Abstract
The diagnosis of Horner's syndrome (HS) can be difficult, as patients rarely present with the classic triad of ptosis, miosis, and anhydrosis. Frequently, there are no associated symptoms to help determine or localise the underlying pathology. The onset of anisocoria may also be uncertain, with many cases referred after incidental discovery on routine optometric assessment. Although the textbooks discuss the use of cocaine, apraclonidine, and hydroxyamphetamine to diagnose and localise HS, in addition to reported false positive and negative results, these pharmacological agents are rarely available during acute assessment or in general ophthalmic departments. Typically, a week is required between using cocaine or apraclonidine for diagnosis and localisation of HS with hydroxyamphetamine, leaving the clinician with the decision of which investigations to request and with what urgency. Modern imaging modalities have advanced significantly and become more readily available since many of the established management algorithms were written. We thus propose a practical and safe combined clinical and radiological diagnostic protocol for HS that can be applied in most clinical settings.
Keywords: Horner's syndrome, carotid dissection, magnetic resonance imaging, computed tomography
Introduction
Horner's syndrome (HS) is a combination of clinical signs, classically of ipsilateral ptosis, pupillary miosis, and facial anhydrosis (Table 1), secondary to the interruption of the oculosympathetic pathway.
Table 1. Anatomical pathway, pathological processes, and localising clinical features.
| Neuronal order | Location | Pathology | Associated clinical signs |
|---|---|---|---|
| First order (central) | Posterior hypothalamus | Pituitary tumour | |
| Brainstem | Stroke—Wallenberg syndrome, pontine haemorrhage Demyelination | Vertigo Altered facial sensation Contralateral CN IV palsy Crossed motor/sensory signs | |
| C-spine Intermediolateral grey substance C8-T2 | Arnold-chiari Cervical spondylosis Syringomyelia Neck trauma | Radicular signs | |
| Second order (intermediate) | C8–T2 ventral nerve roots | Cervical rib | Neck/arm pain and weakness |
| Brachial plexus injury | |||
| Apex of the lung | Tumours - Pancoast tumour - mesothelioma | Signs of lung disease | |
| Mediastinum | Cardiothoracic procedure Aortic aneurysm or dissection | ||
| Cervical sympathetic chain | Subclavian artery aneurysm Thyroid tumour Post neck dissection | Vocal cord paralysis Anhydrosis of face and neck | |
| Third order (post ganglionic) | Superior cervical ganglion at C2-3 | Jugular venous ectasia | |
| Carotid artery | Carotid - dissection - aneurysm - arteritis | Facial pain Stroke Ocular ischaemia | |
| Cavernous sinus | Base of skull tumours | Abducens palsy | |
| Inflammatory mass | |||
| Orbit | Herpes zoster |
Establishing the diagnosis begins with a detailed clinical history with use of adjunctive pharmacological testing and diagnostic imaging. The clinical signs may be subtle and it is not common for patients to present with the classic triad of signs. Associated presenting clinical symptoms can assist in localising a lesion and guide imaging studies. However, patients who are referred with asymptomatic HS pose the greatest diagnostic challenge.
In the majority of cases, the cause of an isolated HS will be benign or from a previously identified cause, for example, postsurgical. However, it is important to consider carotid dissection or neoplasm as an underlying cause. The long anatomical pathway of the oculosympathetic chain makes it vulnerable to many pathological processes. Imaging the entire pathway may require multiple imaging modalities, ionising radiation, intravenous contrast agents, and a substantial length of time. Therefore, this approach is not practical in every patient.
With the variety of clinical presentations, pharmacological testing agents, and recent advances in diagnostic imaging, as well as the variable provision of these resources with different health-care infrastructures, there is a need for a clear combined clinical, pharmacological, and imaging guide to the investigative process in HS. The authors thus review the diagnostic difficulties encountered through this diagnostic process and propose a combined investigative algorithm applicable in the majority of clinical settings.
