Abstract
Peritoneal dialysis (PD) solution contains high concentrations of glucose and glucose degradation products (GDPs). One of several GDPs—3,4-dideoxyglucosone-3-ene (3,4-DGE)—was recently identified as the most reactive and toxic GDP in PD fluids. In vitro, 3,4-DGE has been shown to induce mesothelial cell damage; however, its role in peritoneal fibrosis in vivo remains unclear. In the present study, we intraperitoneally administered chlorhexidine gluconate (CG) for mild peritoneal injury, and we then injected 3,4-DGE [38 μmol/L (low concentration) or 145 μmol/L (high concentration)] 5 times weekly for 4 weeks. Significant thickening of the parietal peritoneal membrane was observed only when treatment with low or high concentrations of 3,4-DGE occurred after CG administration, but not when either CG or 3,4-DGE alone was given. The combination of CG and 3,4-DGE also caused upregulation of messenger RNA expression of transforming growth factor β1, connective tissue growth factor, fibronectin, collagen type 1 α1 chain, alpha smooth muscle actin (α-SMA), vascular endothelial growth factor 164, NADPH oxidase 1 and 4, p22phox, p47phox, and gp91phox in peritoneal tissue. Treatment with CG alone was sufficient to cause significant F4/80-positive macrophage infiltration, appearance of α-SMA-positive cells, and vessel formation in the submesothelial layer. Addition of 3,4-DGE markedly enhanced those changes and induced apoptosis, mainly in leukocytes. The concentration of 3,4-DGE in the abdominal cavity declined more rapidly in CG-treated mice than in PBS-treated mice. Peritoneal membrane permeability determined by peritoneal equilibration test showed high transport conditions in peritoneum treated with both CG and 3,4-DGE. These results indicate that, when mild peritoneal damage is already present, 3,4-DGE causes peritoneal thickening and fibrosis, resulting in deterioration of peritoneal membrane function.
Key words: Peritoneum; mesothelial cells; 3,4-DGE; apoptosis; macrophages; angiogenesis; chlorhexidine gluconate
Peritoneal dialysis (PD) is a well-established method of home dialysis for patients with end-stage renal failure. During long-term PD, the peritoneal membrane develops peritoneal fibrosis in response to a variety of injuries, including bioincompatible PD solutions, peritonitis, uremia, and chronic inflammation (1,2). Solutions for PD contain high concentrations of glucose, which result in glucose degradation products (GDPs) during the process of heat sterilization. Some GDPs identified in PD fluid (3,4) include acetaldehyde, 3-deoxyglucosone, formaldehyde, 2-furaldehyde, glyoxal, 5-hydroxymethylfurfural, methylglyoxal (MGO), and 3,4-dideoxyglucosone-3-ene (3,4-DGE). Among those GDPs, the highly reactive 3,4-DGE is a toxic substance in PD fluid (4,5). Recently, PD fluid with a neutral pH and lower GDPs has shown improved performance, as indicated by reduced levels of inflammatory markers in effluent and of circulating advanced glycation endproducts (6).
Fluids containing high GDP levels are relevant to peritoneal fibrosis and loss of ultrafiltration (7,8). Although the mechanisms of GDP cytotoxicity are not fully understood, 3,4-DGE has been shown to affect the cytotoxicity of acidic, heat-sterilized PD fluid on human peritoneal mesothelial cells (9). In particular, 3,4-DGE induces apoptosis and epithelial-mesenchymal transition (EMT) in peritoneal mesothelial cells (10). The concentration of 3,4-DGE in conventional PD fluids is normally 10-38 μmol/L (4,5), enough to promote mesothelial cell apoptosis (10). The high reactivity of 3,4-DGE is responsible for depletion of total intracellular glutathione (9), suggesting that 3,4-DGE can enhance oxidative stress in peritoneal mesothelial cells (11). Infusion of conventional PD solution containing an intermediate level of GDPs and lipopolysaccharide (compared with low-GDP solution and lipopolysaccharide) induced high peritoneal transport in rats (12,13). Low-GDP solution caused less peritoneal injury and submesothelial vascularization in rats (14).
Peritoneal dialysis fluid containing GDPs is closely associated with EMT of peritoneal mesothelial cells in vivo (15). However, it is not clear whether 3,4-DGE plays a role in peritoneal damage in vivo, because no report has shown that 3,4-DGE induces peritoneal fibrosis in that situation. We therefore used two doses of 3,4-DGE—38 μmol/L, the highest found in conventional PD fluid, and 145 μmol/L, higher than the amount found in PD fluid—to examine the role of 3,4-DGE in peritoneal fibrosis and inflammation in vivo. We also examined whether pre-existing chlorhexidine gluconate (CG)-induced peritoneal injury increases 3,4-DGE-induced peritoneal injury.
METHODS
ANIMAL MODELS
All animal experiments were approved by the animal experimentation committee of Kyoto University Graduate School of Medicine. Purification of 3,4-DGE was performed as previously described (11). We treated C57BL/6J mice weighing approximately 26 g with intraperitoneal injections of 0.3 mL 0.1% CG in 15% ethanol and 85% phosphate-buffered saline (PBS) every other day for 1 week; they were then injected with 38 μmol/L or 145 μmol/L 3,4-DGE dissolved in 1 mL PBS every weekday for 4 weeks without antibiotics. Control mice received PBS only. Mice were assigned randomly to one of the following groups:
Group 1: initial PBS and subsequent PBS without 3,4-DGE [PBS+3,4-DGE(-)], n = 10
Group 2: initial PBS and subsequent 38 μmol/L 3,4-DGE (PBS+38 μmol/L 3,4-DGE), n = 8
Group 3: initial PBS and subsequent 145 μmol/L 3,4-DGE (PBS+145 μmol/L 3,4-DGE), n = 5
Group 4: initial CG and subsequent PBS without 3,4-DGE [CG+3,4-DGE(-)], n = 8
Group 5: initial CG and subsequent 38 μmol/L 3,4-DGE (CG+38 μmol/L 3,4-DGE), n = 11
Group 6: initial CG and subsequent 145 μmol/L 3,4-DGE (CG+145 μmol/L 3,4-DGE), n = 5
QUANTITATIVE ANALYSIS OF 3,4-DGE IN PLASMA AND PERITONEAL EFFLUENT
The concentration of 3,4-DGE in the abdominal cavity was evaluated. Mice were injected with PBS (n = 8) or 0.1% CG (n = 7) every other day for 1 week. Then, using an 18G needle, 4 mL 3,4-DGE diluted in PBS was injected into the peritoneal cavity of the mice. Peritoneal solution was collected at 1, 10, 20, and 30 minutes after injection. At 30 minutes after injection, blood samples were collected using heparin-coated capillary tubes from mice given 145 μmol/L 3,4-DGE (n = 2 from the PBS group, n = 3 from the CG group). Blood samples were centrifuged and plasma components were separated.
