Abstract
OBJECTIVES
The pleural membrane of the lower pleural cavity has a greater ability to recycle fluid than the pleural membrane of the upper pleural cavity. During lobectomy, the visceral pleura is removed with the lobe, whereas the parietal pleura is traumatized during manipulation. This study investigates variations of the drainage according to the type of lobectomy and its relation to effusion-related complications.
METHODS
Data of upper and lower lobectomy patients were compared with those of wedge resection patients. All patients were suctioned until totally dry before closure, and one chest tube was left in the hemithorax. The amount of fluid drained per day, the duration of drainage, the length of hospital stay and the morbidity were noted. Student's paired t-test and Mann–Whitney U-test were used for comparison; P < 0.05 was defined as statistically significant.
RESULTS
Patients after lower lobectomy had more fluid drained when compared with patients after upper lobectomy or wedge resection on the first (636 ± 90, 268 ± 75 and 225 ± 62 ml, respectively; P = 0.002) and second postoperative day (464 ± 94, 237 ± 90 and 220 ± 62 ml, respectively; P = 0.046). The drainage tube was removed earlier in patients with upper lobectomy procedures than in patients with lower lobectomy procedures (4.6 ± 0.9 vs 8.1 ± 1.4 days; P = 0.014). Effusion-related complications developed in lower lobectomies with a higher output from the second postoperative day.
CONCLUSIONS
A larger amount of fluid is drained after removal of the lower lobes, possibly because the important fluid-recycling ability of the lower parts of the cavity is malfunctioning. Early drainage tube removal after lower lobectomy may be reconsidered when taking into account the possibility of effusion-related complications.
Keywords: Lobectomy, Drainage, Effusion, Complications
INTRODUCTION
The lower parts of the pleural cavity have been shown to play a greater role in the recycling of pleural fluid, a phenomenon that is attributed to the ability of mesothelial cells to transport water and electrolytes via cellular transporters [1, 2] and to the more pronouced presence of the lymphatic network on the lower lobe visceral pleura and the stomata diaphragmatic parietal pleura [3–5]. This ability is hindered by surgical trauma and manipulations carried out within the pleural cavity [6, 7]. During lobectomies, the lobe is removed along with the covering visceral pleura and inevitably, the relevant cavity parietal pleura, with its lymphatic drainage, is traumatized during procedural manipulations. It may be assumed that the important postoperative fluid recycling ability varies after upper lobectomy compared with lower lobectomy and, therefore, postoperative drainage may be different. This finding may be important to identify what type of lobectomy may present a predisposition for effusion-related complications in an era with a trend towards early chest drainage removal [8, 9].
This study investigates the differential in postoperative fluid drainage appearing in upper and lower lobectomies and their relation with effusion-related postoperative complications.
PATIENTS AND METHODS
The data of consecutive patients subjected to upper or lower lobectomy over a period of 3 months were analysed. These data included, apart from the demographic details of the patients, the side of hemithorax lobectomy, the amount of fluid drained per day, the duration of drainage (chest tube removal), morbidity, mortality and the duration of stay in hospital. The postoperative outcome of lobectomy patients was compared with the outcome of patients undergoing wedge resection via mini-thoracotomy for biopsy reasons as an indicator of minimal visceral pleura excision and parietal pleura trauma. All patients participating in the study provided a signed consent form.
All patients were suctioned until totally dry before closure, and one chest tube was left in the hemithorax, positioned posteriorly and apically (according to our institute's practice). Patients with suspicion of postoperative haemorrhage or air leak >5 days were not included in the present study [10]. The chest tube was left in suction immediately postoperatively in all cases for the first 2 days and under water seal until no air leak could be observed, and it was removed when fluid (not turbid) <200 ml per day was drained (according to our institute's practice). The patients were all discharged on the day following the removal of the chest tube, unless indicated otherwise.
Statistical analysis was performed with the SPSS version 10.0 for Windows (SPSS Inc., Chicago, IL, USA). Student's paired t-test was used for the comparison of the means of numerical data, whereas ANOVA (with Bonferroni post hoc test) was used for comparison between groups. The non-parametric Mann–Whitney U-test was used for comparison of the categorical data.