Anatomical localisation in relation to clinical signs
The underlying pathology of the HS will be apparent in over 80% of patients at the time of the first neuro-ophthalmic consultation, based on history or clinical localisation of the lesion.1 Thus, despite the course of the oculosympathetic three-neuron pathway traversing several anatomical locations, clinical examination may localise the HS to one segment of the pathway in the latter group (summarised in Table 1). Figures 1, 2, 3 demonstrate imaging examples of the first (FON)-, second (SON)-, and third (TON)-order neuron HS pathologies.
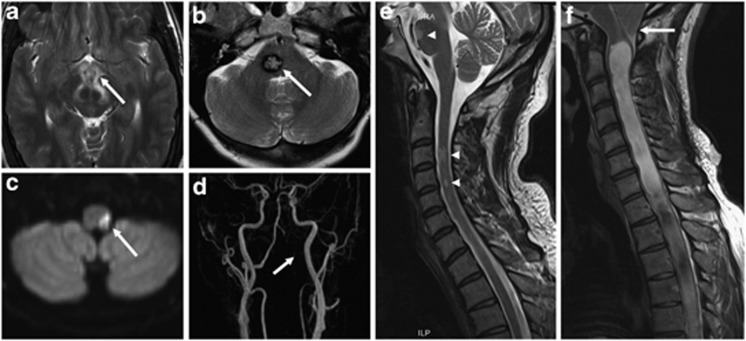
Figure 1.
Examples of first-order neuron lesions on magnetic resonance imaging (MRI). (a) Axial T2-weighted image of a hypothalamic inflammatory lesion (white arrow), (b) axial T2-weighted image of a right pontine cavernoma (white arrow), (c) diffusion-weighted imaging demonstrating restriction in a lateral medullary infarct (white arrow), and (d) corresponding subtracted 3D maximum-intensity projection time-of-flight (TOF) magnetic resonance angiogram (MRA) showing absence of signal in the V3 and V4 segments of the left vertebral artery (white arrow) from arterial dissection resulting in the left posterior inferior cerebellar artery (PICA) infarct seen in (c). Sagittal cervical spine T2-weighted acquisitions demonstrating (e) multiple intramedullary demyelinating lesions (white arrowheads) in a patient with multiple sclerosis and (f) a Chiari I malformation causing crowding at the craniocervical junction (white arrow) and an extensive syringomyelia.
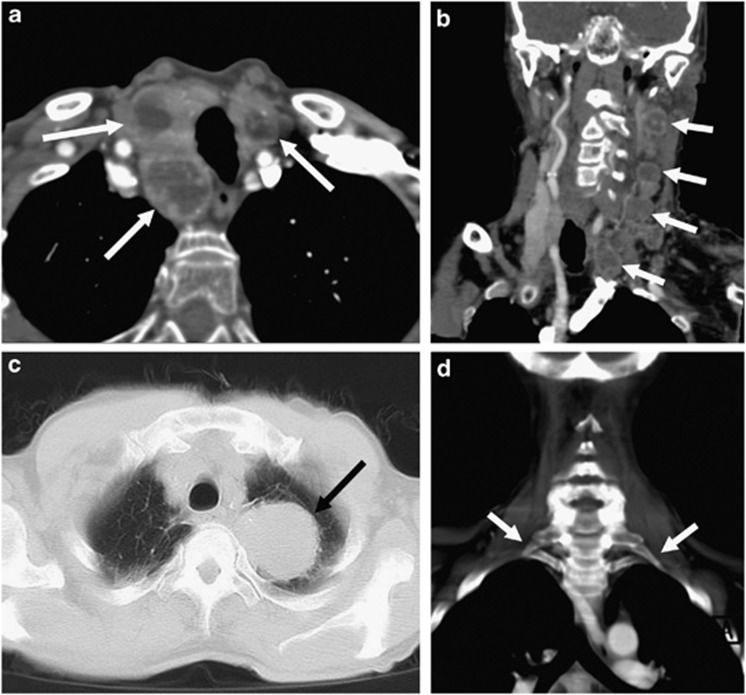
Figure 2.
