Abstract
PURPOSE
Autoclaves and UV sterilizers have been commonly used to prevent cross-infections between dental patients and dental instruments or materials contaminated by saliva and blood. To develop a dental sterilizer which can sterilize most materials, such as metals, rubbers, and plastics, the sterilization effect of an atmospheric pressure non-thermal air plasma device was evaluated.
MATERIALS AND METHODS
After inoculating E. coli and B. subtilis the diamond burs and polyvinyl siloxane materials were sterilized by exposing them to the plasma for different lengths of time (30, 60, 90, 120, 180 and, 240 seconds). The diamond burs and polyvinyl siloxane materials were immersed in PBS solutions, cultured on agar plates and quantified by counting the colony forming units. The data were analyzed using one-way ANOVA and significance was assessed by the LSD post hoc test (α=0.05).
RESULTS
The device was effective in killing E. coli contained in the plasma device compared with the UV sterilizer. The atmospheric pressure non-thermal air plasma device contributed greatly to the sterilization of diamond burs and polyvinyl siloxane materials inoculated with E. coli and B. subtilis. Diamond burs and polyvinyl siloxane materials inoculated with E. coli was effective after 60 and 90 seconds. The diamond burs and polyvinyl siloxane materials inoculated with B. subtilis was effective after 120 and 180 seconds.
CONCLUSION
The atmospheric pressure non-thermal air plasma device was effective in killing both E. coli and B. subtilis, and was more effective in killing E. coli than the UV sterilizer.
Keywords: Sterilization, Cross Infections, Non-thermal Atmospheric Pressure Plasma, Bacteria
INTRODUCTION
Dental treatments can frequently induce cross-contamination between dental patients and dentists through instruments and materials as well as between impression materials and dental technicians.1-6 Dentists and their assistants should consider a counterplan to protect themselves from pathogenic microorganisms when they treat dental patients with such microorganisms.1,2,7
Generally, dental materials made of metal can be disinfected by autoclave sterilization, whereas rubbers or plastics can be sterilized by either chemical methods using glutaldehyde or physical methods using ultrasound and ultraviolet.8 Autoclave sterilization is specified by medical procedural law as being effective in the prevention of cross-contamination by removing and destroying pathogens.9 However, the wet technique can reduce the durability of instruments by corroding their surfaces, while the dry technique requires more time for sterilization and can blunt knife edges.8,10 Ethylene oxide sterilizers, which are generally used in hospitals where many surgeries are performed, have the drawbacks of being impractical in terms of size, price and safety in clinical practice.8,10,11 The sterilization efficacy of autoclaving is well verified. However, to prepare dental instruments for individual dental patients, dentists should possess a corresponding number of instruments, such as high-speed and low-speed hand pieces attached to the dental treatment unit. Dentists face problems of reduction in performance and durability of the instruments due to repeated autoclave sterilization.8,10 Thus, the necessity for new sterilization devices that are acceptably safe, efficient and economically feasible has been proposed.7 Moisan et al. applied plasma which was produced at low-pressure to B. subtilis and chemical radicals such as oxygen atom or excited oxygen molecules appeared to contribute to sterilization. Moreover, numbers of studies have shown the characteristic of plasma that deactivates microorganism including spores, gram negative and positive bacteria, yeast, and virus. Since such low-pressure plasma was being produced in vacuum state, complex and costly equipment was required. However, there has been development of technology which enables the production of stable and non-thermal plasma under atmospheric pressure. The atmospheric plasma can produce numbers of chemical substances constantly, has no toxicity, and is a new biomedical application and method at a low cost. Recent interest has been focused on the studies of interactions between non-thermal plasma and viable tissues, as well as their applications in the medical field.12-14 In clinical practice, we could potentially use sterilization devices utilizing the plasma principle to remove toxic materials from surfaces7,11,15 and tooth bleaching techniques using non-thermal atmospheric pressure plasma.16
This study was conducted to verify the sterilization efficacy and safety of a non-thermal atmospheric pressure air plasma device.
MATERIALS AND METHODS
The assessment for the sterilization efficacies of an atmospheric pressure non-thermal air plasma device which was specially designed for this experiment (Fig. 1) and an ultraviolet (UV) sterilizer (Dentistar SHIELD, Hallim Dentech, Japan) (Fig. 2) in decontaminating dental instruments was performed. Table 1 and 2 show the dimensions of each instrument.
Fig. 1.
