluorescence, and an unstained sample was included to account for autofluorescence. The cells were incubated for 20 min in the dark at 4°C. The cells were washed twice with staining buffer, followed by fixation in 500 μl, 2% paraformaldehyde. Cells were analyzed on a BD Biosciences FACScan flow cytometer using CellQuest software.
Proteomics
The MR-specific C-20 antibody was used for immunoprecipitation from cell lysates of 43 cells and 43MR cells. Gels were stained with Coomassie, and bands of highest intensity were excised and subjected to in-gel digestion with trypsin. Peptide sequence analysis was performed by microcapillary reverse-phase HPLC nanoelectrospray tandem MS on a ThermoFinnigan LTQ LC-MS/MS system in the Proteomics Core Facility at Vanderbilt University. Proteomic analysis was performed in the VUMC Mass Spectrometry Research Center Proteomics Laboratory. Peptide sequences obtained from the LC-MS/MS were analyzed using the Sequest algorithm (The Scripps Research Institute, La Jolla, CA, USA). Matched (%) indicates peptide sequence coverage compared with the MR protein.
Construction of GST-MRCT and GST pull-down assay
The WT and RR-AA mutant cytoplasmic tail, including the transmembrane domain of the MR (MRCT), were subcloned into pGEX5X-1, obtained from GE Life Sciences (Piscataway, NJ, USA), using the EcoRI and XhoI restriction sites. The coding region of GST-MRCT was subcloned into pCMVTag2B. The RR-AA mutant was generated by site-directed mutagenesis, according to the manufacturer's instructions. Oligonucleotides used to create the GST-MRCT RR-AA mutation were obtained from Integrated DNA Technologies (Coralville IA, USA). Oligonucleotide sequences were as follows: MRCT RR-AA forward: 5′-CGA ATT CAT GTA CAA GAA AGC GGC GGT GCA CCT GCC CCA GGA AG-3′; MRCT RR-AA reverse: 5′-CTT CCT GGG GCA GGT GCA CCG CCG CTT TCT TGT ACA TGA ATT CG-3′. All reagents used for the GST pull-down assay were obtained from GE Life Sciences. GST pull-down assays were performed as follows: GST-MRCT or GST-MRCT-RR-AA was incubated with GST-Sepharose beads at 4°C for 15 min. Beads were washed twice in buffer to remove excess unbound GST-MRCT or GST-MRCT RR-AA. HeLa cell lysate was added to the beads, and the mixture was incubated at 4°C for 2 h. Following incubation, the beads were washed twice and prepared for gel separation and Western blot analysis.
RESULTS
Immunoprecipitation of the MR and identification of coprecipitated proteins
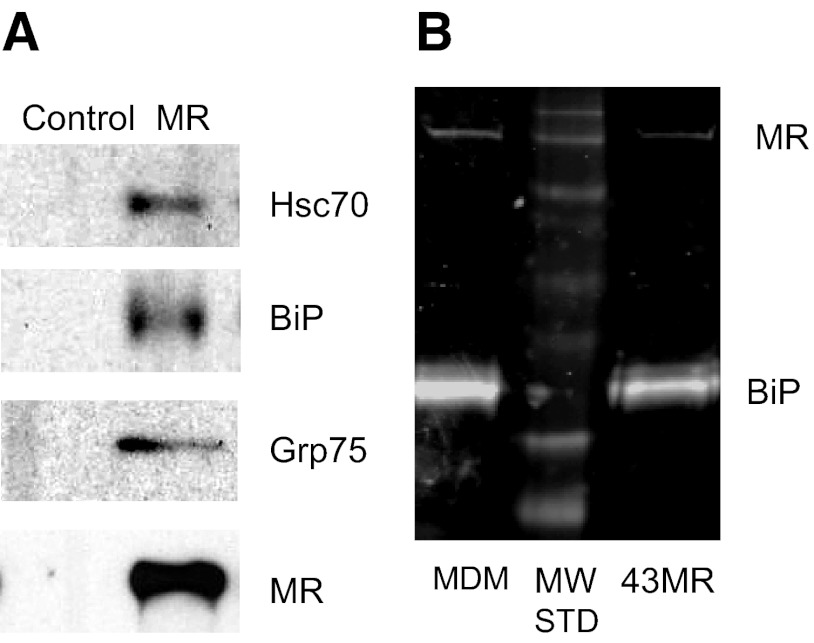
The initial goal of this study was to identify binding partners that interact with the cytoplasmic domain of the MR. MR protein complexes were immunoprecipitated from 43MR cells using the C-20 anti-MR antibody, and proteins were separated by SDS-PAGE. Protein bands were visualized using colloidal blue staining and then excised for in-gel digestion with trypsin. The digested bands were subjected to HPLC and tandem MS. Human protein databases were searched using a Sequest algorithm, and several potential MR binding proteins were identified, including proteins involved in regulation of macrophage function (calpastatin, peroxiredoxin 1, IQGAP1, and eEF1α2). Surprisingly, several proteins in the HSP70 family of proteins, including Grp75, BiP, and Hsc70, showed a high percentage of matched peptide sequences (Fig. 1). Based on these results, further studies were conducted to confirm the specificity of HSP70–MR interactions.
