Abstract
Purpose
To assess the prevalence of astigmatism and its relationship with biometric optic components in preterm school children with diode laser-treated threshold retinopathy of prematurity (ROP).
Methods
A prospective, cross-sectional study in which cycloplegic keratometry, refraction, and ultrasound biometric measurement of optic components were performed on 24 consecutive preterm children with diode laser-treated threshold ROP at the age of 9 years. The study results were compared with data on 1021 age-matched full-term control children from a national survey.
Results
The laser-treated eyes had a mean astigmatism of 3.47 D, with a mean spherical equivalent of −4.49 D. Of the 46 eyes studied, 98% of eyes showed astigmatism ≥0.5 D and 50% had high astigmatism (>3.0 D). Most astigmatic eyes (97.7%) showed with-the-rule astigmatism, with the mean plus cylinder axis at 89.30o. Further correlation analysis showed the astigmatism in refraction was highly correlated with the corneal astigmatism (r=0.921, P<0.001) and the vertical corneal curvature (r=0.405, P=0.005). There was significantly steeper vertical corneal curvature (P=0.003) and flatter horizontal corneal curvature (P=0.031) in eyes with laser-treated ROP when compared with age-matched full-term controls. The eyes with laser-treated ROP also show significantly thicker lens (3.93 mm) and shallower anterior chamber depth (ACD; 2.92 mm) than full-term controls (P<0.001).
Conclusions
There is significantly higher prevalence and greater magnitude of astigmatism in eyes with laser-treated threshold ROP compared with full-term controls. The steeper vertical corneal curvature component contributes to the increased astigmatism in eyes with laser-treated ROP.
Keywords: astigmatism, biometry, diode laser, optic components, retinopathy of prematurity
Introduction
Retinopathy of prematurity (ROP) is an important cause of infant blindness and visual impairment worldwide.1 Visual outcome of premature infants with regressed ROP is attributed to both structural and functional outcomes. Astigmatism and myopia are common findings in premature infants and incidences of which increases with increasing prematurity and presence of ROP.2, 3 In a population-based study, Holmstrom et al3 reported that 52% of premature infants with birth body weight (BW) of ≤1500 g developed astigmatism (≥1.0 D) at 6 months corrected age and 18% had high astigmatism (≥2.0 D). In addition, both lower birth BW and presence of ROP were significantly associated with higher incidence of astigmatism.3 Quinn et al4 also reported that the incidence of myopia and high myopia was substantially higher in infants with astigmatism or anisometropia than in infants without these refractive abnormalities. A higher incidence of astigmatism was associated with more severe ROP.4 However, it is not clear yet which ocular element is most involved in the development of astigmatism and myopia in patients with prematurity and ROP. Various hypotheses, including anomalies of corneal diameter and steep curvature, shallow anterior chamber, increased axial length (AL), and increased lens thickness, have previously been suggested.4, 5, 6, 7 Clearly, more studies of biometric measurements are needed to clarify the role of optic components in determining the refractive abnormalities of astigmatism and myopia in preterm patients.
Most studies concerning the refractive error (RE) and optic components have focused on infants or children of preschool age.2, 3, 4, 5, 7 However, the emmetropization process of ocular development continues after the age of 5 years. Few studies with recent data concerning the long-term biometric optic components in school age children with threshold ROP after laser therapy in Asia are available.8 The purpose of this study was to investigate the prevalence, amount, and axis of astigmatism and their relationships with biometric optic components of premature infants with threshold ROP treated with diode laser who reached 9 years of age, and the study results were compared with those for age-matched full-term control children from a national survey. To the best of our knowledge, there is a relative paucity of studies on astigmatism and its relationship with biometric ocular data up to 9 years of age in the literature. In this study, we evaluated the effect of preterm birth on ocular development, and we highlighted the biometric optic components and their relationships with RE in children with laser-treated ROP.
Patients and methods
The study protocol was approved by the Institutional Review Board of Taipei Veterans General Hospital. From January 1997 to December 2000, premature infants <2000 g birth BW or <34 weeks of gestational age were screened for ROP in the neonatal newborn room or intensive care unit at Taipei Veterans General Hospital by a single ophthalmologist (CY). At the first ophthalmic examination, the eyes of high-risk premature infants were screened at postnatal age of 4–6 weeks under indirect ophthalmoscope.9, 10, 11 The stage and severity of ROP was classified according to the International Classification of ROP.12 Threshold disease of ROP was defined by the Cryotherapy for Retinopathy of Prematurity Cooperative Group (CRYO-ROP study) as ≥5 contiguous or 8 cumulative clock-hours of extraretinal neovascularization stage 3 ROP and associated with plus disease within zones I or II.13 Plus disease represented the dilatation and tortuosity of retinal blood vessels in the posterior pole. The indication for laser treatment is development of threshold ROP as defined by CRYO-ROP study and similar to the conventional laser group in the Early Treatment for Retinopathy of Prematurity Cooperative Group (ET-ROP) study.14 Infants with threshold disease received retinal ablation laser photocoagulation therapy within 72 h of diagnosis. Thirty-seven premature babies received diode laser (810 nm wavelength) treatment (Iris Medical Instruments, Inc., Mountain View, CA, USA) therapy under laser indirect ophthalmoscope for threshold ROP. The setting of laser treatment was 250–300 mW at the creamy-white laser intensity, near confluence pattern, and placed to the retina avascular zone anterior to the fibrovascular ridge.
