CASE REPORT
Dr. Ang: A 31-year-old woman sought medical attention for fever, malaise, anorexia, weight loss, and diarrhea after recently giving birth to her third child. She was found to have hepatomegaly on physical exam, and a computed tomography (CT) scan revealed a 14 × 10 cm heterogeneous mass in the left hepatic lobe. No additional details regarding the imaging characteristics of the mass were available. No serum α-fetoprotein (AFP) level was obtained.
Dr. Abou-Alfa: Dr. Farran, what is the differential diagnosis of a liver mass in a young female patient?
Dr. Farran: Focal nodular hyperplasia (FNH) and hemangiomas are the 2 most common benign liver tumors in this population followed by hepatocellular carcinoma (HCC). Patients should be asked about oral contraceptive use as this is strongly linked to HCCs and hemangiomas, though its role in FNH is less clear.1 Given that this patient had a fever, it would have been interesting to know if the mass could have represented an abscess, indicating an infectious work-up.
Dr. Ang: A left hepatic lobectomy was performed for 14.5 cm mass. I will ask Dr. Boulos to comment on the pathology.
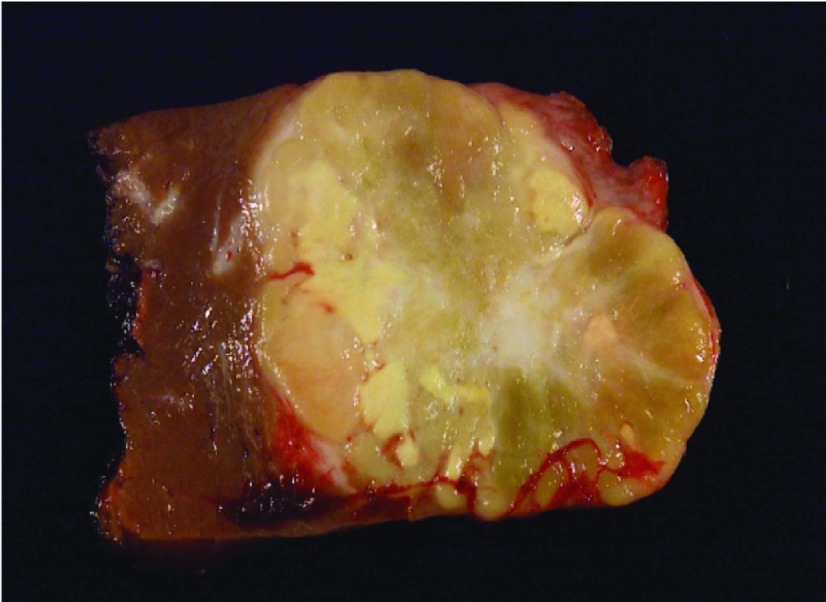
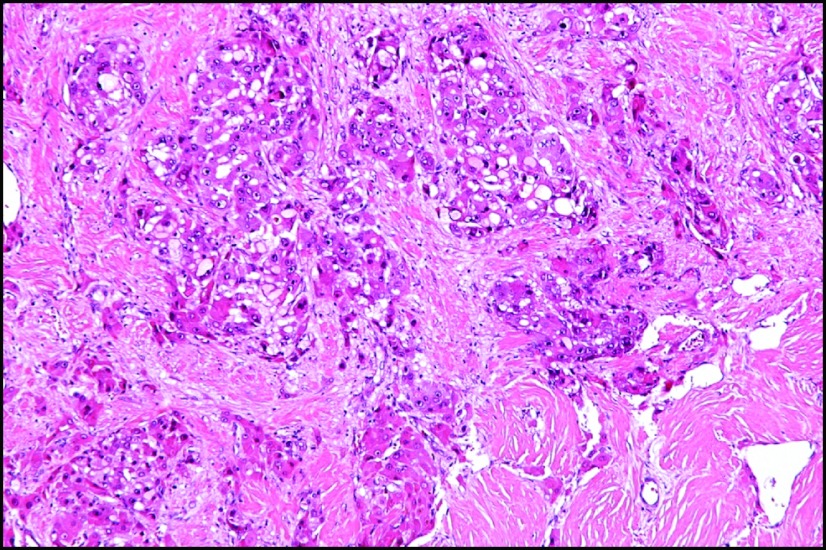
Dr. Boulos: The resected tumor showed moderately differentiated fibrolamellar carcinoma (FLC) with lymphovascular invasion and clean margins. The tumor contained dark, round intracytoplasmic inclusions with linear and whorled structures that were consistent with α-1-antitrypsin (AAT). On gross pathology, FLCs are large, lobulated, and well-circumscribed lesions with a fibrous central scar and outward projections like the spokes of a bicycle wheel (Figure 1). On microscopy, FLC cells are large, polygonal, with large amounts of eosinophilic cytoplasm. The cells form islands or cords that are separated by bands of lamellar fibrosis2 (Figure 2). Immunoreactivity for hepatocyte and biliary markers such as cytokeratin 7, HepPar-1, pCEA, and epithelial membrane antigen are common in FLC.3
Figure 1.
