Abstract
Oesophagopleural fistula is a recognized complication of pneumonectomy. Surgical repair with direct closure and reinforcement with omentum, muscle or pleural flap is the mainstay of definitive treatment. However, this option might not be suitable for all patients. The authors report on the case of a 63-year old female refusing surgical repair of an oesophagopleural fistula after left pneumonectomy. A novel approach (using an Amplatzer atrial septal occluder device) for fistula closure was attempted. Despite a promising initial result, the procedure failed. This is the first report on the use of a septal occluder to try and close an oesophagopleural fistula.
Keywords: Oesophagopleural fistula, Pneumonectomy, Oesophagus, Pleura
INTRODUCTION
An oesophagopleural fistula is a dreaded complication of pneumonectomy with an incidence of 0.2–1% [1, 2]. The initial experience in the treatment of this complication carried a mortality between 49 and 63%. The mortality was the highest in the group of patients treated with extensive surgery (including oesophagectomy and gastric or colonic interposition) or the most conservative approach (chest drainage and gastrostomy) [1, 2]. The following surgical reports on direct closure and reinforcement with omentum, intercostal muscle flap or pleural flap significantly improved the outcome of the patients with a success rate of 86% and became the mainstay of treatment as demonstrated in a literature review by Sethi and Takaro [3]. However, these procedures still represent significant surgical interventions and would not be suitable for fragile patients or for those refusing surgery. An alternative, less-invasive treatment for these groups of patients is required.
CASE REPORT
A 63-year old female with history of hiatal hernia and gastro-oesophageal reflux was diagnosed with bronchopulmonary carcinoid extending from the medial aspect of the distal left main bronchus to the left lower lobe bronchus and adjacent lower lobe lung parenchyma (cT2aN0M0). She underwent a left pneumonectomy after attempted left lower lobe sleeve resection (reverse sleeve), which was unsuccessful due to kinking of the airway (a recognized problem with reverse sleeve resections).
The left main bronchus stump was stapled and cut flush with the carina, whereby the stapled left main bronchus stump disappeared behind the aortic arch, thus preventing the need to cover and reinforce the stump. The patient had an uneventful postoperative course and was discharged on the sixth postoperative day.
She presented with a low-grade temperature, nausea and diarrhoea 3 weeks later. Her clinical examination was unremarkable, and the chest X-ray showed a decreased air–fluid level in the post-pneumonectomy space. The computed tomography (CT) scan revealed a 10-mm defect in the distal oesophagus communicating with the left pneumonectomy space (Fig. 1A). A contrast study confirmed these findings. A left thoracic window with resection of the fifth, sixth and seventh ribs and Eloesser flap was performed to drain the empyema, and the cavity was irrigated three times daily with 1 l of warm saline. A nasojejunal feeding tube was inserted to optimize the caloric intake. The surgical options to close the fistula and post-pneumonectomy space were discussed and refused by the patient. A 3-month follow-up CT scan revealed a significantly reduced post-pneumonectomy cavity, but unchanged size of the fistula (Fig. 1B). The edges of the fistula fully epithelialized as seen on thoracoscopy and oesophagoscopy (Fig. 1C). A new conservative approach using a 12-mm Amplatzer septal occluder (AGA Medical Corp., Plymouth, MN, USA) was proposed. Institutional Review Board approval was obtained. The patient was informed and counselled about the untested use of the occluder for closure of the oesophagopleural fistula and its possible complications.
Figure 1:
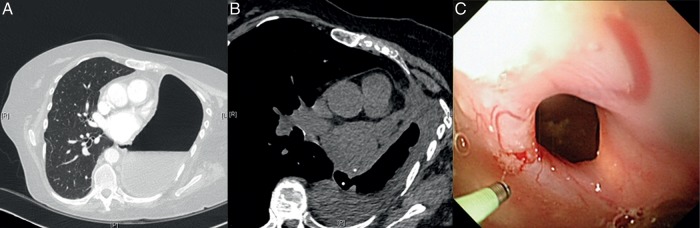
(A) CT scan 3 weeks after surgery showed a 10-mm oesophageal defect communicating with the post-pneumonectomy space. (B) At four-month follow-up, CT scan illustrated a decrease in size of the cavity, but the size of the fistula remained unchanged. (C) Thoracoscopy confirmed fully epithelialized edges of the fistula.