Of the 37 children with laser-treated threshold ROP, one infant who had zone I rush disease rapidly progressed to stage 5 despite photocoagulation. Unfortunately, the patient died before the age of 6 months, and this was not attributed to ROP laser treatment. Twelve patients were excluded from this study because they were either lost to follow-up or unable to undergo the complete ocular examinations because of cognitive issue. Overall, a total of 24 children were enrolled in this study with informed parental consent. The patients were recalled at the age of 9 years to the eye clinic at Taipei Veterans General Hospital for complete ophthalmological assessment by the clinician (CY). The premature birth history and data were collected from the medical records, including the gestational age (GA), birth (BW, and perinatal neurological events, such as intraventricular hemorrhage, periventricular leucomalacia, and cerebral palsy.
Patients underwent the complete ophthalmological assessment, including best-corrected visual acuity, cycloplegic refraction, biometric measurement of optic components, and indirect ophthalmoscopic examination. The best-corrected visual acuity was determined by Snellen chart at 6 m. To include all data in the statistical analysis and comparison, visual acuity was converted as the log of the minimum angle of resolution (log MAR) value. Cycloplegic refraction of the eyes was determined with a desktop computer auto kerato-refractometer (Topcon KR-8100, Tokyo, Japan) after instillation of 1% cyclopentolate hydrochloride eye drops twice at a 10-min interval. RE was converted to the spherical equivalent (SE). The astigmatism cylinder, corneal curvature (presented as vertical and horizontal corneal radius (CR)), corneal astigmatism amplitude, and steep axis of astigmatism were also measured. The biometric optic components, including anterior chamber depth (ACD), lens thickness and AL, were measured with A-scan ultrasound (Sonomed, model A-1500, Lake Success, NY, USA) after cycloplegia. Three measurements were recorded for each procedure, with the mean±SD calculated from the three separate measurements.
Astigmatism and high astigmatism were defined as cylinder ≥0.5 D and >3.0 D, respectively. Myopia was defined as SE <−0.5 D, hyperopia as SE >+0.5 D. Astigmatism was further classified as with-the-rule (WTR; axis 75o–105o), against-the-rule (axis 0o–15o and 165o–180o), and oblique astigmatism (axis 16o–74o and 106o–164o). Anisometropia was defined as the difference of SE between the eyes being ≥1.5 D.
To evaluate the effect of prematurity and ROP on astigmatism, we compared the astigmatism, SE, and optic components in the eyes of threshold ROP patients with those of age-matched full-term controls. The data obtained from a national survey of general population children in Taiwan, including 1021 school children at the age of 9 years, were used as age-matched full-term control.15
Statistical analysis
Statistical analysis of the data was performed using SPSS software (version 18.0, SPSS Inc., Chicago, IL, USA). A P-value <0.05 was considered statistically significant. Independent Student's t-test or Wilcoxon's rank-sum test were used for comparing mean values of continuous outcomes between the laser-treated eyes and age-matched control eyes. Chi-square test and Fisher's exact test were used to compare categorical outcomes. Pearson correlation analyses were used to assess the association between the prematurity status and optic components and the association between the astigmatism in refraction and the corneal astigmatism. For the purpose of comparison, the two eyes of the same patient were used as independent variables. Linear regression analysis was performed with generalized estimating equation to control for between-eye correlation.16
Results
From the 24 children recruited for this study, a further 2 eyes were excluded as assessment of ocular tests was not possible: one with retinal detachment due to zone I stage 5 ROP, the other with dense cataract and retinal fold involving the fovea. Therefore, a total of 46 laser-treated eyes were included in this analysis.
The baseline characteristics of patients with laser-treated threshold ROP are shown as follows. The mean GA at birth was 28.80±2.35 weeks, and the mean birth BW was 1256±315 g. The mean age at the last follow-up was 9.2±0.5 years (range: 8.6–9.8 years). The mean LogMAR visual acuity was 0.20±0.24 (equivalent to Snellen VA=6/10). Five (20.8%) patients were treated for clinical diagnosis of amblyopia. Among them, strabismic amblyopia was noted in one patient, anisometropic amblyopia in three patients, and amblyopia with extremely high myopia (<−10.0 D) in one. Six (25%) patients had strabismus, with three cases of esotropia and three of exotropia. The occurrence of perinatal neurological events was noted in 9 patients, including intraventricular hemorrhage in 5 patients, periventricular leucomalacia in 5 cases, and cerebral palsy in 3 children. One eye had retinal vessel strengthening with mild macular dragging involving the fovea on fundoscopy. None of the remaining 45 laser-treated eyes showed any evidence of macular ectopia, disc dragging, or other sign of poor structural outcome.