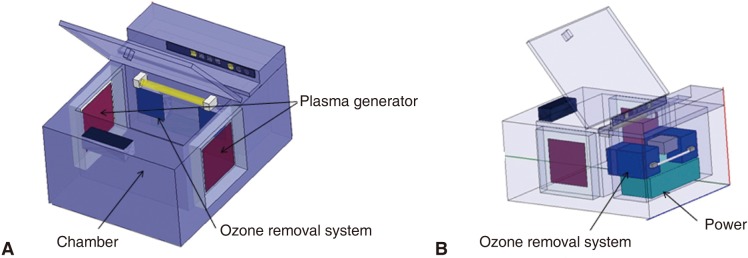
Schematic view of an atmospheric pressure non-thermal air plasma device. A: The frontal view, B: The rear view.
Fig. 2.
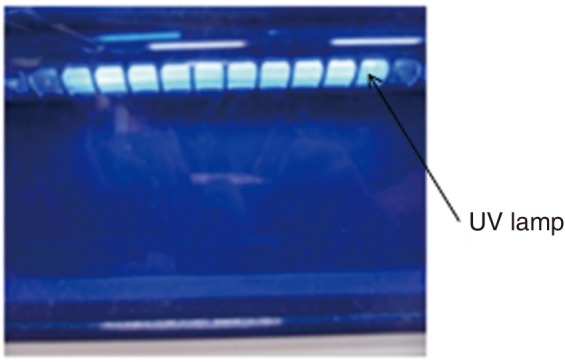
Schematic view of a UV sterilizer.
Table 1.
Specification of experimental atmospheric pressure non-thermal air plasma device
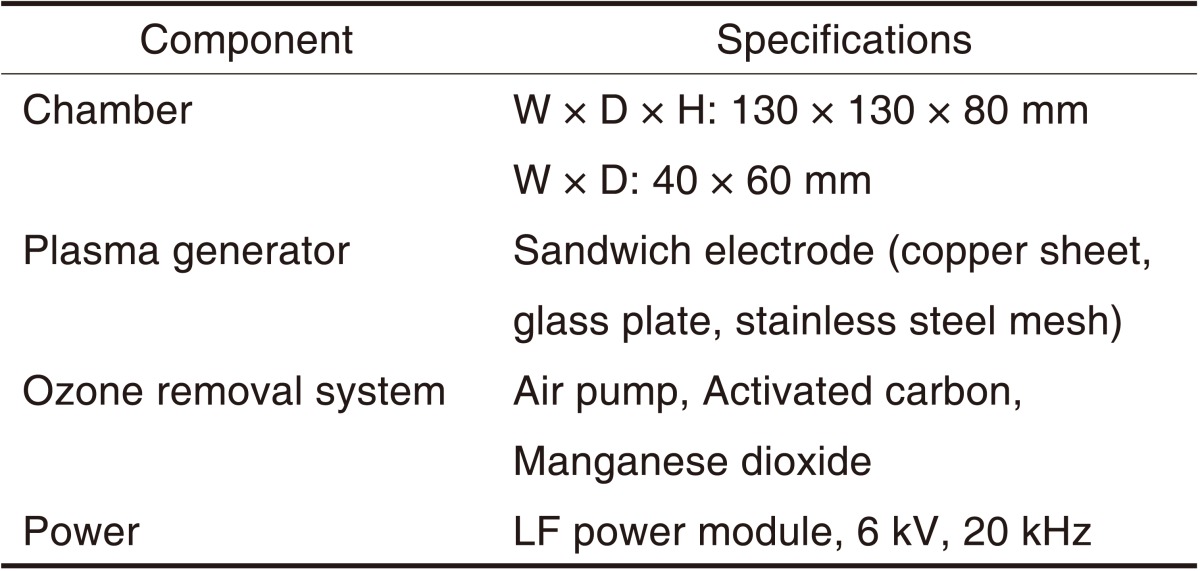
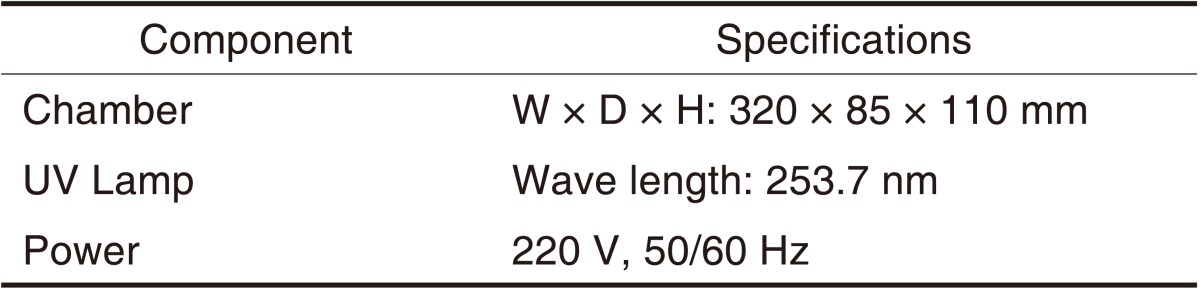
Table 2.
