Abstract
OBJECTIVES
In current practice, donors and recipients are not matched for gender in lung transplantation. However, some data have suggested a possible effect of gender combinations on lung transplant outcomes. We examined whether donor–recipient (D/R) gender mismatch is related to adverse outcomes after lung transplantation in terms of early and long-term graft function and survival.
METHODS
We reviewed 256 donors and lung transplant recipients over a 14-year period. Patients were distributed into four groups: Group A (D/R: female/female), Group B (D/R: male/male), Group C (D/R: female/male), Group D (D/R: male/female). Donor and recipient variables were compared among groups, including early graft function, 30-day mortality, freedom from bronchiolitis obliterans syndrome (BOS), and long-term survival.
RESULTS
Group A: 57 (22%), Group B: 99 (39%), Group C: 62 (24%), Group D: 38 (15%) transplants (P = 0.001). Donor age was 29 ± 14, 27 ± 12, 33 ± 13 and 23 ± 12 years for Groups A, B, C and D, respectively (P = 0.004). Recipient age was 31 ± 15, 44 ± 17, 42 ± 16 and 30 ± 16 years for Groups A, B, C and D, respectively (P = 0.000). PaO2/FiO2 (mmHg) 24 h post-transplant was: Group A: 276 ± 144, Group B: 297 ± 131, Group C: 344 ± 133 and Group D: 238 ± 138 (P = 0.015). Primary graft dysfunction developed in 23, 14, 17 and 21% of recipients from Groups A, B, C and D, respectively (P = 0.45). Operative mortality was 4.4, 6.5, 5.2 and 2%, for recipients from Groups A, B, C and D, respectively (P = 0.66). Freedom from BOS was 73, 59 and 36% for gender-matched transplants vs 76, 67 and 40% for gender-mismatched transplants at 3, 5 and 10 years, respectively (P = 0.618), without differences among groups. A non-significant survival benefit was observed for female recipients, irrespective of the donor gender.
CONCLUSIONS
Donor–recipient gender mismatch does not have a negative impact on early graft function and mortality following lung transplantation. There is a trend towards a survival benefit for female recipients, irrespective of the donor gender.
Keywords: Lung transplantation, Donors, Gender, Primary graft dysfunction, Bronchiolitis obliterans syndrome, Survival
INTRODUCTION
Lung transplantation is an established treatment option for patients with end-stage disease. However, the success of lung transplant procedures is limited by a significant perioperative mortality [1]. The major causes of death are primary graft dysfunction (PGD), cardiac-related death and infections.
Appropriate recipients for donor lungs are chosen according to ABO compatibility, need for single- or double-lung transplant and body measurements. On the contrary, age, gender, race or cytomegalovirus (CMV) status is not matched between donors and recipients.
Data from liver [2], kidney [3] and heart transplants [4] have shown that female-to-male combinations are associated with poor survival. However, the influence of donor and recipient gender on the outcome of lung transplantation is not clear. While Roberts et al. [5] from the Massachusetts General Hospital reported improved survival for gender-mismatched transplants, with longer freedom from bronchiolitis obliterans syndrome (BOS) for female-to-male transplants, a French multicentre study reported poor survival for female-to-male transplants [6]. Similarly, the group of Toronto, using data from the Registry of the International Society for Heart and Lung Transplantation (ISHLT), observed poor early and long-term outcomes for female-to-male transplants, with better results for female-to-female pairs [7]. The most recent report from the ISHLT Registry showed a modest survival advantage for female recipients, with female-to-male transplants as a borderline protective factor for 5-year survival. On the contrary, for those recipients surviving 1 year, male-to-female transplants presented higher risk of mortality at 5 years [8]. Given these controversial data from the literature, the present study was designed to assess whether donor–recipient (D/R) gender mismatch is associated with poor early and long-term outcomes after lung transplantation, focusing on four variables: PGD, 30-day mortality, freedom from BOS and long-term survival.
PATIENTS AND METHODS
We analysed all lung transplants performed at the Reina Sofia Hospital from January 1994 to December 2009 (n = 314). For the purposes of this study, 45 cases of size mismatch requiring lobar transplantations or pulmonary tailoring were excluded from the analysis. Also, 4 multiorgan transplants and 9 retransplants were excluded. Finally, 256 lung transplants were entered in the study.
Study design
Transplants were distributed into four groups according to donor and recipient gender: Group A (female donor to female recipient—FF), Group B (male donor to male recipient—MM), Group C (female donor to male recipient—FM) and Group D (male donor to female recipient—MF). Prospective data collected from our transplant registry were recorded and analysed retrospectively, and comparisons between matched and mismatched pairs, and among the four study groups were done.
Donors
Heart-beating donors were used for transplantation in these series. In general, donors accepted for transplantation met the standard criteria for donor acceptability [9]: age <55 years, PaO2/FiO2 ratio above 300 mmHg, clear chest radiographs, tobacco history <20 pack-years, the absence of purulent secretions or aspiration on bronchoscopy and the absence of macroscopic lung abnormalities at the time of retrieval. However, in selected cases, donors older than 55 years, with mild unilateral abnormalities in chest radiographs, with a presumed smoking habit above 20 pack-years, with some amount of secretions in the airways, or limited signs of pulmonary parenchymal contusion were also deemed suitable for transplantation. In any case, lungs with PaO2/FiO2 <300 mmHg at the time of retrieval were considered acceptable for transplantation.
Organ allocation
The decision regarding organ allocation to recipients was made according to the severity of each patient, donor and recipient measurements and age (younger donors to younger recipients). In terms of size, 20% of variations in height and weight for calculation of estimated predicted total lung capacity (pTLC), and 10% of variations in radiographic measurements were accepted between donor and recipient. In the event of two potential recipients for a particular donor with similar criteria, time on the waiting list of the candidates was considered to allocate the donor. No attempt was made to match the donor and recipient on the basis of gender or CMV status.
To estimate donor and recipient measurements, from baseline height and age, we used reference equations to calculate the pTLC. For adult male donors, TLC = 0.094 × height (cm) − 0.015 × age (years) − 9.167. For adult female donors, TLC = 0.079 × height (cm) − 0.008 × age (years) − 7.49 [10].
Lung transplant protocol
The donor lung procurement was performed following the standard technique of combined cardiopulmonary extraction [11]. Immediately after lung harvesting, an additional retrograde second flushing of the preservation solution was given to optimize lung preservation by perfusing the bronchial circulation [12]. The preservation solution used was modified Eurocollins® until the year 2001, from when Perfadex® solution (Vitrolife, Göteborg, Sweden) was introduced routinely.
In the recipients, either single- or double-lung transplantation was performed through a posterolateral thoracotomy or a clamshell incision, respectively. Cardiopulmonary bypass was instituted in case of the inability to maintain the recipient on one lung during pneumonectomy or implantation, or in case of graft dysfunction after the first lung was implanted. After completion of the transplantation, a fibreoptic bronchoscopy was performed to assess the viability of the bronchial anastomoses and to aspirate secretions in the airways.