Examples of second-order neuron lesions on computed tomographic angiography (CTA). (a) Axial image of multiple enhancing nodules within the thyroid gland (white arrows), (b) coronal reformatted image of multiple enlarged enhancing jugular chain lymph nodes in a patient with known disseminated carcinoma (white arrows), (c) axial image on lung windows showing a left apical bronchogenic carcinoma/Pancoast tumour (black arrow), and (d) coronal reformatted image on bone windows demonstrating bilateral cervical ribs (white arrows).
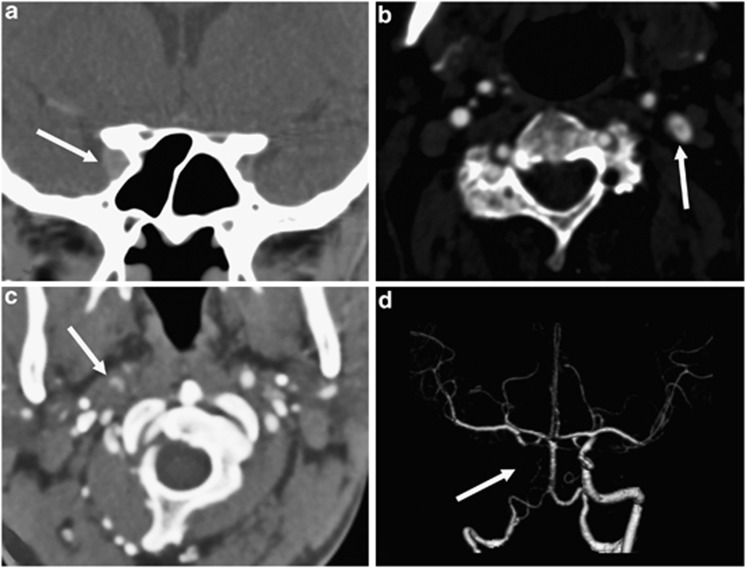
Figure 3.
Examples of third-order neuron lesions on computed tomographic angiography (CTA). (a) Coronal reformatted image demonstrating a right anterior cavernous sinus lesion (white arrow), (b) axial image of a dissection flap in the left internal carotid artery (white arrow), (c) axial image of crescentic contrast within the false lumen of the right internal carotid artery with increased soft tissue surrounding both compared with the left (white arrow), and (d) corresponding surface rendered volume reconstruction of the distal carotid circulation demonstrating absence of contrast in the distal cervical and intracranial internal carotid artery (white arrow).
A painful HS is the most common ocular sign of internal carotid artery (ICA) dissection, and has been reported in up to 58% of cases of ICA dissection,2, 3 with only one case report presenting as a painless HS.4 If a HS is associated with ipsilateral orbital, face, or neck pain of acute onset, this should be considered an ICA dissection until proven otherwise.5 Ocular signs to alert clinicians to the presence of an ICA dissection are listed in Table 2. Cluster headaches with associated signs of acute oculosympathetic palsy are seen in 5–22% of patients.6, 7
Table 2. Clinical features of Horner's syndrome and internal carotid artery dissection.
| Classic Horner's syndrome triad | Other Horner's syndrome features | Other ocular signs of carotid dissection |
|---|---|---|
| Mild ipsilateral ptosis (<2 mm) Ipsilateral miosis, with dilation lag Ipsilateral facial anhydrosis | Upside down ptosis Ocular hypotony Conjunctival hyperaemia | Transient monocular vision loss (30%) Positive phenomena (scintillations) Ocular motor nerve palsy (2.6%) Central retinal artery occlusion |
Challenges of the clinical examination
The classic triad of ptosis, miosis, and anhydrosis are rarely all present, and the findings are often subtle. The value of examining old photographs, to help clarify the clinical appearance and its apparent duration, should not be underestimated.
Ptosis may be very slight if present, of 1–2 mm only, and is reportedly absent in 12% of HS.8 Similarly, sympathetically innervated muscle is found in the lower lid, and therefore the lower lid may be elevated, producing an ‘upside-down ptosis'. With overall palpebral aperture reduction, the globe can appear enophthalmic.
