Abstract
OBJECTIVES
To evaluate thoracic endovascular aortic repair (TEVAR) as emergency therapy despite suspected aortic infection.
METHODS
Within a 5-year period, we treated 6 patients with a strategy of primary TEVAR despite suspected aortic infection in patients with symptomatic or already ruptured thoracic aortic pathology.
RESULTS
In-hospital mortality was 16.7%. The reason for death was septic multiorgan failure. During follow-up, 2 patients were converted to secondary open surgery in a stable elective setting. The median follow-up was 42.5 months. All surviving patients are not receiving continuing antibiotic therapy. Freedom from infection is 100% to date.
CONCLUSIONS
TEVAR as emergency therapy despite suspected aortic infection is feasible and may well serve as a definite treatment option in selected cases. As recurring infection cannot be entirely excluded, life-long clinical and morphological surveillance remains mandatory.
Keywords: Thoracic aortic endovascular repair, Aortic infection, Secondary open surgery
INTRODUCTION
The entity of native aortic infection is fortunately rare, representing 2.6% of all aortic pathology, with the thoracic aorta being the least common site of occurrence [1]. The underlying pathology in primary infections—nearly without exception—is a disruption of intimal integrity and thereby bacteriemic seeding [2]. The standard treatment in infected aortic pathology consists of antibiotic therapy, open surgical debridement and restoration of vascular continuity [3–5]. This procedure in an apparently very frail subset of patients is still associated with an aggregate mortality and morbidity rate of up to 43% [6].
The aim of this study was to evaluate thoracic endovascular aortic repair (TEVAR) as emergency therapy despite suspected aortic infection.
PATIENTS AND METHODS
Patients
Within a 5-year period, we treated 6 patients with a strategy of primary TEVAR in native aortic infection. Patient demographics are shown in Table 1.
Table 1:
Descriptive characteristics of the cohort
| N = 6 (overall) | |
|---|---|
| Demographics | |
| Age, mean (standard deviation) | 71 (19.8) |
| Female, n (%) | 5 (83) |
| Chronic health conditions | |
| Hypertension, n (%) | 2 (33.4) |
| chronic obstructive pulmonary disease, n (%) | 1 (16.7) |
| Peripheral artery occlusive disease, n (%) | 1 (16.7) |
| Coronary artery disease, n (%) | 2 (33.4) |
Unless otherwise indicated, data are numbers (percentages).
Diagnostic and treatment algorithm
The diagnosis of native aortic infection was made according to clinical findings, blood sample testing and imaging. We used serum leucocyte count and C-reactive protein serum levels. Computed tomography (CT) findings were interpreted in combination with clinical findings as the image-guided diagnosis of native aortic infection is difficult. TEVAR was used as a primary treatment strategy in all cases. The rationale for primary TEVAR in all 6 patients was the circumstance of urgency or emergency. Emergency open surgery in thoracic aortic pathology is known to be associated with a high remaining operative risk due to many factors, not only surgery itself. As the anatomy was suitable, this was the reason to choose primary TEVAR because the option for secondary open surgical repair still remained.
Differentiation between infection and inflammation
The situation of culture-negative aortitis is comparable with the situation of culture-negative endocarditis where the clinical course and the morphological findings strongly suggest an infective process; however, no clear germ identification can be made. By this judgement algorithm, the diagnosis was determined in our culture-negative cases.
Antibiotic treatment algorithm
Regarding antibiotic therapy, we followed the guidelines of the European Society for Cardiology addressing active infective prosthetic endocarditis due to the common patterns of aortic infections in both culture-positive and culture-negative situations [7]. The most appropriate antibiotic treatment was applied when the type of bacteria was identified. In all patients, antibiotics were discontinued during follow-up. The timepoint was chosen according to the individual course encompassing clinical evolution (absence of fever or weight loss), returning to normal values of infectious parameters (C-reactive protein serum levels, white blood cell count) and normalized imaging.