RESULTS
During the period of study, 38 lobectomy cases in total fulfilled the inclusion criteria, of which 20 were upper and 18 lower lobectomies. The mean age was 69.8 ± 9.5 years old, and 20 patients (52.6%) were males. The mean age of the 15 wedge resection patients included in the study was 68.2 ± 7.9 years, seven patients (46.6%) were males, and most cases involved metastatic tumours. The demographics of patients who underwent upper and lower lobectomies as well as wedge resections are shown in Table 1.
Table 1:
Demographics of n = 53 lobectomy and wedge resection patients
| Characteristic | Upper (n = 20) | Lower (n = 18) | Wedge (n = 15) |
|---|---|---|---|
| Age (years) | 71.4 ± 8.5 | 69.8 ± 10.5 | 68.2 ± 7.9 |
| Males | 11 (55%) | 9 (50%) | 7 (46.6%) |
| Comorbidities | 18 (90%) | 16 (88.8%) | 12 (80%) |
| Malignancy | 12 (80%) | ||
| Squamous | 18 (90%) | 17 (94.4%) | – |
| Adenocarcinoma | 1 (5%) | 1 (5.5%) | – |
| Other | 1 (5%) | – | – |
| Benign | – | – | 3 (20%) |
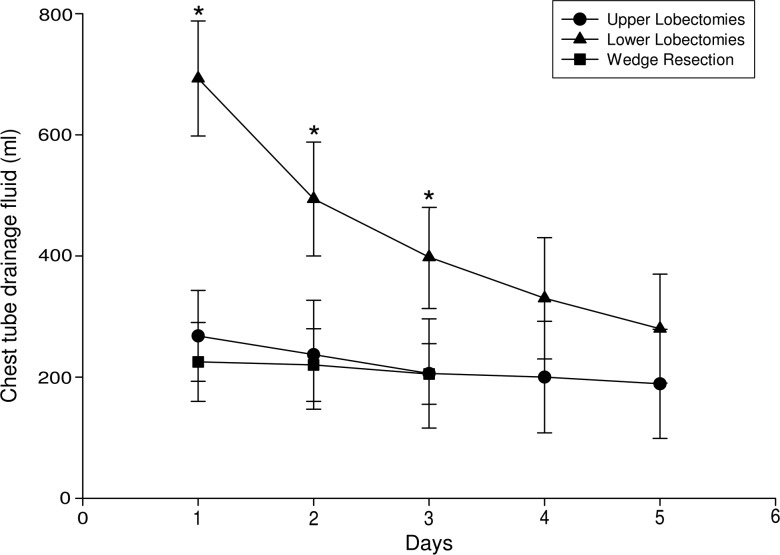
Patients recovering from lower lobectomy procedures presented a larger volume of fluid drainage (636 ± 93 ml) when compared with patients who underwent upper lobectomy (268 ± 75 ml; P = 0.002) and wedge resection (225 ± 62 ml; P = 0.002) on the first postoperative day (Fig. 1). The same observation was also made for the second postoperative day [494 ± 94 ml for lower vs 237 ± 90 ml for upper lobectomy (P = 0.046) and vs 220 ± 62 ml for wedge resection patients (P = 0.048); Fig. 1] as well as the third postoperative day [398 ± 85 ml for lower vs 206 ± 90 ml for upper lobectomy (P = 0.023) and vs 205 ± 52 ml for wedge resection patients (P = 0.028); Fig. 1].
Figure 1:
Volume of postoperative fluid drainage per day in upper (n = 20) and lower lobectomies (n = 18) and in wedge resections (n = 15). Values are expressed as the mean drainage (in millilitres) ± SD; *P < 0.05 for lower when compared with upper lobectomies and wedge resections.
Patients who underwent right lower lobectomies (n = 11) had a tendency to have a larger volume of fluid drainage in comparison with the volume of fluid collected from left lower lobectomy patients (n = 7) on the first postoperative day (508 ± 65 vs 662 ± 75 ml, respectively; P = 0.058). In right and left upper lobectomy patients, the fluid drainage postoperatively was of a similar volume (250 ± 95 vs 287 ± 82 ml, respectively; P = 0.933).
Complications were noted in two of the cases of upper and seven of the cases of lower lobectomy; in the two cases of upper and another two cases of lower lobectomy, atrial fibrillation developed and required treatment. In the remaining five lower lobectomy cases (27.7%), effusion-related complications developed that necessitated antibiotic treatment and prolonged drainage, which led in one case to loculation formation and computed tomography (CT)-guided drainage (Table 2). The effusion-related complications were noted after the third postoperative day (Table 2).