Anisocoria is more pronounced in the dark, when the iris dilator should be acting, and may be most obvious within the first 5 s of darkness. After 10–15 s in the dark, anisocoria will be less apparent (known as dilation lag). The actual degree of anisocoria varies with the resting size of the pupil, alertness of the patient, and sympathetic drive.
The distribution of anhydrosis varies from the entire half face to a small patch on the forehead, depending on the lesion location. However, as most people live and work in temperature-controlled environments, and the assessment area will most often be air-conditioned, it is easy to miss disturbances of sweating. Starch-iodine or a friction test can be used to examine for anhydrosis9 in the setting of an autonomic dysfunction clinic. However, these are rarely performed in an emergency department or ophthalmic clinic.
Challenges of pharmacological localisation
Pharmacological testing is limited because of poor availability of the reagents and associated false positive and negative rates. These rates are higher in the very acute stages of HS as the test relies on denervation hypersensitivity or transmitter depletion, which may not have occurred.
Cocaine has been shown to be highly effective at separating HS patients from controls, with a postcocaine anisocoria of >0.8 mm giving an odds ratio of 1050 : 1.10 Cocaine confirms the diagnosis, but does not localise the lesion to a particular site, and thus cannot guide imaging.
Apraclonidine can similarly confirm the diagnosis but is unable to localise a site for targeted imaging. The overall sensitivity of Apraclonidine is 87%,11 which is comparable to cocaine testing with a positive test reported within 36 h of onset of symptoms.12 However, the results are yet to be validated in a large study and there are several reports of false negative tests.13, 14
There are many limitations to the use of Hydroxyamphetamine, with false negative results occurring particularly within the first week of HS onset.15 It is necessary to wait at least 24–48 h before using hydroxyamphetamine, if cocaine or apraclonidine was used. In addition, the drops need to be requested through a hospital pharmacy, which can take several days even in a dedicated eye hospital. As an alternative, the more readily available phenylephrine 1% has been shown to be as effective, causing mydriasis in postganglionic HS due to denervation hypersensitivity.16 However, it is still necessary to wait for >24 h if cocaine or apraclonidine has been instilled. Given that a nonreversible ocular or hemispheric stroke occur in 18% of all ICA dissections over 1 h to 31 days, with the majority occurring within the first 2 weeks,5 it may not be appropriate to wait for 1–2 days to use hydroxyamphetamine in a patient with an acute onset, pain, or a history consistent with trauma, particularly if there has been a delay in presentation.
Current imaging in HS
Imaging plays an important role in the diagnosis of an underlying cause of HS, particularly in the absence of localising signs, especially pathologies that carry a substantial morbidity and mortality. At present, there is no firm consensus on clear imaging guidelines, with many still advocating traditional modalities such as carotid Doppler ultrasound, chest radiographs, as well as flexion and extension views of the cervical spine.17 The fundamental considerations of which imaging modality to use are dependent upon the sensitivity and specificity of the test, its availability, and the associated dose of ionising radiation. These considerations have evolved considerably over time with the advancement of technology, resulting in not only a reduction in radiation dose and increased sensitivity and specificity, but also increased availability of cross-sectional modalities in most health-care institutions.
Limitations of chest radiography (CXR) and carotid ultrasonography (US)
Traditionally, the CXR has been part of the imaging diagnostic repertoire in HS to exclude apical lung/Pancoast tumours and aortic dissections that extend to the carotid circulation. Even with modern day digital radiography, bronchogenic carcinomas are missed on CXR on average in 19% of cases, with a range of between 12 and 90% in various studies. This rate has been shown to increase if the lesion is peripheral or in the upper lobe (as in the case of an apical tumour), constituting 85 and 72%, respectively, of all misses by location in one study.18 Similarly, the subtle radiographic signs have long been acknowledged as not adequate in excluding aortic dissection,19 with the assessment of acute aortic emergencies being dominated by computed tomography (CT) assessment.