Gross appearance of fibrolamellar carcinoma tumor.
Figure 2.
Hematoxylin & eosin stain of fibrolamellar carcinoma.
Dr. Al-Olayan: What is FLC? How is it different from typical hepatocellular carcinoma (HCC)?
Dr. Abou-Alfa: FLC is a rare primary liver malignancy that affects adolescents and young adults who are otherwise healthy without any chronic liver disease or cirrhosis. According to SEER data from 1986–1999, FLC represents <1% of all primary liver cancers.4 While typical HCC occurs more frequently in males, FLC has been shown to affect females and males equally with some data suggesting that it may be slightly more common in females.5,6 Like many patients with FLC, our patient had no history of exposure to hepatitis B or C and no known metabolic disorders, alcohol abuse, or contact with other hepatotoxins.4,7 FLC is often associated with a normal serum AFP and demonstrates a higher propensity for lymph node metastases than typical HCC.7,8
Dr. Haidar: Are there any typical imaging characteristics of FLC? And is there a preferred imaging modality?
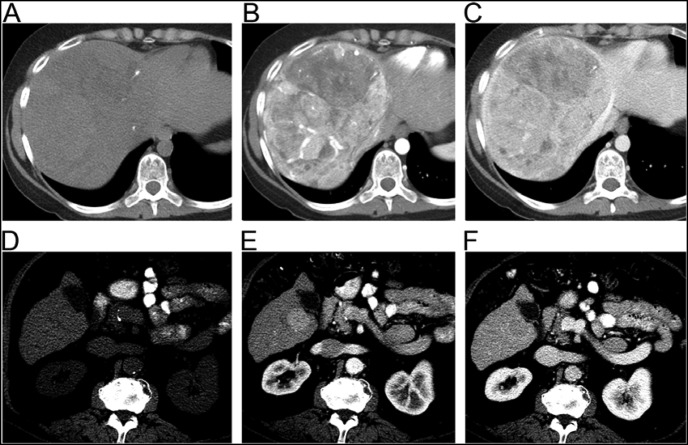
Dr. Do: On a triple phase CT FLC appears as a large, arterially enhancing lesion with central scarring that contains calcifications (Figure 3).9 The presence of calcifications helps to distinguish FLC from FNH, which can also have a central scar but without calcifications. HCCs also appear hyperdense on arterial phase but can be lower in attenuation on precontrast images due to triglyceride content. Central scars are uncommon in adenomas.10 At the present time, no data suggest that magnetic resonance imaging is more sensitive for the detection and characterization of FLC than a high-quality triple phase CT.
Figure 3.
Fibrolamellar carcinoma (A–C) vs. typical hepatocellular carcinoma (D–F). Left to right: precontrast (A and D), arterial (B and E), and venous phase (C and F) images.