Refractive outcome
The detailed information of visual outcome and RE of the patients with laser-treated ROP is shown in Table 1. The mean magnitude of the astigmatism cylinder in eyes with laser-treated ROP was 3.47±1.92 D compared with 0.08±0.90 D in controls (P<0.001). The average SE in eyes with laser-treated ROP was −4.49±3.76 D; these eyes were significantly myopic when compared with the mean SE of −0.44±1.48 D in controls (P<0.001). The details of comparisons of astigmatism cylinder, axis, and corneal curvature components between eyes of preterm children with laser-treated threshold ROP and age-matched full-term controls are shown in Table 2. Compared with control subjects, there was higher prevalence of astigmatism (98 vs 41%) and higher prevalence of myopia in eyes with laser-treated ROP (93 vs 32%). Significant anisometropia (≥1.5 D) in eyes with laser-treated ROP was also noted in five (20.8%) patients. The advanced REs such as high myopia (<−6.0 D) and high astigmatism (>3.0 D) in eyes with laser-treated ROP were 28.3 and 50%, respectively. No significant difference in the prevalence of astigmatism or high astigmatism was found between eyes with normal-appearing macula or macular dragging (ectopia).
Table 1. Visual outcome and refractive error of the patients with laser-treated threshold retinopathy of prematurity.
| Patient No. | GA (wk) | BW (g) |
BCVA |
Spherical equivalent (D) |
Astigmatism cylinder (D) |
Duration of oxygen therapy (days) | Perinatal neurological events | |||
|---|---|---|---|---|---|---|---|---|---|---|
| OD | OS | OD | OS | OD | OS | |||||
| 1 | 27 | 1150 | 6/6 | 6/7.5 | −4.75 | −7.50 | −2.25 | −1.50 | 53 | IVH |
| 2 | 26 | 950 | 6/6.7 | 6/7.5 | −2.53 | −1.13 | −5.75 | −5.75 | 37 | PVL,CP |
| 3 | 26 | 1090 | 6/8.6 | 6/8.6 | −3.50 | −2.00 | −4.00 | −4.50 | 12 | |
| 4 | 26 | 1230 | 6/8.6 | 6/10 | −1.00 | −1.00 | −3.00 | −2.50 | 10 | PVL |
| 5 | 29 | 1011 | 6/8.6 | 6/15 | −5.50 | −7.50 | −7.25 | −7.50 | 50 | IVH |
| 6a | 27 | 1000 | NLP | 6/15 | NA | −10.50 | NA | −5.00 | 20 | |
| 7 | 28 | 1100 | 6/6 | 6/6 | −3.75 | −3.50 | −1.75 | −1.50 | 25 | PVL,CP |
| 8 | 26 | 900 | 6/6.7 | 6/60 | −4.63 | −9.63 | −2.75 | −3.25 | 45 | |
| 9 | 26 | 760 | 6/12 | 6/20 | −14.25 | −14.50 | −3.00 | −4.25 | 54 | |
| 10 | 25 | 760 | 6/6 | 6/6.7 | −5.50 | −6.50 | −1.75 | −1.75 | 45 | IVH |
| 11 | 33 | 1618 | 6/15 | 6/30 | −2.75 | −2.25 | −1.00 | −2.50 | 5 | |
| 12b | 28 | 1250 | 1/60 | 6/12 | NA | −9.50 | NA | −4.75 | 28 | |
| 13 | 31 | 1900 | 6/6.7 | 6/6.7 | −1.75 | −1.75 | −4.20 | −2.00 | 12 | IVH,PVL |
| 14 | 31 | 1600 | 6/6 | 6/8.6 | −0.50 | −1.25 | −1.50 | −1.25 | 15 | |
| 15 | 30 | 1800 | 6/6 | 6/7.5 | −1.25 | −3.25 | −3.25 | −3.75 | 3 | |
| 16 | 30 | 1130 | 6/6 | 6/6 | +0.75 | +1.00 | −2.50 | −1.75 | 35 | |
| 17 | 30 | 1618 | 6/20 | 6/15 | −13.50 | −7.13 | −8.00 | −7.75 | 15 | |
| 18 | 29 | 1010 | 6/6 | 6/6 | −1.75 | −1.50 | −0.50 | −0.25 | 3 | |
| 19 | 30 | 1400 | 6/6 | 6/6 | −2.25 | −1.75 | −4.50 | −5.00 | 23 | |
| 20 | 29 | 1018 | 6/6.7 | 6/6.7 | −3.75 | −4.25 | −5.88 | −5.13 | 30 | PVL,CP |
| 21 | 34 | 1380 | 6/10 | 6/12 | −6.50 | −5.13 | −4.00 | −2.75 | 9 | |
| 22 | 29 | 1450 | 6/60 | 6/7.5 | −9.00 | −7.00 | −4.75 | −3.00 | 12 | IVH |
| 23 | 29 | 1460 | 6/8.6 | 6/7.5 | −2.50 | −2.50 | −3.25 | −3.25 | 10 | |
| 24 | 31 | 1420 | 6/15 | 6/12 | −3.50 | −3.00 | −2.25 | −1.75 | 17 | |
Abbreviations: CP, cerebral palsy; IVH, intraventricular hemorrhage; NA, not applicable as assessment of visual acuity and ocular examination is not possible; PVL, periventricular leucomalacia.