Table 2:
Details of n = 5 patients with effusion-related complications
| Gender | Males | 3 (0.6%) |
| Age (years) | 70.2 ± 9.6 | |
| Malignancy | Squamous | 4 (80%) |
| Adenocarcinoma | 1 (20%) | |
| Comorbidities | Diabetes mellitus | 1 (20%) |
| Chronic obstructive pulmonary disease | 3 (60%) | |
| Hypertension | 3 (60%) | |
| Main findings | Pyrexia (no other causes) | 5 (100%) |
| Turbid drainage fluid | 5 (100%) | |
| Days after surgery (mean ± SD) | Development of effusion-related complication | 3.2 ± 1.0 |
| Radiographic findings | Effusion | 4 (80%) |
| Consolidation | 1 (20%) | |
| Loculations | 2 (40%) | |
| Thoracic CT performed | Postoperative evaluation | 2 (40%) |
| Effusion culture | Positive | 5 (100%) |
| Treatment | Suctioned drainage | 5 (100%) |
| Antibiotics | 5 (100%) | |
| Repositioning of chest tube | 1 (20%) | |
| CT-guided drainage | 1 (20%) |
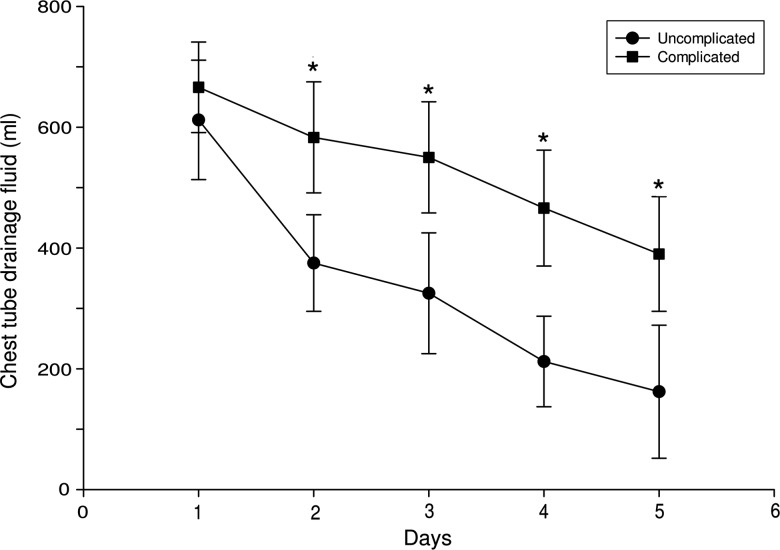
In lower lobectomies, the volume of drainage in effusion-complicated cases was greater than in uncomplicated cases from the second postoperative day (583 ± 102 vs 375 ± 95 ml, respectively; P = 0.035; Fig. 2). This greater drainage was maintained until at least the fifth postoperative day (Fig. 2).
Figure 2:
Volume of postoperative fluid drainage per day in lower lobectomy effusion-related complicated (n = 5) and uncomplicated cases (n = 13). Values are expressed as the mean drainage (in millilitres) ± SD; *P < 0.05 for the complicated when compared with the uncomplicated cases.
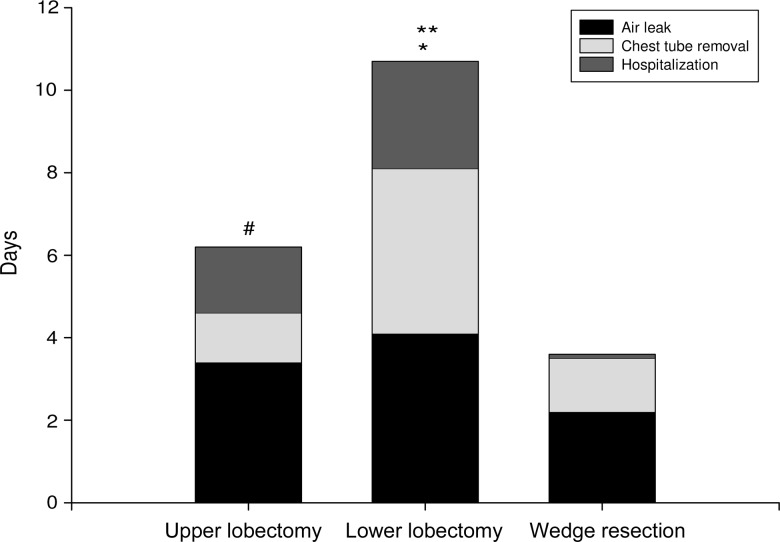
For both upper and lower lobectomy patients, the air leak ceased within the same number of postoperative days (3.4 ± 1.1 vs 4.1 ± 1.2 days, respectively; P = 0.689; Fig. 3). The chest tube was removed after 4.6 ± 0.9 days for upper and 8.1 ± 1.4 days for lower lobectomy patients (P = 0.014; Fig. 3). In the wedge resection group, the air leak ceased after 2.2 ± 1.2 days (P = 0.07 vs upper and P = 0.058 vs lower lobectomy patients), while the chest tube was removed after 3.5 ± 1.3 days (P = 0.045 vs upper and P = 0.002 vs lower lobectomy patients; Fig. 3). The duration of chest tube drainage was more prolonged for effusion-complicated cases than for uncomplicated cases (10.67 ± 0.9 vs 6.25 ± 0.9 days, respectively; P = 0.008).