The concentrations of 3,4-DGE in plasma and peritoneal effluent were analyzed by liquid chromatography-mass spectrometry as a quinoxaline derivative after a reaction with 2,3-diamino naphthalene. Briefly, 50 μL of sample or standard solution was diluted with an equivalent amount of 0.2 mol/L sodium phosphate buffer (pH 7.4). Then 100 μL of 0.05% 2,3-diamino naphthalene (Tokyo Chemical Industry, Tokyo, Japan) in acetonitrile was added to the solution and carefully mixed. For deproteinization, the mixture was centrifuged (6000g for 10 minutes), after which an aliquot of the supernatant was incubated at 25°C for 20 hours under dark conditions for derivative formation. The reaction solution was assayed by reverse-phase high-performance liquid chromatography using a Symmetry column (Waters, Milford, MA, USA) with 25% - 65% gradient elution of acetonitrile containing 0.1% formic acid. The 3,4-DGE-quinoxaline derivative was then detected as protonated molecular ion at 267.1 Da [C16H14N2O2 + H]+ by electrospray positive-ionization mass spectrometry.
The preliminary experiment confirmed that the blood component inhibited the reaction between 3,4-DGE and 2,3-diaminonaphthalene, and therefore, to minimize the inhibitory effect, the calibration standards were prepared using serum. For analysis of the peritoneal fluid, aqueous solutions of 3,4-DGE were used as calibration standards. All standard solutions were treated using identical derivatization processing.
MODIFIED PERITONEAL EQUILIBRATION TEST
Before the mice were humanely euthanized, a modified peritoneal equilibration test was performed to determine peritoneal permeability as previously described (16). Briefly, the mice (group 1: n = 3; group 2: n = 3; group 4: n = 3; group 5: n = 5) were given 3-mL intraperitoneal injections of 7% glucose dialysis solution (Perisate: JMS, Hiroshima, Japan). After a 2-hour dwell, effluents were collected and blood samples were drawn. Serum and dialysate creatinine and urea nitrogen levels were measured by the enzymatic method (SRL, Tokyo, Japan). Calculation of the mass transfer-area coefficient (MTAC) of urea was calculated as previously described (17). Mice were euthanized under anesthesia at 5 weeks after CG-treatment, and samples were collected for histologic and biochemical analyses.
HISTOLOGY AND IMMUNOHISTOCHEMICAL STUDY FOR THE PERITONEUM
Anterior abdominal walls containing parietal peritoneum were fixed with 4% buffered paraformaldehyde and embedded in paraffin. We measured the thickness of the fibrotic submesothelial zone above the abdominal muscle layer in cross-sections as previously described (18).
For immunohistochemical analyses of F4/80, CD31, and cytokeratin, the sections were processed as described, with some modifications (18,19). After 0.1% trypsin-mediated antigen retrieval, the samples were incubated with rat monoclonal anti-F4/80 antibody (Serotec, Oxford, UK), rat monoclonal anti-CD31 antibody (BD Biosciences, San Diego, CA, USA), or rabbit polyclonal anti-cytokeratin antibody (Dako, Glostrup, Denmark). After incubation with biotin-conjugated secondary anti-rat immunoglobulin G antibody (Vector Laboratories, Burlingame, CA, USA), the specimens were treated with streptavidin-conjugated horse-radish peroxidase (Dako) and then developed using 3,3′-diaminobenzidine tetrahydrochloride (Dako). For immunohistochemical analyses of alpha smooth muscle actin (α-SMA), the sections were processed using microwave-mediated antigen retrieval and were then incubated with rabbit polyclonal anti-α-SMA antibody (Abcam, Cambridge, UK).
REAL-TIME POLYMERASE CHAIN REACTION ANALYSIS
Quantitative real-time polymerase chain reaction was performed using Premix Ex Taq (Takara Bio, Shiga, Japan) on an Applied Biosystems 7300 real-time polymerase chain reaction system (Applied Biosystems, Foster City, CA, USA) or a StepOnePlus system (Applied Biosystems) as previously described, with some modifications (18,19). Gene-specific primers and probes were then used to determine the expression levels of mouse transforming growth factor β1 (TGF-β1), connective tissue growth factor (CTGF), fibronectin, collagen type 1 alpha 1 chain (COL1A1), α-SMA, vascular endothelial growth factor 164 (VEGF164), NADPH oxidase 1 (NOX1) and 4 (NOX4), p22phox, p47phox, and gp91phox (Table 1). Expression of each messenger RNA (mRNA) was normalized to GAPDH mRNA (TaqMan rodent GAPDH control reagents: Applied Biosystems).
TABLE 1.
TaqMan primers and probe sequencesa
ASSESSMENT OF APOPTOSIS
Apoptosis was quantified using a terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay with in situ cell death detection kit and fluorescein (Roche, Basel, Switzerland) as previously described (20). To detect apoptotic cell types, triple staining for CD45, TUNEL, and DAPI was performed. Paraffin-embedded peritoneal sections 4 μm in thickness were deparaffinized and treated with microwaves for antigen retrieval. Sections were then processed with rabbit polyclonal anti-CD45 antibody (Abcam) for 1 hour at room temperature. After incubation with DyLight 549 conjugated donkey anti-rabbit antibody (Jackson ImmunoResearch, West Grove, PA, USA), the sections were treated with 0.1% Triton X in 0.1% sodium citrate buffer and then incubated with TUNEL reaction mixture.
STATISTICAL ANALYSIS
Data are expressed as mean ± standard error of the mean. The statistical analysis was performed using one-way analysis of variance or the Student t-test, as appropriate. A p value of less than 0.05 was considered statistically significant.
RESULTS
PRETREATMENT WITH CG ENHANCES 3,4-DGE-INDUCED PERITONEAL FIBROSIS
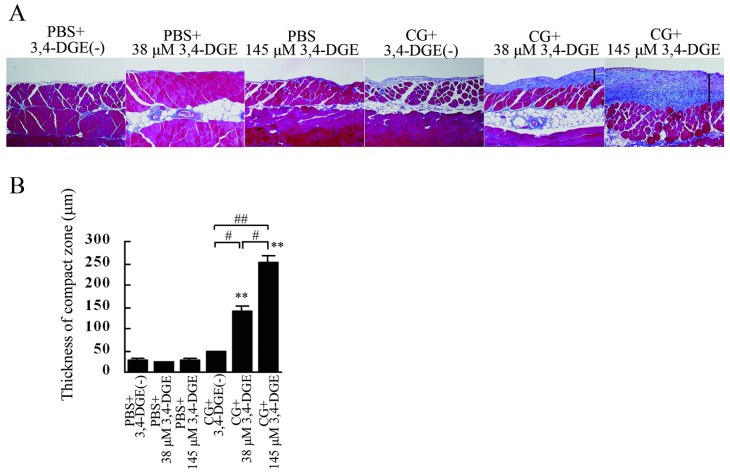
Mice received intraperitoneal injections of PBS every other day for 1 week (3 times) and then injections of low (38 μmol/L) or high (145 μmol/L) concentrations 3,4-DGE every weekday for 4 weeks. Mice treated with 3,4-DGE or CG (or both) and mice receiving PBS+3,4-DGE(-) all had similar body weights (Figure 1). The thickness of the peritoneal membrane was analyzed using Masson trichrome staining. The PBS+3,4-DGE(-) and 38 μmol/L or 145 μmol/L PBS+3,4-DGE mice showed almost normal peritoneal tissues. Notably, the thickness of the peritoneal membrane was greater for CG+38 μmol/L 3,4-DGE mice than for CG+3,4-DGE(-) mice. The CG+145 μmol/L 3,4-DGE mice showed even more pronounced peritoneal membrane thickness: PBS+3,4-DGE(-), 27.9 ± 4.8 μm; PBS+38 μmol/L 3,4-DGE, 23.5 ± 1.6 μm; PBS+145 μmol/L 3,4-DGE, 26.8 ± 4.1 μm; CG+3,4-DGE(-), 47.2 ± 1.7 μm; CG+38 μmol/L 3,4-DGE, 142 ± 11 μm; CG+145 μmol/L 3,4-DGE, 253 ± 16 μm (Figure 2).
