Abstract
In recent years, the number of obese population in Korea has been growing up along with the economic development, environmental factors, and the change in life style. Considering the growth of obese population and the adverse effect of obesity on health, it is getting more important to prevent and diagnose the obesity with the quantitative measurement of body fat that has become an important indicator for obesity. In this study, we proposed a procedure for the automated fat assessment from computed tomography (CT) data using image processing technique. The proposed method was applied to a single-CT image as well as CT-volume data, and results were correlated to those of dual-energy X-ray absorptiometry (DEXA) that is known as the reliable method for evaluating body fat. Using single-CT images, correlation coefficients between DEXA and the automated assessment and DEXA and the manual assessment were 0.038 and 0.058, respectively (P > 0.05). Hence, there was no significant correlation between three methods using the proposed method with single-CT images. On the other hand, in case of CT-volume data, the above correlation coefficients were increased to 0.826, 0.812, and 0.805, respectively (P < 0.01). Thus, DEXA and the proposed methods with CT-volume data showed highly significant correlation with each other. The results suggest that the proposed automated assessment using CT-volume data is a reliable method for the evaluation of body fat. It is expected that the clinical application of the proposed procedure will be helpful to reduce the time for the quantitative evaluation of patient’s body fat.
Keywords: Body fat, Fat assessment, Computed tomography (CT), DEXA, Separation mask
Introduction
Obesity is the status of a body with excessive body fat. Since obesity often becomes the cause of diverse chronic diseases, it is an emerging serious problem among public health issues. The number of obese population has dramatically been increasing all over the world including US along with the economic development and changes in environmental factors and life style. During 20 years from 1988 to 2008, the level of obesity in American population has been increased by 12 % in male population and 10.1 % in female population [1]. Considering the growth of obese population and the adverse effect of obesity on health, it is getting more important to prevent and diagnose the obesity with the quantitative measurement of body fat that has become an important indicator for obesity.
There are some methods available for the measurement of body fat including body mass index (BMI), waist–hip ratio (WHR), bioelectrical impedance (BIA), computed tomography (CT)-based measurement, and dual-energy X-ray absorptiometry (DEXA). Although BMI, WHR, and BIA are widely used to calculate body fat, results from these methods do not adequately represent the actual amount of body fat and are affected by many factors including inter- and intra-observer variability [2–5]. DEXA, the most commonly used method to estimate bone mineral density, is used to measure the accurate amount of body fat by excluding measured bone mass. DEXA is becoming more popular in Korea since it utilizes a low dose of radiation and provides validated accurate measurement of body fat. However, the drawback of DEXA is that it cannot provide the information on the distribution of body fat such as discriminating between subcutaneous and visceral body fat [5, 6]. Clinically, visceral obesity is different form subcutaneous obesity, because the visceral obesity has been known to be related with metabolic syndrome [7].
Although CT uses higher dose of X-rays compared with DEXA, CT-based measurement of body fat can directly compute the amount of body fat and discriminate visceral fat from subcutaneous fat from results. Radiation exposure can be minimized by using low-dose CT scanning, or additional radiation exposure can be avoided by utilizing CT taken for other purposes such as health screening. However, CT-based fat delineation is a time-consuming procedure since clinicians have to manually define the regions of body fat [2, 6]. To overcome such drawback, many studies have been reported to automate the quantification of body fat using CT images with the image processing technology [8–13]. To differentiate between visceral and subcutaneous fat in CT images, Bandekar et al. (2005) [8] have proposed automatic fat analysis in computed tomography based on fuzzy affinity and active shape model. The performance of this method was evaluated by measures of accuracy and sensitivity compared with results of manual quantification by experts. This method showed the accuracy of 98.29 ± 0.62 % for subcutaneous fat and 97.66 ± 0.98 % for visceral fat. In case of sensitivity, it was 90.01 ± 3.77 % for subcutaneous fat and 86.14 ± 7.25 % for visceral fat [8]. Zhao et al. (2006) [13] have presented a method to automatically quantify visceral and subcutaneous fat distribution on volumetric computed tomographic (CT) data using pixel information along radii drawn from the center of the body at an increment of 3°. When results using this method and manual measurement by radiologist for nine subjects were compared, the differences between automatic and manual methods were 1.54 % for visceral fat and 0.65 % for subcutaneous fat [13].
Studies mentioned above only reported methods of automatic body fat evaluation based on image processing technique and results in comparison with manual assessment using same CT data. To our knowledge, there is no study to compare the performance of automatic evaluation of body fat using image processing with those of various fat assessment methods, except manual segmentation of fat using CT. Thus, in the present study, we propose the analytic method based on CT volume for the measurement of body fat amount and distribution, which was compared with manual assessment method using CT and DEXA.