Figure 3:
Duration of air leak, chest tube drainage and hospitalization in upper (n = 20) and lower lobectomies (n = 18) and in wedge resections (n = 15). Values are expressed as the mean duration (in days) ± SD; *P < 0.05 for drainage duration between lower vs upper lobectomies and wedge resections; **P < 0.05 for duration of hospitalization between lower vs upper lobectomies and wedge resections; and #P < 0.05 for drainage duration and hospitalization between upper lobectomies vs wedge resections.
The duration of hospitalization was shorter for upper lobectomy than for lower lobectomy patients (6.1 ± 0.9 vs 10.43 ± 1.3 days; P = 0.032; Fig. 3) and the duration of hospital stay for wedge lobectomy patients was 3.50 ± 1.3 days (P = 0.026 vs upper and P = 0.008 vs lower lobectomy patients; Fig. 3). Lower lobectomy effusion-complicated cases remained in hospital for longer than uncomplicated cases (15 ± 0.9 vs 7 ± 0.8 days, respectively; P = 0.001).
No mortality was noted in upper or lower lobectomy or wedge resection cases.
The follow-up of all upper lobectomy patients was unremarkable, in contrast to follow-up for lower lobectomy patients, in whom recurrent effusion occurred in two cases, necessitating repeated thoracocentesis and aspiration but without the need for readmission.
DISCUSSION
The main finding of the present study is that a greater amount of fluid is drained postoperatively after lower lobectomies, and especially after right lower lobectomies, compared with the volume collected after upper ones, indicating that lower lobectomy significantly hinders the ability of the lower pleural parts to redistribute fluid. Cases of effusion-related complication developed only after lower lobectomies, in which there appeared to be a larger volume of drainage after the second postoperative day, when compared with uncomplicated lower lobectomies. Lower lobectomy patients required more prolonged chest tube drainage and were hospitalized for a longer period, especially in cases of effusion-related complication. It is suggested that in lower lobectomies a prolonged high drainage output after the second postoperative day may be associated with effusion-related complications and therefore an early removal of the inserted chest tube should be reconsidered.
The mesothelial cells of the visceral and parietal pleura have the ability to transport fluid and electrolytes [7, 11, 12]. This ability is considered to be an additional mechanism in pleural fluid recycling, operating continuously in the normal pleural cavity [7, 13]. It has been shown to be more pronounced in the mesothelium over the visceral pleura of the lower lobe and over the parietal pleura of the caudal and diaphragmatic regions, where the lymphatic network and the stomata are more abundant [1–8]. Additionally, in order for this transportation ability of the mesothelial cells to be operational, an intact, unharmed and functional mesothelium is necessary [3, 4]. During surgery, the mesothelium is traumatized in different ways, leading to the impairment of its transportation ability to an extent dependent on the site of surgical trauma, i.e. the lower parts of the pleural cavity and the diaphragm may be more traumatized during a lower lobectomy rather than during an upper lobectomy.