Figure 1. Analysis of potential MR BiPs.
The MR-specific C-20 antibody was used for immunoprecipitation from cell lysates of 43MR cells. Following staining and in-gel digestion, peptide sequence analysis was performed by microcapillary reverse-phase HPLC nanoelectrospray tandem MS on a ThermoFinnigan LTQ LC-MS/MS system in the Proteomics Core Facility at Vanderbilt University. Peptide sequences obtained from the LC-MS/MS were analyzed using the Sequest algorithm (The Scripps Research Institute). Match (%) indicates peptide sequence coverage compared with the MR protein, and matching peptides are indicated in red in the three panels.
Specificity of MR–HSP70 interaction
To verify the specificity of HSP70–MR binding, coimmunoprecipitation and Western blot analysis were performed using MR-negative 43 cells, 43MR cells, and MDM cell lysates. MR cell protein complexes were immunoprecipitated from cell lysates and proteins separated by SDS-PAGE and transferred to nitrocellulose. Hsc70, BiP, and Grp75 were visualized using mAb and polyclonal antibodies (Fig. 2A). Cells lacking the MR (43 cells) showed similar levels of BiP, Hsc70, and Grp75 in whole cell lysates but no detectable MR, and no HSP70 proteins were coimmunoprecipitated with anti-MR antibodies (data not shown). To ensure that MR and BiP were coimmunoprecipitated in primary macrophages, human MDM cell lysates were used in similar coimmunoprecipitation experiments. As shown in Fig. 2B, BiP–MR complexes were coprecipitated with the appropriate anti-MR antibody in 43MR cells and MDM lysates.
Figure 2. Analysis of binding of the MR and members of the HSP70 family of proteins.
Whole cell lysates from 43MR (A) or 43MR and MDM cells (B) were incubated with anti-MR antibody for immunoprecipitation. Proteins in the immunoprecipitate were separated by SDS-PAGE and then transferred to nitrocellulose. Blots were incubated with antibodies to the indicated proteins (Hsc70, BiP, Grp75, and MR) and visualized by chemiluminescence (A) or infrared imaging via LI-COR (B). MW STD = molecular weight markers (44 kDa to 250 kDa).
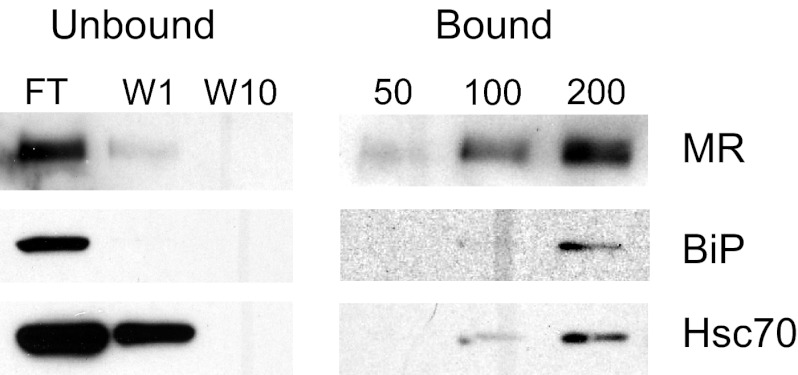
To further define the specificity of binding, pull-down experiments were conducted using fucose-conjugated to agarose beads, a reagent that has been used for purification of the MR [21]. Cell lysates were incubated with immobilized fucose and bound proteins detected using specific antibodies after transfer to nitrocellulose. Results in Fig. 3 show that BiP and Hsc70 copurified with the MR.
Figure 3. Ligand-affinity purification of MR–HSP70 complexes.
Whole cell lysates of 43MR cells were incubated with 0–200 μl fucose-coated agarose beads overnight at 4°C. The beads were pelleted, and unbound, flow-through (FT) was collected. After 10 washes (W10), no additional MR or HSP70 proteins were detected by Western analysis in the flow-through fraction (left panel). Bound complexes were analyzed by Western blot following electrophoretic separation using antibodies specific for MR, BiP, and Hsc70. Bound MR, BiP, and Hsc70 were detected in the lysates that had been incubated with 100 or 200 μl fucose agarose (right panel).
Confocal analysis of MR colocalization with partner proteins
Confocal analysis was used to examine the intracellular colocalization of MR with HSP70 proteins. HeLa cells were transfected with the opti-MR, and the colocalization of MR with BiP, Grp75, and Hsc70 was determined. As shown in Fig. 4, the MR shows a diffuse pattern of staining, supporting previous reports that the MR is found throughout the cytoplasm of the cell, as well as the cell surface, with ∼20% of the protein on the surface under normal conditions [22]. All three HSP proteins also showed a fairly diffuse pattern of staining, with significant overlap with the MR (Fig. 4A–C, lower right-hand panel). These results suggest that the MR colocalizes with BiP, Hsc70, and Grp75 under nonstress conditions in HeLa cells transiently expressing the MR.