Data collection and follow-up protocol
Data were retrospectively collected. Patients were enrolled into a strict follow-up protocol that required clinical, laboratory and CT scan evaluations at 3, 6 and 12 months and annually thereafter.
Statistical methods
Normally distributed continuous data are presented as mean ± standard deviation. Categorical variables are presented as absolute and relative frequencies. Overall survival and freedom from reinfection were calculated according to the method of Kaplan and Meier. All calculations were performed with SPSS 19.0 software for MacOSX (IBM, Inc., Somers, NY, USA).
RESULTS
In-hospital mortality
In-hospital mortality was 16.7% (n = 1). This patient died due to septic multiorgan failure after emergency TEVAR for contained rupture of a huge penetrating atherosclerotic ulcer in the distal aortic arch followed by emergency conversion to open surgery due to pre-existing aorto-bronchial fistulation.
Germs and estimated infection entry sites
Germs were isolated in 4 of 6 patients and are shown in Table 2. In the culture-negative patients, a history of continuing antibiotic therapy due to ipsilateral pneumonia to the infection site and, in the other case, due to urinary tract infection was observed. In another patient with culture-positive findings, an ipsilateral pneumonia was diagnosed.
Table 2:
Preoperative details and adjunctive procedures
| Patient no. | Age | Indication for intervention | Time diagnosis to intervention (days) | Time antibiotic therapy to intervention (days) | Germs detected | Body temperature before intervention | Adjunctive procedures | Additional surgery during hospitalization | In-hospital death |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 76 | Ruptured false aneurysm | 1 | 6 | E. coli, Staphylococcus aureus | 37.0 | Adhesiolysis, small bowel resection, endarterectomy of distal aorta. An 8-mm expanded polytetrafluoroethylene conduit, left thoracic drainage | Pleural decortications, resection of left inferior lobe, evacuation of haematoma | No |
| 2 | 78 | Ruptured false aneurysm | 0 | 0 | S. aureus | 38.4 | No | No | No |
| 3 | 83 | False aneurysm with aortopulmonic fistula | 1 | 21 | No | 37.0 | Selective occlusion of left subclavian artery | Reintervention due to Type I endoleakage, open repair with self-tailored pericardial prosthesis | Yes |
| 4 | 29 | Ruptured aneurysm | 0 | 0 | Bordetella holmesii, Mycobacterium tuberculosis HIV | 36.8 | No | No | No |
| 5 | 84 | Contained rupture | 0 | 8 | S. aureus | 38.0 | Carotid-subclavian bypass | Coiling subclavian artery due to Type II endoleakage, percutaneous transluminal angioplasty Type I endoleakage | No |
| 6 | 64 | Symptomatic aneurysm | 1 | 21 | No | 37.6 | No | No | No |
Timepoint of treatment
The clinical situation in combination with imaging urged an emergent treatment approach in all patients. Clinical findings were dominated by new and recent onset of thoracic or chest pain. Two patients had a new onset of haemoptysis.
Secondary surgical procedures after TEVAR
Secondary surgical procedures were performed in 2 patients. One patient underwent emergency conversion on the second day after TEVAR due to persisting compression of the left main bronchus. This patient underwent a complete removal of the infected native and prosthetic material with aortic reconstruction using a bovine pericardial patch as a neoaorta [3]. Finally, the bronchial defect was treated by sleeve resection of the left main bronchus. However, this patient died due to septic multiorgan failure after 15 days.
In another patient, radical surgical debridement was performed by pleural decortication, resection of the left inferior lobe and evacuation of a haematoma. Figures 1–4 depict the morphological course of a patient undergoing TEVAR for active infective thoracic aortic pathology followed by elective conversion using a bovine pericardial patch as a neoaorta.