From all the above discussion, it can be assumed that in cases of surgery interfering with the lower pleural cavity parts, as in lower lobectomies, the relevant pleura is traumatized (during manipulations) and removed (the visceral part with the lobe), leading to loss of the main recycling potential of the mesothelium (which is largely performed by the lower pleural cavity parts). The ability to redistribute the postoperative fluids is therefore lost, resulting in accumulation of fluid, which is eventually drained through the chest tube. This finding should be considered by surgeons when choosing early drainage removal or the number of chest tubes inserted [8, 9, 14]. Additionally, the volume of fluid drained in right lower lobectomies had a tendency to be greater than in left lower lobectomies. This finding may be explained by the presence of the heart in the left hemithorax, which diminishes the area of the pleural fluid recycling, or because in general the right-sided lobectomies are considered more laborious and consequently, the pleural surfaces are traumatized to a greater extent; however, this observation requires further clarification.
The time of removal of the chest tube, after air leakage has ceased, has been a subject of argument between thoracic surgeons [8, 9]. This argument is intensified by the lack of specific guidelines concerning the safe timing of chest tube removal [8]; the more conservative surgeons do not remove the chest tube earlier than with a daily drainage of 200–250 ml, fearing effusion complications, whereas others remove the chest tube immediately after the air leakage has ceased, regardless of the amount of drainage per day, even in cases with high-output drainage. It is considered that in video asisted thoracic surgery (VATS) wedge procedures, a chest tube may even not be needed [15, 16]. The removal of drains with high fluid output is considered by some authors to be a safe practice in well-selected patients and the rate of readmissions due to effusions is acceptably low [8, 9]. Conversely, a lower lobe lesion and the number of segments removed were correlated with increased morbidity and higher complications in thoracoscopic lobectomy patients, whereas the duration of drainage was not [9]. However, no information exists about possible differences of the morbidity outcomes mentioned above according to the type or site of lobectomy. In our study, all effusion-related complications developed after the third postoperative day following lower lobectomies that retained a high output after the second postoperative day. This study, therefore, suggests that the type of lobectomy should be considered when early removal of the drainage is planned, because after lower lobectomies the drainage is significantly greater and effusion complications develop more frequently, suggesting that the fear of effusion-related complications because of fluid entrapment may be valid.
Of course, it must be noted that an increased fluid output after lower lobectomies does not mean that more effusion-related complications appear and, despite the fact that the chest tube was not removed early, development of effusion-related complications was not avoided in some patients. A high fluid output may indicate non-functional pleura, which is unable to redistribute postoperative fluids and consequently, early chest tube removal may predispose to fluid retention and effusion-related complications. An early removal of the chest tube, according to the present study, may be suggested after intraoperative care of the pleural surfaces, consideration of the site of lobectomy (preferable in upper lobectomies) and when a decreasing volume of drainage is measured postoperatively (not if there is prolonged high output) [17].
The main limitation of the present study is that the number of patients included in the study was relatively small, because its main purpose was to investigate the volume of drainage after each type of lobectomy. More research will be needed in the future to clarify possible relations of the type of lobectomy (and VATS) with the number of effusion-related complications developing after surgery. Additionally, more research is needed concerning the positioning of the tube (i.e. posterior vs anterior or basal vs apical), the laterality of the operated hemithorax (left vs right) and other factors in the variation of postoperative drainage of the pleural fluid.
In conclusion, drainage after lower lobectomies is greater, necessitating a longer duration of drainage and possibly longer hospitalization, and is possibly associated with more effusion-related complications. This event could be attributed to the fact that the normal process of electrolyte and fluid transportation of the mesothelium and the lymphatics functioning over the lower pleural cavity parts is hindered because of the removal of lower lobe visceral pleura and trauma induced by the surgical manipulations of the lower cavity pleura. All these findings should be considered when early removal of lower lobectomy chest drainage is planned.
Conflict of interest: none declared.