Figure 1.
— Time course of body weight in mice. Mice were treated with intraperitoneal injections of 0.1% chlorhexidine gluconate (CG) or phosphate buffered saline (PBS) every other day for a week. They were then injected with 38 μmol/L or 145 μmol/L 3,4-dideoxyglucosone-3-ene (3,4-DGE) or PBS every weekday for 4 weeks. Initial PBS followed by PBS without 3,4-DGE (n = 10, open circles); initial PBS followed by PBS plus 38 μmol/L 3,4-DGE (n = 8, open triangles); initial PBS followed by PBS with 145 μmol/L 3,4-DGE (n = 5, open squares); initial CG followed by PBS without 3,4-DGE (n = 8, closed circles); initial CG followed by PBS with 38 μmol/L 3,4-DGE (n = 11, closed triangles); CG plus 145 μmol/L followed by PBS with 3,4-DGE (n = 5, close squares). All mice were compared with mice receiving PBS without 3,4-DGE; no significant differences were observed.
Figure 2.
— Histologic appearance of the peritoneum in each group. (A) Compared with mice treated with phosphate-buffered saline (PBS) plus 38 μmol/L 3,4-dideoxyglucosone-3-ene (3,4-DGE), mice treated with chlorhexidine gluconate (CG) plus 38 μmol/L 3,4-DGE showed thickened peritoneum. In mice treated with CG+145 μmol/L 3,4-DGE, peritoneal fibrosis was more pronounced. Arrows indicate the submesothelial compact zone (Masson trichrome stain). (B) Thickness of the peritoneal membrane (mean ± standard error of the mean) in mice under various conditions: PBS without 3,4-DGE [PBS+3,4-DGE(-), n = 7]; PBS+38 μmol/L 3,4-DGE (n = 5); PBS+145 μmol/L 3,4-DGE (n = 5); CG+3,4-DGE(-) (n = 5); CG+38 μmol/L 3,4-DGE (n = 6); CG+145 μmol/L 3,4-DGE (n = 5). ** p < 0.01 versus mice treated with PBS and same dose of 3,4-DGE; # p < 0.05; ## p < 0.01.
EFFECT OF CG AND 3,4-DGE ON GENE EXPRESSION IN PERITONEAL INJURY
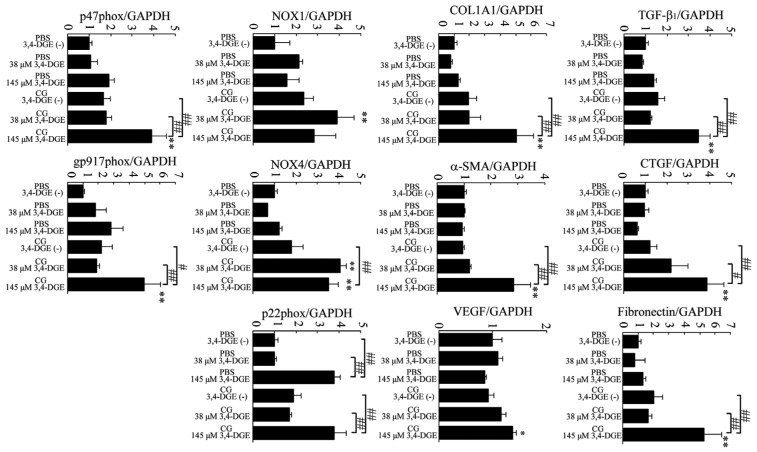
We next examined gene expression of TGF-β1, CTGF, fibronectin, COL1A1, α-SMA, and VEGF164 in peritoneum (Figure 3). Administration of 38 μmol/L or 145 μmol/L 3,4-DGE in PBS-treated mice did not increase mRNA expression of TGF-β1, CTGF, fibronectin, COL1A1, α-SMA, and VEGF164. Compared with CG+3,4-DGE(-) mice, the CG+145 μmol/L 3,4-DGE mice showed increased TGF-β1, CTGF, fibronectin, COL1A1, α-SMA, and VEGF164 expression, suggesting that the combination of CG and 3,4-DGE enhances extracellular matrix production.
Figure 3.
— Analysis by real-time reverse-transcriptase polymerase chain reaction of peritoneal expression of messenger RNA for transforming growth factor β1 (TGF-β1), connective tissue growth factor (CTGF), fibronectin, collagen type 1 α1 (COL1A1), alpha smooth muscle actin (α-SMA), vascular endothelial growth factor 164 (VEGF164), NOX1, NOX4, p22phox, p47phox, and gp91phox. GAPDH was used as a control. Number of mice: phosphate buffered saline (PBS) without 3,4-dideoxyglucosone-3-ene (3,4-DGE) (n = 7); PBS+38 μmol/L 3,4-DGE (n = 5); PBS+145 μmol/L 3,4-DGE (n = 5); CG without 3,4-DGE (n = 5); CG+38 μmol/L 3,4-DGE (n = 6); and CG+145 μmol/L 3,4-DGE (n = 5). All values: mean ± standard error of the mean. * p < 0.05; ** p < 0.01 versus mice receiving PBS with the same dose of 3,4-DGE; # p < 0.05; ## p < 0.01.
Next, we examined the expression of NOX1, NOX4, p22phox, p47phox, and gp91phox, which are essential membrane components of NAD(P)H oxidase, in peritoneum. Compared with PBS+3,4-DGE(-) mice, the PBS+38 μmol/L 3,4-DGE mice did not show increases in mRNA expression for those components. Compared with PBS+3,4-DGE(-) mice, the PBS+145 μmol/L 3,4-DGE mice showed increased mRNA expression of p22phox. The CG+38 μmol/L 3,4-DGE mice showed higher expression of NOX1 and NOX4 than did CG+3,4-DGE(-) mice. In CG+145 μmol/L 3,4-DGE mice, NOX4, p47phox, and gp91phox mRNA expression were higher than the expression observed in PBS+3,4-DGE(-) mice. These results suggest that the combination of CG and 3,4-DGE can increase oxidative stress.