Immunosuppression consisted of the standard triple therapy: cyclosporine or tacrolimus, azathioprine or mycophenolate mofetil and steroids. Methylprednisolone administration was begun intravenously in the operating room (10 mg/kg before reperfusion). Immediately after completion of the lung transplantation, cyclosporine (Sandimmun®; Novartis, Basle, Switzerland) was started at sufficient doses to achieve blood levels of 350–400 ng/ml, and methylprednisolone was maintained at diminishing doses until the fourth postoperative day, to be switched to deflazacort (Dezacor®; Hoechst Marion Roussel, Barcelona, Spain) (1.5 mg/kg/day). Azathioprine (Imurel®; Medeva Pharma, Madrid, Spain) (2 mg/kg/day) was started 48–72 h postoperatively after obtaining donor and recipient cultures. However, mycophenolate mofetil (Cellcept®; Roche Lab., Inc., Nutley, NJ, USA) (2–3 g/day) instead of azathioprine was given for some patients. Patients with recurrent acute rejection episodes, cyclosporine-related toxicity or those who developed BOS, were switched from cyclosporine to tacrolimus (Prograf®; Fujisawa, Killorglin Co., Kerry, Ireland), at sufficient doses to achieve blood levels of 10–20 ng/ml. No cytolitic therapy was systematically used.
Antimicrobial therapy was administered based on antibiotic sensitivities from preoperative sputum cultures of the recipient and from the donor bronchoaspirate. Postoperative bronchoscopies were performed 24–48 h post-transplant, at the time of extubation and at discharge, and thereafter whenever a clinical suspicion of infection or rejection appeared. Late postoperative routine surveillance bronchoscopies were not performed.
The use of different lung preservation solutions and immunosuppression regimens were equally distributed among the four study groups.
Definitions
PGD was defined and graded according to the recommendations of the Working Group on PGD of the ISHLT [13]. To rule out possible cases with low oxygenation ratios within the first 24 h post-transplant that could improve early thereafter (reperfusion injury, post-transplant pulmonary oedema), we only selected those cases with persistent low PO2 and pulmonary infiltrates in chest radiographs at 72 h post-transplant (PGD Grades 2–3 at 72 h) (Table 1).
BOS was graded and defined following the criteria of the ISHLT [14]. For the purposes of this study, BOS grades 2 or 3 were considered for the analysis (Table 2).
Extended donors were defined as those having at least two of the following: age older than 55 years, mild abnormalities in chest X-ray, evidence of mucopurulent secretions at bronchoscopy, smoking habit above 20 pack-years [15].
Table 1:
Grading of primary graft dysfunction (PGD) according to the report of the ISHLT working group on PGD [13]
| PGD grades | PaO2/FiO2 | Rx infiltrates (oedema) |
|---|---|---|
| 0 | >300 | Absent |
| 1 | >300 | Present |
| 2 | 200–300 | Present |
| 3 | <200 | Present |
Table 2:
Grading of bronchiolitis obliterans syndrome (BOS) according to the year–2003 ISHLT classification [14]
| BOS 0 | FEV1 > 90% of baseline and FEV25–75 > 75% of baseline |
| BOS 0p | FEV1 81–90% of baseline and/or FEV25–75 ≤ 75% of baseline |
| BOS 1 | FEV1 66–80% of baseline |
| BOS 2 | FEV1 51–65% of baseline |
| BOS 3 | FEV1 ≤ 50% of baseline |
Data collection
Donor-related variables included age, gender, ABO blood group, PaO2/FiO2, cause of death, CMV status and optimal/extended criteria.
Transplant-related variables included type of transplant (single- or double-lung transplant), graft ischaemic times and need for bypass.
Recipient-related variables included age, gender, ABO blood group, CMV status, transplant indication, PaO2/FiO2 24 h post-transplant, ICU stay, length of post-transplant intubation, hospital stay, number of acute rejection episodes, development of PGD, 30-day mortality, freedom from BOS and survival.
Statistics
Comparisons between gender-matched and gender-mismatched lung transplants were performed by univariate analysis. χ2 test and Fisher's exact test were used to assess differences between categorical variables. Unpaired t-test was used to compare means between two quantitative variables from normally distributed data, and Mann–Whitney test for non-normally distributed data.
Comparisons of continuous variables among the four groups of gender pairs were done by analysis of variance and post-hoc Scheffe test.
Survival rates and freedom from BOS were calculated in the overall group, between gender-matched and gender-mismatched transplants and among the four groups of gender pairs, by using the Kaplan–Meier method and log-rank test. Additional analyses were done conditional to 1-year survival, and stratified by indication of transplant [chronic obstructive pulmonary disease (COPD), pulmonary fibrosis and cystic fibrosis (CF)].
Continuous variables are expressed as mean ± standard deviation. Categorical variables are expressed as counts and proportions, with 95% confidence intervals (95% CI). Differences with P-values <0.05 were considered significant. The statistical analysis was performed using SPSS (SPSS 11.0 for Windows: SPSS Inc., Chicago, IL, USA).
RESULTS
Study population
Two hundred and fifty-six lung transplants met the selection criteria for the study. There were 161 double-lung transplants (63%), and 95 single-lung transplants (37%). The indications for lung transplantation were CF in 80 (31%), pulmonary fibrosis in 70 (27%), COPD in 67 (26%) and other indications in 39 (16%) patients. The mean age of the recipients was 38 ± 17 years (5–67 years. One hundred and sixty-one recipients were male and 95 were female. Fifty-one patients (20%) required cardiopulmonary bypass (CPB) during the operation, and 10 were placed on extracorporeal membrane oxygenation perioperatively. Preoperatively, 33 patients (13%) were under invasive mechanical ventilation at the time of transplantation.
In terms of blood groups, 95 recipients were blood Group O, 120 were blood Group A, 29 were blood Group B and 12 were blood Group AB. D/R CMV status was positive/positive in 156 cases, negative/positive in 49, positive/negative in 24 and negative/negative in 23 cases (data not available in four donors).
Donor demographics
The age of donors was 29 ± 13 years (8–62). There were 137 males and 119 females. Causes of death were traumatic in 136 (53%), cerebrovascular accident in 115 (45%) and other causes in 5 (2%) donors. One hundred and five donors were blood Group O, 101 donors were blood Group A, 25 donors were blood Group B and 6 donors were blood Group AB. Forty-eight donors (19%) were considered extended donors.
Donor–recipient gender match vs mismatch
One hundred and fifty-six (61%) were gender-matched transplants, and 100 (39%) were gender-mismatched transplants. Group B (MM) accounted for 39% of all transplants (99 transplants), as opposed to 57 (22%) of Group A (FF), 62 (24%) of Group C (FM) and 38 (15%) of Group D (MF) (P = 0.001).
No significant differences were observed between gender-matched and gender-mismatched lung transplants in terms of donor and recipient demographics, transplant indication, type of transplant, ischaemic times and early outcomes. Only traumatic donors accounted for the majority of gender-matched transplants (60%; 95% CI: 53–67, P = 0.013) (Table 3).