Dr. Shamseddine: Back to the pathology: do we know of any association between AAT and FLC? Was there anything in this patient's history that suggested an AAT deficiency?
Dr. Ang: There are 3 known case reports of patients with FLC linked with AAT deficiency, 2 in association with the PiMZ genotype.11–13 AAT is a serine protease inhibitor of neutrophil elastase that is produced and secreted by hepatocytes. Patients with AAT deficiency accumulate AAT glycoproteins in the endoplasmic reticulum of hepatocytes, resulting in liver injury and cirrhosis.12 Our patient had a history of well-controlled seasonal asthma and a sister who had a benign liver tumor resected, though further details were not available. Serum AAT levels were not checked in our patient, and blood was not sent for genotyping.
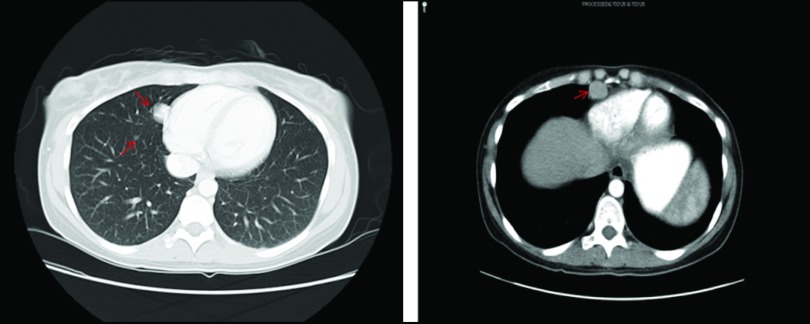
Dr. Ang: The patient was managed expectantly after surgery without any evidence of disease recurrence until a year later when a CT scan showed numerous enlarging bilateral pulmonary nodules, a 2.8 × 1.8 cm right pericardial mass without an effusion, and a small supradiaphragmatic lymph node, all suspicious for recurrent FLC (Figure 4). She presented to Memorial Sloan-Kettering Cancer Center (MSKCC) for a consultation at this time. A lung biopsy was performed confirming metastatic FLC.
Figure 4.
CT scan showing metastatic FLC to the lungs and pericardium.
Dr. Kelsen: What would you recommend as systemic therapy?
Dr. Ang: Given the lack of a standard of care, the patient was offered a phase I clinical trial of cisplatin, irinotecan, and flavopiridol, achieving stable disease for 2 years. Due to cumulative toxicities from cisplatin, systemic therapy was discontinued, and she subsequently underwent bilateral pulmonary wedge resections. Her disease-free interval unfortunately did not last long, and within a few months a new CT scan showed worsening disease.
Dr. Naghy: By that time, sorafenib was already approved for HCC. Was this recommended?
Dr. Ang: Yes, indeed, sorafenib 400 mg PO BID was given, but her disease progressed. She participated in 2 clinical trials testing a histone deacetylase inhibitor followed by inhaled sargramostin. Since FLC has been shown to overexpress oncogenic drivers such as the epidermal growth factor receptor (EGFR),14,15 it is possible that genetic silencing by histone deacetylase inhibition could be effective. The patient's disease continued to progress, and she returned to MSKCC with new onset dysphagia, dull headaches, and dyspnea that was worse when supine, but not interfering with daily activities.
Dr. Abou-Alfa: Dr Wehbe, what could be going on? What is the differential diagnosis of this presentation?
Dr. Wehbe: Her symptoms are worrisome for mediastinal pathology such as lymphadenopathy or thrombus causing superior vena cava (SVC) syndrome. Cross-sectional chest imaging is warranted.
Dr. Ang: A CT was performed. Dr. Haidar, can you please elaborate on what the scan shows?
Dr. Haidar: The scan (Figure 5) shows an extensive, confluent lymph node mass at the level of the ascending aorta, which is compressing the pulmonary artery and, presumably, the esophagus as well. The SVC is compressed anteriorly and to the left of the aortic arch. These radiographic findings, along with correlating symptoms, support a diagnosis of SVC syndrome. The presence of collateral or accessory veins is another common radiographic feature in SVC syndrome, but none are seen on these images.