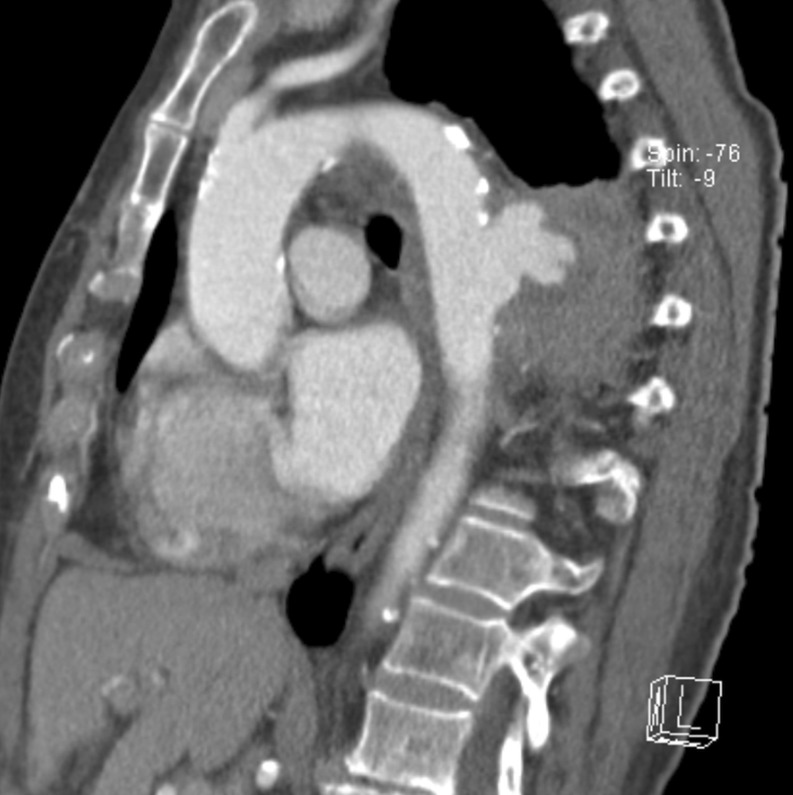
Figure 1:
Ruptured mycotic aneurysm at the time of diagnosis.
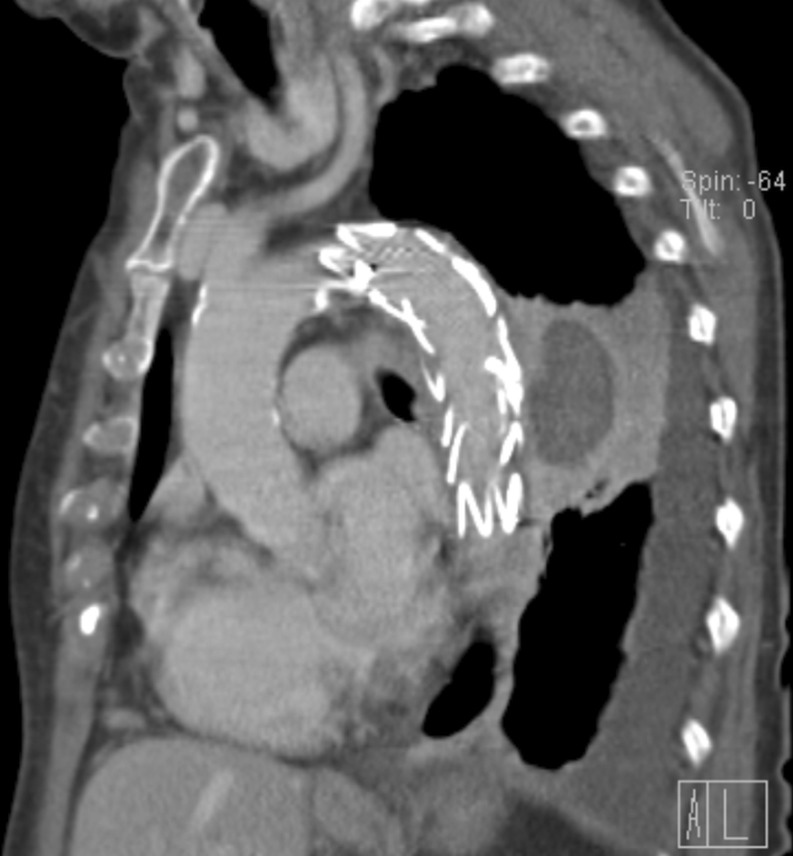
Figure 2:
Completion computed tomography angiography 2 days after treatment.