Table 3:
Comparative data between donor/recipient (D/R) gender-matched and gender-mismatched lung transplants
| D/R gender match (n = 156) | 95% CI | D/R gender mismatch (n = 100) | 95% CI | P-value | |
|---|---|---|---|---|---|
| Donor age (years) | 28 ± 13 | 24–30 | 29 ± 13 | 25–31 | 0.499 |
| Donor cause of death | 0.013 | ||||
| Trauma | 92 (60) | 53–67 | 44 (45) | 35–55 | |
| Cerebrovascular | 61 (39) | 32–46 | 54 (54) | 44–64 | |
| Other | 3 (1) | 0–2 | 2 (1) | 0–3 | |
| Donor intubation (h) | 35 ± 33 | 20–38 | 41 ± 43 | 26–44 | 0.234 |
| Donor PaO2/FiO2 (mmHg) | 471 ± 80 | 461–503 | 460 ± 93 | 450–492 | 0.315 |
| Extended donor | 26 (16) | 10–22 | 22 (22) | 14–30 | 0.183 |
| Recipient age (years) | 39 ± 17 | 37–45 | 37 ± 17 | 35–43 | 0.382 |
| Transplant indication | 0.588 | ||||
| COPD | 42 (28) | 21–35 | 25 (26) | 18–34 | |
| Pulmonary fibrosis | 45 (30) | 23–37 | 25 (26) | 18–34 | |
| Cystic fibrosis | 49 (32) | 25–39 | 31 (32) | 23–41 | |
| Other | 15 (10) | 5–15 | 15 (15) | 8–22 | |
| Type of transplant | 0.188 | ||||
| Single | 62 (39) | 32–46 | 33 (33) | 24–42 | |
| Double | 95 (61) | 54–68 | 66 (67) | 58–76 | |
| Need of bypass | 33 (21) | 15–27 | 17 (17) | 10–24 | 0.262 |
| Ischaemic time first graft (min) | 323 ± 55 | 304–334 | 327 ± 59 | 308–338 | 0.622 |
| Ischaemic time second graft (min) | 468 ± 69 | 443–491 | 468 ± 71 | 443–491 | 0.956 |
| Recipient PaO2/FiO2 24 h (mmHg) | 290 ± 135 | 234–319 | 303 ± 144 | 247–332 | 0.537 |
| Primary graft dysfunction | 26 (17) | 11–23 | 18 (19) | 11–27 | 0.428 |
| Thirty-day mortality | 27 (17) | 11–23 | 18 (19) | 11–27 | 0.486 |
COPD: chronic obstructive pulmonary disease.
Quantitative variables are expressed as mean ± standard deviation. Qualitative variables are expressed as frequencies and proportions in parenthesis (% within each study group).
Comparisons among the four groups of gender pairs showed that traumatic donors were more frequently male (Group B: 75%, 95% CI: 67–83%; Group D: 71%, 95% CI: 57–85%), as opposed to female donors, who died more frequently from cerebrovascular accident (Group A: 65%, 95% CI: 53–77%; Group C: 71%, 95% CI: 60–82%) (P = 0.000) (Table 4). The oldest donors were females from Group C (mean age 33 ± 13 years, 95% CI: 30–37) and the youngest were males from Group D (mean age 23 ± 12 years, 95% CI: 20–28) (P = 0.004). Male recipients were significantly older than female recipients: Group B recipients, 44 ± 17 years (95% CI: 40–47), and Group C recipients, 42 ± 16 years (95% CI: 38–46), as opposed to Group A recipients, 31 ± 15 years (95% CI: 28–36), and Group D recipients, 30 ± 16 years (95% CI: 24–35) (P = 0.000) (Table 4).
Table 4:
Comparative analysis of donor, recipient, operative and postoperative variables among the four groups of donor/recipient (D/R) gender pairs
| Group A (FF) | 95% CI | Group B (MM) | 95% CI | Group C (FM) | 95% CI | Group D (MF) | 95% CI | P-value | |
|---|---|---|---|---|---|---|---|---|---|
| Donor age (years)a | 29 ± 14 | 26–33 | 27 ± 12 | 25–30 | 33 ± 13 | 30–37 | 23 ± 12 | 20–28 | 0.004 |
| Donor cause of death | <0.001 | ||||||||
| Trauma | 19 (34) | 22–46 | 73 (75) | 67–83 | 17 (28) | 17–39 | 27 (71) | 57–85 | |
| Cerebrovascular | 36 (65) | 53–77 | 25 (25) | 17–33 | 43 (71) | 60–82 | 11 (29) | 15–43 | |
| Other | 3 (1) | 0–3 | 0 | 2 (1) | 0–3 | 0 | |||
| Donor smoking habit | 12 (22) | 11–33 | 22 (25) | 17–33 | 8 (14) | 6–22 | 9 (27) | 13–41 | 0.388 |
| Donor intubation (h) | 37 ± 36 | 27–47 | 34 ± 32 | 28–41 | 43 ± 48 | 31–55 | 38 ± 33 | 27–49 | 0.576 |
| Donor PaO2/FiO2 (mmHg) | 482 ± 80 | 461–503 | 465 ± 79 | 449–481 | 460 ± 92 | 436–483 | 461 ± 96 | 429–493 | 0.476 |
| Extended donor | 9 (16) | 7–25 | 17 (17) | 10–24 | 14 (22) | 12–32 | 8 (21) | 8–34 | 0.748 |
| Recipient age (years)b | 31 ± 15 | 28–36 | 44 ± 17 | 40–47 | 42 ± 16 | 38–46 | 30 ± 16 | 24–35 | <0.001 |
| Recipient under mechanical ventilation | 10 (17) | 7–27 | 7 (7) | 2–12 | 9 (15) | 6–24 | 7 (19) | 7–31 | 0.160 |
| Transplant indication | <0.001 | ||||||||
| COPD | 4 (7) | 1–13 | 38 (40) | 31–49 | 19 (33) | 22–44 | 6 (16) | 5–27 | |
| Pulmonary fibrosis | 17 (30) | 18–48 | 28 (29) | 20–38 | 19 (33) | 22–44 | 6 (16) | 5–27 | |
| Cystic fibrosis | 26 (46) | 33–59 | 23 (24) | 16–32 | 13 (22) | 12–32 | 18 (47) | 32–62 | |
| Other | 9 (16) | 7–25 | 6 (6) | 2–10 | 7 (12) | 4–20 | 8 (21) | 8–34 | |
| Type of transplant | 0.013 | ||||||||
| Single | 15 (27) | 16–38 | 47 (46) | 36–56 | 25 (40) | 28–52 | 8 (21) | 9–30 | |
| Double | 42 (73) | 62–84 | 53 (54) | 44–64 | 35 (60) | 48–72 | 31 (79) | 67–91 | |
| Need of bypass | 19 (34) | 22–46 | 14 (14) | 7–21 | 9 (15) | 6–24 | 8 (21) | 8–34 | 0.018 |
| Ischaemic time first graft (min) | 328 ± 57 | 312–346 | 320 ± 54 | 308–331 | 328 ± 60 | 312–346 | 324 ± 60 | 302–346 | 0.785 |