Materials and Methods
CT data were collected from ten subjects (five male and five female patients; age range, 22.2–62.8 years) who were in- or outpatients at the Seoul National University Hospital. The experimental protocol was approved by the institutional review board of Seoul National University Hospital, and all subjects gave written informed consent for the study before participation.
CT scan were performed with all subjects in the supine position using Sensation 16 (SIEMENS, Germany; field of view of 320 × 320 mm, matrix of 512 × 512, slice thickness of 3 mm, 120 kVp, 58 mAs, 1,524 pixel per mm resolution).
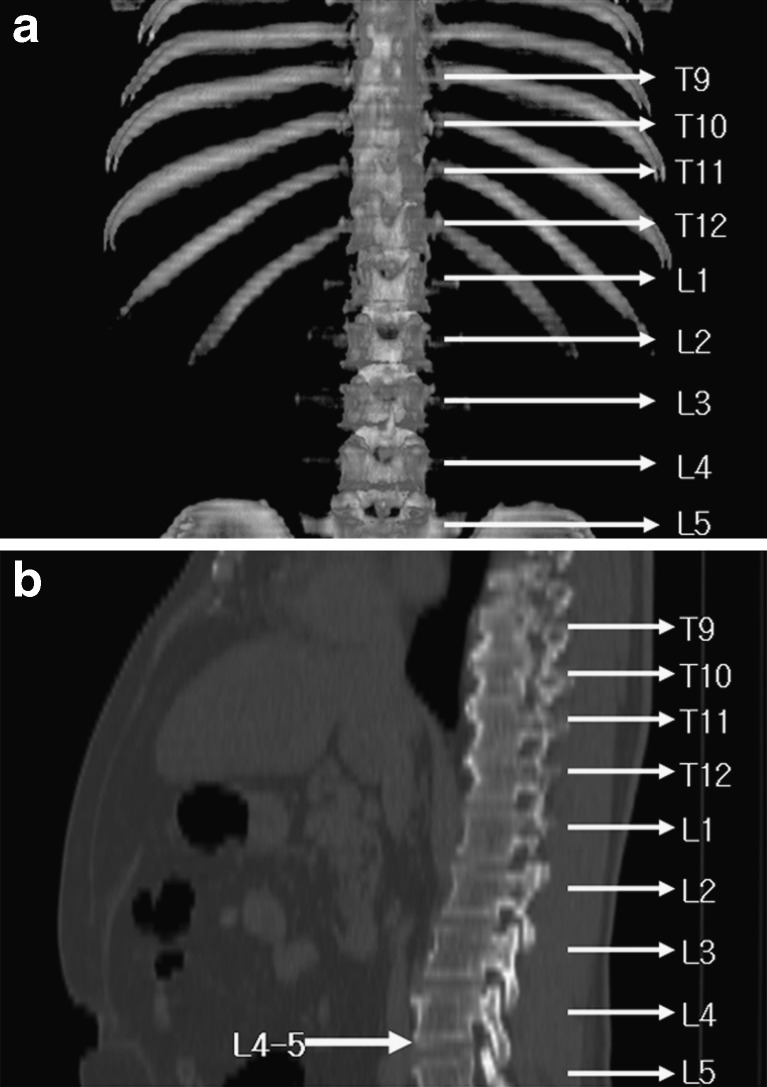
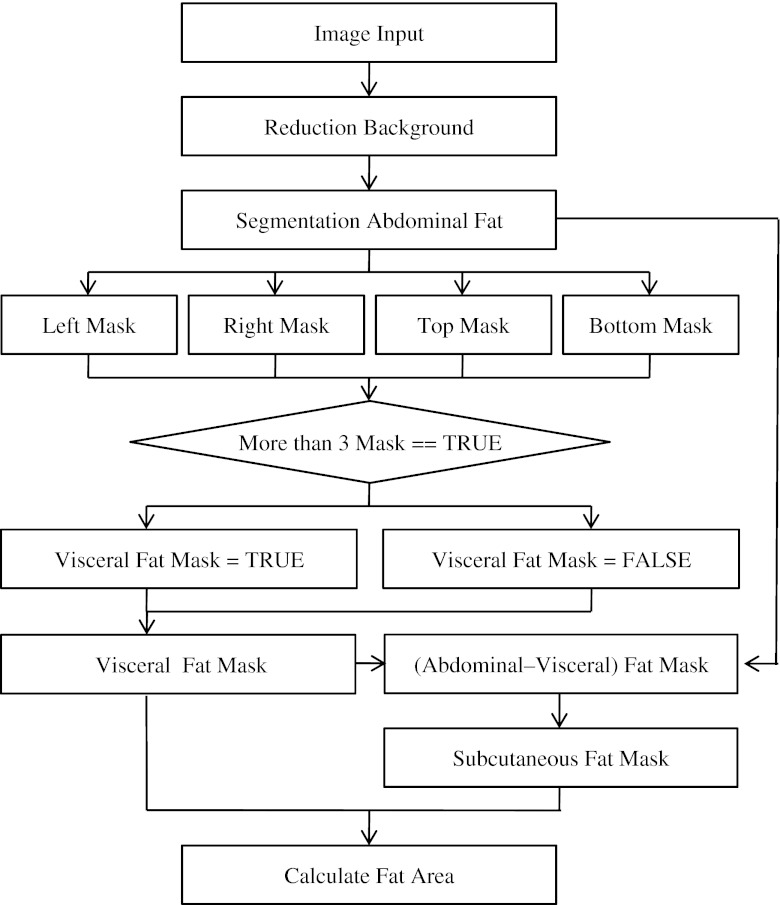
From each patient, one slice of CT data was collected at the location of umbilicus between fourth and fifth lumbar vertebrae (L4–5) as shown in Fig. 1. For CT-volume data, six additional CT images were collected from above and below the umbilicus of each patient, respectively. Collected CT data were applied to both of manual and automatic body fat assessment methods. For manual assessment, an expert delineated the regions of visceral and subcutaneous fat using ImageJ (ver. 1.60, National Institute of Health, USA). The three-compartment DEXA, which separates body composition into bone, lean body mass, and fat materials, was applied to the CT data using the whole-body DEXA scanner, LUNAR Prodigy Vision scanner (software version 9.30, GE Healthcare, USA). It means that the same patients who had CT scans were scanned using DEXA. The analysis tool for automatic body fat assessment from the CT data was developed using Microsoft Visual Studio (Ver. 2005, Microsoft, USA). The automated assessment of body fat from CT data was performed using a protocol described in Fig. 2.
-
Subtracting background