Carotid Doppler US is still widely used to assess stenosis and pathologies predominantly centred on the carotid bifurcation, but its ability to assess the proximal carotid and distal cervical/intracranial vasculature is limited because of interference from the thoracic inlet, mandible, and skull base. In the context of carotid artery dissection, flow pattern aberrations because of dissection-related stenosis may be identified in >90% of patients; however, these are nonspecific findings. The more specific findings of segmental dilatation, double lumen with flap, eccentric haematoma surrounding a narrowed lumen, and low/absent flow velocity in the dissected false lumen are observed in less than one-third of cases, with almost all patients eventually requiring further cross-sectional imaging or conventional angiography.20, 21
Proposed imaging protocol in HS
Imaging assessment of first-order neuron HS or HS with concurrent central nervous system signs (eg, a HS plus a contralateral ataxic hemiparesis or upperlimb paraesthesiae) is best achieved with magnetic resonance imaging (MRI). FON lesions are usually associated with neurological localising signs that will help guide the MRI protocol. If a patient has a FON HS without brain or brainstem signs, then the MRI protocol should include cervical and upper thoracic spinal cord imaging.22, 23
Imaging of patients with SON and TON HS is performed using a CT angiogram protocol to include the circle of Willis and the orbits, down to the level of the aortic arch at about T4–T5. This is the equivalent of a CT angiogram of the aortic arch, carotid arteries, and intracranial vessels, with additional image processing to visualise the lung apices and the orbits. Patients with a TON HS or HS with no localising clinical signs but with a reliable history of acute onset, pain, associated with trauma or malignancy should have the CT angiogram protocol on the day of clinical assessment.
This protocol has several advantages over other modalities. It provides extensive coverage of the entire pathway including the gold-standard imaging of the lung apices, with a minimal amount of imaging time, and hence it is less prone to movement artefacts and variations in body habitus. Although MRI provides superior contrast resolution, CT with contrast, in addition to be widely available in most institutions, is arguably as effective and in many ways complementary in the assessment of the head and neck region.24 The arterial phase contrast administered in this CT protocol is essential for the assessment of the arterial system of the neck. CTA has been demonstrated to be the preferred cross-sectional modality for cervical arterial dissections, as although MR imaging exhibits exceptional contrast resolution, the spatial and temporal resolution is relatively lower.25
The limitations include the use of iodinated contrast and ionising radiation, the latter of the order of 4–6 mSv.26 In cases where there is a contraindication to iodinated contrast, then fat-suppressed MRI and arterial phase contrast enhanced MR angiography (CEMRA) is a suitable but lengthier and less available alternative. Estimation of the degree of increased lifetime risk of cancer from a single-exposure examination is highly technical and dependent on several factors including age of exposure, but may be estimated at between 20 and 30 per 100 000.27 Although the relative radiation dose is much higher than that of a conventional CXR, the diagnostic yield is far greater than all other imaging modalities.
In HS patients with no localising signs, pain, trauma, malignancy, or non-acute onset, no urgent imaging is required. The patient can be brought to clinic for review of old photographs and pharmacological testing. Any imaging can be requested on the basis of these results. In the absence of further localization, the CT angiogram protocol should be performed within 6 weeks, unless the HS is confirmed to have been present for over a year. If the HS has been present for over a year, pupillometry may assist in localisation, and imaging can be deferred indefinitely or be requested on a nonurgent basis at the clinician's discretion.