| Ischaemic time second graft (min) | 477 ± 70 | 452–501 | 462 ± 70 | 441–482 | 477 ± 74 | 450–503 | 458 ± 67 | 429–486 | 0.608 |
| Recipient PaO2/FiO2 24 h (mmHg)c | 276 ± 144 | 228–324 | 297 ± 131 | 265–329 | 345 ± 133 | 303–387 | 238 ± 138 | 182–294 | 0.015 |
| Recipient ICU stay (days) | 18 ± 23 | 10–26 | 12 ± 14 | 9–16 | 11 ± 11 | 8–15 | 13 ± 10 | 9–17 | 0.281 |
| Recipient intubation (h) | 91 ± 204 | 21–161 | 136 ± 321 | 56–216 | 85 ± 157 | 38–132 | 87 ± 133 | 35–139 | 0.638 |
| Recipient hospital stay (days) | 38–29 | 28–48 | 30–30 | 28–43 | 34–25 | 26–42 | 39–31 | 27–52 | 0.841 |
| Acute rejection episodes (n) | |||||||||
| Overall | 1.37 ± 1.49 | 0.88–1.86 | 1.02 ± 1.10 | 0.74–1.29 | 1.30 ± 1.16 | 0.94–1.66 | 1.41 ± 1.35 | 0.90–1.93 | 0.369 |
| 1 month | 0.90 ± 0.82 | 0.63–1.16 | 0.52 ± 0.58 | 0.38–0.66 | 0.70 ± 0.67 | 0.49–0.91 | 0.66 ± 0.76 | 0.36–0.95 | 0.063 |
| 2–3 months | 0.18 ± 0.39 | 0.04–0.32 | 0.27 ± 0.51 | 0.14–0.40 | 0.18 ± 0.45 | 0.03–0.33 | 0.43 ± 0.57 | 0.21–0.65 | 0.158 |
| >3 months | 0.39 ± 0.80 | 0.09–0.68 | 0.26 ± 0.62 | 0.09–0.43 | 0.48 ± 0.59 | 0.28–0.67 | 0.40 ± 0.50 | 0.19–0.61 | 0.431 |
| Primary graft dysfunction | 13 (23) | 12–34 | 13 (14) | 7–21 | 10 (17) | 8–26 | 8 (21) | 8–34 | 0.459 |
| 30-day mortality | 11 (19) | 9–29 | 16 (16) | 9–23 | 13 (22) | 12–32 | 5 (13) | 3–23 | 0.665 |
| Causes of death | 0.641 | ||||||||
| Primary graft dysfunction | 4 (7) | 1–13 | 3 (3) | 0–6 | 2 (3) | 0–6 | 0 | ||
| Cardiac failure | 2 (4) | 0–9 | 7 (7) | 2–12 | 6 (10) | 3–17 | 3 (8) | 0–16 | |
| Sepsis | 2 (4) | 0–9 | 5 (5) | 1–9 | 1 (2) | 0–5 | 2 (5) | 0–10 | |
| Surgical | 1 (2) | 0–5 | 0 | 2 (3) | 0–6 | 0 | |||
| Other | 2 (4) | 0–9 | 0 | 2 (3) | 0–6 | 1 (2) | 0–6 | ||
COPD: chronic obstructive pulmonary disease.
Quantitative variables are expressed as mean ± standard deviation. Qualitative variables are expressed as frequencies and proportions in parenthesis (% within each study group). FF: female-to female (n = 57); MM: male-to-male (n = 99); FM: female-to male (n = 62); MF: male-to-female (n = 38).
aDonor age: C vs D (P = 0.007).
bRecipient age: A vs B (P < 0.001); B vs D (P < 0.001); C vs D (P = 0.007).
cRecipient PaO2/FiO2 (24 h): C vs D (P = 0.023).
Pulmonary fibrosis and COPD were the most frequent indications in male recipients, as opposed to CF, which was the most frequent indication in females (P = 0.000) (Table 4). Cardiopulmonary bypass was most frequently needed in female-to-female transplants (Group A: 34%, 95% CI: 22–46%) (P = 0.018), and double-lung transplants were more frequently indicated in female recipients: 73% of recipients from Group A (95% CI: 62–84%), and 79% of recipients from Group D (95% CI: 67–91%) (P = 0.013). Recipients' PaO2/FiO2 ratio 24 h post-transplant was better in female-to-male transplants (Group C: 345 ± 133 mmHg, 95% CI: 303–387) than in male-to-female transplants (Group D: 238 ± 138 mmHg, 95% CI: 182–294) (P = 0.023).
Thirty-day mortality
Overall 30-day mortality was 17% (45 patients). Causes of death were haemodynamic/cardiac failure in 18 (7%), sepsis in 10 (4%), PGD in 9 (3%), surgical in 3 (1%) and other in 5 (2%) patients. Thirty-day mortality for gender-matched transplants was 17% (95% CI: 11–23%), and 19% for gender-mismatched transplants (95% CI: 11–27%) (P = 0.486) (Table 3). Thirty-day mortality was 19% (95% CI: 9–29%) for Group A, 16% (95% CI: 9–23%) for Group B, 22% (95% CI: 12–32%) for Group C and 13% (95% CI: 3–23%) for Group D (P = 0.665) (Table 4).
Primary graft dysfunction
Forty-four patients developed PGD (17%), with a PGD-related mortality of 27%. PGD was present in 26 recipients (17%) of gender-matched transplants (95% CI: 11–23%), and in 18 recipients (19%) of gender-mismatched transplants (95% CI: 11–27%) (P = 0.428) (Table 3). When we compared the four groups of gender pairs, no differences in the onset of PGD were observed among groups: 23% for Group A (95% CI: 12–34%), 14% for Group B (95% CI: 7–21%), 17% for Group C (95% CI: 8–26%) and 21% for Group D (95% CI: 8–34%) (P = 0.459) (Table 4). PGD patients exhibited significantly lower PaO2/FiO2 values at 24 h post-transplant than those without PGD (131 ± 59 vs 339 ± 119 mmHg, respectively; P < 0.001).
Bronchiolitis obliterans syndrome
Freedoms from BOS for the overall group of patients were 74, 60, 38 and 16% at 3, 5, 10 and 15 years post-transplant, respectively (Fig. 1A). No differences were observed in freedom from BOS between gender-matched (half-life: 7.8 years) and gender-mismatched lung transplants (half-life: 8 years) (P = 0.618) (Fig. 1B). When comparing freedom from BOS among the four groups of gender pairs, no differences were observed (P = 0.488) (Fig. 1C).