Specification of UV sterilizer
The experimental materials were diamond burs (EX-26, Mani Inc., Japan) and silicon impression materials (Imprint, 3M ESPE, USA) (Fig. 3). Each silicon impression specimen was cut into a regular hexahedron of 10 × 10 × 10 mm, whose one surface was roughened using 100-grit SiC paper for the inoculation of bacterial suspensions. Each of the diamond burs and silicon impression specimens was immersed in 7% ethanol solution before inoculation with bacterial suspension.
Fig. 3.
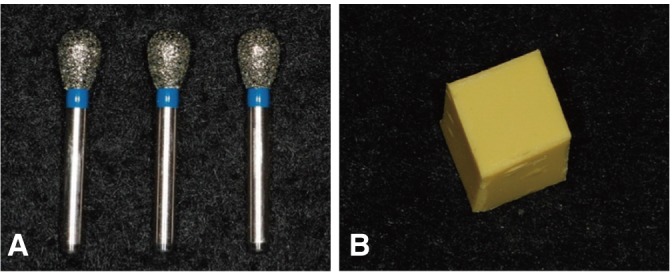
Inoculated material. A: Diamond bur, B: Polyvinyl siloxane.
Gram-negative Escherichia coli (E. coli, KCTC1611) and gram-positive Bacillus subtilis (B. subtilis, KCTC 1396) were used for this study. E. coli was cultured in Luria-Bertani media (1% sodium chloride, 0.5% yeast extract and 1% tryptone) at 37℃ at 200 rpm. B. subtilis was cultured in nutrient broth (0.5% peptone and 0.3% beef extract) in the same manner as described above.
After the diamond burs and silicone impression materials were inoculated using micropipettes within the testing bench with 5 and 10 µL of E. coli suspension, respectively, they were sterilized with the plasma device for 30, 60, 90 and 120 seconds. The same procedures were performed using the B. subtilis suspension for 60, 120, 180 and 240 seconds. Each specimen was immersed in a phosphate-buffered saline (PBS) solution. The PBS solution containing surviving bacteria was diluted at 1,000 times and was smeared on an agar plate. The plate was cultured at 37℃ for 15 hours in a CO2 incubator (Forma Scientific, International MI/SS Inc, USA) The colony-forming units (CFU) of the cultured bacteria were counted. Sterilization efficacy was assessed by CFU per the unit volume of the PBS. Each experiment at each time point was repeated 4 times (Table 3).
Table 3.
Performance tests
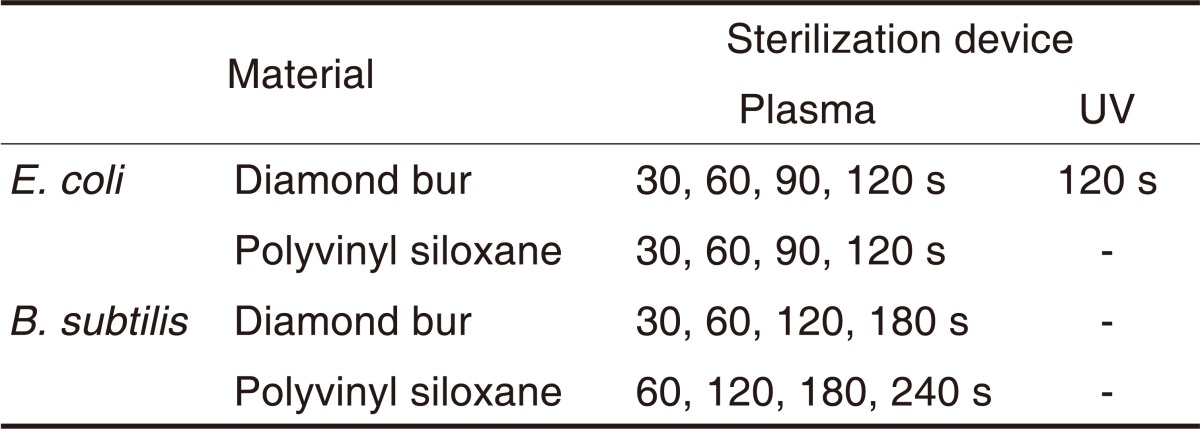
The E. coli suspension and the diamond burs were used for these comparisons. They were sterilized using the plasma device and the UV sterilizer. Sterilization efficacy of these 2 sterilizers was assessed in the same manner as mentioned above (Table 3).