Patient No 6 had stage 5 ROP in right eye.
Patient No. 12 had dense cataract and a retinal fold involving the fovea of right eye.
Table 2. Comparisons of astigmatism cylinder, spherical equivalent (SE), and corneal curvature components between eyes of preterm children with laser-treated threshold retinopathy of prematurity and age-matched full-term controls.
|
ROP eyes |
Age-matched controls |
P-value | |
|---|---|---|---|
| (N=46) | (N=1021) | ||
| Astigmatism cylinder*, D | 3.47±1.92 | 0.08±0.90 | <0.001 |
| Spherical equivalent (SE)*,D | −4.49±3.76 | −0.44±1.48 | <0.001 |
| Vertical CR (VCR)**, mm | 7.46±0.38 | 7.67±0.35 | 0.003 |
| Horizontal CR (HCR)***, mm | 7.98±0.35 | 7.88±0.28 | 0.031 |
| Mean corneal radius (CR), mm | 7.73±0.34 | 7.76±0.45 | 0.650 |
| Astigmatism steep axis,o | 89.30±7.24 | 83.63±21.49 | 0.08 |
P-value <0.05 is statistically significant.
Data are presented as the mean±SD.
*P value <0.001.
**P value <0.01.
***P value <0.05.
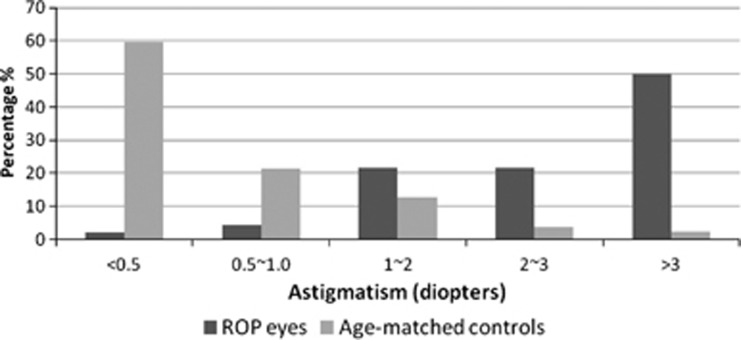
The distribution of astigmatism between eyes with laser-treated ROP and age-matched controls is shown in Figure 1. In control population, >80% of eyes had <1.0 D astigmatism. Conversely, the distribution of astigmatism in eyes with laser-treated threshold ROP showed >80% with >1.0 D astigmatism, and peaked at >3.0 D astigmatism (50%).
Figure 1.
Distribution of astigmatism between eyes of children with diode laser-treated threshold ROP and age-matched control (ROP eyes, N=46; age-matched controls, N=1021).
Keratometric studies showed that the vertical CR of laser-treated ROP eyes was 7.46±0.38 mm, horizontal CR 7.98±0.35 mm, and mean CR 7.73±0.34 mm. The mean corneal astigmatism amplitude was 2.89±1.22 D. Most eyes (97.7%) with astigmatism showed WTR astigmatism (axis 75o–105o) with the mean plus cylinder axis at 89.30±7.24o, and <3% had oblique astigmatism (axis 16o–74 o and 106o–164o). Further correlation analysis showed the astigmatism in refraction was highly correlated with the corneal astigmatism (r=0.921, P<0.001) and the vertical corneal curvature(r=0.405, P=0.005). Also, the astigmatism cylinder was highly correlated with the SE (r=0.383, P<0.01).
Biometric optic components
There was significantly steeper vertical corneal curvature (P=0.003) and flatter horizontal corneal curvature (P=0.031) in eyes with laser-treated ROP when compared with age-matched full-term controls (Table 2). A comparison of the biometric optic components between eyes of preterm children with laser-treated ROP and controls in detail is shown in Table 3. Compared with those of age-matched control subjects, the eyes with laser-treated ROP showed significantly thicker lens (P<0.001) and shallower ACD (P<0.001) in optic component measurement, but no difference in terms of AL between two groups (P=0.700).