REFERENCES
- 1.Kouritas VK, Hatzoglou CH, Foroulis CN, Gourgoulianis KI. Human parietal pleura present electrophysiology variations according to location in pleural cavity. Interact CardioVasc Thorac Surg. 2008;7:544–7. doi: 10.1510/icvts.2007.172007. [DOI] [PubMed] [Google Scholar]
- 2.Kouritas VK, Hatzoglou C, Gourgoulianis KI, Molyvdas PA. Pleural electrophysiology variations according to location in pleural cavity. Interact CardioVasc Thorac Surg. 2009;9:391–4. doi: 10.1510/icvts.2009.203356. [DOI] [PubMed] [Google Scholar]
- 3.Wang NS. The regional difference of pleural mesothelial cells in rabbits. Am Rev Respir Dis. 1974;110:623–33. doi: 10.1164/arrd.1974.110.5.623. [DOI] [PubMed] [Google Scholar]
- 4.Wang NS. The preformed stomas connecting the pleural cavity and the lymphatics in the parietal pleura. Am Rev Respir Dis. 1975;111:12–20. doi: 10.1164/arrd.1975.111.1.12. [DOI] [PubMed] [Google Scholar]
- 5.Lai-Fook SJ. Pleural mechanics and fluid exchange. Physiol Rev. 2004;84:385–410. doi: 10.1152/physrev.00026.2003. [DOI] [PubMed] [Google Scholar]
- 6.Kouritas VK, Tepetes K, Christodoulides G, Ioannou M, Spyridakis M, Gourgoulianis KI, et al. Permeability alterations after surgical trauma in normal rabbit peritoneum. Eur Surg Res. 2010;45:113–9. doi: 10.1159/000318146. [DOI] [PubMed] [Google Scholar]
- 7.Kouritas VK, Foroulis CN, Ioannou M, Kalafati G, Tsilimingas N, Gourgoulianis KI, et al. Pleural electrophysiology alterations in spontaneous pneumothorax patients. Interact CardioVasc Thorac Surg. 2010;10:958–61. doi: 10.1510/icvts.2009.214262. [DOI] [PubMed] [Google Scholar]
- 8.Cerfolio RJ, Bryant AS. Results of a prospective algorithm to remove chest tubes after pulmonary resection with high output. J Thorac Cardiovasc Surg. 2008;135:269–73. doi: 10.1016/j.jtcvs.2007.08.066. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi R, Fujino Y, Yamashita T, Oka S. A prospective study of the association between drainage volume within 24 h after thoracoscopic lobectomy and postoperative morbidity. J Thorac Cardiovasc Surg. 2009;137:1394–9. doi: 10.1016/j.jtcvs.2008.10.035. [DOI] [PubMed] [Google Scholar]
- 10.Kouritas VK, Hatzoglou C, Foroulis CN, Hevas A, Gourgoulianis KI, Molyvdas PA. Low glucose level and low pH alter the electrochemical function of human parietal pleura. Eur Respir J. 2007;30:354–7. doi: 10.1183/09031936.00047106. [DOI] [PubMed] [Google Scholar]
- 11.Hatzoglou CH, Gourgoulianis KI, Molyvdas PA. Effects of SNP, ouabain and amiloride on electrical potential profile of isolated sheep pleura. J Appl Physiol. 2001;90:1565–9. doi: 10.1152/jappl.2001.90.4.1565. [DOI] [PubMed] [Google Scholar]
- 12.Sarkos S, Hatzoglou CH, Dahabre J, Gourgoulianis KI, Molyvdas PA. Effect of amiloride in human and sheep parietal pleura. Respir Physiol. 2002;132:233–7. doi: 10.1016/s1569-9048(02)00077-0. [DOI] [PubMed] [Google Scholar]
- 13.Zocchi L, Agostoni E, Cremaschi D. Electrolyte transport across the pleura of rabbits. Respir Physiol Neurobiol. 1991;86:125–38. doi: 10.1016/0034-5687(91)90044-j. [DOI] [PubMed] [Google Scholar]
- 14.Okur E, Baysungur V, Tezel C, Sevilgen G, Ergene G, Gokce M, et al. Comparison of the single or double chest tube applications after pulmonary lobectomies. Eur J Cardiothorac Surg. 2009;35:32–5. doi: 10.1016/j.ejcts.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 15.Watanabe A, Watanabe T, Ohsawa H, Mawatari T, Ichimiya Y, Takahashi N, et al. Avoiding chest tube placement after video-assisted thoracoscopic wedge resection of the lung. Eur J Cardiothorac Surg. 2004;25:872–6. doi: 10.1016/j.ejcts.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 16.Russo L, Wiechmann RJ, Magovern JA, Szydlowski GW, Mack MJ, Naunheim KS, et al. Early chest tube removal after video-assisted thoracoscopic wedge resection of the lung. Ann Thorac Surg. 1998;66:1751–4. doi: 10.1016/s0003-4975(98)00946-1. [DOI] [PubMed] [Google Scholar]
- 17.Cerfolio RJ, Bryant AS. The management of chest tubes after pulmonary resection. Thorac Surg Clin. 2010;20:299–405. doi: 10.1016/j.thorsurg.2010.04.001. [DOI] [PubMed] [Google Scholar]