MACROPHAGE RECRUITMENT, A-SMA-POSITIVE CELLS, AND VASCULAR VESSELS
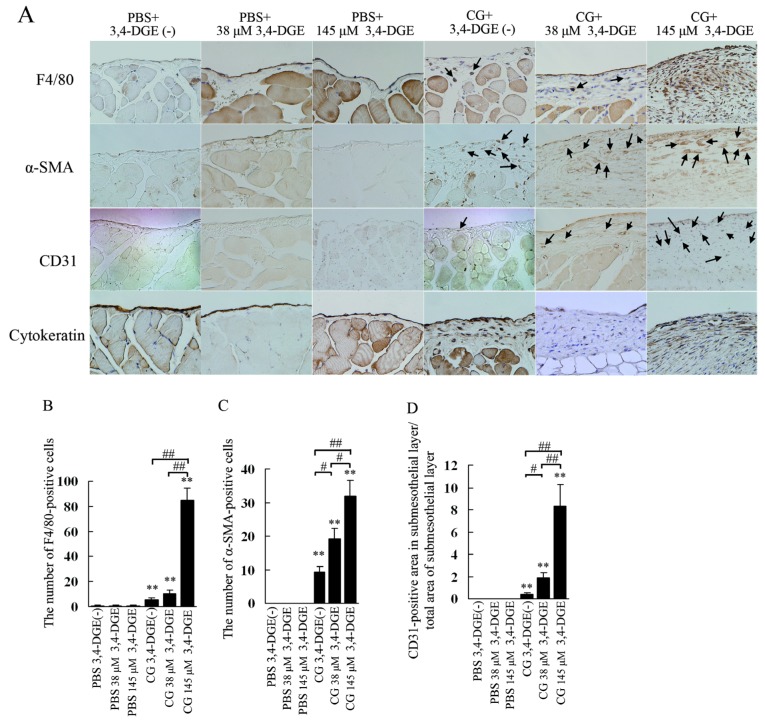
Macrophage infiltration was assessed by immunohistochemistry for F4/80 [Figure 4(A)]. The number of macrophages in peritoneum was very small in PBS+3,4-DGE(-) and 38 μmol/L or 145 μmol/L PBS+3,4-DGE mice [Figure 4(B)]. In CG+3,4-DGE(-) mice, significantly increased numbers of F4/80-positive cells were observed around the submesothelial compact zone. Although the presence of 38 μmol/L 3,4-DGE did not change the number of infiltrating macrophages, the numbers of those cells were markedly increased in CG+145 μmol/L 3,4-DGE mice compared with CG+3,4-DGE(-) mice (85.1 ± 9.4 vs 5.8 ± 1.1). These results indicate that 3,4-DGE alone did not increase macrophage infiltration at the concentrations tested, but that 3,4-DGE augmented macrophage infiltration in the presence of pre-existing peritoneal injury.
Figure 4.
— (A) Immunohistochemical study for F4/80, alpha smooth muscle actin (α-SMA), CD31, and cytokeratin in the peritoneum. Mice receiving chlorhexidine gluconate (CG) plus 38 μmol/L or 145 μmol/L 3,4-dideoxyglucosone-3-ene (3,4-DGE) showed increased F4/80-positive cells (arrows) and α-SMA positive cells (arrows). Vessels positive for CD31 were increased in mice treated with CG plus 38 μmol/L or 145 μmol/L 3,4-DGE. Cytokeratin staining showed that mesothelial cells were detached in mice treated with CG plus 38 μmol/L or 145 μmol/L 3,4-DGE. (B) Number of F4/80-positive cells in the submesothelial area. (C) Number of α-SMA-positive cells in the submesothelial area. (D) Ratio of the area in the submesothelial layer positive for CD31 to the total area of the submesothelial layer. Number of mice: phosphate buffered saline (PBS) without 3,4-DGE [PBS+3,4-DGE(-), n = 7]; PBS+38 μmol/L 3,4-DGE (n = 5); PBS+145 μmol/L 3,4-DGE (n = 5); CG+3,4-DGE(-) (n = 5); CG+38 μmol/L 3,4-DGE (n = 6); CG+145 μmol/L 3,4-DGE (n = 5). All values: mean ± standard error of the mean. * p < 0.05; ** p < 0.01 versus mice treated with PBS and the same dose of 3,4-DGE; # p < 0.05; ## p < 0.01.
We next examined α-SMA-positive cells using immunohistochemistry [Figure 4(A)]. The expression of α-SMA was confined to vascular smooth muscle cells in PBS-treated mice. In CG-treated mice, α-SMA-positive cells were localized in the submesothelial compact zone. The numbers of α-SMA-positive cells were significantly higher in both 38 μmol/L and 145 μmol/L CG+3,4-DGE mice than in CG+3,4-DGE(-) mice [20.2 ± 2.2 and 32 ± 4.5 vs 9.4 ± 1.4, Figure 4(C)].
To investigate vascular changes, we performed an immunohistochemical study for CD31 [Figure 4(A)]. In PBS-treated mice, no CD31-positive vessels were observed in the submesothelial layer. The CG+3,4-DGE(-) mice showed increased CD31-positive vessels in the compact zone, an increase that was further augmented in 38 μmol/L and 145 μmol/L CG+3,4-DGE mice.
The area of CD31-positive vessels in the submesothelial compact zone was also quantified [Figure 4(D)]. The area of these blood vessels was greater in 38 μmol/L and 145 μmol/L CG+3,4-DGE mice than in CG+3,4-DGE(-) mice, and the effect was dose-dependent (1.8% ± 0.45% and 8.3% ± 1.9% vs 0.42% ± 0.11%). These results demonstrate that 3,4-DGE can increase CD31-positive vessels in injured peritoneum.
We next examined the presence of mesothelial cells by cytokeratin staining. All PBS-treated mice, including those receiving 145 μmol/L 3,4-DGE showed almost intact mesothelial cells [Figure 4(A)]. Although CG+3,4-DGE(-) mice showed no change in mesothelial cells, mice treated with of 3,4-DGE in addition to CG showed severe detachment of mesothelial cells from the peritoneal membrane [Figure 4(A)].