Table 3. Comparisons of mean biometric optic components between eyes of children with laser-treated threshold retinopathy of prematurity and age-matched controls.
|
ROP eyes |
Age-matched controls |
P-value | |
|---|---|---|---|
| (N=46) | (N=1021) | ||
| LT*, mm | 3.93±0.32 | 3.39±0.24 | <0.001 |
| ACD*, mm | 2.92±0.35 | 3.58±0.35 | <0.001 |
| AL, mm | 23.31±1.26 | 23.24±0.97 | 0.700 |
Abbreviations: ACD, anterior chamber depth; AL, axial length; LT, lens thickness.
*P-value <0.05 is statistically significant.
Data are presented as the mean±SD.
To clarify the correlation of prematurity status with optic components in ROP eyes, Pearson correlation analysis showed that preterm children with lower birth BW or younger GA tended to have shallower ACD, thicker lens, and steeper cornea (Table 4). In preterm children, lower birth BW was correlated with steeper cornea (r=0.358, P=0.015) and shallower ACD (r=0.368, P=0.012); younger GA was correlated with shallower ACD (r=0.313, P=0.034) and thicker lens (r=−0.295, P=0.046). However, there was no correlation between prematurity status and AL.
Table 4. Correlation analysis of the prematurity status (BW or GA) with optic components (ACD, CR, LT and AL) in eyes of children with laser-treated threshold retinopathy of prematurity (N=46).
| BW (gm) | GA (wk) | |
|---|---|---|
| ACD, mm | R=0.368 (P=0.012)* | R=0.313 (P=0.034)* |
| CR, mm | R=0.358 (P=0.015)* | R=0.064 (P=0.672) |
| LT, mm | R=−0.258 (P=0.083) | R=−0.295 (P=0.046)* |
| AL, mm | R=0.129 (P=0.394) | R=−0.055 (P=0.718) |
Abbreviations: ACD, anterior chamber depth; AL, axial length; BW, birth weight; CR, corneal radius; GA, gestational age; LT, lens thickness.
R: Pearson correlation coefficient.
*P-value <0.05 is statistically significant.
Discussion
This study presents the long-term status of astigmatism and its relationship with biometric optic components of laser-treated threshold ROP in children at the age of 9 years. Comparing with control subjects, there were significantly higher prevalence and greater magnitude of astigmatism in eyes with laser-treated ROP. Most eyes showed WTR astigmatism with the mean plus cylinder axis at 89.30o. The magnitude of astigmatism in preterm school children with laser-treated ROP is well correlated with the corneal astigmatism and vertical corneal curvature. The above findings are well compatible with the significantly steeper vertical corneal curvature and flatter horizontal corneal curvature in keratometric measurement of eyes with laser-treated ROP when compared with age-matched full-term controls. We demonstrated that the difference in corneal curvature components contributed to the increased astigmatism in eyes with laser-treated ROP in the current study. Preterm children with laser-treated ROP also showed significantly thicker lens and shallower ACD in biometric measurements when compared with age-matched full-term controls. However, there was no difference in terms of AL between the two groups.
Most premature infants develop astigmatism, and children with advanced ROP tend to have more astigmatism. Holmstrom et al3 reported that 52% of premature infants with birth BW of ≤1500 g developed astigmatism (≥1.0 D) at 6 months corrected age and 18% had high astigmatism (≥2.0 D). In addition, both lower birth BW and presence of ROP were significantly associated with higher incidence of astigmatism.3 Compared with the age-matched controls, the results of the present study showed that the laser-treated threshold ROP eyes had significantly higher prevalence and severity of astigmatism at 9 years of age. In the current study, 98% of the laser-treated eyes had ≥0.5 D astigmatism, and 50% had high astigmatism of ≥3.0 D. Davitt et al,17 in a longitudinal study of ET-ROP, reported that nearly 43% of their laser-treated premature (<1251 g) infants, either early-treated or under conventional laser management, developed astigmatism (≥1.0 D) by age 3 years and 20% had high astigmatism (≥2.0 D). The decreases in prevalence of astigmatism were reported as being from 6 to 30 months of corrected age in three previous studies of preterm infants.3, 18, 19 Conversely, a recent study, in which 293 children with birth BW <1701 g underwent refractive measurement at 10–12 years, reported little change in astigmatism power or axis, with 75% of children showing an increase in cylinder power within 0.75 D when compared with the measurement at 6 months.20 Theng et al21 similarly reported an increase in astigmatism during the first 3 years of life in preterm children. Recently, Davitt et al22 also found astigmatism progression by 6 years of age; 60% of eyes with laser-treated threshold ROP developed astigmatism of ≥1.0 D, and nearly 30% of eyes had high astigmatism (≥2.0 D). They reported that there was a trend toward higher prevalence of astigmatism and high astigmatism in eyes with ROP residua, either straightened vessels or macular heterotopia.22 The prevalence of astigmatism or high astigmatism was not influenced by the macular dragging in our study, although this conclusion is limited by the small number of eyes with macular ectopia.