Statistical analyses were performed using the SPSS statistical package program (Release 12.0, SPSS Inc, Chicago, IL, USA). The sterilization efficacy of the atmospheric pressure non-thermal air plasma device was examined by one-way ANOVA, and significance was assessed by the LSD post hoc test at P<.05 level of significance. Comparisons of sterilization efficacy between the 2 sterilizers were made using the paired t test.
RESULTS
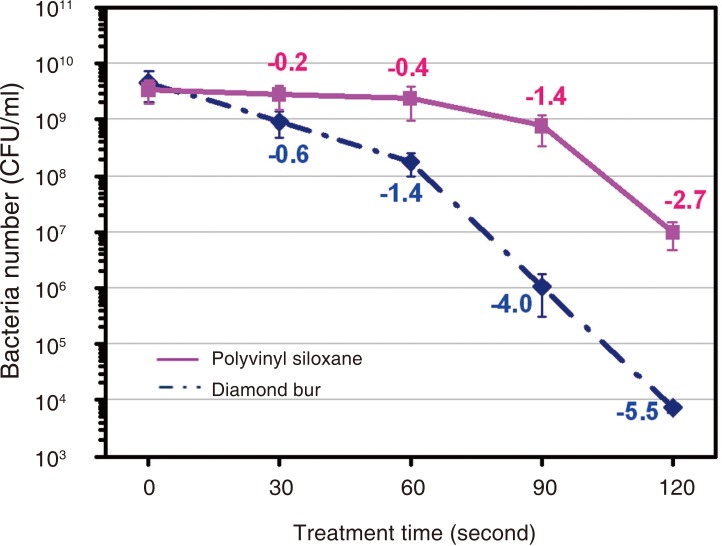
Table 4 presents the results of the E. coli cultures. The CFU for E. coli was greater than 109/mL before plasma sterilization, which was decreased after plasma sterilization. Table 4 shows the logarithms of the mean (± standard deviation) CFU values in order to examine sterilization efficacy against the E. coli suspension at different time points. CFU was significantly decreased from second 60 onward (P<.05) (Table 5). The logarithmic sterilization efficacy curves are shown in Fig. 4.
Table 4.
Mean values and standard deviations of E. coli surviving (CFU/mL) after treatment with an atmospheric pressure non-thermal air plasma device
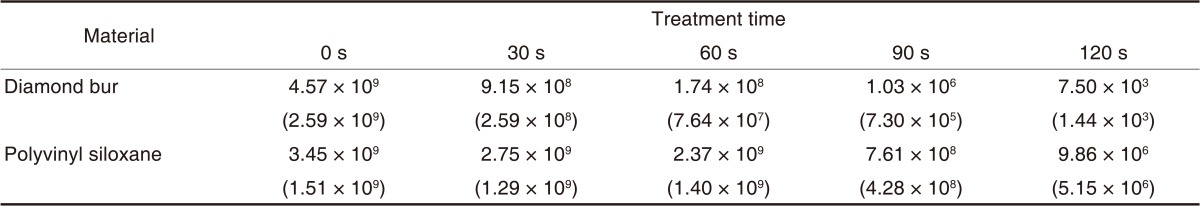
Numbers in parentheses are standard deviations.
Table 5.
Mean values and standard deviations of E. coli surviving on diamond bur and polyvinyl siloxane after treatment with an atmospheric pressure non-thermal air plasma device (LogN/N0)
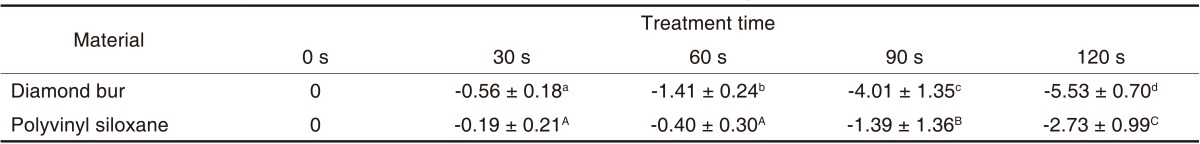
Fig. 4.
Survival curve (CFU/mL) of E. coli after treatment with an atmospheric pressure non-thermal air plasma device as a function of treatment time.