Figure 5.
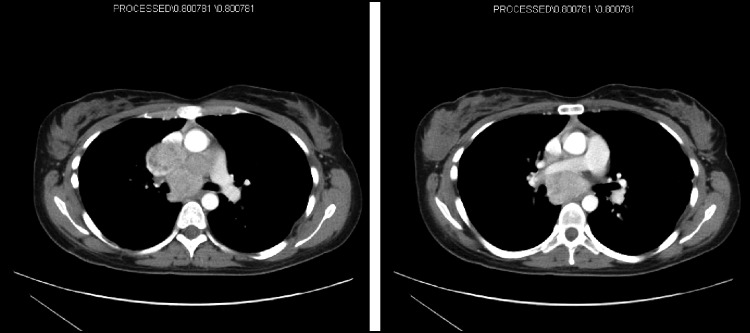
CT scan showing SVC compression by metastatic FLC.
Dr. Sibai: Is there a role for SVC stenting in this situation?
Dr. O'Reilly: SVC stenting is generally reserved as a salvage procedure in patients with recurrent or persistent symptoms despite chemotherapy and/or radiotherapy. Hemodynamic complications as well as stent occlusion and migration are major concerns,16 especially in a young patient. I would not recommend stenting as a first-line intervention in this instance unless the patient shows clear signs of SVC syndrome.
Dr. Ang: Stenting was indeed deferred, and the patient was treated with a course of palliative radiotherapy despite the fact that little is known about how FLC responds to radiation therapy. Radiation is an accepted treatment modality for SVC syndrome17 and was felt to be the best option since multiple systemic therapies had failed to control her disease. The patient had good symptom resolution, although the mediastinal mass did not significantly decrease in size. A year later, the patient developed similar symptoms with mild lightheadedness and a pressure-like sensation around her neck when supine.
Dr. Faraj: Was surgery contemplated?
Dr. Ang: The mediastinal mass was resected. A few months later, a right breast mass was discovered and resected, revealing a benign phyllodes tumor.
Dr. Kelsen: Is there any known association with phyllodes tumors of the breast and FLC? Can FLC metastasize to the breast?
Dr. Ang: There is no known association between phyllodes tumors and FLC. Breast metastases from FLC have not been previously reported. The most common sites of FLC metastases are the upper abdominal and thoracic lymph nodes, lungs, and peritoneum.7,8 The patient continued to be observed for slowly growing but asymptomatic recurrent mediastinal disease until a year later when she developed recurrent symptoms of SVC compression. Under cardiopulmonary bypass, she underwent debulking of bilateral paratracheal masses with macroscopic residual disease left behind. Her symptoms improved, but she again presented 4 months later with headaches, pressure around her neck, dysphagia, weight loss, and dyspnea. In view of her functional decline and lack of effective therapies, best supportive care was favored.
Dr. Shamseddine: This patient survived 10 years with her disease. What do we know about the prognosis of FLC, and how does it compare with typical HCC?
Dr. Abou-Alfa: The prognosis of FLC is a subject of debate and can best be described as highly variable. In the surgical and transplant literature, median survival is as high as 112 months, and up to 70% of patients can survive 5 and even 10 years,7,18 leading to the perception that this is a much more indolent disease with better outcomes compared to typical HCC. Patients with unresectable FLC have much worse outcomes; 5-year and median survival are 0%–5% and approximately 12 months.5,7 Even after curative surgery, up to 100% of patients relapse with significant morbidity and mortality from their disease.19 Recent data indicate that the prognosis of FLC is similar to that of typical HCC not associated with cirrhosis. Aside from unresectable disease, factors associated with worse survival in FLC include advanced stage disease and the presence of lymph node metastases. Recent data from a large series of FLC patients suggest that females have a shorter survival than males.6 Dr. Ang, can I ask you please to tell the group about our efforts in treating FLC.