Astigmatism has been known to be associated with ROP.2 However, the exact mechanism of astigmatism associated with prematurity and ROP is not well understood.3 Corneal curvature (CR or keratometric power) is one of the determinant factors in RE. In normal development, most growth of the eye takes place in the first year of life; the refractive state changes as AL increases, and the cornea and lens flatten after birth.23 However, prematurity may affect the emmetropization process of ocular development in the postnatal stage, so-called myopia of prematurity secondary to the arrested development of the ocular anterior segment.24 Corneal curvature is usually steep in newborn infants and steeper in premature infants.25 Donzis et al26 observed a longitudinal decrease and rapid flattening in the corneal curvature in eyes of six premature babies born at 28–34 weeks GA, starting from the last few months of gestation and continuing for the first 3 months of life. Yamamoto et al27 also reported a mean corneal curvature of 50.75 D in premature infants compared with 48.06 D in mature babies, with an increased keratometric power concomitant with an increased severity of ROP. In the current study, we found that there was significantly steeper vertical corneal curvature in eyes with laser-treated ROP at the age of 9 years compared with full-term age-matched controls. We demonstrated that the premature characteristics of anterior segment components in preterm infants may persist up to school age. Fledelius,28 in a 7 –10-year follow-up study, also found that the CR was lower (steeper curvature) in premature babies with or without ROP. They suggested that the development of the ocular anterior segment was significantly influenced (a relative arrest) by the preterm delivery. Additionally, the ROP insult or postnatal stress may also influence postnatal ocular development.3, 29 We hypothesize that there is incomplete postnatal development of the cornea, anterior sclera, and anterior segment, as evidenced by shallowing of the anterior chamber and smaller CR in the current study. Fielder et al24 have suggested that the ROP insult retards that part of the globe which is undergoing maximal growth, and this effect will, in turn, mechanically inhibit anterior segment development. In the current study, further analysis also showed that the status of prematurity (younger GA or lower birth BW) was well correlated to steeper cornea and shallower ACD. Therefore, we confirm that the severity of prematurity (indicated by lower GA or BW) may ultimately influence the astigmatism of preterm children through its influence on corneal curvature.
We demonstrated that the magnitude of astigmatism in refraction was well correlated with the corneal astigmatism and vertical corneal curvature measured by keratometry. In the current report, most eyes showed WTR astigmatism, with steeper vertical corneal curvature and plus cylinder axis at 89.30o. We hypothesize that there is some correlation between where the laser was placed vs the axis of astigmatism. Most of the laser treatment is placed at temporal meridian in the current study; it would suggest that the temporal laser treatment might have affected the natural course of corneal curvature change in the horizontal axis. The predominant WTR astigmatism in eyes with laser-treated ROP in the current report supports previous studies' findings that most preterm infants had WTR astigmatism.19, 30, 31 Conversely, high astigmatism with a predominant against-the-rule has been reported in children with retinal residua and cryotherapy.3, 32 Whether treatment of ROP affects the development of astigmatism has been an issue to be studied. Quinn et al33 compared cryotherapy-treated eyes with untreated eyes with threshold ROP and found a tendency for a greater proportion of treated than control eyes to have astigmatism of ≥1.00 D at the age of 10 years. The authors concluded that the differences were likely due to cryotherapy's effectiveness in preserving eyes with significant REs that otherwise would have progressed to retinal detachment. In some studies comparing the refractive outcome of threshold ROP after different treatments, the results showed no difference in the prevalence of astigmatism between cryotherapy and laser-treated eyes.32, 34, 35 The only difference that has been found is that patients in the cryotherapy group are more likely to have against-the-rule astigmatism than those in the laser photocoagulation group.32 Trans-scleral cryotherapy is reported to be more traumatic with diffuse tissue destruction of choroid and sclera compared with eyes treated with transpupillary laser therapy in threshold ROP.34, 35 Cryo spots are large and easily confluent. Laser spots are small and discrete with spared interval. Laser therapy may therefore be less likely to interfere with the corneal and anterior scleral growth. All our patients were treated by laser photocoagulation for acute ROP, and most patients had good structural outcome without ROP residua.
Some potential limitations of our study include its nonrandomized study design and lack of a preterm non-severe ROP patients as control set. Some patients were excluded because they were unable to cooperate with biometric assessment due to cognitive impairment. Longitudinal data at different age points were not available, thus serial change of optic components could not be studied. Nevertheless, this study followed a cohort of preterm children for a long period and provided valuable information regarding the long-term astigmatism and biometric optic components following diode laser-treated threshold ROP up to 9 years of age. Furthermore, the optic components of laser-treated ROP were compared with the age-matched full-term control children from a national survey.
In conclusion, there are higher prevalence and greater magnitude of astigmatism in preterm children with laser-treated threshold ROP. Compared with age-matched full-term control subjects, these eyes show significantly steeper vertical corneal curvature, thicker lens, and shallower anterior chamber in optic component analysis but no difference in AL. Refractive status of preterm children with ROP is mainly related to altered anterior segment components. High astigmatism may lead to an increased risk of refractive amblyopia. We emphasize that preterm children with laser-treated ROP should undergo regular follow-up for early identification and timely treatment of high astigmatism during the amblyogenic period of ocular development.