Figure 4. Confocal analysis of colocalization of MR with partner proteins.
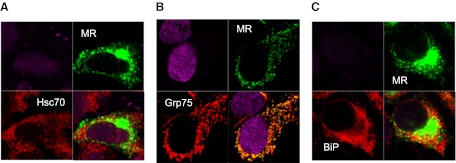
HeLa cells were plated in 35-mm Mattek dishes and transiently transfected with the opti-MR plasmid or with a control pcDNA4.0 vector. Cells were fixed, permeabilized, and incubated with primary antibody (MR, Hsc70, Grp75, or BiP) for 1 h. Cells were washed and stained with fluorescently labeled secondary antibody for 30 min. Cells were mounted in Vectashield mounting media between coverslips and examined using a Zeiss LSM 510 confocal microscope with a 63× oil-immersion objective. Serial z-sections, were taken and representative images were collected simultaneously on a z-plane of focus. Images are shown at the midpoint of the cell (3.0–4.0 μ). Image processing was performed using Adobe Photoshop CS imaging software. Data are representative of three independent experiments, with similar results in 43MR and 293 cells expressing the opti-MR (data not shown). In each panel (A–C), the upper-left quadrant are cells stained with control antibody alone and TO-PRO-3 dye for nuclear staining. Fluorescent staining of MR (green) and HSP70 proteins (red) is shown in the upper-right and lower-left quadrants, respectively. The merged images are shown in the lower-right quadrant in each panel.
Effect of BiP inhibition of association with the MR
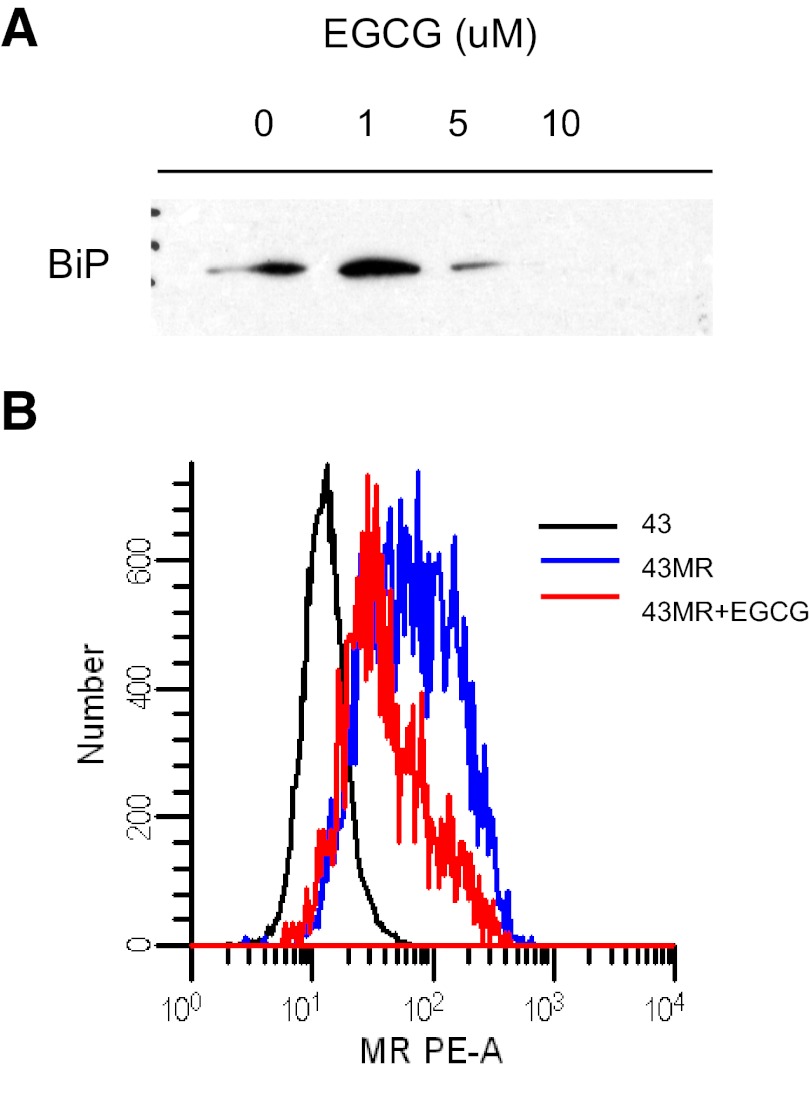
The ASGPR is a cell-surface receptor that shares a number of properties with the MR, including clearance of extracellular glycoproteins, interaction with the endocytic clathrin machinery, recycling kinetics, and expression of endocytic motifs in the cytoplasmic domain. Huang et al. [15] recently reported that BiP regulates appropriate trafficking of the ASGPR and that inhibition of BiP prevents the ASGPR from returning to the cell surface. The effect of BiP inhibition on cell-surface expression of the MR was tested using EGCG, which has been reported to bind to the ATPase site of BiP and prevent its association with intracellular binding partners [23]. 43MR cells were incubated with increasing concentrations of EGCG (0–10 μM) overnight, followed by immunoprecipitation using anti-MR antibodies and Western blot analysis for BiP expression. As shown in Fig. 5A, incubation of cells with EGCG at 10 μM completely blocked the BiP–MR association. To examine the effect of EGCG on MR surface expression, 43MR cells were incubated with 10 μM EGCG, followed by analysis of surface MR by FACS. EGCG reduced MR expression by ∼50% (Fig. 5B).