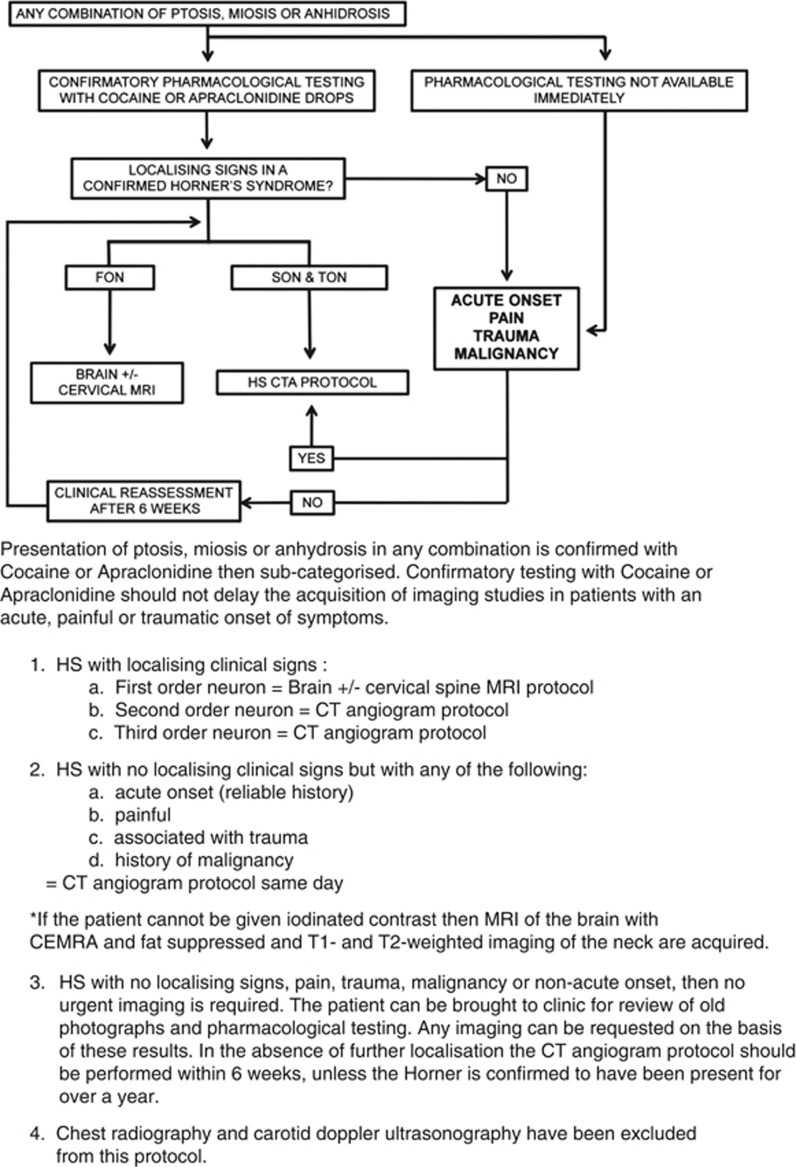
Table 3 summarises the technical aspects of imaging protocol and Figure 4 details the combined algorithm.
Table 3. Technical aspects of the imaging protocol.
| HS neuronal order | Modality and imaging time | Acquisition parameters |
|---|---|---|
| First order | MRI brain (15–18 min) | Sagittal T1-weighted |
| Axial T2-weighted | ||
| Coronal FLAIR | ||
| Axial T2*-weighted | ||
| Diffusion-weighted Imaging (DWI) | ||
| MRI cervical and upper thoracic spine (13–16 min) | Sagittal T2-weighted | |
| Sagittal T1-weighted | ||
| Axial MERGE | ||
| Axial T2-weighted | ||
| Second and third order | CT angiogram (16–18 s) | Multidetector CT 90 ml iodinated contrast at 4 ml/s Spiral isotropic volumetric data 0.625 mm slice thickness Arch of aorta to Circle of Willis |
| Second and third order (iodinated contrast contraindication) | MR protocol (17–20 min) | Axial T1-weighted fat-suppressed Axial T2-weighted fat-suppressed CEMRA 20 ml gadolinium at 3 ml/s |
Figure 4.
Diagrammatic representation and summary points of the HS combined investigation algorithm.
A congenital HS can present at any age and is usually evident because of heterochromia. Readers are refered to a recent review of paediatric HS in relation to neuroblastoma.28
Summary
Our proposed protocol provides a simple combined clinical, pharmacological, and imaging algorithm that is applicable to most institutions. This protocol calls for the same investigation for pre- and post-ganglionic HS, with the exception of FON lesions, therefore precluding the need for localisation with hydroxyamphetamine or phenylephrine 1% drops in the acute setting, particularly as this may delay definitive imaging.
