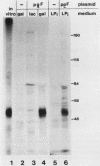
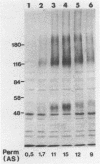
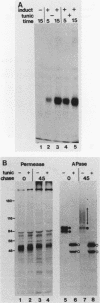
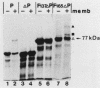
Abstract
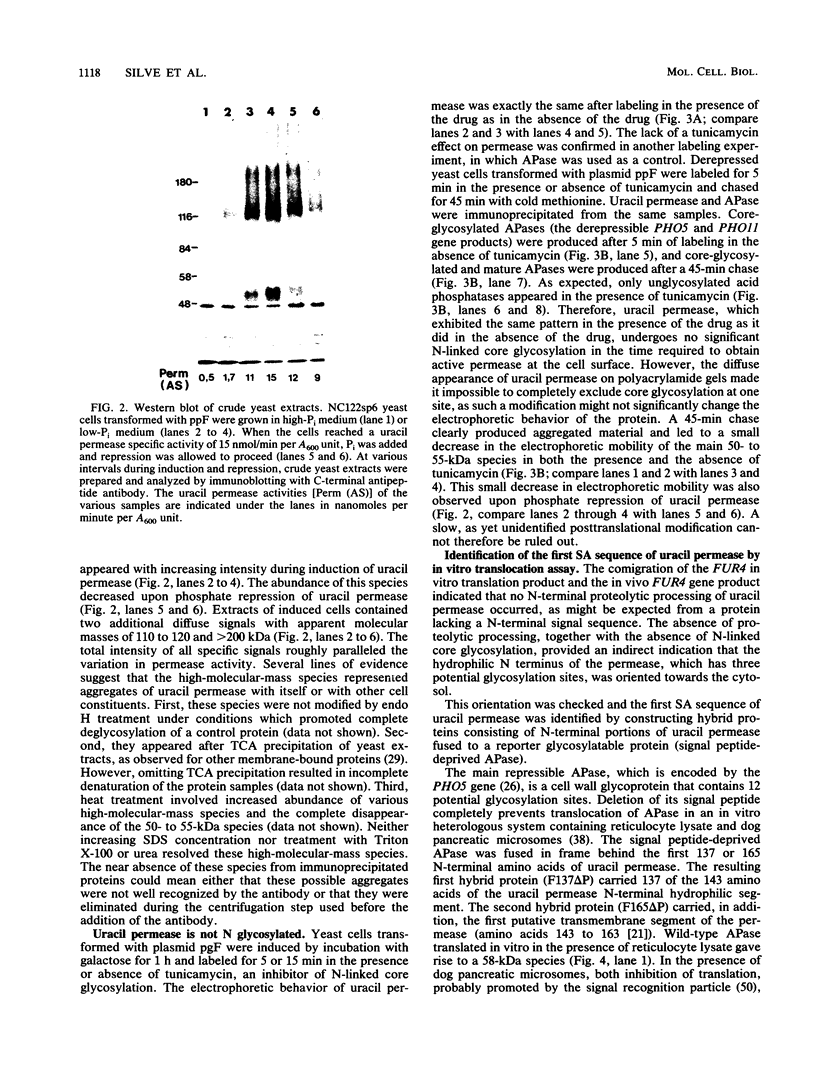
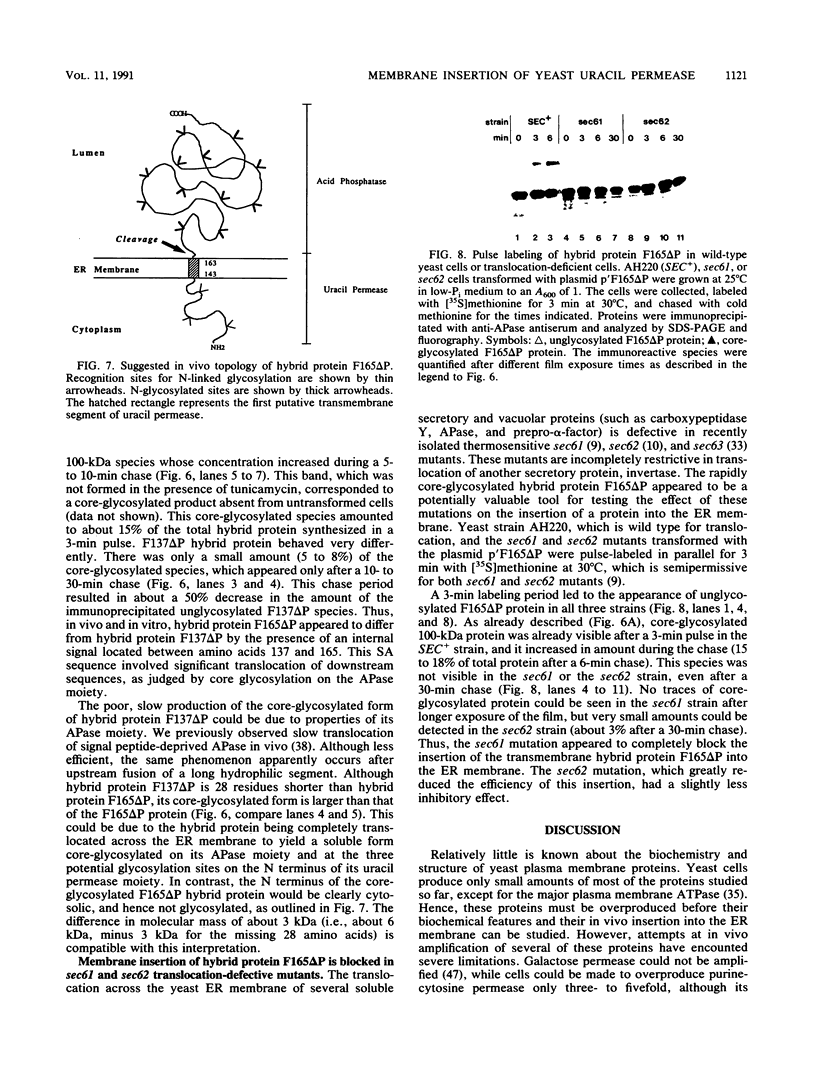
Uracil permease is a multispanning protein of the Saccharomyces cerevisiae plasma membrane which is encoded by the FUR4 gene and produced in limited amounts. It has a long N-terminal hydrophilic segment, which is followed by 10 to 12 putative transmembrane segments, and a hydrophilic C terminus. The protein carries seven potential N-linked glycosylation sites, three of which are in its N-terminal segment. Overexpression of this permease and specific antibodies were used to show that uracil permease undergoes neither N-linked glycosylation nor proteolytic processing. Uracil permease N-terminal segments of increasing lengths were fused to a reporter glycoprotein, acid phosphatase. The in vitro and in vivo fates of the resulting hybrid proteins were analyzed to identify the first signal anchor sequence of the permease and demonstrate the cytosolic orientation of its N-terminal hydrophilic sequence. In vivo insertion of the hybrid protein bearing the first signal anchor sequence of uracil permease into the endoplasmic reticulum membrane was severely blocked in sec61 and sec62 translocation mutants.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmad M., Bussey H. Yeast arginine permease: nucleotide sequence of the CAN1 gene. Curr Genet. 1986;10(8):587–592. doi: 10.1007/BF00418125. [DOI] [PubMed] [Google Scholar]
- Beltzer J. P., Wessels H. P., Spiess M. Signal peptidase can cleave inside a polytopic membrane protein. FEBS Lett. 1989 Aug 14;253(1-2):93–98. doi: 10.1016/0014-5793(89)80937-8. [DOI] [PubMed] [Google Scholar]
- Blumer K. J., Reneke J. E., Thorner J. The STE2 gene product is the ligand-binding component of the alpha-factor receptor of Saccharomyces cerevisiae. J Biol Chem. 1988 Aug 5;263(22):10836–10842. [PubMed] [Google Scholar]
- Celenza J. L., Marshall-Carlson L., Carlson M. The yeast SNF3 gene encodes a glucose transporter homologous to the mammalian protein. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2130–2134. doi: 10.1073/pnas.85.7.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y. D., Dickson R. C. Primary structure of the lactose permease gene from the yeast Kluyveromyces lactis. Presence of an unusual transcript structure. J Biol Chem. 1988 Nov 15;263(32):16696–16703. [PubMed] [Google Scholar]