Dr. Ang: There are no known effective systemic therapies for FLC that cannot be resected. Only 2 prospective chemotherapy trials have included FLC patients as part of a larger cohort of patients with typical HCC.20,21 One study compared 2 cisplatin-based chemotherapy regimens after surgery or biopsy in patients who are naive to systemic therapy. Median survival in the FLC cohort was 13.6 months, which was not significantly different from typical HCC.20 Although a median survival of 23.1 months and an objective response rate of 62.5% were reported with 5-fluorouracil and interferon α-2b; this regimen was very toxic.21 To date, there are no reports regarding the use of sorafenib in FLC with the exception of a retrospective review in which 10 patients were treated, none of whom had a durable response.6
Dr. O'Reilly: Is there any genetic target one might consider?
Dr. Ang: Markers for both hepatocellular and biliary differentiation as well as chromosomal aberrations common to both lineages have been reported in FLC.3,22 Up-regulation of NFκB,23 EGFR, the Ras/Raf/MAPK and PI3K/Akt/mTOR oncogenic signaling cascades, and their downstream effectors15,24 have also been reported in FLC. Mutations of β-catenin, p53, c-Met, and HGF, which are common in typical HCC, are absent in FLC.22,24 Overall, FLCs contain fewer epigenetic changes and are more genetically stable than HCC.25
Several case reports have described young males with FLC who present with gynecomastia and high serum estradiol levels that normalize following tumor resection.26–29 In females, FLC has been associated with the use of oral contraception and pregnancy.30,31 In addition, FLC tumors have been shown to overexpress aromatase.15,26–29 These observations suggest a potential pathogenic role for estrogen. Based on these observations, we have developed a phase II clinical trial that tests the antineoplastic activity of estrogen suppression with leuprolide and letrozole and mTOR inhibition with everolimus (www.clinicaltrials.gov, NCT01642186). Patients will be randomized to estrogen deprivation therapy, everolimus, or a combination of everolimus plus estrogen deprivation therapy. The trial also includes correlative studies looking at novel tumor markers for FLC such as neurotensin,32 vitamin B12 binding capacity,33,34 and des-γ-carboxyprothrombin.35 Estrogen and testosterone levels will also be followed and correlated with outcome. Tumor specimens will also be analyzed for aromatase and estrogen receptor expression, Ki67, and markers of mTOR pathway activity. This trial will be conducted by the Fibrolamellar Carcinoma Consortium, whose institutional members include MSKCC, the University of California San Francisco, Johns Hopkins University, and Harvard-affiliated hospitals.
Dr. Abou-Alfa: Thank you, Dr. Ang. That was a very insightful case and presentation. Can you please help summarize the key points?
Dr. Ang: FLC should be part of the differential diagnosis of a liver tumor in children, adolescents, and young adults who are otherwise healthy. At the present time, the pathogenesis of FLC is unknown, and efforts aimed at elucidating its molecular characteristics are a research priority. Surgery can provide long-term disease control and should be performed where possible, even in patients with low-volume extrahepatic disease. Liver transplantation and ablative therapies may be considered, although there are no prospective data to guide their use. Patients with unresectable disease should be enrolled in clinical trials as there are no effective systemic options.
Acknowledgments
This case was presented at the MSKCC/American University of Beirut/ National Guard Hospital, Riyadh, case conference in February 2012. This conference is supported by an endowment gift of Mrs. Mamdouha El- Sayed Bobst and the Bobst Foundation.