APOPTOSIS
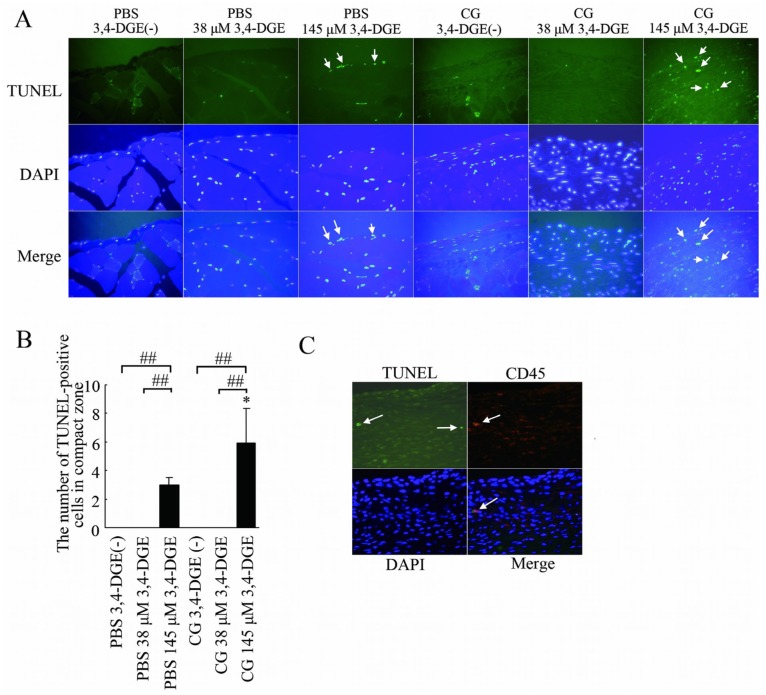
We next used TUNEL staining to examine apoptosis in peritoneal injury. Almost no apoptotic cells were detected without 3,4-DGE or with 38 μmol/L 3,4-DGE even in CG-treated mice. In CG+3,4-DGE(-) mice, apoptotic cells were increased only in the abdominal rectus muscles, not in the submesothelial compact zone [Figure 5(A)]. The PBS+145 μmol/L 3,4-DGE and CG+145 μmol/L 3,4-DGE mice both showed pronounced apoptotic cells in peritoneum [Figure 5(A)].
Figure 5.
— Apoptotic cells in the peritoneum. (A) TUNEL staining (green), DAPI staining (blue), and merged images. Mice treated with phosphate-buffered saline (PBS) plus 145 μmol/L 3,4-dideoxyglucosone-3-ene (3,4-DGE) showed TUNEL-positive cells (arrows). Mice treated with chlorhexidine gluconate (CG) plus 145 μmol/L 3,4-DGE showed more TUNEL-positive cells than did mice treated with PBS+145 μmol/L 3,4-DGE (arrows). DAPI was used as nuclear staining. Most TUNEL-positive cells were also positive for DAPI. Number of mice: PBS without 3,4-DGE [PBS+3,4-DGE(-), n = 7]; PBS+38 μmol/L 3,4-DGE (n = 5); PBS+145 μmol/L 3,4-DGE (n = 5); CG+3,4-DGE(-) (n = 5); CG+38 μmol/L 3,4-DGE (n = 6); CG+145 μmol/L 3,4-DGE (n = 5). (B) Ratio of TUNEL-positive cells to DAPI-positive cells in the submesothelial area in mice. All values: mean ± standard error of the mean. * p < 0.05 versus mice treated with PBS and the same dose of 3,4-DGE; ## p < 0.01. (C) Triple staining for TUNEL (green), CD45 (red), and DAPI (blue) in mice (n = 5) treated with CG+145 μmol/L 3,4-DGE. Some of TUNEL-positive cells are leukocytes (arrows).
For nuclear staining, DAPI was used. Most TUNEL-positive cells were also positive for DAPI, confirming that the TUNEL signals derived specifically from nuclei. The mean number of TUNEL-positive cells in PBS+3,4-DGE and CG+3,4-DGE mice was 3.0 and 5.9, respectively [Figure 5(B)], indicating that 3,4-DGE is relevant to apoptosis in vivo in the peritoneum.
To detect the cell type of the apoptotic cells, triple staining for TUNEL, DAPI, and CD45 (a leukocyte marker) was performed in CG+3,4-DGE mice [Figure 5(C)]. Some TUNEL-positive cells were positive for CD45, indicating that some of apoptotic cells were leukocytes.
ELIMINATION OF 3,4-DGE FROM THE PERITONEAL CAVITY AND APPEARANCE OF 3,4-DGE IN PLASMA
We next examined the rate at which 3,4-DGE was eliminated from the peritoneal cavity in PBS- or CG-treated mice, because apoptosis was not detected in the 38 μmol/L 3,4-DGE group, a result that is not consistent with a previous in vitro study that showed induction of mesothelial cell apoptosis even in the presence of 38 μmol/L 3,4-DGE.
We speculated that the 3,4-DGE injected into the peritoneal cavity was rapidly eliminated, especially in injured perineum. The residual concentration of 3,4-DGE in PBS-treated mice was 53% [Figure 6(A)]. In contrast, CG-treated mice showed a much reduced residual level of 3,4-DGE [10%, Figure 6(A)]. Interestingly, in mice given 145 μmol/L 3,4-DGE, the plasma concentration of 3,4-DGE was much higher in the CG-treated group than in the PBS-treated group [Figure 6(B)]. These results indicate that injured peritoneum causes 3,4-DGE to disappear from the peritoneal cavity rapidly and that some 3,4-DGE is absorbed into the systemic circulation.
Figure 6.
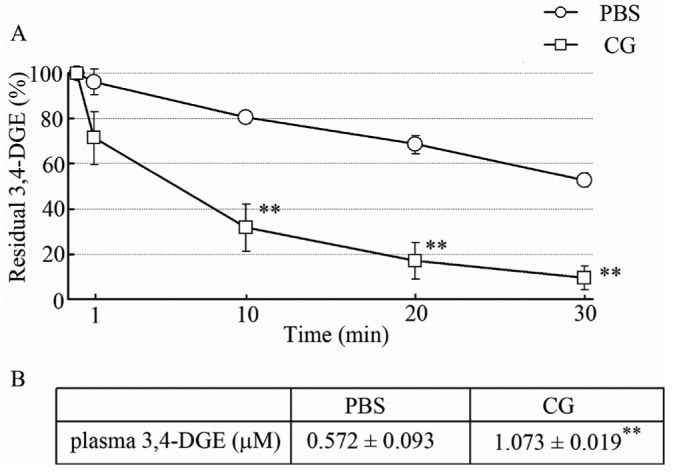
— Elimination rate of 3,4-dideoxyglucosone-3-ene (3,4-DGE) from the peritoneal cavity and appearance rate of 3,4-DGE in plasma. Mice were treated with phosphate buffered saline (PBS) or chlorhexidine gluconate (CG) 3 times in 1 week, and then 4 mL 3,4-DGE-containing PBS was injected into the peritoneal cavity. Peritoneal fluid was collected at 1, 10, 20, and 30 minutes after injection. (A) Residual concentration of 3,4-DGE in peritoneal fluid. Mice treated with CG showed rapid elimination of 3,4-DGE from the peritoneal cavity (PBS-treated: n = 8; CG-treated: n = 7). (B) Plasma level of 3,4-DGE in PBS- or CG-treated mice at 30 minutes after injection of 145 μmol/L 3,4-DGE. Compared with PBS-treated mice, those treated with CG showed high plasma levels of 3,4-DGE (PBS-treated: n = 2; CG-treated: n = 3). ** p < 0.01 versus PBS-treated mice.