The algorithm targets the specific pathologies and anatomical sites, matching it with the best available modality for assessment and diagnosis. The choice of a CTA protocol means that imaging, where warranted to exclude the acute pathologies that carry significant morbidity and mortality, can be requested and performed in a timely manner without the delays and lengthy protocols of other modalities. The CXR and carotid Doppler US have been omitted from the proposed protocol, as it may provide false reassurance, and delay acquisition of definitive diagnostic imaging.
The protocol also allows time for full clinical assessment in less urgent cases; however, imaging is still recommended when duration of HS of over 1 year cannot be proven in the hope that cases of malignancy would still be detected in a timely manner.
The authors declare no conflict of interest.
References
- Almog Y, Gepstein R, Kesler A. Diagnostic value of imaging in horner syndrome in adults. J Neuroophthalmol. 2010;30 (1:7–11. doi: 10.1097/WNO.0b013e3181ce1a12. [DOI] [PubMed] [Google Scholar]
- Mokri B. Traumatic and spontaneous extracranial internal carotid artery dissections. J Neurol. 1990;237 (6:356–361. doi: 10.1007/BF00315659. [DOI] [PubMed] [Google Scholar]
- Guillon B, Levy C, Bousser MG. Internal carotid artery dissection: an update. J Neurol Sci. 1998;153 (2:146–158. doi: 10.1016/s0022-510x(97)00287-6. [DOI] [PubMed] [Google Scholar]
- Venketasubramanian N, Singh J, Hui F, Lim MK. Carotid artery dissection presenting as a painless Horner's syndrome in a pilot: fit to fly. Aviat Space Environ Med. 1998;69 (3:307–310. [PubMed] [Google Scholar]
- Biousse V, Touboul PJ, D'Anglejan-Chatillon J, Levy C, Schaison M, Bousser MG. Ophthalmologic manifestations of internal carotid artery dissection. Am J Ophthalmol. 1998;126 (4:565–577. doi: 10.1016/s0002-9394(98)00136-6. [DOI] [PubMed] [Google Scholar]
- Riley FC, Moyer NJ. Oculosympathetic paresis associated with cluster headaches. Am J Ophthalmol. 1971;72 (4:763–768. doi: 10.1016/0002-9394(71)90015-8. [DOI] [PubMed] [Google Scholar]
- Grimson BS, Thompson HS. Raeder's syndrome. A clinical review. Surv Ophthalmol. 1980;24 (4:199–210. doi: 10.1016/0039-6257(80)90041-7. [DOI] [PubMed] [Google Scholar]
- Maloney WF, Younge BR, Moyer NJ. Evaluation of the causes and accuracy of pharmacologic localization in Horner's syndrome. Am J Ophthalmol. 1980;90 (3:394–402. doi: 10.1016/s0002-9394(14)74924-4. [DOI] [PubMed] [Google Scholar]
- Rosenberg ML. The friction sweat test as a new method for detecting facial anhidrosis in patients with Horner's syndrome. Am J Ophthalmol. 1989;108 (4:443–447. doi: 10.1016/s0002-9394(14)73315-x. [DOI] [PubMed] [Google Scholar]
- Kardon RH, Denison CE, Brown CK, Thompson HS. Critical evaluation of the cocaine test in the diagnosis of Horner's syndrome. Arch Ophthalmol. 1990;108 (3:384–387. doi: 10.1001/archopht.1990.01070050082036. [DOI] [PubMed] [Google Scholar]
- Koc F, Kavuncu S, Kansu T, Acaroglu G, Firat E. The sensitivity and specificity of 0.5% apraclonidine in the diagnosis of oculosympathetic paresis. Br J Ophthalmol. 2005;89 (11:1442–1444. doi: 10.1136/bjo.2005.074492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebas M, Seror J, Debroucker T. Positive apraclonidine test 36 hours after acute onset of Horner syndrome in dorsolateral pontomedullary stroke. J Neuroophthalmol. 2010;30 (1:12–17. doi: 10.1097/WNO.0b013e3181b1b41f. [DOI] [PubMed] [Google Scholar]