Table 6 shows the mean (± standard deviation) of CFU for B. subtilis at the different time points. The CFU was decreased after plasma sterilization.
Table 6.
Mean values and standard deviations of B. subtilis surviving (CFU/mL) after treatment with an atmospheric pressure non-thermal air plasma device
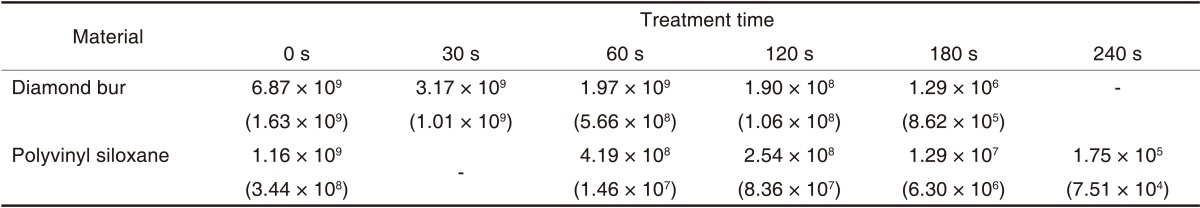
Numbers in parentheses are standard deviations.
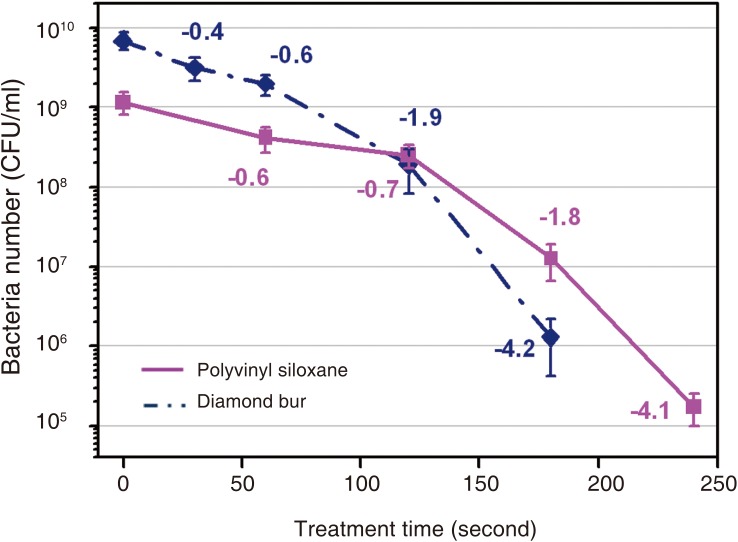
Table 7 shows the logarithms of the means (± standard deviation) of the CFU values in order to examine sterilization efficacy at different time points. CFU was significantly decreased from second 120 onward for diamond burs (P<.05), which was significantly decreased from second 180 onward for silicone impression materials (P<.05) (Table 8). Fig. 5 depicts logarithmic CFU curves for sterilization efficacy.
Table 7.
Mean values and standard deviations of B. subtilis surviving on diamond bur and polyvinyl siloxane after treatment with an atmospheric pressure non-thermal air plasma device (LogN/N0)
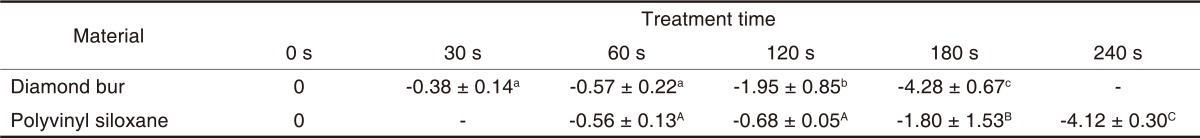
Identical superscripted letters indicate that values are not significantly different (P>.05).
Table 8.