Figure 5. Effect of BiP inhibition on BiP–MR interaction and MR surface expression.
(A) 43MR cells were treated with EGCG (0–10 μM) overnight. Cells lysates were then incubated with anti-MR antibody and protein A/G agarose beads (Santa Cruz Biotechnology). The immune complexes were separated by SDS-PAGE and analyzed by Western blot with anti-BiP antibody. (B) 43MR cells (1×106) were incubated with 10 μM EGCG for 5 h. Following incubation, the cells were harvested and incubated with PE-conjugated, isotype-matched control or PE-conjugated anti-MR antibody. Black, Untreated 43MR stained with the isotype control; blue, 43MR cells treated with vehicle alone (DMSO) and incubated with PE-anti-MR; red, 43MR cells treated with EGCG and incubated with PE-anti-MR. The number of events acquired for each sample was 3 × 104. PE-A designates the use of standard Ex/Em filter set for PE.
Association of BiP with the MRCT
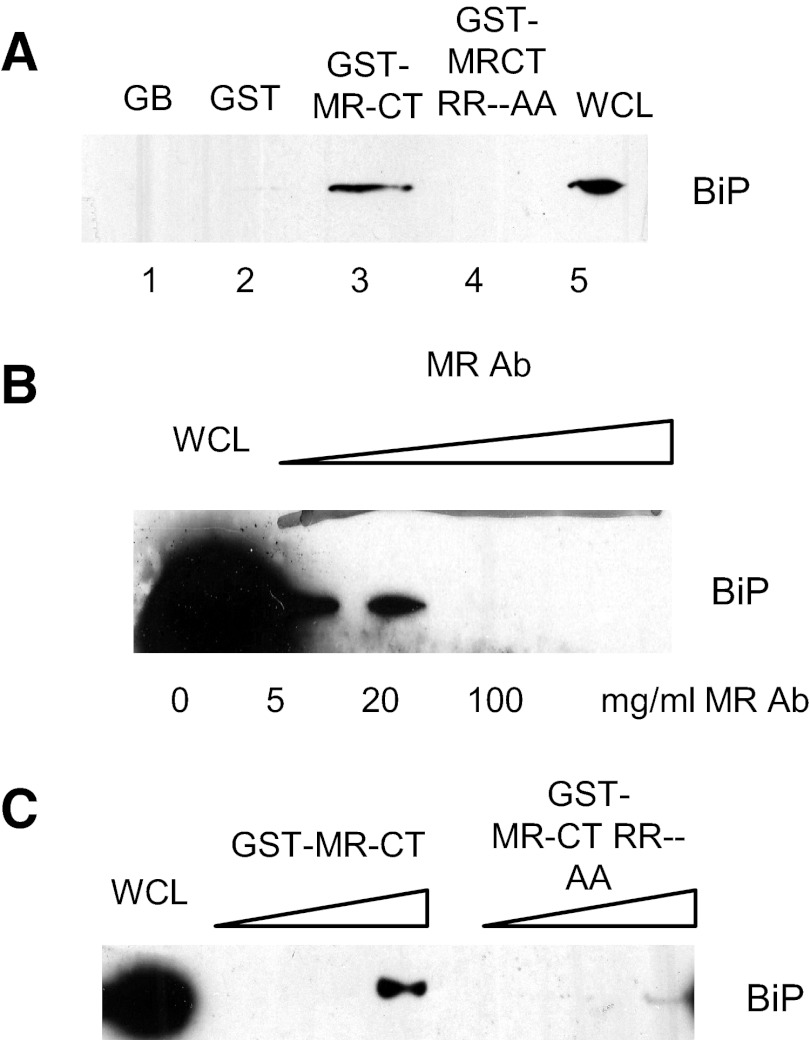
Based on previous reports that several endocytic receptors bind BiP through receptor cytoplasmic domains [15, 16], experiments were conducted to examine the role of the MRCT in mediating the MR–BiP interaction and to identify potential amino acid sequences required for this interaction. First, the interaction of the MRCT with BiP was examined by GST pull-down experiments. The coding region of MRCT from the opti-MR cDNA was subcloned into a pGEX-5X-1 plasmid to generate GST-fused MRCT. Bacterial expressed GST-MRCT or GST alone proteins were purified by incubation with GBs. GB-bound GST-MRCT or GST alone proteins were incubated with HEK293 or HeLa cell lysates. The results of Western blot analysis showed that GST-MRCT could interact with BiP (Fig. 6B, lane 3), whereas GST (lane 2) or beads alone (lane 1) failed to bind BiP. Next, to determine the specificity of the MRCT–BiP interaction, GST-MRCT bound to GB was incubated with cell lysates of HeLa cells in the presence of increasing concentrations of anti-MR antibody (0–100 μg/ml). Results of a Western blot analysis showed that BiP binding to GST-MRCT was blocked by the MR antibody in dose-dependent manner (Fig. 6C).