Figure 1:
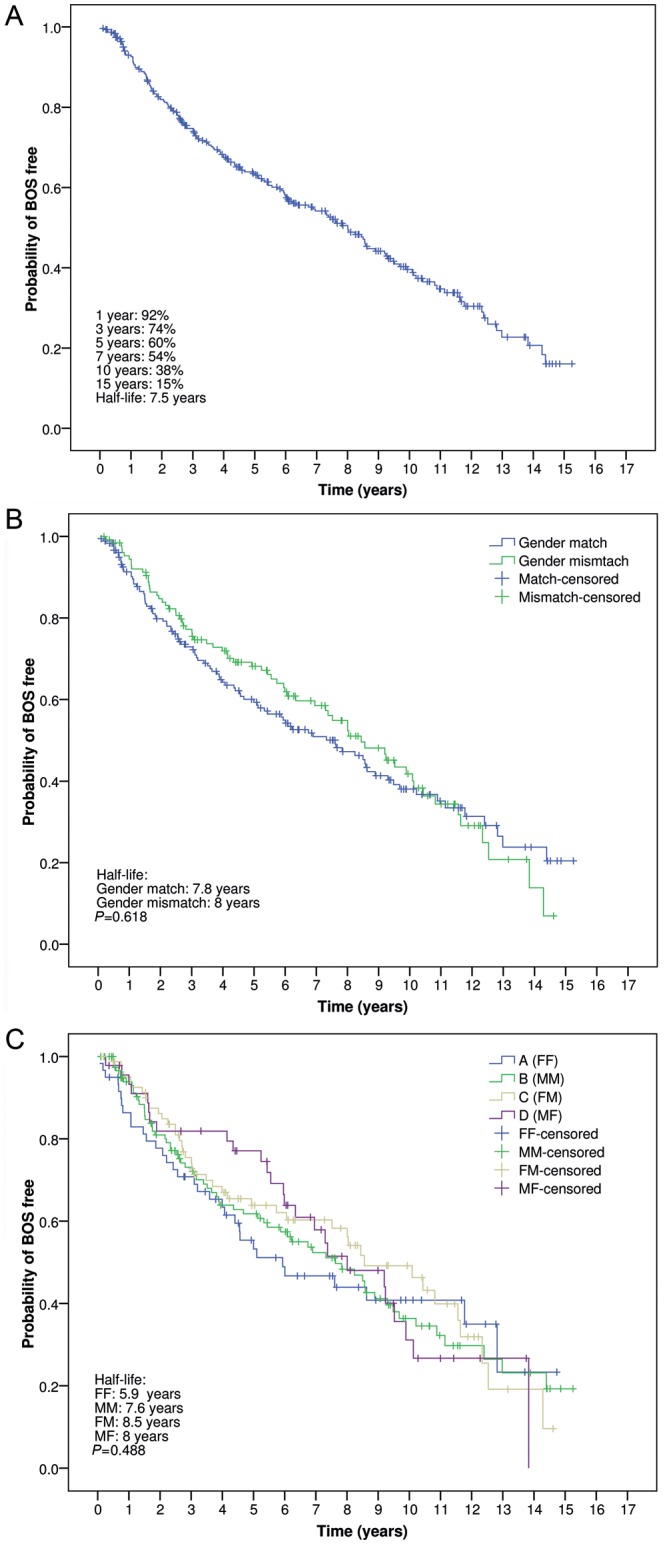
(A) Probability of freedom from BOS in the overall series. (B) Probability of freedom from BOS between gender-matched and gender-mismatched lung transplants. (C) Probability of freedom from BOS according to donor and recipient gender combinations. FF: female-to-female; MM: male-to-male; FM: female-to-male; MF: male-to-female (Kaplan–Meier and log-rank test).
Figure 2 depicts the probability of freedom from BOS according to donor and recipient gender combinations, and stratified by transplant indication. No differences were observed among groups for COPD, CF and pulmonary fibrosis patients.
Figure 2:
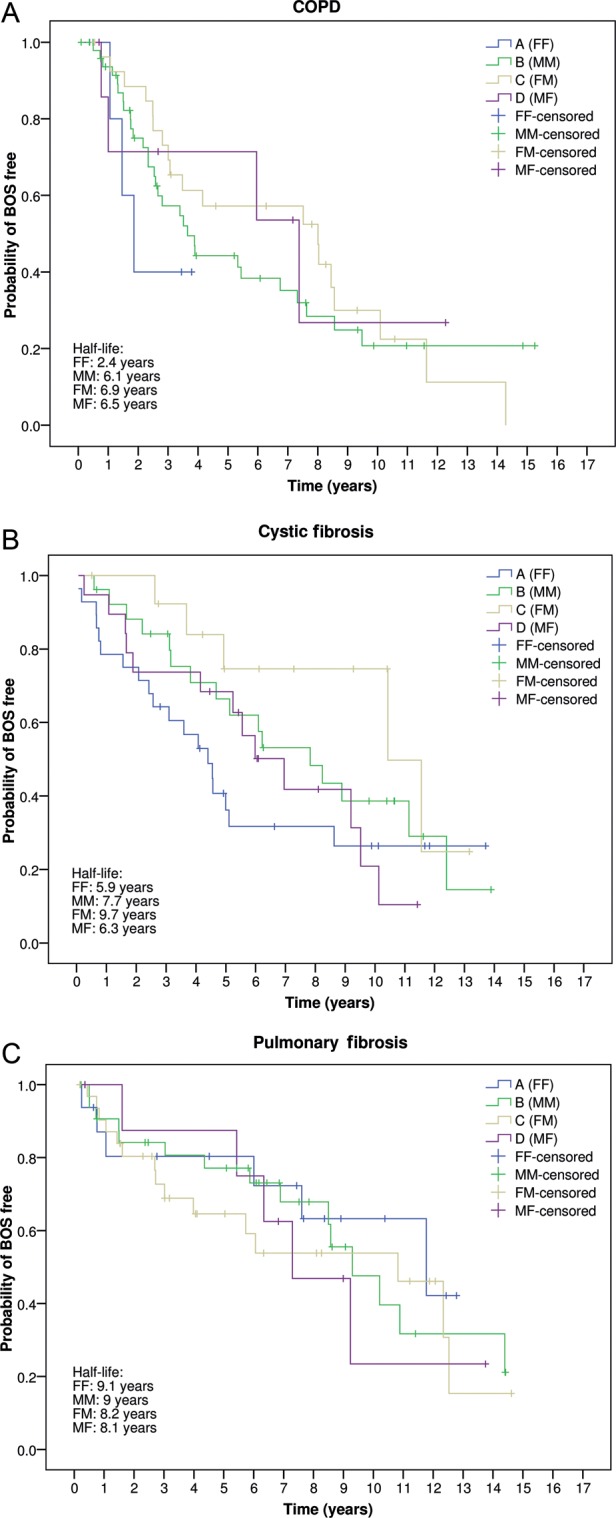
Probability of freedom from BOS, according to donor and recipient gender combinations, and stratified by transplant indication (P = 0.534). A: COPD; B: Cystic fibrosis; C: Pulmonary fibrosis FF: female-to-female; MM: male-to-male; FM: female-to-male; MF: male-to-female (Kaplan–Meier and log-rank test).