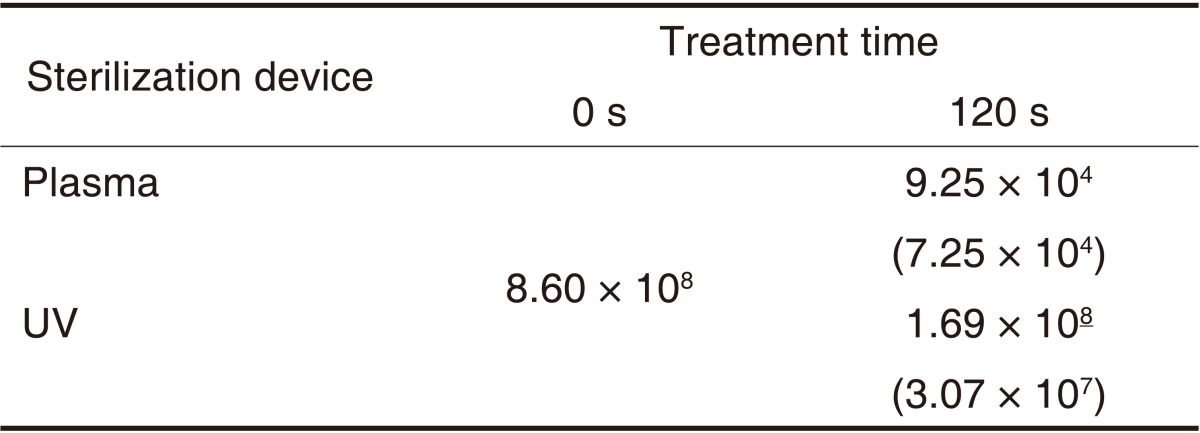
Mean values and standard deviations of E. coli surviving (CFU/mL) after treatment with an atmospheric pressure non-thermal air plasma device and UV sterilizer on diamond burs
Numbers in parentheses are standard deviations.
Fig. 5.
Survival curve (CFU/mL) of B. subtilis after treatment with an atmospheric pressure non-thermal air plasma device as a function of treatment time.
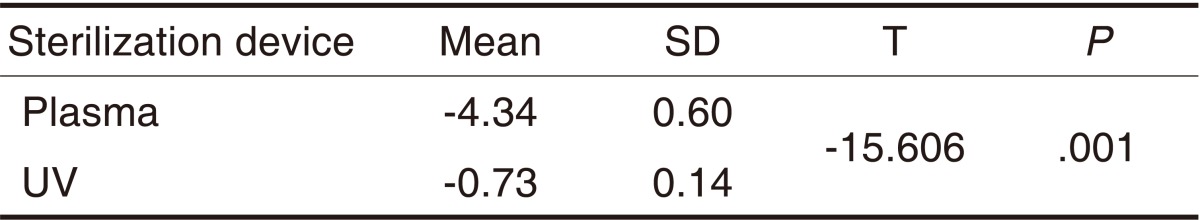
The means (± standard deviation) of CFU for E. coli obtained 120 seconds after the UV and plasma sterilization are shown in Table 8. The logarithms of the means (± standard deviation) of the CFU values are shown in Table 9. There were significant differences in sterilization efficacy between the 2 sterilizers (P<.05) (Table 9). The CFU value was significantly lower in the atmospheric pressure non-thermal air plasma device than in the UV sterilizer.
Table 9.
Mean values and standard deviations of E. coli surviving after treatment with an atmospheric pressure non-thermal air plasma device and UV sterilizer on diamond burs (LogN/N0)
DISCUSSION
Infectious diseases, such as hepatitis B, hepatitis C and tuberculosis, can be transmitted to other dental patients or dental professionals during dental treatment through either direct contact with blood and saliva of patients or through indirect contact with contaminated instruments.2,8,17 For this reason, it is important to prevent cross-contamination between dental patients and dental professionals.1,8,18
The importance of infection control at dental clinics has been recognized since the actual state of infections at dental clinics was broadcast through mass media.1 Consequently, the Korean Ministry of Health and Welfare has prepared the criteria for dental infection control during dental treatment and has established a legislation for the disinfection and sterilization of dental instruments and materials.9 The term "disinfection" means reduction in the number of pathogenic microorganisms excluding spores, while the term "sterilization" means reduction in the number of pathogenic microorganisms including spores.8,9 Liquid chemicals are commonly used for disinfection, but ultrasound and UV light are sometimes used. Sterilization methods include autoclaving, gas, dry heat, hydrogen peroxide gas plasma and liquid chemical sterilizations.9,14,15,19 Because inadequate disinfection and sterilization are the main causes of infections at dental clinics, dental instruments and materials should be completely disinfected or sterilized to prevent cross-contamination at dental clinics.