The authors declare no conflict of interest.
References
- Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity. Three-month outcome. Arch Ophthalmol. 1990;108:195–204. doi: 10.1001/archopht.1990.01070040047029. [DOI] [PubMed] [Google Scholar]
- Dobson V, Fulton AB, Manning K, Salem D, Petersen RA. Cycloplegic refractions of premature infants. Am J Ophthalmol. 1981;91:490–495. doi: 10.1016/0002-9394(81)90238-5. [DOI] [PubMed] [Google Scholar]
- Holmstrom M, el Azazi M, Kugelberg U. Ophthalmological long-term follow up of preterm infants: a population based, prospective study of the refraction and its development. Br J Ophthalmol. 1998;82 (11:1265–1271. doi: 10.1136/bjo.82.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Cryotherapy for Retinopathy of Prematurity Cooperative Group. Quinn GE, Dobson V, Repka MX, Reynolds J, Kivlin J, Davis B, et al. Development of myopia in infants with birth weights less than 1251 grams. Ophthalmology. 1992;99:329–340. doi: 10.1016/s0161-6420(92)31968-2. [DOI] [PubMed] [Google Scholar]
- Gordon RA, Donzis PB. Myopia associated with retinopathy of prematurity. Ophthalmology. 1986;93:1593–1598. doi: 10.1016/s0161-6420(86)33523-1. [DOI] [PubMed] [Google Scholar]
- McLoone EM, O'Keefe M, McLoone SF, Lanigan BM. Long-term refractive and biometric outcomes following diode laser therapy for retinopathy of prematurity. J AAPOS. 2006;10:454–459. doi: 10.1016/j.jaapos.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Cryotherapy for Retinopathy of Prematurity Cooperative Group The natural ocular outcome of premature birth and retinopathy. Status at 1 year. Arch Ophthalmol. 1994;112:903–912. doi: 10.1001/archopht.1994.01090190051021. [DOI] [PubMed] [Google Scholar]
- Connolly BP, Ng EY, McNamara JA, Regillo CD, Vander JF, Tasman W. A comparison of laser photocoagulation with cryotherapy for threshold retinopathy of prematurity at 10 years: part 2. Refractive outcome. Ophthalmology. 2002;109:936–941. doi: 10.1016/s0161-6420(01)01015-6. [DOI] [PubMed] [Google Scholar]
- Yang CS, Chen SJ, Lee FL, Hsu WM, Liu JH. Retinopathy of prematurity: screening, incidence and risk factors analysis. J Chin Med Assoc. 2001;64:706–712. [PubMed] [Google Scholar]
- Yang CS, Chen SJ, Lee FL, Hsu WM, Liu JH. Clinical survey of retinopathy of prematurity. Taiwan J Ophthalmol. 2002;41:51–58. [Google Scholar]
- Yang CS, Wang AG, Sung CS, Hsu WM, Lee FL, Lee SM. Long-term visual outcome of laser-treated threshold retinopathy of prematurity: a study of refractive status at 7 years. Eye. 2010;24:14–20. doi: 10.1038/eye.2009.63. [DOI] [PubMed] [Google Scholar]
- The Committee for the Classification of Retinopathy of Prematurity An international classification of retinopathy of prematurity. Arch Ophthalmol. 1984;102:1130–1134. doi: 10.1001/archopht.1984.01040030908011. [DOI] [PubMed] [Google Scholar]
- Cryotherapy for Retinopathy of Prematurity Cooperative Group Multicenter trial of cryotherapy for retinopathy of prematurity: ophthalmological outcome at 10 years. Arch Ophthalmol. 2001;119:1110–1118. doi: 10.1001/archopht.119.8.1110. [DOI] [PubMed] [Google Scholar]
- Early Treatment for Retinopathy of Prematurity Cooperative Group Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–1696. doi: 10.1001/archopht.121.12.1684. [DOI] [PubMed] [Google Scholar]
- Shih YF, Chiang TH, Lin LL. Lens thickness changes among schoolchildren in Taiwan. Invest Ophthalmol Vis Sci. 2009;50:2637–2644. doi: 10.1167/iovs.08-3090. [DOI] [PubMed] [Google Scholar]
- Chen TC, Tsai TH, Shih YF, Yeh PT, Yang CH, Hu FC, et al. Long term evaluation of refractive status and optical components in eyes of children born prematurely. Invest Ophthalmol Vis Sci. 2010;51:6140–6148. doi: 10.1167/iovs.10-5234. [DOI] [PubMed] [Google Scholar]
- Early Treatment for Retinopathy of Prematurity Cooperative Group. Davitt BV, Dobson V, Quinn GE, Hardy RJ, Tung B, Good WV, et al. Astigmatism in the Early Treatment for Retinopathy of Prematurity Study: findings to 3 years of age. Ophthalmology. 2009;116:332–339. doi: 10.1016/j.ophtha.2008.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders KJ, McCulloch DL, Shepherd AJ, Wilkinson AG. Emmetropisation following preterm birth. Br J Ophthalmol. 2002;86:1035–1040. doi: 10.1136/bjo.86.9.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson EK, Holmström GE. Development of astigmatism and anisometropia in preterm children during the first 10 years of life: a population-based study. Arch Ophthalmol. 2006;124:1608–1614. doi: 10.1001/archopht.124.11.1608. [DOI] [PubMed] [Google Scholar]