Although acquired CT images can broadly be divided into two compartments of body and air, they also include unnecessary background such as bed and sheets near the body. Since these unnecessary parts may affect the assessment of body fat, they were removed using thresholding and labeling techniques before the body fat determination. The pixel value of air in HU (Hounsfield unit) is known to be as low as about −1,000 [14]. Thus, considering the background noise of CT images, all regions below −900 HU were subtracted to remove air compartments. Then, unnecessary areas such as bed and sheets were removed by labeling all regions followed by removing all regions except the largest region, the body. With all background removed, the segmentation and assessment of body fat were performed only in the area of upper body.
-
Thresholding
Prior to the delineation between visceral fat and subcutaneous fat, a binary image representing regions of body fat was made by thresholding. The CT image taken between the fourth and fifth lumbar vertebrae includes the areas of skin, muscle, bone, intestine, and fat. It has been reported that the ranges of pixel values of body fat is between −190 and −30 HU [15]. Hence, pixels with values in the range between −190 and −30 HU were detected as body fat during the thresholding process. The segmented body fat was then represented as a binary image by assigning label ‘T’ to the fat area (value = 255) and label ‘F’ to the non-fat area (value = 0).
-
Differing the visceral fat from the subcutaneous fat
In this study, a segmentation mask was made from the thresholded binary image and used to differentiate visceral fat from subcutaneous fat. A thresholded binary image (Fig. 3) shows visceral fat surrounded by subcutaneous fat with bones and other organs removed by thresholding process. However, it was observed that visceral and subcutaneous fat were connected to each other in some cases, and the patterns of such connection were not consistent. In this study, the segmentation mask of non-subcutaneous fat area was made to separate regions of visceral fat and subcutaneous fat.
Fig. 1.
Location of vertebrae lumbales L4–5; a coronal view; b sagittal view
Fig. 2.