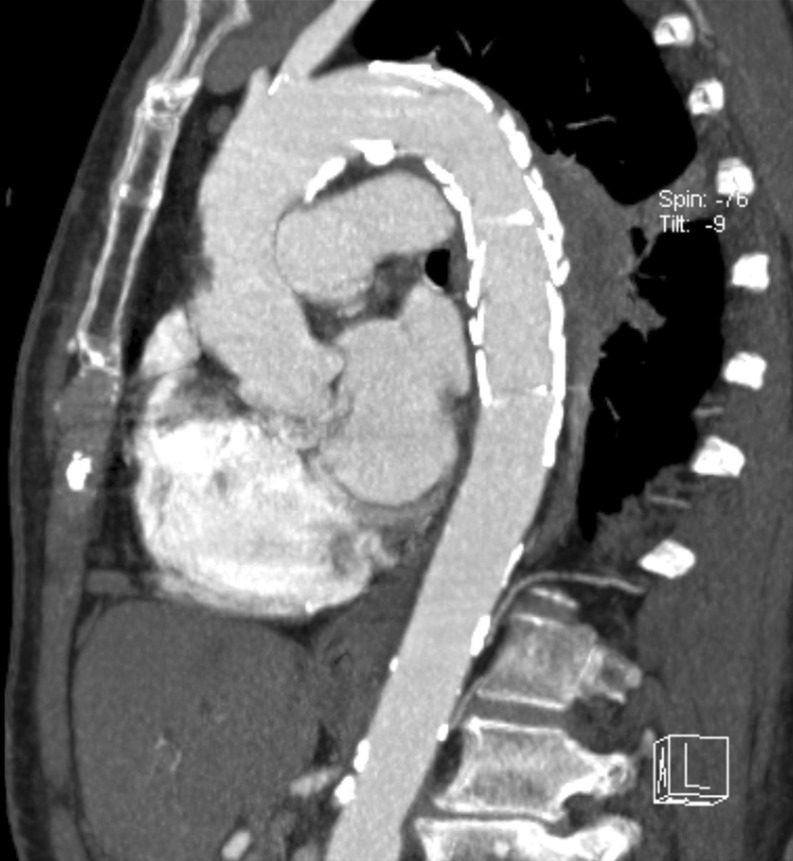
Figure 3:
Completion computed tomography angiography after 3 months.
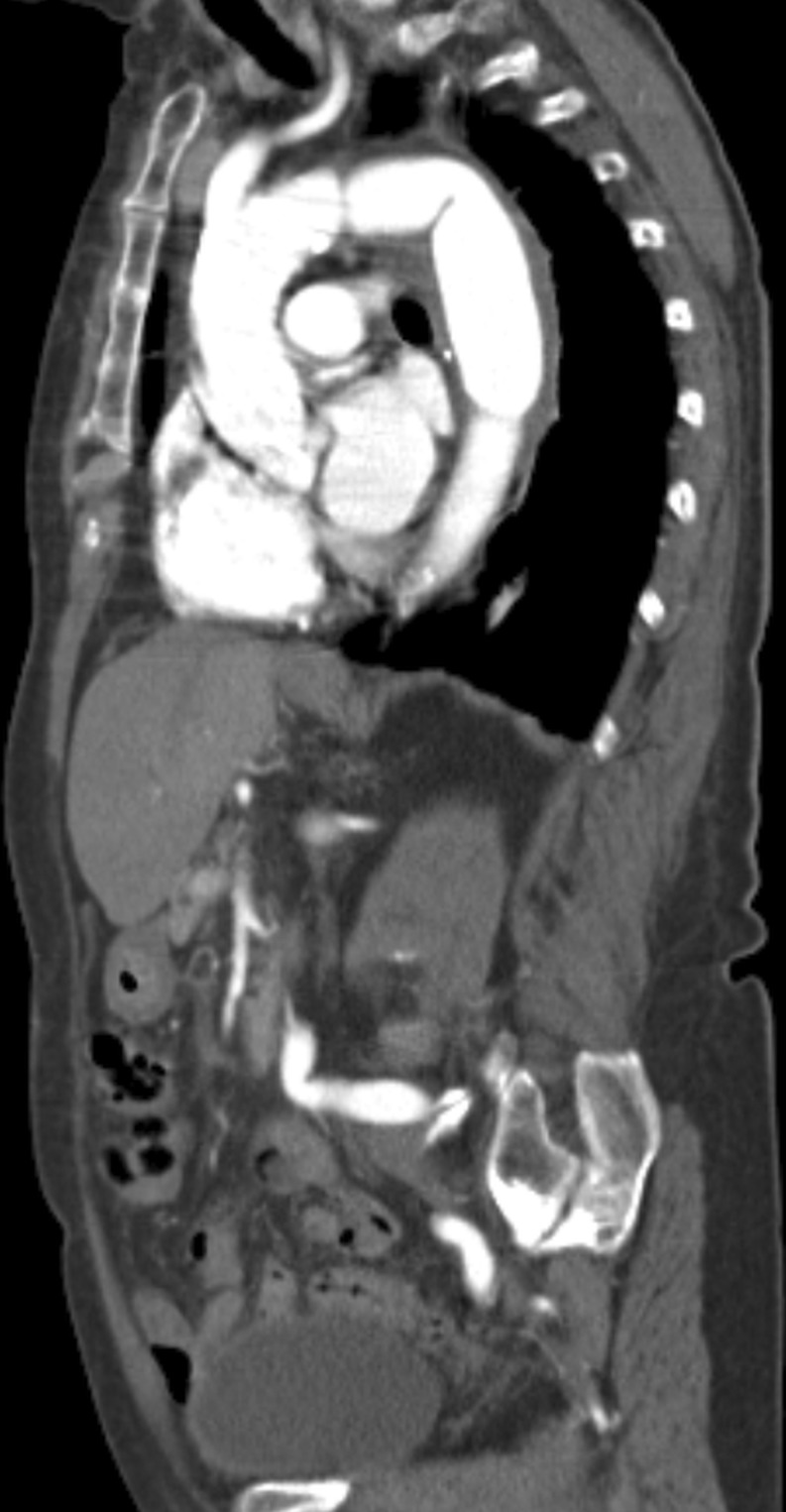
Figure 4:
Completion computed tomography angiography after elective conversion and orthotopic reconstruction with the bovine neoaorta.
Secondary surgical conversions after TEVAR
Two patients underwent secondary surgical conversions as a prophylactic approach during follow-up after 3 and 8 months (Table 3). The main reason for conversions was a good clinical state in combination with anticipation of the potential for recurring infection. Another patient refused elective conversion. The remaining patients were deemed unfit.
Table 3:
Postoperative details and laboratory parameters
| Patient no. | C-reactive protein (mg/l) pre-/postintervention | White blood cells (g/l) pre-/postintervention | Postoperative antibiotic therapy (months) | Follow-up (months) | Bridge to open repair |
|---|---|---|---|---|---|
| 1 | 397/12 | 14.4/8.4 | 24 | 56 | No |
| 2 | 300/57 | 20/11.4 | 7.5 | 38 | Conversion after 3 months |
| 3 | 187/83 | 11.9/11.4 | 1 | 0 | Conversion during hospital stay |
| 4 | 66/12 | 10/11.4 | 9 | 25 | Conversion after 8 months |
| 5 | 177/148 | 15.6/10.1 | 12 | 14 | No |
| 6 | 139/73 | 14.1/7.7 | 3 | 47 | No |
Follow-up
The median follow-up was 42.5 months. Neither in patients after secondary surgical conversions, nor in patients with TEVAR was recurrence of infection observed. One patient underwent major abdominal surgery due to rectal carcinoma with a favourable outcome.