MODIFIED PERITONEAL EQUILIBRATION TEST
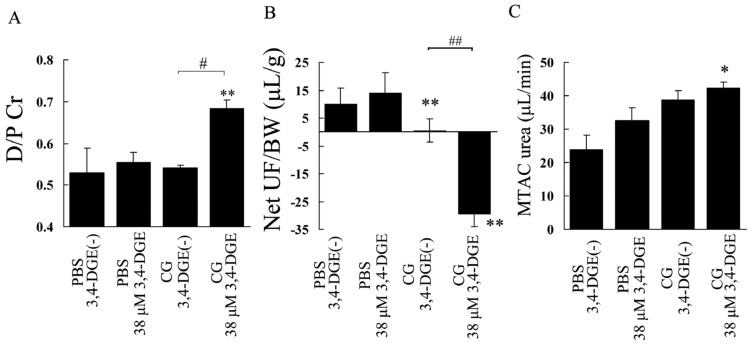
Peritoneal equilibration tests were performed to examine the functional role of 3,4-DGE in peritoneal fibrosis. Figure 7(A) shows that the dialysate-to-plasma ratio of creatinine (D/P Cr) in PBS+38 μmol/L 3,4-DGE mice was not different from that in PBS+3,4-DGE(-) mice. In contrast, the D/P Cr was significantly higher in CG+38 μmol/L 3,4-DGE mice than in PBS+38 μmol/L 3,4-DGE or CG+3,4-DGE(-) mice (0.68 vs 0.55 and 0.54 respectively). By analyzing the ratio of net ultrafiltration to body weight, CG-treated mice not receiving 3,4-DGE showed reduced ultrafiltration and CG+38 μmol/L 3,4-DGE mice showed net ultrafiltration failure. The MTAC urea also indicated that, compared with PBS+38 μmol/L 3,4-DGE mice, CG+3,4-DGE mice showed increased urea transport. These results suggest that 3,4-DGE is associated with high peritoneal transport in peritoneal fibrosis.
Figure 7.
— Modified peritoneal equilibration test. (A) The creatinine (Cr) concentration in 7% glucose dialysate effluent (D) divided by the Cr concentration in plasma (P) in mice at 2 hours. (B) Net ultrafiltration (UF) / body weight (BW). (C) Mass transfer-area coefficient (MTAC). Compared with mice treated with phosphate buffered saline (PBS) plus 38 μmol/L 3,4-dideoxyglucosone-3-ene (3,4-DGE), mice treated with chlorhexidine gluconate (CG) plus 38 μmol/L 3,4-DGE showed high peritoneal transport. Net UF / BW and MTAC urea indicated that CG treatment induced ultrafiltration failure and high urea transport. Number of mice: PBS without 3,4-DGE [PBS+3,4-DGE(-), n = 3]; PBS+38 μmol/L 3,4-DGE (n = 3); CG+3,4-DGE(-) (n = 3); CG+38 μmol/L 3,4-DGE (n = 5). All values: mean ± standard error of the mean. *p < 0.05; **p < 0.01 versus PBS-treated mice receiving the same dose of 3,4-DGE; # p < 0.05; ## p < 0.01.
DISCUSSION
Although previous reports showed that GDP-containing conventional PD solutions induce peritoneal fibrosis with enhanced peritoneal permeability even after 5 weeks in vivo (21), it is not clear whether 3,4-DGE is the sole cause of peritoneal changes. In the present study, we infused 3,4-DGE dissolved in PBS into the peritoneal cavities of mice to examine the effects of that compound on peritoneal fibrosis. Although there is some controversy about the use of CG to induce an encapsulating peritoneal sclerosis model (22), we used this existing model of CG-induced peritoneal injury to investigate the role of 3,4-DGE. Vlijm et al. reported that the condition of chronic renal failure can be a “first hit” in peritoneal damage and that perhaps other stimuli can substitute for CG in damaging mesothelial cells (22). Infusion of 3,4-DGE alone did not induce peritoneal changes in conditions of uninjured peritoneum. The peritoneal damage caused by 3,4-DGE alone may be different from that caused by PD solution because of the difference of glucose concentration. The combination of CG and 3,4-DGE caused thickening of the submesothelial zone, suggesting that 3,4-DGE is an aggravating factor in peritoneal fibrosis when peritoneal damage is already present.
The infusion of GDP-containing conventional dialysis solution with lipopolysaccharide induces a high peritoneal transport rate, with mild thickening of the peritoneal membrane (12). In the present study, we used 38 μmol/L or 145 μmol/L 3,4-DGE-containing PBS. The 3,4-DGE alone did not elicit peritoneal fibrosis, probably because half the 3,4-DGE is eliminated from the peritoneal cavity at 30 minutes. In peritoneal mesothelial cells, VEGF-A, which enhances vascular permeability and angiogenesis, has been shown to be increased by MGO and 3,4-DGE (23,24). Blockade of VEGF by anti-VEGF monoclonal antibody prevents hyperglycemia-induced structural and functional peritoneal microvascular alterations in rats, indicating that VEGF plays a role in permeability to small molecules and angiogenesis (25). The present study shows that only CG+145 μmol/L 3,4-DGE mice showed increased levels of VEGF, leading to high peritoneal permeability as indicated by peritoneal equilibration tests. Antiangiogenic reagents TNP-470 and endostatin have been shown to ameliorate peritoneal fibrosis and permeability (26,27). In the present study, increased numbers of CD31-positive vessels were most often noted in CG+145 μmol/L 3,4-DGE mice, which is consistent with increased peritoneal permeability.
Expression of genes associated with extracellular matrix (TGF-β1, CTGF, fibronectin, and COL1A1) was significantly increased in the peritoneum of CG+145 μmol/L 3,4-DGE mice. In cultured human peritoneal mesothelial cells, stimulation with 3,4-DGE has been shown to increase TGF-β secretion (24). Our study indicates that administration of 3,4-DGE without CG did not upregulate TGF-β1 mRNA expression. Taken together, these findings suggest that injured peritoneum expresses increased TGF-β mRNA because of stimulation by 3,4-DGE.
Although the precise mechanism of upregulated TGF-β expression in CG+145 μmol/L 3,4-DGE mice is not clear, we speculate that oxidative stress is one factor affecting TGF-β expression in our model. Reduction of oxidative stress with l-2-oxothiazolidine-4-carboxylic acid (28), a glutathione precursor, or N-tert-butyl-α-phenylnitrone (29) lowered the TGF-β level in cultured mesothelial cells, indicating that, in peritoneal mesothelial cells, oxidative stress can increase TGF-β, which is one of the well-known inducers of EMT in mesothelial cells (30,31). Epithelial-mesenchymal transition is characterized by downregulation of E-cadherin and cytoskeletal rearrangement with expression of α-SMA. Our results show that the number of α-SMA-positive cells was increased in the submesothelial zone. Although CG treatment alone or CG plus 38 μmol/L 3,4-DGE increased α-SMA-positive cells, mRNA expression of α-SMA in those groups of mice was not increased. Further investigation is needed into this discrepancy between mRNA and protein levels.