Survival
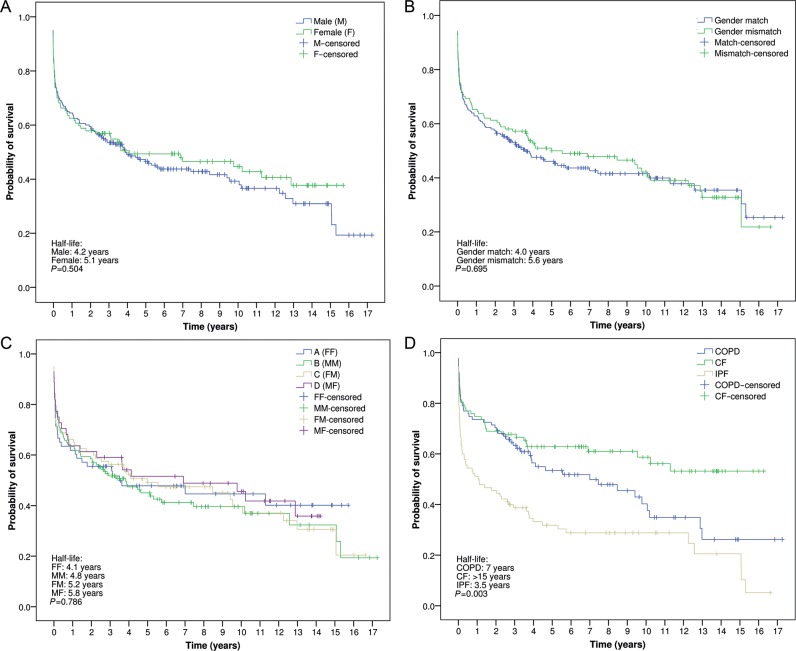
The overall survivals of the patient series were 58, 51, 41 and 35% at 3, 5, 10 and 15 years, respectively. Comparing recipient gender, there was no difference between female recipients and male recipients. However, there was a trend towards better long-term survival for females (half-life for males: 4.2 years vs half-life for females: 5.1 years; P = 0.504) (Fig. 3A). No differences were observed when comparing survival between gender-matched and gender-mismatched transplants (half-life for gender-matched: 4.0 years vs half-life for gender mismatched: 5.6 years; P = 0.695) (Fig. 3B). In addition, the comparative analysis among the four groups of gender pairs demonstrated no survival differences. However, there was a trend towards better long-term survival in female recipients, irrespective of the donor's gender (Fig. 3C). Finally, Fig. 3D depicts the probability of survival in the three main indications for transplantation, with poor short- and long-term survivals for fibrotic patients (half-life: 3.5 years), as opposed to COPD (half-life: 7 years) and CF patients (half-life: >15 years) (P = 0.003).
Figure 3:
(A) Probability of survival comparing recipient gender. (B) Probability of survival comparing gender-matched and gender-mismatched lung transplants. (C) Comparative survival according to donor and recipient gender combinations. (D) Probability of survival by transplant indication. FF: female-to-female; MM: male-to-male; FM: female-to-male; MF: male-to-female; COPD: chronic obstructive pulmonary disease; CF: cystic fibrosis; IPF: idiopathic pulmonary fibrosis (Kaplan–Meier and log-rank test).
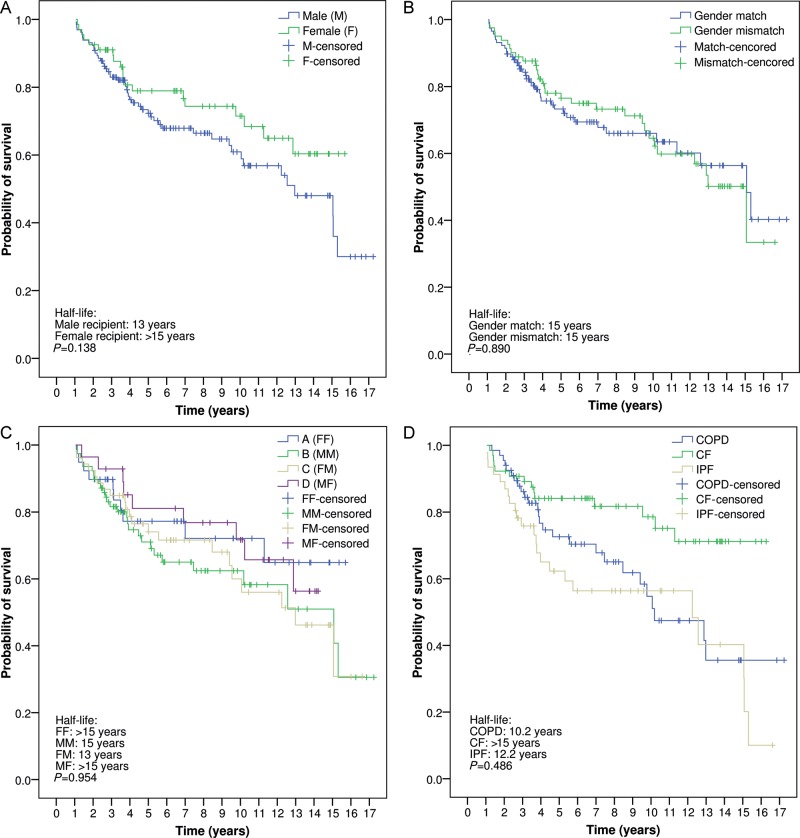
To elucidate whether early post-transplant mortality could have an influence on long-term survival, an additional survival analysis was done on 1-year survivors. A non-significant survival benefit persisted for female recipients surviving 1 year post-transplant (half-life for males: 13 years vs half-life for females: >15 years; P = 0.138) (Fig. 4A). No differences were observed when comparing survival between gender-matched and gender-mismatched transplants surviving 1 year post-transplant (half-life: 15 years) (Fig. 4B), and a non-significant improved long-term survival persisted for female recipients (Groups A and D), irrespective of the donor gender (differences not significant) (Fig. 4C). On the contrary, differences in survival according to transplant indication disappeared when analysing only 1-year survivors, although a trend persisted towards improved survival for CF patients (P = 0.486) (Fig. 4D).
Figure 4:
(A) Probability of survival, conditional to 1-year survival, according to recipient gender. (B) Probability of survival, conditional to 1-year survival, comparing gender-matched and gender-mismatched lung transplants. (C) Comparative survival, conditional to 1-year survival, according to donor and recipient gender combinations. (D) Probability of survival, conditional to 1-year survival, according to transplant indication. FF: female-to-female; MM: male-to-male; FM: female-to-male; MF: male-to-female; COPD: chronic obstructive pulmonary disease; CF: cystic fibrosis; IPF: idiopathic pulmonary fibrosis (Kaplan–Meier and log-rank test).