REFERENCES
- 1. Giannitrapani L, Soresi M, La Spada E, et al. : Sex hormones and risk of liver tumor. Ann N Y Acad Sci 1089:228–236, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Craig JR, Peters RL, Edmondson HA, et al. : Fibrolamellar cancer of the liver: a tumor of adolescents and young adults with distinctive clinico-pathologic features. Cancer 45:372–379, 1980 [DOI] [PubMed] [Google Scholar]
- 3. Ward SC, Huang J, Tickoo SK, et al. : Fibrolamellar carcinoma of the liver exhibits immunohistochemical evidence of both hepatocyte and bile duct differentiation. Mod Pathol 23:1180–1190, 2010 [DOI] [PubMed] [Google Scholar]
- 4. El Serag HB, Davila JA: Is fibrolamellar carcinoma different from hepatocellular carcinoma? A US population-based study. Hepatology 39:798–803, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Moreno-Luna LE, Arrieta O, Garcia-Leiva J, et al. : Clinical and pathologic factors associated with survival in young adult patients with fibrolamellar hepatocarcinoma. BMC Cancer 5:142, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ang C, Venook AP, Choti MA, et al. : Clinical/pathologic features and survival of patients with fibrolamellar hepatocellular carcinoma (FLL-HCC): data from the Fibrolamellar Hepatocellular (FLL-HCC) Consortium. J Clin Oncol 29, 2011. (suppl; abstr 4089) [Google Scholar]
- 7. Stipa F, Yoon SS, Liau KH, et al. : Outcome of patients with fibrolamellar hepatocellular carcinoma. Cancer 106:1331–1338, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Malouf GG, Brugieres L, Le Deley MC, et al. : Pure and mixed fibrolamellar hepatocellular carcinomas differ in natural history and prognosis after complete surgical resection. Cancer 118:4981–4990, 2012 [DOI] [PubMed] [Google Scholar]
- 9. Ichikawa T, Federle MP, Grazioli L, et al. : Fibrolamellar hepatocellular carcinoma: pre- and posttherapy evaluation with CT and MR imaging. Radiology 217:145–151, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Ruppert-Kohlmayr AJ, Uggowitzer MM, Kugler C, et al. : Focal nodular hyperplasia and hepatocellular adenoma of the liver: differentiation with multiphasic helical CT. Am J Roentgenol 176:1493–1498, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Al Bugami MM, Farahat KL, Al-Ashgar HI, et al. : Fibrolamellar hepatocellular carcinoma with alpha-1-antitrypsin liver disease. Saudi J Gastroenterol 10:92–95, 2004 [PubMed] [Google Scholar]
- 12. Rabinovitz M, Gavaler JS, Kelly RH, et al. : Lack of increase in heterozygous alpha-1-antitrypsin deficiency phenotypes among patients with hepatocellular and bile duct carcinoma. Hepatology 15:407–410, 1992 [DOI] [PubMed] [Google Scholar]
- 13. Govindarajan S, Ashcavai M, Peters RL: Alpha-1-antitrypsin phenotypes in hepatocellular carcinoma. Hepatology 1:628–631, 1981 [DOI] [PubMed] [Google Scholar]
- 14. Patonai A, Erdélyi-Belle B, Korompay A, et al. : Molecular characteristics of fibrolamellar hepatocellular carcinoma. Pathol Oncol Res, in press [DOI] [PubMed] [Google Scholar]
- 15. Muramori K, Taguchi S, Taguchi T, et al. : High aromatase activity and overexpression of the epidermal growth factor receptor in fibrolamellar hepatocellular carcinoma in a child. J Pediatr Hematol Oncol 33:e195–197, 2011 [DOI] [PubMed] [Google Scholar]
- 16. Nguyen NP, Borok TL, Welsh J, et al. : Safety and effectiveness of vascular endoprosthesis for malignant superior vena cava syndrome. Thorax 64:174–178, 2009 [DOI] [PubMed] [Google Scholar]
- 17. Wilson LD, Detterbeck FC, Yahalom J: Clinical practice: superior vena cava syndrome with malignant causes. N Engl J Med 356:1862–1869, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Hemming AW, Langer B, Sheiner P, et al. : Aggressive surgical management of fibrolamellar hepatocellular carcinoma. J Gastrointest Surg 1:342–346, 1997 [DOI] [PubMed] [Google Scholar]