Figure 6. Binding of BiP to the cytoplasmic tail region of the MR.
The coding region of the MRCT from the opti-MR cDNA was subcloned into the pGEX-5X-1 plasmid to generate GST-fused MRCT. Bacterial-expressed GST-MRCT or GST-alone proteins were purified by incubation with GBs. GB-bound GST-MRCT or GST alone was incubated with HeLa cell lysates overnight at 4°C and proteins separated by SDS-PAGE. Western blot analysis showed that BiP bound to GST-MRCT constructs (A, lane 3) but not to GST alone (A, lane 2). To determine specificity of BiP binding to the MRCT, HeLa cell extracts were incubated with GST-MRCT in the presence of increasing concentrations of anti-MR antibody (0–100 μg/ml). Proteins were separated by SDS-PAGE and bound BiP analyzed by Western blot (B). To determine the potential motifs in the MRCTs that bind BiP, the RR amino acids in the KKRR sequence were mutated to AA, and the resulting, mutated MRCT was subcloned into pGEX-5X-1 to generate the GST-MRCT RRAA construct. GST-MRCT-RRAA or GST-MRCT bound to glutathione beads was incubated with HEK293 cell lysates, and BiP binding was examined by Western blot analysis. As shown in A, lane 4, mutation of the RR residues eliminated BiP binding MR tail. To further examine the role of the RR sequence in BiP binding, HeLa lysates were incubated with increasing concentrations of GST-MRCT or GST-MRCT RRAA, and proteins bound to glutathione beads were analyzed by SDS-PAGE and Western blot. As shown in C, BiP binding to the mutated MRCT was reduced significantly. WCL, Whole cell lysates.
A recent study by Takenaka et al. [24] showed that peptides containing hydrophobic sequences, followed by dibasic amino acids, had the highest affinity for HSP70 proteins. The MR contains a dibasic motif—KKRR—in the juxtamembrane region of the cytoplasmic tail (Fig. 6A). To determine the role of this motif in MR-BiP binding, the two arginine residues were replaced with alanine by site-directed mutagenesis. The mutant GST-MRCT-RRAA or WT GST-MRCT was expressed and purified as described and tested for its ability to bind BiP. GST-MRCT-RRAA or GST-MRCT bound to glutathione beads was incubated with HEK293 cell lysates, and BiP binding was examined by Western blot analysis. As shown in Fig. 6C, lane 4, mutation of the RR residues eliminated BiP binding to the MRCT. Incubation of HEK293 cell lysates with an increasing amount of GST-MRCT resulted in significant BiP binding, whereas incubation with increasing levels of GST-MRCT-RRAA showed reduced binding.
DISCUSSION
The MR has long been recognized as a cell-surface receptor that plays a critical role in regulating extracellular proteins and enzymes containing high mannose oligosaccharide groups [1, 3]. Additional studies have suggested that the MR also binds and facilitates entry of pathogens through phagocytosis, as well as activating intracellular signaling pathways leading to production of cytokines [2, 25]. When the identification of the primary structure of this receptor was first reported, it was found that a large portion of this type I endocytic receptor was in the extracellular domain, with only 45 out of a total of 1456 aa comprising the cytoplasmic tail [5]. Early reports showed that removal of the entire cytoplasmic domain, as well as the mutation of the tyrosine residue in the FENTLY motif, decreased phagocytosis of yeast particles and endocytosis by ∼50% [6]. What is not known are the cytoplasmic partners that contribute to these endocytic, phagocytic, and signaling pathways. In the current report, studies were conducted to begin defining cellular binding partners involved in these functions.
Proteomic analysis revealed several predictable partners, such as IQGAP1, involved in phagocytic cup formation and eEF1a2, which plays a role in macrophage deactivation [11, 12]. The surprising finding was the binding of several members of the HSP family, including Grp75, GRP78/BiP, and Hsc70. Further studies revealed that these proteins could be identified as a part of a complex with the MR using immunoprecipitation with anti-MR antibodies, ligand precipitation using fucose-agarose beads, and colocalization using immunostaining and confocal microscopy. A possible binding site in the MR cytoplasmic domain for BiP was identified further through mutation of a multibasic motif (KKRR).