Algorithm flowchart
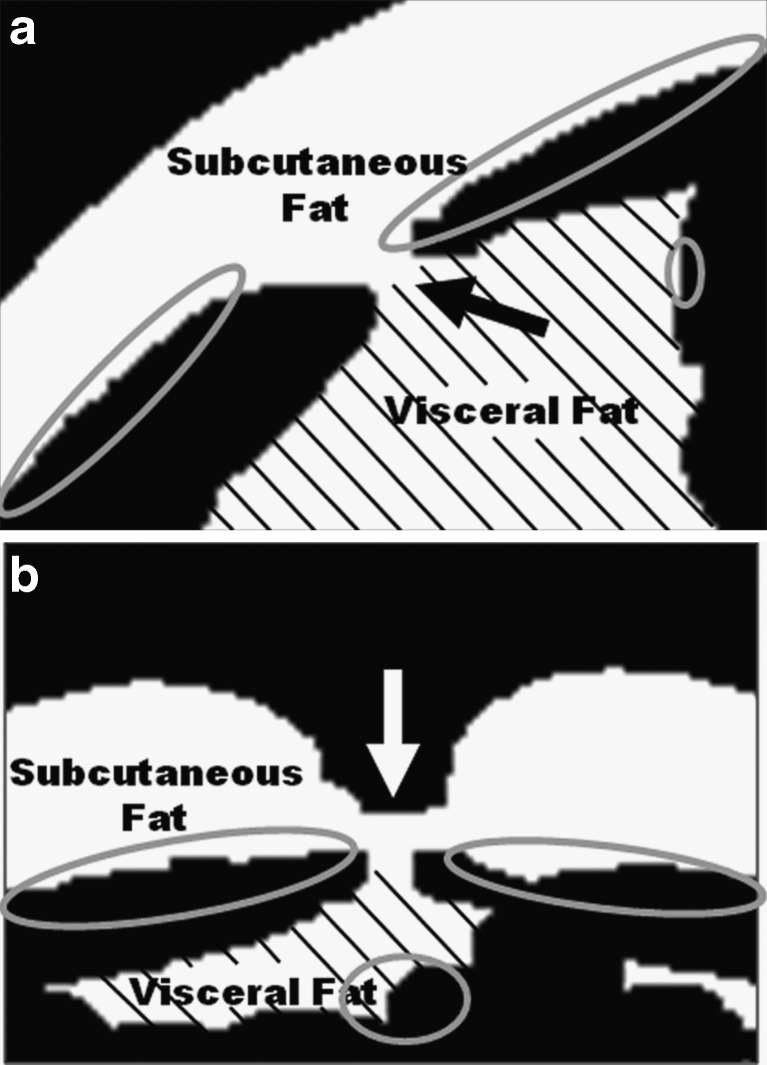
Fig. 3.
Initial point of scanning on segmentation mask; a left to right direction, b top to bottom direction
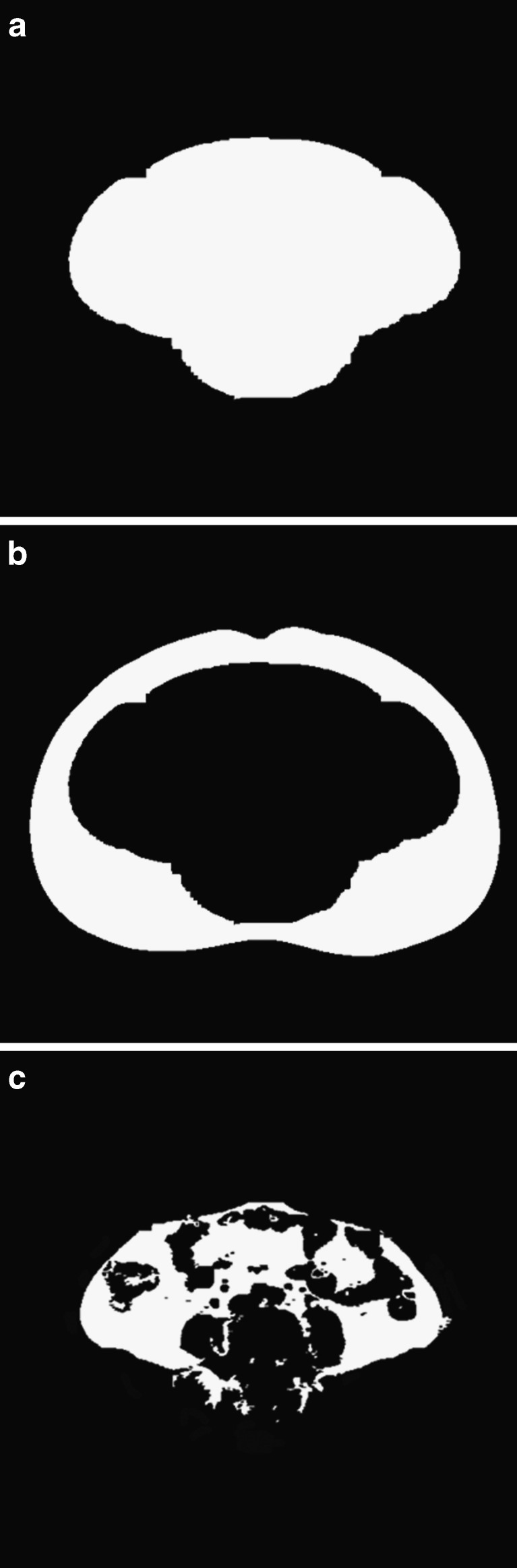
The segmentation mask was computed from four preliminary masks with different scanning directions, left to right, right to left, top to bottom, and bottom to top. Each preliminary mask was created by assigning ‘True’ value from the first ‘False’ pixel after subcutaneous fat was detected in the binary image to the last pixel in the designated scanning direction. When subcutaneous fat and visceral fat are connected, detected borders between subcutaneous and visceral fat in some primary masks were located in the regions of visceral fat as shown in Fig. 3. To resolve this problem, the segmentation mask was created using a modified bit-AND operation of four primary masks. The modified bit-AND operation of matching pixels from four binary images is defined to be true if three or more pixels have true value, while the original bit-AND operation of four variables is true only if all variables have true value. With the modified bit-AND operation, the segmentation mask surrounding areas of visceral fat was made from four preliminary masks (Fig. 4a).
Fig. 4.
a The segmentation mask using modified AND operation; b the subcutaneous fat by subtracting the segmentation mask; c the visceral fat by applying the threshold limits in the area of the segmentation mask