- 19. Maniaci V, Davidson BR, Rolles K, et al. : Fibrolamellar hepatocellular carcinoma: prolonged survival with multimodality therapy. Eur J Surg Oncol 35:617–621, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Katzenstein HM, Krailo MD, Malogolowkin MH, et al. : Fibrolamellar hepatocellular carcinoma in children and adolescents. Cancer 97:2006–2012, 2003 [DOI] [PubMed] [Google Scholar]
- 21. Patt YZ, Hassan MM, Lozano RD, et al. : Phase II trial of systemic continuous fluorouracil and subcutaneous recombinant interferon alfa-2b for treatment of hepatocellular carcinoma. J Clin Oncol 21:421–427, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Ward SC, Waxman S: Fibrolamellar carcinoma: a review with focus on genetics and comparison to other malignant primary liver tumors. Semin Liver Dis 31:61–70, 2011 [DOI] [PubMed] [Google Scholar]
- 23. Li W, Tan D, Zenaii M, et al. : Constitutive activation of nuclear factor-kappa B (NF-κB) signaling pathway in fibrolamellar hepatocellular carcinoma. Int J Clin Exp Pathol 3:238–243, 2010 [PMC free article] [PubMed] [Google Scholar]
- 24. Kannangai R, Vivekanandan P, Martinez-Murillo F, et al. : Fibrolamellar carcinomas show overexpression of genes in the RAS, MAPK, PIK3 and xenobiotic degradation pathways. Hum Pathol 38:639–644, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Vivekanandan P, Torbenson M: Epigenetic instability is rare in fibrolamellar carcinomas but common in viral-associated hepatocellular carcinomas. Mod Pathol 21:670–675, 2008 [DOI] [PubMed] [Google Scholar]
- 26. Muramori K, Taguchi S, Taguchi T, et al. : High aromatase activity and overexpression of epidermal growth factor receptor in fibrolamellar hepatocellular carcinoma in a child. J Pediatr Hematol Oncol 33:e195–197, 2011 [DOI] [PubMed] [Google Scholar]
- 27. Agarwal VR, Takayama K, Van Syk JJ, et al. : Molecular basis of severe gynecomastia associated with aromatase expression in a fibrolamellar hepatocellular carcinoma. J Clin Endocrinol Metab 83:1797–1800, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Hany MA, Betts DR, Schmugge M, et al. : A childhood fibrolamellar hepatocellular carcinoma with increased aromatase activity and a near triploid karyotype. Med Pediatr Oncol 28:136–138, 1997 [DOI] [PubMed] [Google Scholar]
- 29. McCloskey JJ, Germain-Lee EL, Perman JA, et al. : Gynecomastia as a presenting sign of fibrolamellar carcinoma of the liver. Pediatrics 82:379–382, 1988 [PubMed] [Google Scholar]
- 30. Louie-Johnson MW, Hewitt PM, Perera DS, et al. : Fibrolamellar hepatocellular carcinoma in pregnancy. HPB 5:191–193, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gemer O, Segal S, Zohav E: Pregnancy in a patient with fibrolamellar hepatocellular carcinoma. Arch Gynecol Obstet 255:211–212, 1994 [DOI] [PubMed] [Google Scholar]
- 32. Collier NA, Weinbren K, Bloom SR, et al. : Neurotensin secretion by fibrolamellar carcinoma of the liver. Lancet 1(8376):538–540, 1984 [DOI] [PubMed] [Google Scholar]
- 33. Paradinas FJ, Melia WM, Wilkinson ML, et al. : High serum vitamin B12 binding capacity as a marker of the fibrolamellar variant of hepatocellular carcinoma. BMJ 283:840–842, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Waxman S, Gilbert HS: A tumor related vitamin B12 binding protein in adolescent hepatoma. N Engl J Med 289:1053–1056, 1973 [DOI] [PubMed] [Google Scholar]
- 35. Nakao A, Virji A, Iwaki Y, et al. : Abnormal prothrombin (des-g-carboxyprothrombin) in hepatocellular carcinoma. Hepatogastroenterology 38:450–453, 1991 [PMC free article] [PubMed] [Google Scholar]