Several recent reports have extended the role of HSPs to include regulation of receptor-mediated uptake of macromolecules from the extracellular space, a role that the MR plays in removal of hydrolases and other high mannose-containing glycoproteins. Huang et al. [15] reported that disruption of the HSP complex in HuH cells resulted in a 50% reduction in the surface levels of the ASGPR. The authors further demonstrated that binding of the HSP complex was required for interaction of the ASGPR with adaptor proteins within the clathrin complex, allowing for appropriate endocytosis. The authors concluded that HSP70 binding to the cytoplasmic domain of the ASGPR was required prior to adaptor protein binding. These studies were conducted in a mutant HuH cell line that also showed defective HSP-mediated transferrin receptor and MR trafficking, suggesting that HSP binding to the cytoplasmic region is shared by several prototypic recycling receptors. Vega et al. [16] reported that heat shock increased transferrin endocytosis through accelerated internalization of ligand-receptor complexes and increased receptor recycling. Although they did not examine direct HSP70 transferrin receptor binding, the results from the current MR study as well as the work of Huang et al. [15] suggest that HSP70 may play an important role in connecting endocytic receptors to the internalization machinery within the cell. In a more general role, Newmyer and Schmid [26] reported that Hsc70, another member of the HSP70 family, is involved in modulation of clathrin dynamics during the clathrin-coated vesicle cycle and demonstrated that mutants of Hsc70 blocked transferrin receptor recycling. The MR appears to follow a traditional recycling receptor pathway, suggesting that HSP family members and the MR may interact at the early endosomal stage, where clathrin assembly and disassembly are required for efficient movement of the receptor to the next stage (i.e., late endosome and recycling to the plasma membrane).
The identity of the binding motif for HSPs within the cytoplasmic domain of the recycling receptors has not been reported. Takenaka et al. [24] screened a 15-mer phage display random peptide library with Hsc70 and showed a significant enrichment in peptides containing KK, KR, or KKRR sequences. Peptides containing hydrophobic sequences followed by the dibasic amino acids had the highest affinity for Hsc70. The MR contains a KKRR sequence in the juxtamembrane region of the cytoplasmic domain. When the RR sequence was mutated to AA in the current study, BiP binding to the MR cytoplasmic domain was lost, implicating this region in MR-BiP binding. Although not investigated in the current study, Ha et al. [27] reported the involvement of a key serine residue in the C-terminal domain of aquaporin that appeared to be important for the direct aquaporin–Hsc70 interaction. Other studies have similarly shown that serine phosphorylation in the ASGPR tail appears to play a role in the interaction with Hsp70.
The MR exists on the cell surface and intracellularly, with steady-state levels at ∼20% and 80% [22]. Although this intracellular pool functions, in part, to replenish surface MR, depleted by proteolysis or lost during the endocytic and/or phagocytic processes, the role of the remaining intracellular MR pool is not known. Recent reports have highlighted the expanding role of lectin chaperones in the proper targeting and transport of newly synthesized proteins [28]. One possible function for this intracellular MR pool might be to assist in the trafficking of high mannose-containing glycoproteins as they are processed within and transported from the ER. Supporting this hypothesis is the recent finding by Nawa et al. [29] that the intracellular lectin VIP36 specifically interacts with BiP and may play a role in delivering misfolded glycoproteins to BiP. In addition, Nonaka et al. [30] reported that MBL, which shares a high degree of homology with the MR carbohydrate recognition domain, is found intracellularly in hepatocytes and appears to function in proper trafficking of newly synthesized glycoproteins. At least one endogenous ligand for MBL is lysosomal-associated membrane protein 1, a lysosomal protein that has been shown to be dependent on attachment of high mannose chains in the ER for proper delivery to lysosomes [31]. Defining this molecule as an intracellular cargo receptor lends further important support and evidence to the hypothesis that intracellular MR plays a similar function as a cargo receptor in macrophages. These observations suggest that MBL assists in transport of proteins to the lysosomal compartment, a function that the MR may play as well.
Although the current study focused primarily on the interaction of the MRCT with HSP70, interaction with other domains in the MR cannot be ruled out. For example, a number of recent reports have suggested that although HSP70 functions primarily as an intracellular molecular chaperone, under certain circumstances, it is released from cells into the extracellular environment, where it mediates intracellular signaling on target cells [14]. Several cell-surface receptors have been reported to bind and internalize the released HSP70. Although the exact identification of the primary receptor is not known, Theriault et al. [32] reported in 2006 that members of the scavenger receptor family and C-type lectins of the NK family were involved in binding and internalization of Hsp70. The authors suggested that there might be a wide range of cell-surface HSP70-binding structures, thus permitting intracellular responses to extracellular Hsp70 that are cell-specific and Hsp70 family member-specific. The MR, which is found predominantly on macrophages and DCs, might play this role for cells of the innate immune system.
The results in the current study extend the function and role of HSPs in regulation of cellular function and implicate HSP–MR interaction in regulation of entry of extracellular glycoproteins and/or ingestion of pathogens by macrophages. Although the exact role of this interaction is not yet known, it is likely that HSPs bind to the MR and modulate entry and/or trafficking mediated by this C-type lectin. Future studies from this laboratory will focus on identifying a role for HSP–MR interaction in macrophages, the location of this interaction within the cell, and the residues in the HSP70 and MR molecules involved in the binding.