The autoclaving and dry heat sterilization methods are difficult to use for heat-labile instruments, while the low-temperature gas sterilization method raises problems of safety and expense.15,19 In addition, since these methods require given time intervals, rapid sterilization is difficult to perform. Although sterilization methods using r-rays, electronic beams and UV light have been proposed, these methods have some limitations due to inadequate performance, instability and high costs. Thus, further studies on new sterilization methods are warranted. There have been numerous studies on non-thermal plasma sterilization at room temperature.11,14,20,21 The mechanisms by which the non-thermal plasma device inactivate bacteria have been suggested.15,22 Plasma is a state in which all materials are completed ionized.10 Abundant ions, free radicals and UV light generated by non-thermal plasma have various effects: killing cancer cells, tooth bleaching, hemostasis and the eradication of microorganisms.19 Laroussi23 have also reported that factors inactivating microorganisms, which are generated by non-thermal plasma, include free radicals, charged particles and UV light. Non-thermal plasma was initially synthesized in a vacuum condition. However, as the problems with inhomogeneity and instability of electric discharge have been solved, atmospheric pressure non-thermal air plasma technologies have been applied to the disinfection and sterilization of dental instruments.10,20,22
E. coli and B. subtilis have frequently been used in previous experimental studies and thus were chosen for this study.15,17 E. coli is a pathogen related to focal or systemic infections, is easily cultured as a gram-negative rod and has a significant resistance to various external factors.15,17 B. subtilis is a spore-forming gram-positive rod, has a higher resistance to sterilization than gram-negative bacteria and is currently used as a biological indicator of sterilization efficacy.8,20 Dental burs and silicone impression materials are often contaminated with the blood and saliva of dental patients during treatment.6 Thus, these burs and materials were chosen for this study. The atmospheric pressure non-thermal air plasma device decreased the CFUs for both E. coli and B. subtilis (Tables 4 and 6). The procedure significantly decreased CFU in the diamond burs from second 60 onward for E. coli (Table 5) and from second 120 onward for B. subtilis (Table 7). In the silicone impression materials, the CFU was significantly decreased from second 90 onward for E. coli (Table 5) and from second 180 onward for B. subtilis (Table 7). In general, E. coli showed quicker reductions in CFU than B. subtilis. The atmospheric pressure non-thermal air plasma showed better sterilization rates than the UV sterilizer (Tables 8 and 9). The UV sterilizer had sterilization efficacy only in its contact areas, whereas the non-thermal air plasma was effective in all areas in contact with the air due to the dispersion of the plasma through the air.7 The plasma device contained oxygen free radicals, hydroxyl free radicals, hydrogen peroxide and ozone. Among them, ozone has both a high sterilization efficacy and a potent oxidizing activity.24 A maximum ozone concentration of 0.1 ppm is allowed in workplaces.7 To remove ozone from the plasma device, activated charcoal and manganese dioxide was passed through the plasma device using an air pump.7 Three minutes after the use of the air pump, the ozone concentration reached the values of ≤.025 ppm and thus the effect of ozone was excluded from our experiment results.
This study has some limitations. First, a complete sterilization efficacy of the plasma device was observed but was not obtained in this study. Further studies on this issue are required. Second, there are numerous pathogens other than E. coli and B. subtilis, including Streptococcus mutans (a cariogenic flora), Candida albicans (a causative agent of denture stomatitis) and hepatitis B virus (which 10% of the dentists are infected by).4,5 More research on these causative microorganisms is needed to support our results. In addition, a detailed mechanism for the effects of plasma on sterilization efficacy remains to be elucidated.
CONCLUSION
In the diamond burs and silicone impression materials, the CFU was significantly reduced for both E. coli and B. subtilis after treatment with atmospheric pressure nonthermal air plasma. The CFU was also more significantly reduced for E. coli by the atmospheric pressure non-thermal air plasma device than by the UV sterilizer. The results of this study provide the basis for the development of a new simpler, cheaper and more convenient atmospheric pressure non-thermal air plasma device for clinical practice.
Footnotes
This work was supported by a 2-Year Research Grant of Pusan National University.