DISCUSSION
In this single-centre experience, we did not find a negative effect of D/R gender mismatch on early and long-term outcomes after lung transplantation. Long-term survival was not affected by gender mismatch, although we found trends for increased survival for all female recipients, likely related to their underlying disease (i.e. CF). Also, early postoperative mortality did not differ among gender groups and D/R mismatch was not associated either with the onset of PGD or with the development of BOS after transplantation.
Differences in transplant outcomes based on donor and recipient characteristics have been identified in other solid-organ transplantations. However, the possible influence of gender on the outcome of lung transplantation has received little attention. There are several reasons to expect a difference when transplanting across organ and recipient gender. Gender-associated differences could play a role in terms of graft survival, organ size, metabolic demands, circulating hormones and receptors.
In kidney transplantation, Inoue et al. [16] demonstrated better graft survival for female recipients, with female-to-female transplants as the combination with the best graft survival, whereas male-to-male transplantations had the worst. On the contrary, other investigators found a worse prognosis for female kidneys transplanted into male recipients, when compared with male kidneys transplanted into female recipients [3]. Several theories have been proposed to explain these gender-related differences, such as the concept of nephron under-dosing: since male kidneys have more nephrons than female kidneys, an assumption about the relative benefit of a male donor is simply that male kidneys have greater nephron mass. Several studies suggested that male renal allografts may function better than grafts from female donors, but clear experimental support for this explanation of nephron under-dosing with female donor grafts is lacking. Many other factors are likely to play a role in these differences, such as different immunological and hormonal mechanisms that could influence graft survival [17].
Similarly, poor transplant outcomes for female-to-male transplants have been described in hepatic [2] and cardiac transplantation [4] as well, but again, no consistent explanation for such association was given in any case.
For lung transplantation, the data are even more confusing and some theories for the differential outcome of lung transplant recipients based on gender differences have been advocated, including immunity and tolerance theories [18] and hormonal changes [19]. Gender-related hormonal differences have been proposed as factors in the prevalence and outcome of several pulmonary disorders, such as COPD [20]. As of now, there are no published animal studies that evaluate the effect of gender on graft function and survival, and very few data have been published from the clinical scenario. Roberts et al. [5], in a series of 98 patients, found that D/R gender matching significantly affected the development of BOS and survival after lung transplantation. They found trends to increased survival for all female recipients, and for all gender-mismatched transplant recipients. Data from the ISHLT Registry [8] have suggested the best results among female-to-male recipient grafts with stronger survival among FF. Thabut et al. [6] observed that donor gender was significantly associated with long-term survival. On the contrary, other investigators have proposed that gender has no influence on survival among lung transplant recipients, which is consistent with the findings reported here [7, 21, 22].
In our study, we have attempted to minimize the potentially deleterious effects of size mismatch by excluding from the analysis all recipients with significant size mismatch requiring pulmonary tailoring or lobar transplants. When comparing survival among the four groups of gender pairs, no differences were observed. However, a trend towards increased long-term survival was found for female recipients, irrespective of the donor gender (Figs 3 and 4). This finding could be related to the number of female recipients with CF who exhibited the best long-term survival in our series, with a half-life for CF, COPD and pulmonary fibrosis of 15, 7 and 3.5 years, respectively (Figs 3D and 4D), while the ISHLT Registry reports half-life of 7.4, 5.3 and 4.5 years for these transplant indications [8].
PGD developed in 17% of patients in our series, with a PGD-related mortality of 27%, which is similar to the reported 10–25% incidence in other large series [23]. PGD was defined and graded according to the recommendations of the Working Group on PGD of the ISHLT [13]. To rule out possible cases with low oxygenation within the first 24 h post-transplant that could improve early thereafter (reperfusion injury, post-transplant pulmonary oedema), we only selected those cases with persistent low oxygenation ratios and pulmonary infiltrates in chest radiographs at 72 h post-transplant, to be sure that those cases defined as having PGD were real PGD cases. In this sense, it has been reported that PGD Grade 3, as opposed to Grades 1 and 2, had the ability to predict mortality and other transplant outcomes [24].
PGD was more frequent in Groups A and D (female recipients) (23 and 21%, respectively). However, this contrasts with the lower 30-day mortality rate for these groups (4.4 and 2%, respectively). This contradictory data may be related to both the higher proportion of CF patients in these groups, and the greater need for CPB in Group A (34%). It has been reported that the use of CPB is a risk factor for the development of PGD. However, CF was related to low early and long-term mortality rates. Similarly, Christie et al. [23] analysed the effect of organ and recipient gender on the incidence of PGD following lung transplantation. In their study, there was a highest rate of PGD among female-to-female lung transplants (23%), and among female-to-male transplants (20%), with an unadjusted risk ratio of 3.99 (95% CI: 1.74–9.11; P = 0.001), with little evidence of gender mismatching driving this relationship (the differences were not significant after adjusting for donor gender). The possible mechanisms that could explain these findings are unclear.
We did not assess PGD stratifying on type of transplant (single vs bilateral procedures). It seems clear that the type of transplant may influence oxygenation, and some of the conclusions of the Working Group on PGD [13] suggest grading PGD for single- and double-lung transplants separately: probably those patients undergoing single-lung transplants for pulmonary fibrosis are not comparable with those COPD or CF patients undergoing bilateral procedures, because the native lung may artificially lower the oxygenation ratio early post-transplant.
In the present series, freedom from BOS did not differ among the four groups of gender pairs, either in the overall series (Fig. 1) or when stratifying by transplant indication (Fig. 2). This contrasts with the findings reported by Roberts et al. [5] suggesting a decreased freedom from BOS in female recipients of transplants from male donors. The most recent report of the ISHLT Registry [8] does not support these differences, and, to date, no consistent explanation has been given to explain the differences in lung transplant outcomes on the basis of gender combinations.
Several limitations must be considered when evaluating the results reported here. The study was retrospective, with the usual limitations of this design. Although the data were recorded prospectively, the necessary redefinition of PGD (mainly for the early transplant era), required revision of charts and assignment of PGD grades on the basis of the previously recorded data, that could be biased by the subjectivity of the members in-charge of collecting data. The same limitation applies for the definitions of causes of death in the patient series. In addition, the defining criteria of extended donors, even though following the general criteria of previous reports, were chosen arbitrarily.
This study was not designed to determine the independent risk factors of poor outcomes after lung transplantation that could be related to D/R gender combinations, but to analyse the influence of such combinations on four major end-points: 30-day mortality, PGD, BOS and long-term survival. Although we aimed at constructing a homogeneous study group (adjusting for size and diagnosis), other confounding factors might have had an influence. These circumstances might have biased the results to some degree but not, in our opinion, to an extent that may invalidate the main conclusions drawn from the study.
To summarize, this single-centre, retrospective analysis found that D/R gender mismatch does not have a negative impact on short-term and long-term outcomes after lung transplantation. The survival benefit for female patients might be associated with the predominance of CF in this particular group of recipients. Therefore, gender should not be considered a significant enough issue in considering the way the lungs are allocated, because of the constraints of shortage of donor organs and the more significant effects of other donor factors including age and underlying disease. For these reasons, D/R gender matching should not be taken into consideration when allocating donors to specific lung transplant candidates. Additional investigations are required to corroborate these results.