The subcutaneous fat was segmented by subtracting the segmentation mask from the thresholded binary image (Fig. 4b). Due to variations among individuals, the distribution of visceral fat surrounding inner organs is hard to be quantified with the standard thresholding value for the detection of body fat. Thus, the threshold values for the visceral fat were separately determined using the distribution of pixel values in the region of the subcutaneous fat. Using the mean and standard deviation (SD) of pixel values in the subcutaneous fat, the upper and lower limits of thresholding value were quantified as mean ± (2 × SD), respectively [16]. The region of visceral fat was segmented by applying the upper and lower threshold limits to CT images in the area of the segmentation mask (Fig. 4c).
Results
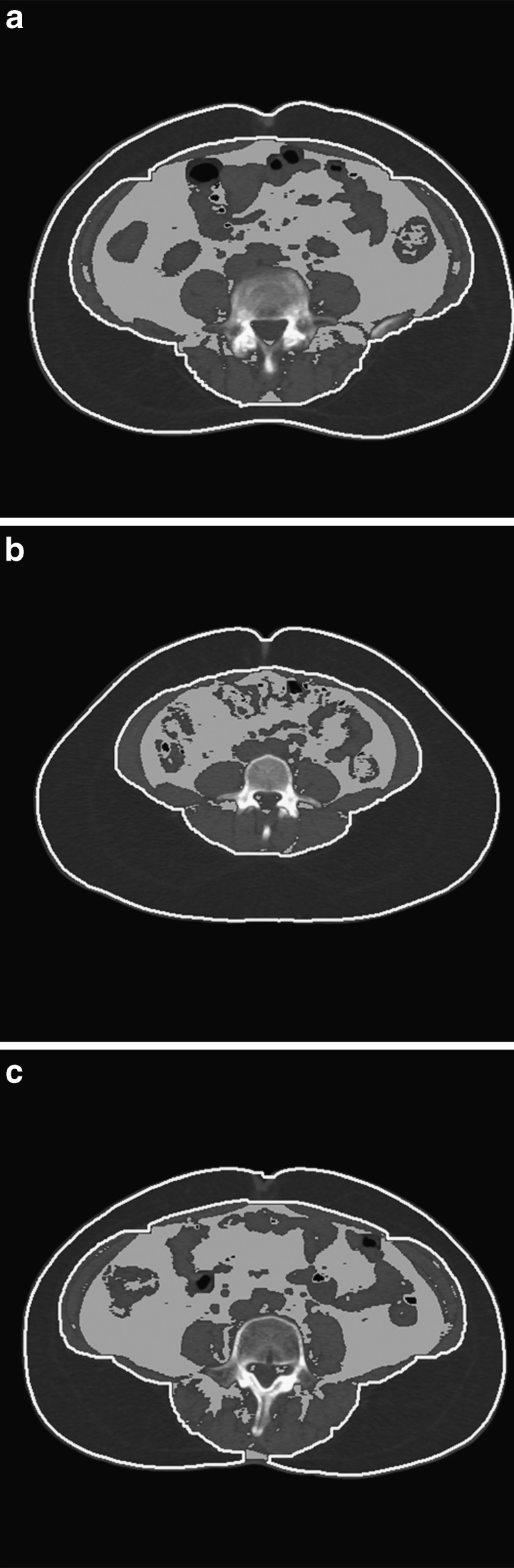
In this study, we developed a method to make the segmentation mask for differentiating subcutaneous and visceral fat from thresholded binary images of background-extracted CT images. Using the segmentation mask, the subcutaneous fat was efficiently segmented from a CT image. Then, the visceral fat was detected and measured using the information of pixels in the subcutaneous fat. Figure 5 shows representative results of the segmentation of subcutaneous and visceral fat using the proposed method. Regions of interest outlined by white line indicates regions of subcutaneous fat, and gray-scale areas wrapped by subcutaneous fat are areas where visceral fat were detected.