ACKNOWLEDGMENTS
This work was supported, in part, by a grant from the U.S. Department of Veterans Affairs (to V.L.S.). Flow cytometry experiments were performed in the VMC Flow Cytometry Shared Resource, which is supported by the Vanderbilt University Ingram Cancer Center (P30 CA68485) and the Vanderbilt University Digestive Disease Research Center (DK05 8404). Confocal imaging and analysis were performed, in part, through the use of the VUMC Cell Imaging Shared Resource (supported by U.S. National Institutes of Health grants CA68485, DK20593, DK58404, HD15052, DK59637, and EY08126).
Footnotes
- ASGPR
- asialoglycoprotein receptor
- BiP
- binding Ig protein
- eEF1α2
- eukaryotic elongation factor 1 α2
- EGCG
- epigallocatechin gallate
- GB
- glutathione-bound agarose bead
- Grp75/78
- 75/78-kDa glucose-regulated protein
- HEK
- human embryonic kidney
- Hsc70
- heat shock cognate 70
- HSP
- heat shock protein
- LC
- liquid chromatography
- LTQ
- linear trap quadrupole
- MBL
- mannose-binding lectin
- MR
- mannose receptor
- MRCT
- mannose receptor cytoplasmic tail
- MS
- mass spectrometry
- NP-40
- Nonidet P-40
- opti-MR
- optimized mannose receptor
- VUMC
- Vanderbilt University Medical Center
AUTHORSHIP
S.Y. performed experiments, analyzed data, and contributed to writing the manuscript. D.J.V. consulted and contributed to vector construction, experimental design, and writing and finalizing the manuscript. V.L.S is the principal investigator and designed the research, analyzed data, and contributed to writing and finalizing the manuscript.
REFERENCES
- 1. Stahl P., Schlesinger P. H., Sigardson E., Rodman J. S., Lee Y. C. (1980) Receptor-mediated pinocytosis of mannose glycoconjugates by macrophages: characterization and evidence for receptor recycling. Cell 19, 207–215 [DOI] [PubMed] [Google Scholar]
- 2. Underhill D. M., Ozinsky A. (2002) Phagocytosis of microbes: complexity in action. Ann. Rev. Immunol. 20, 825–852 [DOI] [PubMed] [Google Scholar]
- 3. Shepherd V. L., Hoidal J. R. (1990) Clearance of neutrophil-derived myeloperoxidase by the macrophage mannose receptor. Am. J. Respir. Cell Mol. Biol. 2, 335–340 [DOI] [PubMed] [Google Scholar]
- 4. Stahl P. D., Ezekowitz R. A. B. (1998) The mannose receptor is a pattern recognition receptor involved in host defense. Curr. Opin. Immunol. 10, 50–55 [DOI] [PubMed] [Google Scholar]
- 5. Taylor M. E., Conary J. T., Lennartz M. R., Stahl P. D., Drickamer K. (1990) Primary structure of the mannose receptor contains multiple motifs resembling carbohydrate-recognition domains. J. Biol. Chem. 265, 12156–12162 [PubMed] [Google Scholar]
- 6. Kruskal B. A., Sastry K., Warner A. B., Mathieu C. E., Ezekowitz R. A. B. (1992) Phagocytic chimeric receptors require both transmembrane and cytoplasmic domains from the mannose receptor. J. Exp. Med. 176, 1673–1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schweizer A., Stahl P. D., Rohrer J. (2000) A di-aromatic motif in the cytosolic tail of the mannose receptor mediates endosomal sorting. J. Biol. Chem. 275, 29694–29700 [DOI] [PubMed] [Google Scholar]
- 8. Chen J-J., Goldstein J. L., Bronw M. S. (1990) NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J. Biol. Chem. 265, 3116–3123 [PubMed] [Google Scholar]
- 9. Bonifacino J. S., Traub L. M. (2003) Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu. Rev. Biochem. 72, 395–447 [DOI] [PubMed] [Google Scholar]
- 10. Vigerust D. J., Egan B. S., Shepherd V. L. (2005) HIV-1 Nef mediates post-translational down-regulation and redistribution of the mannose receptor. J. Leukoc. Biol. 77, 522–534 [DOI] [PubMed] [Google Scholar]
- 11. Bukau B., Weissman J., Horwich A. (2006) Molecular chaperones and protein quality control. Cell 125, 443–451 [DOI] [PubMed] [Google Scholar]
- 12. Brandt D. B., Marion S., Griffiths G., Watanabe T., Kaibuchi K., Grosse R. (2007) Dia1 and IQGAP1 interact in cell migration and phagocytic cup formation. J. Cell Biol. 178, 193–2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nandan D., Yi T., Lopez M., Lai C., Reiner N. E. (2002) Leishmania EF-1α activates the Src homology 2 domain containing tyrosine phosphatase SHP-1 leading to macrophage deactivation. J. Biol. Chem. 277, 50190–50197 [DOI] [PubMed] [Google Scholar]
- 14. Henderson B. (2009) Integrating the cell stress response: a new view of molecular chaperones as immunological and physiological homeostatic regulators. Cell. Biochem. Func. 28, 1–14 [DOI] [PubMed] [Google Scholar]