- Chevallier M. R. Cloning and transcriptional control of a eucaryotic permease gene. Mol Cell Biol. 1982 Aug;2(8):977–984. doi: 10.1128/mcb.2.8.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. A yeast mutant defective at an early stage in import of secretory protein precursors into the endoplasmic reticulum. J Cell Biol. 1987 Aug;105(2):633–645. doi: 10.1083/jcb.105.2.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshaies R. J., Schekman R. SEC62 encodes a putative membrane protein required for protein translocation into the yeast endoplasmic reticulum. J Cell Biol. 1989 Dec;109(6 Pt 1):2653–2664. doi: 10.1083/jcb.109.6.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehring R., Beyreuther K., Wright J. K., Overath P. In vitro and in vivo products of E. coli lactose permease gene are identical. Nature. 1980 Feb 7;283(5747):537–540. doi: 10.1038/283537a0. [DOI] [PubMed] [Google Scholar]
- Fujiki Y., Hubbard A. L., Fowler S., Lazarow P. B. Isolation of intracellular membranes by means of sodium carbonate treatment: application to endoplasmic reticulum. J Cell Biol. 1982 Apr;93(1):97–102. doi: 10.1083/jcb.93.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaber R. F., Styles C. A., Fink G. R. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol Cell Biol. 1988 Jul;8(7):2848–2859. doi: 10.1128/mcb.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grefrath S. P., Reynolds J. A. The molecular weight of the major glycoprotein from the human erythrocyte membrane. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3913–3916. doi: 10.1073/pnas.71.10.3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann E., Rapoport T. A., Lodish H. F. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones M. N., Nickson J. K. Monosaccharide transport proteins of the human erythrocyte membrane. Biochim Biophys Acta. 1981 Jun 16;650(1):1–20. doi: 10.1016/0304-4157(81)90006-x. [DOI] [PubMed] [Google Scholar]
- Jund R., Chevallier M. R., Lacroute F. Uracil transport in Saccharomyces cerevisiae. J Membr Biol. 1977 Sep 14;36(2-3):233–251. doi: 10.1007/BF01868153. [DOI] [PubMed] [Google Scholar]
- Jund R., Weber E., Chevallier M. R. Primary structure of the uracil transport protein of Saccharomyces cerevisiae. Eur J Biochem. 1988 Jan 15;171(1-2):417–424. doi: 10.1111/j.1432-1033.1988.tb13806.x. [DOI] [PubMed] [Google Scholar]
- Jähnig F. Structure predictions of membrane proteins are not that bad. Trends Biochem Sci. 1990 Mar;15(3):93–95. doi: 10.1016/0968-0004(90)90188-h. [DOI] [PubMed] [Google Scholar]
- Leblanc G., Pourcher T., Bassilana M. Molecular biology and bacterial secondary transporters. Biochimie. 1989 Aug;71(8):969–979. doi: 10.1016/0300-9084(89)90079-5. [DOI] [PubMed] [Google Scholar]