Oxidative stress is a key factor in the development of peritoneal fibrosis. Reactive oxygen species produced after stimulation with high glucose upregulate fibronectin expression through the protein kinase C pathway in human peritoneal mesothelial cells (32). Reactive oxygen species have been shown to play a role in changes of peritoneal membrane structure and function in vivo, and antioxidant prevents those changes (33). We showed upregulation of NOX4 and p47phox mRNA expression in CG+145 μmol/L 3,4-DGE mice compared with CG+3,4-DGE(-) mice. Macrophage infiltration was documented in the peritoneum of 38 μmol/L or 145 μmol/L CG+3,4-DGE mice. The infiltrating macrophages aggravate peritoneal fibrosis by secreting profibrogenic cytokines (34). Peritoneal macrophage infiltration is an independent predictor of baseline peritoneal permeability in PD patients (35). At 38 μmol/L, 3,4-DGE can influence macrophage infiltration and vessel formation.
Conventional PD solutions and GDPs induce apoptosis in mesothelial cells. High concentrations of 3-deoxyglucosone or MGO induce mesothelial cell apoptosis (36,37). Administration of 3,4-DGE within the concentration range seen in conventional PD solutions induces apoptosis in cultured mesothelial cells through caspase- and Bax-dependent pathways (10,38), suggesting that 3,4-DGE mediates, at least in part, the cytotoxicity of conventional PD solutions. The precise mechanism and role of apoptosis in the peritoneum in vivo needs further investigation. We showed that 145 μmol/L 3,4-DGE in combination with CG induced cell apoptosis in the thickened peritoneal compact zone in vivo. As previously reported (39), some of the apoptotic cells were leukocytes. The inability of 38 μmol/L 3,4-DGE to induce apoptosis might be a result of rapid elimination of 3,4-DGE from peritoneal cavity. Peritoneum injured by CG can accelerate that process, elevating plasma levels of 3,4-DGE.
CONCLUSIONS
Our study shows that, when mild peritoneal damage is already present, 3,4-DGE enhances peritoneal injury by augmenting macrophage infiltration and extracellular matrix deposition. These findings help to elucidate the effect of 3,4-DGE in peritoneal fibrosis in vivo.
DISCLOSURES
R. Yamada, S. Namoto, T. Yamamoto, N. Seki, N. Souma, and T. Yamaguchi are employees of JMS Co. Ltd.
Acknowledgments
We gratefully acknowledge Y. Sakashita and other lab members for technical assistance and Ms. A. Yamamoto for secretarial assistance.
References
- 1. Saxena R. Pathogenesis and treatment of peritoneal membrane failure. Pediatr Nephrol 2008; 23:695–703 [DOI] [PubMed] [Google Scholar]
- 2. Yung S, Chan TM. Preventing peritoneal fibrosis—insights from the laboratory. Perit Dial Int 2003; 23(Suppl 2):S37–41 [PubMed] [Google Scholar]
- 3. Nilsson-Thorell CB, Muscalu N, Andrén AH, Kjellstrand PT, Wieslander AP. Heat sterilization of fluids for peritoneal dialysis gives rise to aldehydes. Perit Dial Int 1993; 13:208–13 [PubMed] [Google Scholar]
- 4. Linden T, Cohen A, Deppisch R, Kjellstrand P, Wieslander A. 3,4-Dideoxyglucosone-3-ene (3,4-DGE): a cytotoxic glucose degradation product in fluids for peritoneal dialysis. Kidney Int 2002; 62:697–703 [DOI] [PubMed] [Google Scholar]
- 5. Erixon M, Lindén T, Kjellstrand P, Carlsson O, Ernebrant M, Forsbäck G, et al. PD fluids contain high concentrations of cytotoxic GDPs directly after sterilization. Perit Dial Int 2004; 24:392–8 [PubMed] [Google Scholar]
- 6. Williams JD, Topley N, Craig KJ, Mackenzie RK, Pischetsrieder M, Lage C, et al. The Euro-Balance Trial: the effect of a new biocompatible peritoneal dialysis fluid (Balance) on the peritoneal membrane. Kidney Int 2004; 66:408–18 [DOI] [PubMed] [Google Scholar]
- 7. Schwenger V, Morath C, Salava A, Amann K, Seregin Y, Deppisch R, et al. Damage to the peritoneal membrane by glucose degradation products is mediated by the receptor for advanced glycation end-products. J Am Soc Nephrol 2006; 17:199–207 [DOI] [PubMed] [Google Scholar]
- 8. Mortier S, Faict D, Lameire NH, De Vriese AS. Benefits of switching from a conventional to a low-GDP bicarbonate/lactate-buffered dialysis solution in a rat model. Kidney Int 2005; 67:1559–65 [DOI] [PubMed] [Google Scholar]
- 9. Tomo T, Okabe E, Yamamoto T, Namoto S, Iwashita T, Matsuyama K, et al. Impact of 3,4-dideoxyglucosone-3-ene (3,4-DGE) on cytotoxicity of acidic heat-sterilized peritoneal dialysis fluid. J Artif Organs 2007; 10:47–51 [DOI] [PubMed] [Google Scholar]
- 10. Santamaría B, Ucero AC, Reyero A, Selgas R, Ruiz-Ortega M, Catalán M, et al. 3,4-Dideoxyglucosone-3-ene as a mediator of peritoneal demesothelization. Nephrol Dial Transplant 2008; 23:3307–15 [DOI] [PubMed] [Google Scholar]
- 11. Yamamoto T, Tomo T, Okabe E, Namoto S, Suzuki K, Hirao Y. Glutathione depletion as a mechanism of 3,4-dideoxyglucosone-3-ene-induced cytotoxicity in human peritoneal mesothelial cells: role in biocompatibility of peritoneal dialysis fluids. Nephrol Dial Transplant 2009; 24:1436–42 [DOI] [PubMed] [Google Scholar]
- 12. Park SH, Lee EG, Kim IS, Kim YJ, Cho DK, Kim YL. Effect of glucose degradation products on the peritoneal membrane in a chronic inflammatory infusion model of peritoneal dialysis in the rat. Perit Dial Int 2004; 24:115–22 [PubMed] [Google Scholar]
- 13. Kim CD, Kwon HM, Park SH, Oh EJ, Kim MH, Choi SY, et al. Effects of low glucose degradation products peritoneal dialysis fluid on the peritoneal fibrosis and vascularization in a chronic rat model. Ther Apher Dial 2007; 11:56–64 [DOI] [PubMed] [Google Scholar]
- 14. Wieczorowska-Tobis K, Brelinska R, Witowski J, Passlick-Deetjen J, Schaub TP, Schilling H, et al. Evidence for less irritation to the peritoneal membrane in rats dialyzed with solutions low in glucose degradation products. Perit Dial Int 2004; 24:48–57 [PubMed] [Google Scholar]