- O'Connor AR, Stephenson TJ, Johnson A, Tobin MJ, Ratib S, Fielder AR. Change of refractive state and eye size in children of birth weight less than 1701g. Br J Ophthalmol. 2006;90:456–460. doi: 10.1136/bjo.2005.083535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theng JTS, Wong TY, Ling Y. Refractive errors and strabismus in premature Asian infants with and without retinopathy of prematurity. Singapore Med J. 2000;41:393–397. [PubMed] [Google Scholar]
- Davitt BV, Quinn GE, Wallace DK, Dobson V, Hardy RJ, Tung B, et al. Astigmatism progression in the early treatment for retinopathy of prematurity study to 6 years of age. Ophthalmology. 2011;118:2326–2329. doi: 10.1016/j.ophtha.2011.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon RA, Donzis PB. Refractive development of the human eye. Arch Ophthalmol. 1985;103:785–789. doi: 10.1001/archopht.1985.01050060045020. [DOI] [PubMed] [Google Scholar]
- Fielder AR, Quinn GE. Myopia of prematurity: nature, nurture, or disease. Br J Ophthalmol. 1997;81 (1:2–3. doi: 10.1136/bjo.81.1.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki Y. The rapid change of corneal curvature in the neonatal period and infancy. Arch Ophthalmol. 1986;104:1026–1027. doi: 10.1001/archopht.1986.01050190084044. [DOI] [PubMed] [Google Scholar]
- Donzis PB, Insler MS, Gordon RA. Corneal curvatures in premature infants. Am J Ophthalmol. 1985;99:213–215. doi: 10.1016/0002-9394(85)90240-5. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Bun J, Okuda T. Corneal curvature in children. J Jpn Contact Lens Soc. 1981;23:89–92. [Google Scholar]
- Fledelius HC. Preterm delivery and subsequent ocular development. A 7–10-year follow-up of children screened in 1982–1984 for ROP. 4. Oculometric and other metric considerations. Acta Ophthalmol Scand. 1996;74:301–305. doi: 10.1111/j.1600-0420.1996.tb00097.x. [DOI] [PubMed] [Google Scholar]
- Hellstrom A, Hard AL, Engstrom E, Niklasson A, Andersson E, Smith L, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123 (4:e638–e645. doi: 10.1542/peds.2008-2697. [DOI] [PubMed] [Google Scholar]
- Choi MY, Park IK, Yu YS. Long term refractive outcome in eyes of preterm infants with and without retinopathy of prematurity: comparison of keratometric value, axial length, anterior chamber depth, and lens thickness. Br J Ophthalmol. 2000;84 (2:138–143. doi: 10.1136/bjo.84.2.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varughese S, Varghese RM, Gupta N, Ojha R, Sreenivas V, Puliyel JM. Refractive error at birth and its relation to gestational age. Curr Eye Res. 2005;30:423–428. doi: 10.1080/02713680590959295. [DOI] [PubMed] [Google Scholar]
- Al-Ghamdi A, Albiani DA, Hodge WG, Clarke WN. Myopia and astigmatism in retinopathy of prematurity after treatment with cryotherapy or laser photocoagulation. Can J Ophthalmol. 2004;39:521–525. doi: 10.1016/s0008-4182(04)80142-x. [DOI] [PubMed] [Google Scholar]
- Cryotherapy for Retinopathy of Prematurity Cooperative Group. Quinn GE, Dobson V, Siatkowski R, Hardy RJ, Kivlin J, Palmer EA, et al. Does cryotherapy affect refractive error? Results from treated versus control eyes in the cryotherapy for retinopathy of prematurity trial. Ophthalmology. 2001;108:343–347. doi: 10.1016/s0161-6420(00)00527-3. [DOI] [PubMed] [Google Scholar]
- Algawi K, Goggin M, O'Keefe M. Refractive outcome following diode laser versus cryotherapy for eyes with retinopathy of prematurity. Br J Ophthalmol. 1994;78:612–614. doi: 10.1136/bjo.78.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight-Nanan DM, O'Keefe M. Refractive outcome in eyes with retinopathy of prematurity treated with cryotherapy or diode laser: 3 year follow up. Br J Ophthalmol. 1996;80:998–1001. doi: 10.1136/bjo.80.11.998. [DOI] [PMC free article] [PubMed] [Google Scholar]