Fig. 5.
Result of the segmentation of subcutaneous and visceral fat: a case1, b case2; c case3
To evaluate the performance of the proposed method for the automatic assessment of body fat, we calculated correlations among the amounts of fat detected using the DEXA, the manual assessment, and the proposed automated assessment using CT images.
Table 1 shows amounts of fat (n = 10) measured using DEXA and automatic and manual assessments of fat using CT data. Automatic and manual CT-based methods were applied to each of single-CT image at the location of umbilicus and CT volume data. In CT-based methods, the amounts of body fat were measured in the number of pixels in the regions of visceral and subcutaneous fat, respectively.
Table 1.
Result of assessment (DEXA and CT images)
| Index | Location | CT slice (pixel) | CT Volume (pixel) | DEXA (g) | ||
|---|---|---|---|---|---|---|
| Automatic | Manual | Automatic | Manual | |||
| 1 | Subcutaneous | 46,443 | 42,800 | 506,858 | 470,818 | 20,422 |
| Visceral | 28,764 | 24,420 | 361,673 | 246,277 | ||
| All | 75,207 | 67,220 | 868,531 | 717,095 | ||
| 2 | Subcutaneous | 35,529 | 32,075 | 497,829 | 426,346 | 23,618 |
| Visceral | 27,193 | 23,090 | 460,913 | 271,131 | ||
| All | 62,722 | 55,165 | 958,742 | 697,477 | ||
| 3 | Subcutaneous | 27,139 | 27,550 | 267,394 | 289,604 | 19,531 |
| Visceral | 27,522 | 28,355 | 336,767 | 289,083 | ||
| All | 54,661 | 55,905 | 604,161 | 578,687 | ||
| 4 | Subcutaneous | 34,082 | 31,050 | 547,504 | 382,603 | 25,733 |
| Visceral | 23,714 | 22,601 | 430,804 | 400,953 | ||
| All | 57,796 | 53,651 | 978,308 | 783,556 | ||
| 5 | Subcutaneous | 36,627 | 34,836 | 435,133 | 279,921 | 23,050 |
| Visceral | 30,689 | 23,415 | 436,022 | 338,106 | ||
| All | 67,316 | 58,251 | 871,155 | 618,027 | ||
| 6 | Subcutaneous | 37,865 | 34,344 | 325,419 | 387,105 | 19,823 |
| Visceral | 34,899 | 26,165 | 314,868 | 283,151 | ||
| All | 72,764 | 60,509 | 640,287 | 670,256 | ||
| 7 | Subcutaneous | 40,171 | 38,717 | 388,075 | 455,494 | 24,568 |
| Visceral | 40,324 | 37,265 | 415,728 | 347,766 | ||
| All | 80,495 | 75,982 | 803,803 | 803,260 | ||
| 8 | Subcutaneous | 59,822 | 57,923 | 807,237 | 726,505 | 40,343 |
| Visceral | 13,820 | 9,261 | 282,837 | 124,670 | ||
| All | 73,642 | 67,184 | 1,090,074 | 851,175 | ||
| 9 | Subcutaneous | 31,643 | 29,875 | 272,055 | 346,988 | 16,986 |
| Visceral | 33,555 | 28,553 | 335,219 | 258,133 | ||
| All | 65,198 | 58,428 | 607,274 | 605,121 | ||
| 10 | Subcutaneous | 50,433 | 48,394 | 319,587 | 360,071 | 15,818 |
| Visceral | 30,703 | 26,046 | 214,402 | 201,678 | ||
| All | 81,136 | 7,4440 | 533,989 | 561,749 | ||
Table 2 shows correlations among results from DEXA and automatic and manual assessments using CT volume data. The result of DEXA was most correlated to that of automated assessment using CT volume data (r = 0.826, P < 0.01). Correlation coefficients between DEXA and manual assessment using CT volume data and between automatic and manual assessment using CT volume data were 0.812 and 0.805, respectively (P < 0.01). In case of CT volume data, correlation coefficients between automatic and manual assessment was 0.834 (P < 0.01) for subcutaneous and 0.722 (P < 0.05) for visceral. Results showed that tested three methods were significantly correlated among each other.