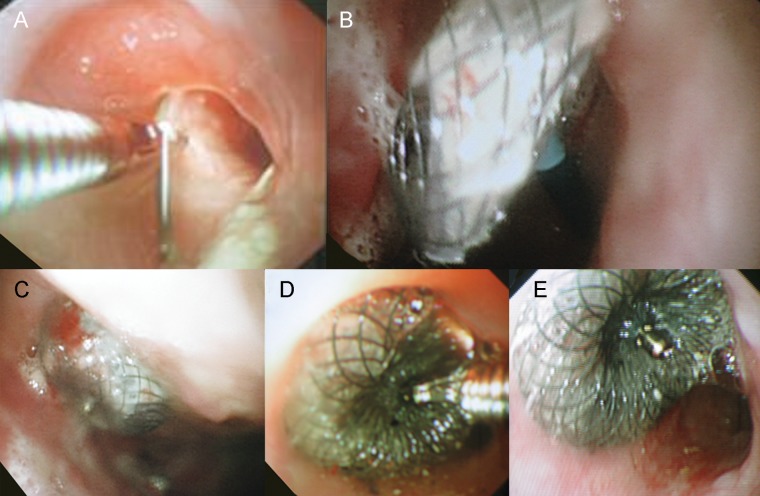
The occluder was inserted using a dual approach via a thoracic window and oesophagus under general anaesthesia. The guide wire was introduced via the fistula using a flexible endoscope in the thoracic window. The second flexible endoscope in the oesophagus helped to further advance the guide wire using an endoscopic grasper (Fig. 2A). The occluder mounted on the delivery system was introduced from the oesophageal side using a Seldinger technique. The large disc of the occluder was deployed in the chest cavity, and the waist of the device was withdrawn into the fistula (Fig. 2B and C). Finally, the smaller disk was deployed in the oesophagus, and the occluder disconnected from the delivery system (Fig. 2D). The satisfactory position of the occluder was confirmed by thoracoscopy, oesophagoscopy and subsequent chest X-ray (Fig. 2E). Discharge from the fistula stopped immediately. An oral intake was resumed by means of increasing fluid intake with the aim of recommencing normal diet after epithelization of the occluder. Unfortunately, the patient reported discharge from the thoracic window 12 days later and oesophagoscopy revealed a tilted, dislodged occluder with an increased size of the fistula. The occluder was therefore removed using biopsy forceps. The injury to the fistula and irritation by nitinol initiated granulation growth and the defect transiently decreased in size to 2 mm. Unfortunately, the defect regained its original size with fully epithelized edges in another 6 months, and the patient finally agreed to surgical closure. The fistula and cavity were repaired by means of an omentoplasty via median laparotomy and previous thoracotomy approach.
Figure 2:
Closure of oesophagopleural fistula with the Amplatzer atrial septal occluder. (A) The guide wire was introduced via a thoracic window and oesophagopleural fistula using a flexible endoscope, and picked up with a grasper via another endoscope in the oesophagus, pulling the wire through and out the oral cavity. (B) The larger disk of the Amplatzer device was deployed in the post-pneumonectomy space. (C) The position of the larger disk was satisfactory and the connector of the disks was consequently seated in the fistula. (D) The smaller disk was deployed in the oesophagus. (E) The well-seated occluder was disconnected from the delivery system. The diameter of oesophageal lumen was not compromised.
A 3-month follow-up barium swallow test confirmed a small oesophageal sinus at the level of previous fistula and full oral intake was commenced. A 9-month clinical follow-up was unremarkable.
DISCUSSION
The conservative approach for the treatment of post-pneumonectomy oesophagopleural fistula with open drainage of the empyema and prolonged nasogastric feeding has been proven feasible in 5 of 7 treated patients reported by Shama and Odell [4]. They suggested that control of the empyema in the post-pneumonectomy space by open drainage and regular irrigations together with prolonged nasogastric feeding led to the closure of the fistula. The conservative treatment as described by Shama and Odell failed in our patient. We believe that the oesophagopleural fistula in our case developed as a consequence of dissection and oesophageal devascularization during attempted sleeve lobectomy. The ischaemic oesophageal defect and severe gastro-oesophageal reflux prevented complete healing of the fistula, and the edges fully epithelialized without any change of the 10-mm size fistula on repeated oesophagoscopies.
Several minimally invasive approaches have been described in the literature for closure of oesophagopleural fistula: insertion of stents, endoscopic clipping, endoscopic suturing and closure with Amplatzer vascular plugs/coils [5–8]. The stents in benign oesophageal diseases are used mainly for postoperative anastomotic leaks, fistulas and oesophageal stenoses. In these cases, the lumen (and motility) of the oesophagus is decreased, reducing the chance for migration of the stent. Tissue ingrowth and subsequent removal of the stent might also be problematic [5]. Moreover, oesophageal stent insertion is not recommended in patients after previous pneumonectomy due to risk of acute compromise of major airways, which are shifted towards the post-pneumonectomy space [9]. Endoscopic clipping and suturing are theoretically attractive alternatives to major surgery, but these techniques are more suitable in small or linear tears, and they cannot be used in ischaemic or inflamed lesions due to tissue fragility issues [6, 7]. The only report on successful closure of an oesophagopleural fistula by Amplatzer vascular plugs and coils dealt with different pathology, i.e. a fistula after oesophageal diverticulectomy without lung resection [8]. None of the above-mentioned procedures was suitable in our case. A new idea of using the Amplatzer septal occluder for the closure of the fistula seemed to be a promising option.