References
- 1.Choi HN, Bae HS, Cho YS. Literature review of dental infection control in Korea. J Dent Hyg Sci. 2010;10:199–209. [Google Scholar]
- 2.Hutchings ML, Vandewalle KS, Schwartz RS, Charlton DG. Immersion disinfection of irreversible hydrocolloid impressions in pH-adjusted sodium hypochlorite. Part 2: Effect on gypsum casts. Int J Prosthodont. 1996;9:223–229. [PubMed] [Google Scholar]
- 3.Leung RL, Schonfeld SE. Gypsum casts as a potential source of microbial cross-contamination. J Prosthet Dent. 1983;49:210–211. doi: 10.1016/0022-3913(83)90503-6. [DOI] [PubMed] [Google Scholar]
- 4.McNeill MR, Coulter WA, Hussey DL. Disinfection of irreversible hydrocolloid impressions: a comparative study. Int J Prosthodont. 1992;5:563–567. [PubMed] [Google Scholar]
- 5.Runnells RR. An overview of infection control in dental practice. J Prosthet Dent. 1988;59:625–629. doi: 10.1016/0022-3913(88)90083-2. [DOI] [PubMed] [Google Scholar]
- 6.Jennings KJ, Samaranayake LP. The persistence of microorganisms on impression materials following disinfection. Int J Prosthodont. 1991;4:382–387. [PubMed] [Google Scholar]
- 7.Morfill GE, Shimizu T, Steffes B, Schmidt HU. Nosocomial infections-a new approach towards preventive medicine using plasmas. New J Phys. 2009;11:115019. [Google Scholar]
- 8.Samaranayake L. Essential Microbiology for dentistry. 3rd ed. Churchill Livingstone: 2007. pp. 385–412. [Google Scholar]
- 9.Guideline of sterilization of appliance and article at medical institution. 2010. Aug 13, Ministry of Health and Welfare Notification No. 2010-61. enactment. [Google Scholar]
- 10.Moreau M, Orange N, Feuilloley MG. Non-thermal plasma technologies: new tools for bio-decontamination. Biotechnol Adv. 2008;26:610–617. doi: 10.1016/j.biotechadv.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 11.Lee K, Paek KH, Ju WT, Lee Y. Sterilization of bacteria, yeast, and bacterial endospores by atmospheric-pressure cold plasma using helium and oxygen. J Microbiol. 2006;44:269–275. [PubMed] [Google Scholar]
- 12.Dobrynin D, Fridman G, Friedman G, Fridman A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J Phys. 2009;11:115020. [Google Scholar]
- 13.Fridman G, Friedman G, Gutsol A, Shekhter AB, Vasilets VN, Fridman A. Applied plasma Medicine. Plasma Process Polym. 2008;5:503–533. [Google Scholar]
- 14.McCombs GB, Darby ML. New discoveries and directions for medical, dental and dental hygiene research: low temperature atmospheric pressure plasma. Int J Dent Hyg. 2010;8:10–15. doi: 10.1111/j.1601-5037.2009.00386.x. [DOI] [PubMed] [Google Scholar]
- 15.Sladek REJ, Stoffels E. Deactivation of Escherichia coli by the plasma needle. J Phys D Appl Phys. 2005;38:1716–1721. [Google Scholar]
- 16.Lee HW, Kim GJ, Kim JM, Park JK, Lee JK, Kim GC. Tooth bleaching with nonthermal atmospheric pressure plasma. J Endod. 2009;35:587–591. doi: 10.1016/j.joen.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 17.Samaranayake LP, Hunjan M, Jennings KJ. Carriage of oral flora on irreversible hydrocolloid and elastomeric impression materials. J Prosthet Dent. 1991;65:244–249. doi: 10.1016/0022-3913(91)90169-w. [DOI] [PubMed] [Google Scholar]
- 18.Smith A, Creanor S, Hurrell D, Bagg J, McCowan M. Management of infection control in dental practice. J Hosp Infect. 2009;71:353–358. doi: 10.1016/j.jhin.2008.11.026. [DOI] [PubMed] [Google Scholar]
- 19.Seo YS, Mohamed AAH, Woo KC, Lee HW, Lee JK, Kim KT. Comparative studies of atmospheric pressure plasma characteristics between He and Ar working gases for sterilization. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 2010;38:1954–1962. [Google Scholar]
- 20.Iza F, Kim GJ, Lee SM, Lee JK, Walsh JL, Zhang YT, Kong MG. Microplasmas: Sources, particle kinetics, and biomedical applications. Plasma Process Polym. 2008;5:322–344. [Google Scholar]
- 21.Laroussi M. Low Temperature Plasma-Based Sterilization: Overview and state-of-the Art. Plasma Process Polym. 2005;2:391–400. [Google Scholar]
- 22.Fridman G, Brooks AD, Balasubramanian M, Fridman A, Gutsol A, Vasilets VN, Ayan H, Friedman G. Comparison of direct and indirect effects of non-thermal atmospheric-pressure plasma on bacteria. Plasma Process Polym. 2007;4:370–375. [Google Scholar]
- 23.Laroussi M. Nonthermal decontamination of biological media by atmospheric-pressure plasma: review, analysis, and prospects. IEEE Trans Plasma Sci IEEE Nucl Plasma Sci Soc. 2002;30:1409–1415. [Google Scholar]
- 24.Suzuki T, Oizumi M, Furuya J, Okamoto Y, Rosenstiel SF. Influence of ozone on oxidation of dental alloys. Int J Prosthodont. 1999;12:179–183. [PubMed] [Google Scholar]