- Lipp J., Flint N., Haeuptle M. T., Dobberstein B. Structural requirements for membrane assembly of proteins spanning the membrane several times. J Cell Biol. 1989 Nov;109(5):2013–2022. doi: 10.1083/jcb.109.5.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiden M. C., Davis E. O., Baldwin S. A., Moore D. C., Henderson P. J. Mammalian and bacterial sugar transport proteins are homologous. Nature. 1987 Feb 12;325(6105):641–643. doi: 10.1038/325641a0. [DOI] [PubMed] [Google Scholar]
- Meyhack B., Bajwa W., Rudolph H., Hinnen A. Two yeast acid phosphatase structural genes are the result of a tandem duplication and show different degrees of homology in their promoter and coding sequences. EMBO J. 1982;1(6):675–680. doi: 10.1002/j.1460-2075.1982.tb01229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Rai R., Genbauffe F. S., Cooper T. G. Structure and transcription of the allantoate permease gene (DAL5) from Saccharomyces cerevisiae. J Bacteriol. 1988 Jan;170(1):266–271. doi: 10.1128/jb.170.1.266-271.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothblatt J. A., Deshaies R. J., Sanders S. L., Daum G., Schekman R. Multiple genes are required for proper insertion of secretory proteins into the endoplasmic reticulum in yeast. J Cell Biol. 1989 Dec;109(6 Pt 1):2641–2652. doi: 10.1083/jcb.109.6.2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Florkiewicz R. Z., Shaw A. S., Rose J. K. An internalized amino-terminal signal sequence retains full activity in vivo but not in vitro. J Biol Chem. 1987 Jun 25;262(18):8889–8895. [PubMed] [Google Scholar]
- Sadler I., Chiang A., Kurihara T., Rothblatt J., Way J., Silver P. A yeast gene important for protein assembly into the endoplasmic reticulum and the nucleus has homology to DnaJ, an Escherichia coli heat shock protein. J Cell Biol. 1989 Dec;109(6 Pt 1):2665–2675. doi: 10.1083/jcb.109.6.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt R., Manolson M. F., Chevallier M. R. Photoaffinity labeling and characterization of the cloned purine-cytosine transport system in Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6276–6280. doi: 10.1073/pnas.81.20.6276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R., Kielland-Brandt M. C., Fink G. R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986 Feb 20;319(6055):689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- Silve S., Monod M., Hinnen A., Haguenauer-Tsapis R. The yeast acid phosphatase can enter the secretory pathway without its N-terminal signal sequence. Mol Cell Biol. 1987 Sep;7(9):3306–3314. doi: 10.1128/mcb.7.9.3306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiess M., Handschin C., Baker K. P. Stop-transfer activity of hydrophobic sequences depends on the translation system. J Biol Chem. 1989 Nov 15;264(32):19117–19124. [PubMed] [Google Scholar]