COMMENT
In-hospital mortality was acceptable and well comparable to recent series. As could be expected, the reason for death in our patient was clearly related to the underlying infectious aortic process and its complication, namely aorto-bronchial fistulation requiring emergency conversion as well as adding another major surgical component to the required orthotopic aortic repair. It has been shown that extensive general or thoracic surgery in addition to aortic repair increases risk in comparable infectious clinical situations [6].
As is known from active prosthetic endocarditis, in the majority of patients Staphylococcus ssp. could be isolated in peripheral blood. This corresponds to findings in early graft infection. Regarding entry sites of infection, we can only speculate, as a clear correlation could only be drawn in 1 patient who recently experienced a urinary tract infection.
The clinical need urged immediate intervention in all cases. As a positive correlation between preoperative antibiotic therapy and success of treatment was known, it would have been preferable to defer treatment. However, the combination of clinical symptoms, predominantly pain, and the dramatic imaging findings prompted us to react. Fortunately, 4 of the 6 patients in our series received broad spectrum antibiotic therapy for more than 6 days.
The diagnosis of mycotic aneurysms is mostly by exclusion and is often mixed with penetrating atherosclerotic ulcers, which may show a similar morphology. Mycotic aneurysms or graft infections without clinical symptoms are nearly mutually exclusive. As such, fever, fatigue, weight loss or even signs of impending rupture such as haemoptysis are important surrogates. New onset of hoarseness, due to injury of the left laryngeal nerve, may be regarded as a sign of rapid expansion in the distal aortic arch. The situation of culture-negative aortitis is comparable with the situation of culture-negative endocarditis where the clinical course and the morphological findings strongly suggest an infective process; however, no clear germ identification can be made.
Imaging techniques in the diagnosis of native and prosthetic aortic infection warrant special emphasis. Multislice CT (MSCT) angiography provides a reliable tool for confirming clinical suspicion. However, milder degrees of inflammation or aortic wall oedema might be missed. Recently, F-18 fluorodeoxyglucose (FDG) PET has appeared to hold promise for the diagnosis of mycotic aneurysms and graft infections by visualizing hypermetabolic activity [7]. In addition to MSCT findings, elevated F-18 FDG uptake within the aortic wall is suggestive of active vascular infection. Finally, response to antibiotic therapy can be adequately monitored, and the change in F-18 FDG uptake within the aortic wall may also be correlated with clinical improvement during continuing antibiotic therapy [8, 9]. Magnetic resonance imaging (MRI) has not been extensively studied as an imaging modality of mycotic aneurysms or graft infections. However, the apparent advantages of magnetic resonance angiography over MSCT angiography may well be used also in this setting with regard to radiation exposure and contrast-related nephropathy. Specific protocols have been developed such as the oedema-weighted technique in order to detect even small changes within and around the aortic wall [2]. Limitations are—as known—availability and already implanted medical devices prohibiting the use of MRI. In our series, due to the urgency of these procedures, we were not able to apply these sophisticated surrogates. One patient received a PET/CT scan during the follow-up.
It has to be discussed in this context whether a primary surgical strategy would have been more adequate in the light of 3 patients undergoing conversion. However, it is our clinical impression that these patients were unfit for open surgery at the point when a strategic decision regarding treatment modality had to be taken. It would be desirable to additionally rely on a scoring system in such situations. However, currently no adequate preoperative scoring system for patients with thoracic or thoracoabdominal aortic pathology is available. This is needed, and would be highly welcome. This should be a task for working groups in the future. In the meantime, the individual decision to deem somebody fit or unfit will remain with the treating physician.
The length of the reported postinterventional antibiotic therapy is controversial and varies between 1 and 8 weeks and lifelong in the literature [4]. In our series, the length of postinterventional antibiotic therapy in the surviving patients was 3–24 months (median 8 months) due to individualized antibiotic therapy and follow-up. So far, there are no signs of reinfection in these patients at a median follow-up of 42.5 months.