Table 2.
Result of correlations among the amounts of fat detected using the DEXA, the manual assessment, and the proposed automated assessment using CT volume
| Method | Avg ± SD | Avg ± SD | Correlation |
|---|---|---|---|
| DEXA and automatic | 22,989.2 ± 6,892.9 | 795,632.4 ± 189,292.6 | 0.826* |
| DEXA and manual | 22,989.2 ± 6,892.9 | 688,640.3 ± 99,968.2 | 0.812* |
| Automatic and manual | |||
| All | 795,632.4 ± 189,292.6 | 688,640.3 ± 99,968.2 | 0.805* |
| Subcutaneous | 436,709.1 ± 164,138.2 | 412,545.5 ± 127,060.9 | 0.834* |
| Visceral | 358,923.3 ± 77,757.8 | 276,094.8 ± 77,813.0 | 0.722** |
*p < 0.01, significant correlation
**p < 0.05, significant correlation
Table 3 shows correlations among results from DEXA and automatic and manual assessment using CT volume data. The result of DEXA was most correlated to that of automated assessment using CT volume data (r = 0.038, P > 0.05). Correlation coefficients between DEXA and manual assessment using CT volume data and between automatic and manual assessment using CT volume data were 0.058 (P > 0.05) and 0.921 (P < 0.01), respectively. In case of CT slice data, correlation coefficients between automatic and manual assessment was 0.991 for subcutaneous and 0.924 for visceral (P < 0.01). Results showed that tested three methods were nonsignificantly correlated among each other, except for result between automatic and manual assessments.
Table 3.
Result of correlations among the amounts of fat detected using the DEXA, the manual assessment, and the proposed automated assessment using CT slice
| Method | Avg ± SD | Avg ± SD | Correlation |
|---|---|---|---|
| DEXA and automatic | 22,989.2 ± 6,892.9 | 69,093.7 ± 9,076.3 | 0.038 |
| DEXA and manual | 22,989.2 ± 6,892.9 | 62,673.5 ± 8,044.6 | 0.058 |
| Automatic and manual | |||
| All | 69,093.7 ± 9,076.3 | 62,673.5 ± 8,044.6 | 0.921* |
| Subcutaneous | 39,975.7 ± 9,715.3 | 37,756.4 ± 9,486.3 | 0.991* |
| Visceral | 29,118.3 ± 7,096.7 | 24,917.1 ± 6,965.4 | 0.924* |
*p < 0.01, significant correlation
Discussion
In this study, we have proposed the automated assessment of body fat using CT data and compared the results with those of DEXA conducted at Seoul National University Hospital as well as with the results of manual assessment using CT data by experts. Results of manual and automated assessment using CT data were correlated to results of DEXA that is a well-accepted method for fat assessment. Using CT-based automated and manual assessment methods, the amounts of body fat were measured using CT-volume data and a CT image at the location of umbilicus and correlated to results from other methods (Tables 2 and 3). Using CT-volume data, results showed significant correlation among the three methods as shown in Table 2. However, using single-CT image, correlations between the assessment methods were significantly low.
Correlation coefficients between DEXA and the automated assessment method were 0.038 (P > 0.05) and 0.826 (P < 0.01) with one CT image and CT-volume data, respectively. Using the same data, correlation coefficients between DEXA and the manual assessment method were 0.058 (P > 0.05) and 0.812 (P < 0.01) with one CT image and CT-volume data, respectively. The rationale for the low correlation between DEXA and the automated or manual assessment method with one CT image is that DEXA utilizes data from the whole-body scanner while a single-CT image only conveys information at one cross-section of the body.
In order to reduce such errors with single-CT images, we applied the automated as well as manual assessment methods to the CT-volume data and compared the results to those of DEXA. Significantly high correlation between the results implies that fat assessment using CT-volume data is more reliable than using a single-CT image. The reliability of the automated assessment method using CT-volume data was confirmed by its high correlation to the manual assessment using CT-volume data (r = 0.805, P < 0.01) as well as DEXA (r = 0.826, P < 0.01).