- Kawasaki A, Borruat FX. False negative apraclonidine test in two patients with Horner syndrome. Klin Monbl Augenheilkd. 2008;225 (5:520–522. doi: 10.1055/s-2008-1027349. [DOI] [PubMed] [Google Scholar]
- Dewan MA, Harrison AR, Lee MS. False-negative apraclonidine testing in acute Horner syndrome. Can J Ophthalmol. 2009;44 (1:109–110. doi: 10.3129/i08-162. [DOI] [PubMed] [Google Scholar]
- Moster ML, Galiani D, Garfinkle W. False negative hydroxyamphetamine test in horner syndrome caused by acute internal carotid artery dissection. J Neuroophthalmol. 2003;23 (1:22–23. doi: 10.1097/00041327-200303000-00005. [DOI] [PubMed] [Google Scholar]
- Danesh-Meyer HV, Savino P, Sergott R. The correlation of phenylephrine 1% with hydroxyamphetamine 1% in Horner's syndrome. Br J Ophthalmol. 2004;88 (4:592–593. doi: 10.1136/bjo.2003.029371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pane A, Burdon M, Miller NR.The Neuro-Ophthalmology Survival Guide1st edMosby: Edinburgh, UK; 2007 [Google Scholar]
- Shah PK, Austin JH, White CS, Patel P, Haramati LB, Pearson GD, et al. Missed non-small cell lung cancer: radiographic findings of potentially resectable lesions evident only in retrospect. Radiology. 2003;226 (1:235–241. doi: 10.1148/radiol.2261011924. [DOI] [PubMed] [Google Scholar]
- Hogg K, Teece S. Best evidence topic report. The sensitivity of a normal chest radiograph in ruling out aortic dissection. Emerg Med J. 2004;21 (2:199–200. [PMC free article] [PubMed] [Google Scholar]
- Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med. 2001;344 (12:898–906. doi: 10.1056/NEJM200103223441206. [DOI] [PubMed] [Google Scholar]
- Ansari SA, Parmar H, Ibrahim M, Gemmete JJ, Gandhi D. Cervical dissections: diagnosis, management, and endovascular treatment. Neuroimaging Clin N Am. 2009;19 (2:257–270. doi: 10.1016/j.nic.2009.01.007. [DOI] [PubMed] [Google Scholar]
- Digre KB, Smoker WR, Johnston P, Tryhus MR, Thompson HS, Cox TA, et al. Selective MR imaging approach for evaluation of patients with Horner's syndrome. AJNR Am J Neuroradiol. 1992;13 (1:223–227. [PMC free article] [PubMed] [Google Scholar]
- Reede DL, Garcon E, Smoker WR, Kardon R.Horner's syndrome: clinical and radiographic evaluation Neuroimaging Clin N Am 200818(2369–385.xi. [DOI] [PubMed] [Google Scholar]
- Ahmad A, Branstetter BF. CT versus MR: still a tough decision. Otolaryngol Clin North Am. 2008;41 (1:1–22. doi: 10.1016/j.otc.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Vertinsky AT, Schwartz NE, Fischbein NJ, Rosenberg J, Albers GW, Zaharchuk G. Comparison of multidetector CT angiography and MR imaging of cervical artery dissection. AJNR Am J Neuroradiol. 2008;29 (9:1753–1760. doi: 10.3174/ajnr.A1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mnyusiwalla A, Aviv RI, Symons SP. Radiation dose from multidetector row CT imaging for acute stroke. Neuroradiology. 2009;51 (10:635–640. doi: 10.1007/s00234-009-0543-6. [DOI] [PubMed] [Google Scholar]
- Committee to Assess Health Risks from Exposure to Low Levels of Ionizing Radiation, National Research Council . Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. National Academies: Washington, DC; 2006. [PubMed] [Google Scholar]
- Hirji N, Shouls G, Ali N. The risk of neuroblastoma in paediatric horner syndromem a systematic reappraisal of the literature. Neuro-Ophthalmology. 2012;36 (6:227–231. [Google Scholar]