Conflict of interest: none declared.
REFERENCES
- 1.Lynch JP, Saggar R, Weigt SS, Ross DJ, Belperio JA. Overview of lung transplantation and criteria for selection of candidates. Semin Respir Crit Care Med. 2006;27:441–69. doi: 10.1055/s-2006-954604. [DOI] [PubMed] [Google Scholar]
- 2.Rustgi VK, Marino G, Halpern MT, Johnson LB, Umana WO, Tolleris C. Role of gender and race mismatch and graft failure in patients undergoing liver transplantation. Liver Transpl. 2002;8:514–8. doi: 10.1053/jlts.2002.33457. [DOI] [PubMed] [Google Scholar]
- 3.McGee J, Magnus JH, Islam TM, Jaffe BM, Zhang R, Florman SS, et al. Donor-recipient gender and size mismatch affects graft success after kidney transplantation. J Am Coll Surg. 2010;210:718–26. doi: 10.1016/j.jamcollsurg.2009.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stehlik J, Edwards LB, Kucheryavaya AY, Benden C, Christie JD, Dipchand AI, et al. The registry of the International Society for Heart and Lung Transplantation: 29th official adult heart transplant report—2012. J Heart Lung Transplant. 2012;31:1052–64. doi: 10.1016/j.healun.2012.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Roberts DH, Wain JC, Chang Y, Ginns LC. Donor-recipient gender mismatch in lung transplantation: impact on obliterative bronchiolitis and survival. J Heart Lung Transplant. 2004;23:1252–59. doi: 10.1016/j.healun.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 6.Thabut G, Mal H, Cerrina J, Dartevelle P, Dromer C, Velly JF, et al. Influence of donor characteristics on outcome after lung transplantation: a multicenter study. J Heart Lung Transplant. 2005;24:1347–53. doi: 10.1016/j.healun.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 7.Sato M, Gutierrez C, Kaneda H, Liu M, Waddell TK, Keshavjee S. The effect of gender combinations on outcome in human lung transplantation: the International Society of Heart and Lung Transplantation Registry experience. J Heart Lung Transplant. 2006;25:634–7. doi: 10.1016/j.healun.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 8.Christie JD, Edwards LB, Kucheryavaya AI, Benden C, Dobbels F, Kirk R, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-eighth adult lung and heart-lung transplant report—2011. J Heart Lung Transplant. 2011;30:1104–22. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 9.Orens JB, Boehler A, de Perrot M, Estenne M, Glanville AR, Keshavjee S, et al. A review of lung transplant donor acceptability criteria. J Heart Lung Transplant. 2003;22:1183–200. doi: 10.1016/s1053-2498(03)00096-2. [DOI] [PubMed] [Google Scholar]
- 10.Goldman HI, Becklake MR. Respiratory function tests. Am Rev Tuberc Pulm Dis. 1959;79:457–67. doi: 10.1164/artpd.1959.79.4.457. [DOI] [PubMed] [Google Scholar]
- 11.Sundaresan S, Trachiotis GD, Aoe M, Patterson GA, Cooper JD. Donor lung procurement: assessment and operative technique. Ann Thorac Surg. 1993;56:1409–13. doi: 10.1016/0003-4975(93)90699-i. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez A, Salvatierra A, Lama R, Algar J, Cerezo F, Santos F, et al. Preservation with a retrograde second flushing of Eurocollins in clinical lung transplantation. Transplant Proc. 1999;31:1088–90. doi: 10.1016/s0041-1345(98)01915-0. [DOI] [PubMed] [Google Scholar]
- 13.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT Working Group on primary graft dysfunction, Part II: definition. A consensus statement of the International Society for Heart and Lung Transplantation. J Heart Lung Transplant. 2005;24:1454–9. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 14.Estenne M, Hertz MI. Bronchiolitis obliterans after human lung transplantation. Am J Respir Crit Care Med. 2002;166:440–4. doi: 10.1164/rccm.200201-003pp. [DOI] [PubMed] [Google Scholar]
- 15.Alvarez A, Moreno P, Espinosa D, Santos F, Illana J, Algar FJ, et al. Assessment of lungs for transplantation: a stepwise analysis of 476 donors. Eur J Cardiothorac Surg. 2010;37:432–9. doi: 10.1016/j.ejcts.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 16.Inoue S, Yamada Y, Kuzuhara K, Ubara Y, Hara S, Ootubo O. Are women privileged organ recipients? Transplant Proc. 2002;34:2775–6. doi: 10.1016/s0041-1345(02)03408-5. [DOI] [PubMed] [Google Scholar]
- 17.Csete M. Gender issues in transplantation. Anesth Analg. 2008;107:232–8. doi: 10.1213/ane.0b013e318163feaf. [DOI] [PubMed] [Google Scholar]
- 18.Simpson E, Scott D, Chandler P. The male-specific histocompatibility antigen. Ann Rev Immunol. 1997;15:39–61. doi: 10.1146/annurev.immunol.15.1.39. [DOI] [PubMed] [Google Scholar]
- 19.Sweezey N, Tchepichev S, Cagnon S, Fertuck K, O'Brodovich H. Female gender hormones regulate mRNA levels and function of the rat lung epithelial Na channel. Am J Physiol. 1998;274:379–86. doi: 10.1152/ajpcell.1998.274.2.C379. [DOI] [PubMed] [Google Scholar]
- 20.Silverman EK, Weiss ST, Drazen JM, Chapman HA, Carey V, Campbell EJ, et al. Gender-related differences in severe, early-onset chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2000;162:2152–8. doi: 10.1164/ajrccm.162.6.2003112. [DOI] [PubMed] [Google Scholar]
- 21.Miñambres E, Llorca J, Subrviola B, Ballesteros MA, Ortiz-Melón F, González-Castro A. Influence of donor-recipient gender mismatch in early outcome after lung transplantation. Transplant Proc. 2008;40:3076–8. doi: 10.1016/j.transproceed.2008.08.122. [DOI] [PubMed] [Google Scholar]
- 22.Fessart D, Dromer C, Thumerel M, Jougon J, Delom F. Influence of gender donor-recipient combinations on survival after human lung transplantation. Transplant Proc. 2011;43:3899–902. doi: 10.1016/j.transproceed.2011.08.101. [DOI] [PubMed] [Google Scholar]
- 23.Christie JD, Kotloff RM, Pochettino A, Arcasoy SM, Rosengard BR, Landis JR, et al. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124:1232–41. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 24.Prekker ME, Nat DS, Walker AR, Johnson AC, Hertz MI, Herrington CS, et al. Validation of the proposed International Society for Heart and Lung Transplantation grading system for primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2006;25:371–8. doi: 10.1016/j.healun.2005.11.436. [DOI] [PubMed] [Google Scholar]