The Amplatzer septal occluder is made of nitinol mesh with two disks joined together with a central connector and polyester fabric within the mesh. It was originally developed for closure of atrial septum defects. The fabric within the device facilitates occlusion and tissue ingrowth, culminating in full epithelization of the device by endocardium within months after insertion. The extracardiac use of this device (or its modifications) has already found its place in general thoracic surgery in the treatment of bronchopleural fistulas with the largest series published by Fruchter et al. [10] reporting on 10 consecutive patients treated with occluders with a closure success rate of 90%. Cases of the closure of oesophagorespiratory fistulae are rare [11]. There is no publication on the use of this device in oesophagopleural fistula. We assume that this could be due to unpublished negative results or possibly because it has never been used in this clinical setting before.
This is the first report on the use of a septal occluder in an attempt to close the oesophagopleural fistula after pneumonectomy. It was expected that the central connector would close the oesophagopleural fistula and the disks would prevent device migration. It was also assumed that the malleable disks and their large contact area with the oesophagus would be suitable even for ischaemic edges of oesophagopleural fistula. The tissue ingrowth and finally disks epithelization would not necessitate the removal of the device and would not impact on oesophageal luminal size and motility. Although initially promising, this attempt finally failed. We assume that this could be due to unfavourable patient selection (severe gastro-oesophageal reflux and ischaemic oesophageal defect) and/or unsuitability of this technique due to non-uniform thickness of the margins of the fistula and thus, potential uneven pressure of the disks on the edges. For the above-mentioned reasons, we cannot recommend the use of atrial septal occluders for closure of post-pneumonectomy oesophagopleural fistulas.
Conflict of interest: none declared.
REFERENCES
- 1.Takarao T, Walkup HE, Okano T. Esophagopleural fistula as a complication of thoracic surgery. A collective review. J Thorac Cardiovasc Surg. 1960;40:179–93. [PubMed] [Google Scholar]
- 2.Evans JP. Post-pneumonectomy oesophageal fistula. Thorax. 1972;27:674–7. doi: 10.1136/thx.27.6.674. doi:10.1136/thx.27.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sethi GK, Takaro T. Esophagopleural fistula following pulmonary resection. Ann Thorac Surg. 1978;25:74–81. doi: 10.1016/s0003-4975(10)63492-3. doi:10.1016/S0003-4975(10)63492-3. [DOI] [PubMed] [Google Scholar]
- 4.Shama DM, Odell JA. Esophagopleural fistula after pneumonectomy for inflammatory disease. J Thorac Cardiovasc Surg. 1985;89:77–81. [PubMed] [Google Scholar]
- 5.Van Boeckel PGA, Sijbring A, Vieggaar FP, Siersema PD. Systematic review: temporary stent placement for benign rupture or anastomotic leak of the oesophagus. Aliment Pharmacol Ther. 2011;33:1292–301. doi: 10.1111/j.1365-2036.2011.04663.x. doi:10.1111/j.1365-2036.2011.04663.x. [DOI] [PubMed] [Google Scholar]
- 6.Sandmann M, Heike M, Faehndrich M. Application of the OTSC system for the closure of fistulas, anastomosal leakages and perforations within the gastrointestinal tract. Z Gastroenterol. 2011;49:981–5. doi: 10.1055/s-0029-1245972. doi:10.1055/s-0029-1245972. [DOI] [PubMed] [Google Scholar]
- 7.Bonin EA, Wong Kee Song LM, Gostout ZS, Bingener J, Gostout CJ. Closure of a persistent esophagopleural fistula assisted by a novel endoscopic suturing system. Endoscopy. 2012;44:E8. doi: 10.1055/s-0031-1291494. [DOI] [PubMed] [Google Scholar]
- 8.Koo JH, Park KB, Choo SW, Kim K, Do YS. Embolization of postsurgical esophagopleural fistula with AMPLATZER vascular plug, coils, and Histoacryl glue. J Vasc Interv Radiol. 2010;21:1905–10. doi: 10.1016/j.jvir.2010.09.004. doi:10.1016/j.jvir.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Farivar AS, Vallières E, Kowdley KV, Wood DE, Mulligan MS. Airway obstruction complicating esophageal stent placement in two post-pneumonectomy patients. Ann Thorac Surg. 2004;78:22–3. doi: 10.1016/j.athoracsur.2003.09.118. doi:10.1016/j.athoracsur.2003.09.118. [DOI] [PubMed] [Google Scholar]
- 10.Fruchter O, Kramer MR, Dagan T, Raviv Y, Abdel-Rahman N, Saute M, et al. Endobronchial closure of bronchopleural fistulae using Amplatzer devices: our experience and literature review. Chest. 2011;139:682–7. doi: 10.1378/chest.10-1528. doi:10.1378/chest.10-1528. [DOI] [PubMed] [Google Scholar]
- 11.Repici A, Presbitero P, Carlino A, Strangio G, Rando G, Pagano N, et al. First human case of esophagus-tracheal fistula closure by using a cardiac septal occluder (with video) Gastrointest Endosc. 2010;71:867–9. doi: 10.1016/j.gie.2009.08.036. doi:10.1016/j.gie.2009.08.036. [DOI] [PubMed] [Google Scholar]