- 15. Oh EJ, Ryu HM, Choi SY, Yook JM, Kim CD, Park SH, et al. Impact of low glucose degradation product bicarbonate/lactate-buffered dialysis solution on the epithelial-mesenchymal transition of peritoneum. Am J Nephrol 2010; 31:58–67 [DOI] [PubMed] [Google Scholar]
- 16. Yokoi H, Kasahara M, Mori K, Ogawa Y, Kuwabara T, Imamaki H, et al. Pleiotrophin triggers inflammation and increased peritoneal permeability leading to peritoneal fibrosis. Kidney Int 2012; 81:160–9 [DOI] [PubMed] [Google Scholar]
- 17. Ni J, Cnops Y, Debaix H, Boisdé I, Verbavatz JM, Devuyst O. Functional and molecular characterization of a peritoneal dialysis model in the C57BL/6J mouse. Kidney Int 2005; 67:2021–31 [DOI] [PubMed] [Google Scholar]
- 18. Yokoi H, Mukoyama M, Mori K, Kasahara M, Suganami T, Sawai K, et al. Overexpression of connective tissue growth factor in podocytes worsens diabetic nephropathy in mice. Kidney Int 2008; 73:446–55 [DOI] [PubMed] [Google Scholar]
- 19. Yokoi H, Mukoyama M, Nagae T, Mori K, Suganami T, Sawai K, et al. Reduction in connective tissue growth factor by antisense treatment ameliorates renal tubulointerstitial fibrosis. J Am Soc Nephrol 2004; 15:1430–40 [DOI] [PubMed] [Google Scholar]
- 20. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 2005; 115:610–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zareie M, Tangelder GJ, ter Wee PM, Hekking LH, van Lambalgen AA, Keuning ED, et al. Beneficial effects of aminoguanidine on peritoneal microcirculation and tissue remodelling in a rat model of PD. Nephrol Dial Transplant 2005; 20:2783–92 [DOI] [PubMed] [Google Scholar]
- 22. Vlijm A, Sampimon DE, de Graaff M, Struijk DG, Krediet RT. Experimental peritoneal sclerosis models should not be based on chlorhexidine gluconate anymore. Nephron Exp Nephrol 2011; 117:e1–8 [DOI] [PubMed] [Google Scholar]
- 23. Lai KN, Leung JC, Chan LY, Li FF, Tang SC, Lam MF, et al. Differential expression of receptors for advanced glycation end-products in peritoneal mesothelial cells exposed to glucose degradation products. Clin Exp Immunol 2004; 138:466–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Leung JC, Chan LY, Li FF, Tang SC, Chan KW, Chan TM, et al. Glucose degradation products downregulate ZO-1 expression in human peritoneal mesothelial cells: the role of VEGF. Nephrol Dial Transplant 2005; 20:1336–49 [DOI] [PubMed] [Google Scholar]
- 25. De Vriese AS, Tilton RG, Stephan CC, Lameire NH. Vascular endothelial growth factor is essential for hyperglycemia-induced structural and functional alterations of the peritoneal membrane. J Am Soc Nephrol 2001; 12:1734–41 [DOI] [PubMed] [Google Scholar]
- 26. Yoshio Y, Miyazaki M, Abe K, Nishino T, Furusu A, Mizuta Y, et al. TNP-470, an angiogenesis inhibitor, suppresses the progression of peritoneal fibrosis in mouse experimental model. Kidney Int 2004; 66:1677–85 [DOI] [PubMed] [Google Scholar]
- 27. Tanabe K, Maeshima Y, Ichinose K, Kitayama H, Takazawa Y, Hirokoshi K, et al. Endostatin peptide, an inhibitor of angiogenesis, prevents the progression of peritoneal sclerosis in a mouse experimental model. Kidney Int 2007; 71:227–38 [DOI] [PubMed] [Google Scholar]
- 28. Ksiazek K, Breborowicz A, Jörres A, Witowski J. Oxidative stress contributes to accelerated development of the senescent phenotype in human peritoneal mesothelial cells exposed to high glucose. Free Radic Biol Med 2007; 42:636–41 [DOI] [PubMed] [Google Scholar]
- 29. Ksiazek K, Mikula-Pietrasik J, Jörres A, Witowski J. Oxidative stress-mediated early senescence contributes to the short replicative life span of human peritoneal mesothelial cells. Free Radic Biol Med 2008; 45:460–7 [DOI] [PubMed] [Google Scholar]
- 30. Yang AH, Chen JY, Lin JK. Myofibroblastic conversion of mesothelial cells. Kidney Int 2003; 63:1530–9 [DOI] [PubMed] [Google Scholar]
- 31. Margetts PJ, Bonniaud P, Liu L, Hoff CM, Holmes CJ, West-Mays JA, et al. Transient overexpression of TGF-β1 induces epithelial mesenchymal transition in the rodent peritoneum. J Am Soc Nephrol 2005; 16:425–36 [DOI] [PubMed] [Google Scholar]
- 32. Lee HB, Yu MR, Song JS, Ha H. Reactive oxygen species amplify protein kinase C signaling in high glucose-induced fibronectin expression by human peritoneal mesothelial cells. Kidney Int 2004; 65:1170–9 [DOI] [PubMed] [Google Scholar]
- 33. Noh H, Kim JS, Han KH, Lee GT, Song JS, Chung SH, et al. Oxidative stress during peritoneal dialysis: implications in functional and structural changes in the membrane. Kidney Int 2006; 69:2022–8 [DOI] [PubMed] [Google Scholar]
- 34. Fieren MW. Mechanisms regulating cytokine release from peritoneal macrophages during continuous ambulatory peritoneal dialysis. Blood Purif 1996; 14:179–87 [DOI] [PubMed] [Google Scholar]
- 35. Sawai A, Ito Y, Mizuno M, Suzuki Y, Toda S, Ito I, et al. Peritoneal macrophage infiltration is correlated with baseline peritoneal solute transport rate in peritoneal dialysis patients. Nephrol Dial Transplant 2011; 26:2322–32 [DOI] [PubMed] [Google Scholar]
- 36. Boulanger E, Wautier MP, Gane P, Mariette C, Devuyst O, Wautier JL. The triggering of human peritoneal mesothelial cell apoptosis and oncosis by glucose and glycoxydation products. Nephrol Dial Transplant 2004; 19:2208–16 [DOI] [PubMed] [Google Scholar]
- 37. Welten AG, Schalkwijk CG, ter Wee PM, Meijer S, van den Born J, Beelen RJ. Single exposure of mesothelial cells to glucose degradation products (GDPs) yields early advanced glycation end-products (AGEs) and a proinflammatory response. Perit Dial Int 2003; 23:213–21 [PubMed] [Google Scholar]
- 38. Lee DH, Choi SY, Ryu HM, Kim CD, Park SH, Chung HY, et al. 3,4-Dideoxyglucosone-3-ene induces apoptosis in human peritoneal mesothelial cells. Perit Dial Int 2009; 29:44–51 [PubMed] [Google Scholar]
- 39. Catalan MP, Santamaría B, Reyero A, Ortiz A, Egido J, Ortiz A. 3,4-Di-deoxyglucosone-3-ene promotes leukocyte apoptosis. Kidney Int 2005; 68:1303–11 [DOI] [PubMed] [Google Scholar]