Two patients underwent elective secondary surgical conversions after TEVAR. Retrospectively, it can be argued that these patients could also have been subjected to a chronic surveillance protocol. It is also obvious that by the refusal of another patient and the non-suitable clinical state of the remaining, knowledge supporting the feasibility of chronic surveillance without adverse events could have been gained. Nevertheless, it cannot be granted that recurring infection will not happen in the future. As a consequence, life-long surveillance, clinically and morphologically, remains imperative.
Limitations of the study
Without doubt, this series is extremely small. However, it is the counter-proof of principle that all infected vascular segments being treated by TEVAR have to be converted and replaced in order to reach an infectious-free status. This report is not meant to support the concept of alloplastic treatment of native aortic infection, but may be seen as an aid in the difficult decision-making of subjecting patients after TEVAR for infectious aortic disease to secondary surgical conversion.
In summary, as emergency therapy despite suspected aortic infection, TEVAR is feasible and may well serve as a definite treatment option in selected cases. As recurring infection cannot be entirely excluded, life-long clinical and morphological surveillance remains mandatory.
Conflict of interest: none declared.
REFERENCES
- 1.Revest M, Decaux O, Cazalets C, Verohye JP, Jego P, Grosbois B. Thoracic infectious aortitis: microbiology, pathophysiology and treatment. Rev Med Interne. 2007;28:108–15. doi: 10.1016/j.revmed.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 2.Gornik HL, Creager MA. Aortitis. Circulation. 2008;117:3039–51. doi: 10.1161/CIRCULATIONAHA.107.760686. doi:10.1161/CIRCULATIONAHA.107.760686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Czerny M, von Allmen R, Opfermann P, Sodeck G, Dick F, Stellmes A, et al. Self-made pericardial tube graft: a new surgical concept for treatment of graft infections after thoracic and abdominal aortic procedures. Ann Thorac Surg. 2011;92:1657–62. doi: 10.1016/j.athoracsur.2011.06.073. doi:10.1016/j.athoracsur.2011.06.073. [DOI] [PubMed] [Google Scholar]
- 4.Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg. 2007;46:906–12. doi: 10.1016/j.jvs.2007.07.025. doi:10.1016/j.jvs.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 5.Tambyraja AL, Wyatt MG, Clarke MJ, Chalmers RT. Autologous deep vein reconstruction of infected thoracoabdominal aortic patch graft. J Vasc Surg. 2003;38:852–4. doi: 10.1016/s0741-5214(03)00614-1. doi:10.1016/S0741-5214(03)00614-1. [DOI] [PubMed] [Google Scholar]
- 6.Razavi MK, Razavi MD. Stent-graft treatment of mycotic aneurysms: a review of the current literature. J Vasc Interv Radiol. 2008;19(6 Suppl):S51–6. doi: 10.1016/j.jvir.2008.02.012. doi:10.1016/j.jvir.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 7.Habib G, Hoen B, Tornos P, Thuny F, Prendergast B, Vilacosta I, et al. Guidelines on the prevention, diagnosis and treatment of infective endocarditis. Eur Heart J. 2009;30:2369–413. doi: 10.1093/eurheartj/ehp285. doi:10.1093/eurheartj/ehp285. [DOI] [PubMed] [Google Scholar]
- 8.Davison JM, Montilla-Soler JL, Broussard E, Wilson R, Cap A, Allen T. F-18 FDG PET-CT imaging of mycotic aneurysm. Clin Nucl Med. 2005;30:483–7. doi: 10.1097/01.rlu.0000167663.17630.0a. doi:10.1097/01.rlu.0000167663.17630.0a. [DOI] [PubMed] [Google Scholar]
- 9.Choi SJ, Lee JS, Cheong MH, Byon SS, Hyun IY. F-18 FDG PET/CT in the management of infected abdominal aortic aneurysm due to salmonella. Clin Nucl Med. 2008;33:492–5. doi: 10.1097/RLU.0b013e31817793a0. doi:10.1097/RLU.0b013e31817793a0. [DOI] [PubMed] [Google Scholar]