- 15. Huang T., Wolkoff A. W., Stockert R. J. (2006) Adaptor heat shock protein complex formation regulates trafficking of the asialoglycoprotein receptor. Am. J. Physiol. Gastrointest. Liver Physiol. 290, G369–G376 [DOI] [PubMed] [Google Scholar]
- 16. Vega V. L., Wisler C., De Maio A. (2010) A new feature of the stress response: increase in endocytosis mediated by Hsp70. Cell Stress Chaperones 15, 517–527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fath S., Bauer A. P., Liss M., Spriestersbach A., Maertens B., Hahn P., Ludwig C., Schafer F., Graf M., Wagner R. (2011) Multiparameter RNA and codon optimization: standardized tool to assess and enhance autologous mammalian gene expression. PLoS One 6, e17596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sperber K., Bauer J., Pizzimenti A., Najfeld V., Mayer L. (1990) Identification of subpopulations of human macrophages through the generation of human macrophage hybridomas. J. Immunol. Methods 129, 31–40 [DOI] [PubMed] [Google Scholar]
- 19. Vigerust D. J., Vick S., Shepherd V. L. (2012) Characterization of a functional mannose receptor in a continuous hybridoma cell line. BMC Immunol. 13, 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Balagopal A., MacFarlane A. S., Mohapatra N., Soni S., Gunn J. S., Schlesinger L. S. (2006) Characterization of the receptor-ligand pathways important for entry and survival of Francisella tularensis in human macrophages. Infect. Immun. 74, 5114–5125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Stephenson J. D., Shepherd V. L. (1987) Purification of the human alveolar macrophage mannose receptor. Biochem. Biophys. Res. Commun. 148, 883–889 [DOI] [PubMed] [Google Scholar]
- 22. Tietze C., Schlesinger P., Stahl P. (1982) Mannose-specific endocytosis receptor of alveolar macrophages: demonstration of two functionally distinct intracellular pools of receptor and their roles in receptor recycling. J. Cell Biol. 92, 417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Tran P., Kim S-A., Choi H. S., Yoon J-H., Ahn S-G. (2010) Epigallocatechin-3-gallate suppresses the expression of HSP70 and HSP90 and exhibits anti-tumor activity in vitro and in vivo. BMC Cancer 10, 276–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Takenaka I. M., Leung S. M., McAndrew S. J., Brown J. P., Hightower L. E. (1995) Hsc70-binding peptides selected from a phage display peptide library that resemble organelle targeting sequences. J. Biol. Chem. 270, 19839–19844 [DOI] [PubMed] [Google Scholar]
- 25. Yamamoto Y., Klein T. W., Friedman H. (1997) Involvement of mannose receptor in cytokine interleukin-1β (IL-1β), IL-6, and granulocyte-macrophage colony-stimulating factor responses, but not in chemokine macrophage inflammatory protein 1β (MIP-1β), MIP-2, and KC responses, caused by attachment of Candida albicans to macrophages. Infect. Immun. 65, 1077–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Newmyer S. L., Schmid S. L. (2001) Dominant-interfering Hsc70 mutants disrupt multiple stages of the clathrin-coated vesicle cycle in vivo. J. Cell Biol. 152, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ha L., Sun T. X., Matsuzaki T., Yi S. H., Eswara J., Bouley R., McKee M., Brown D. (2007) Heat shock protein 70 interacts with aquaporin-2 and regulates its trafficking. J. Biol. Chem. 282, 28721–28732 [DOI] [PubMed] [Google Scholar]
- 28. Pearse B. R., Hebert D. N. (2010) Lectin chaperones help direct the maturation of glycoproteins in the endoplasmic reticulum. Biochim. Biphys. Acta 1803, 684–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nawa D., Shimada O., Kawasaki N., Matsumoto N., Yamamoto K. (2007) Stable interaction of the cargo receptor VIP36 with molecular chaperone BiP. Glycobiology 17, 913–921 [DOI] [PubMed] [Google Scholar]
- 30. Nonaka M., Ma B. Y., Ohtani M., Yamamoto A., Murata M., Totani K., Ito Y., Miwa K., Nogami W., Kawasaki N., Kawasaki T. (2007) Subcellular localization and physiological significance of intracellular mannan-binding protein. J. Biol. Chem. 282, 17908–17920 [DOI] [PubMed] [Google Scholar]
- 31. Kundra R., Kornfeld S. (1999) Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J. Biol. Chem. 274, 31039–31046 [DOI] [PubMed] [Google Scholar]
- 32. Theriault J. R., Adachi H., Calderwood S. K. (2006) Role of scavenger receptors in the binding and internalization of heat shock protein 70. J. Immunol. 177, 8604–8611 [DOI] [PubMed] [Google Scholar]