- Spiess M., Lodish H. F. An internal signal sequence: the asialoglycoprotein receptor membrane anchor. Cell. 1986 Jan 17;44(1):177–185. doi: 10.1016/0092-8674(86)90496-4. [DOI] [PubMed] [Google Scholar]
- Szkutnicka K., Tschopp J. F., Andrews L., Cirillo V. P. Sequence and structure of the yeast galactose transporter. J Bacteriol. 1989 Aug;171(8):4486–4493. doi: 10.1128/jb.171.8.4486-4493.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J., Fink G. R. The histidine permease gene (HIP1) of Saccharomyces cerevisiae. Gene. 1985;38(1-3):205–214. doi: 10.1016/0378-1119(85)90219-7. [DOI] [PubMed] [Google Scholar]
- Teather R. M., Müller-Hill B., Abrutsch U., Aichele G., Overath P. Amplification of the lactose carrier protein in Escherichia coli using a plasmid vector. Mol Gen Genet. 1978 Feb 27;159(3):239–248. doi: 10.1007/BF00268260. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trueheart J., Fink G. R. The yeast cell fusion protein FUS1 is O-glycosylated and spans the plasma membrane. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9916–9920. doi: 10.1073/pnas.86.24.9916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschopp J. F., Emr S. D., Field C., Schekman R. GAL2 codes for a membrane-bound subunit of the galactose permease in Saccharomyces cerevisiae. J Bacteriol. 1986 Apr;166(1):313–318. doi: 10.1128/jb.166.1.313-318.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P., Newman M. J., Foster D. L., Wilson T. H., Kaback H. R. Purification, reconstitution, and characterization of the lac permease of Escherichia coli. Methods Enzymol. 1986;125:429–452. doi: 10.1016/s0076-6879(86)25034-x. [DOI] [PubMed] [Google Scholar]
- Walter P., Blobel G. Translocation of proteins across the endoplasmic reticulum III. Signal recognition protein (SRP) causes signal sequence-dependent and site-specific arrest of chain elongation that is released by microsomal membranes. J Cell Biol. 1981 Nov;91(2 Pt 1):557–561. doi: 10.1083/jcb.91.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber E., Rodriguez C., Chevallier M. R., Jund R. The purine-cytosine permease gene of Saccharomyces cerevisiae: primary structure and deduced protein sequence of the FCY2 gene product. Mol Microbiol. 1990 Apr;4(4):585–596. doi: 10.1111/j.1365-2958.1990.tb00627.x. [DOI] [PubMed] [Google Scholar]
- Yaffe M. P., Schatz G. Two nuclear mutations that block mitochondrial protein import in yeast. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4819–4823. doi: 10.1073/pnas.81.15.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]