The major limitation of this study is the small number of specimens used in this study. It was due to the amount of experimental data obtained by means of DEXA that limited us to only ten cases for the assessment of body fat. For each CT-volume data, we could take only 13 CT images for evaluating body fat. Provided that more data by DEXA as well as the CT-volume data are collected in the future, the correlation between the DEXA and the automated and manual assessment methods is expected to be highly significant. The results obtained in this study suggest that the proposed automated fat assessment is time-saving.
In addition, when lipids accumulated excessively, visceral fat could have an effect on metabolic syndrome, leading to ectopic fat deposition in abnormal locations such as skeletal muscle, liver, heart, etc. [7]. However, a prediction of the effect is not easy because it is difficult to measure the amount of fat mass at specific area of the body by the common methods, including the DEXA method. Consequently, compared with other methods for measuring the amount of fat mass, we expect that our proposed method will contribute more to the clinical diagnosis in that it is possible to segment visceral fat and subcutaneous fat automatically in CT images and to evaluate them quantitatively by accurate measurement of each fat area based on three-dimensional volume reconstruction.
Acknowledgment
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MEST) (2011–0019794) and National Cancer Center No. NCC-1110160.
References
- 1.Ogden CL, Carroll MD: “Prevalence of overweight, obesity, and extreme obesity among adults: United States, Trends 1960–1962 Through 2007–2008,” 2010
- 2.Shin SW, et al. The correlation between simple anthropometric indices and abdominal visceral fat accumulation by computed tomography. Korean Acad Fam Med. 2001;22:316–323. [Google Scholar]
- 3.Kim TKH, Lim SW. A study on the equation for estimating body fat using the body girth. Korea J Sports Sci. 1999;8:439–446. [Google Scholar]
- 4.Yom SKHW, Whang IT, Hong YM. Correlation between body fat percent estimated by bioelectrical impedance analysis and other variable methods. Korean J Pediatr. 2003;46:751–757. [Google Scholar]
- 5.Jung DW, et al: Measuring performance evaluation of body fat measuring instrument applying body measuring value method. Korean J Health Promot Dis Prev 6:79–87, 2006
- 6.Kim JS, et al. Comparison of DEXA and CT for truncal obesity in adult women related to metabolic complications. Korean J Fam Med. 2007;28:675–681. [Google Scholar]
- 7.Despres JP, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28:1039–1049. doi: 10.1161/ATVBAHA.107.159228. [DOI] [PubMed] [Google Scholar]
- 8.Bandekar AN, et al: “Performance evaluation of abdominal fat burden quantification in CT.” Engineering in Medicine and Biology Society, 2005. IEEE-EMBS 2005. 27th Annual International Conference of the, 2005, pp. 3280–3283 [DOI] [PubMed]
- 9.Bandekar AN et al: “Automated pericardial fat quantification in CT data,” Engineering in Medicine and Biology Society, 2006. EMBS '06. 28th Annual International Conference of the IEEE, 2006, pp. 932–935 [DOI] [PubMed]
- 10.Liou T, et al. Fully automated large-scale assessment of visceral and subcutaneous abdominal adipose tissue by magnetic resonance imaging. Int J Obes. 2006;30:844–852. doi: 10.1038/sj.ijo.0803216. [DOI] [PubMed] [Google Scholar]
- 11.Pednekar A, et al: “Automatic segmentation of abdominal fat from CT data.” In: Application of Computer Vision, 2005. WACV/MOTIONS '05 Volume 1. Seventh IEEE Workshops on, 2005, pp. 308–315
- 12.Romero D, et al: “Quantification of subcutaneous and visceral adipose tissue using CT.” In: Medical Measurement and Applications, 2006. MeMea 2006. IEEE International Workshop on, 2006, pp. 128–133
- 13.Zhao B, et al. Automated quantification of body fat distribution on volumetric computed tomography. J Comput Assist Tomogr. 2006;30:777. doi: 10.1097/01.rct.0000228164.08968.e8. [DOI] [PubMed] [Google Scholar]
- 14.Kim SH, et al: "Body fat thresholds in computed tomography image processing." Korean Information Science Society, 1998. 25th Fall Conference of the, 1998, pp. 438–440
- 15.Kvist H, et al. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 16.Yoshizumi T, et al. Abdominal fat: standardized technique for measurement at CT1. Radiology. 1999;211:283. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]