Abstract
♦ Objectives: Switching from peritoneal dialysis (PD) to hemodialysis (HD) is undesirable, because of complications from temporary vascular access, disruption of daily routine, and higher costs. Little is known about the role that social factors play in technique failure.
♦ Design, Setting, Participants, Measurements: We followed for 3 years a nationally representative cohort of US patients who initiated PD in 1996 - 1997. Technique failure was defined as any switch from PD to HD for 30 days or more. We used Cox regression to examine associations between technique failure and demographic, medical, social, and pre-dialysis factors. We estimated hazard ratios (HRs) with 95% confidence intervals (CIs).
♦ Results: We identified an inception cohort of 1587 patients undergoing PD. In multivariate analysis, female sex (HR: 0.78; 95% CI: 0.64 to 0.95) was associated with lower rates of technique failure, and black race [compared with white race (HR: 1.48; 95% CI: 1.20 to 1.82)] and receiving Medicaid (HR: 1.48; 95% CI: 1.17 to 1.86) were associated with higher rates. Compared with patients who worked full-time, those who were retired (HR: 1.49; 95% CI: 1.07 to 2.08) or disabled (HR: 1.38; 95% CI: 1.01 to 1.88) had higher rates of failure. Patients with a systolic blood pressure of 140 - 160 mmHg had a higher rate of failure than did those with a pressure of 120 - 140 mmHg (HR: 1.24; 95% CI: 1.00 to 1.52). Earlier referral to a nephrologist (>3 months before dialysis initiation) and the primary decision-maker for the dialysis modality (physician vs patient vs shared) were not associated with technique failure.
♦ Conclusions: This study confirms that several socio-demographic factors are associated with technique failure, emphasizing the potential importance of social and financial support in maintaining PD.
Key words: Technique failure, modality failure, socio-demographic
INTRODUCTION
Of the more than 397 000 patients on dialysis in the United States in 2009, only about 27 000 (7%) used peritoneal dialysis (PD) as their modality of renal replacement therapy (1). Among incident patients, only 6.1% initiated renal replacement therapy using PD, down from 8.6% a decade earlier. In addition to the already low proportion of patients using PD, many patients are subsequently forced to switch to hemodialysis (HD) because of technique failure. Although rates of technique failure have fallen over the past 10 years, switching from PD to HD still incurs risks, including placement of a temporary vascular access, disruption of the patient’s daily routine, and increased medical costs (2). A cost analysis of Medicare patients found that, when patients switched from PD to HD for at least 60 days, annual expenditures rose by more than $20 000 (3).
Some causes of technique failure have been identified. The leading cause is peritonitis, followed by inadequate dialysis (including ultrafiltration failure) and catheter malfunction (4). Psychosocial factors such as burnout and difficulty learning PD-related tasks may play a role as well.
The search for predictors of PD technique failure has yielded mixed results. Most studies have focused on demo graphic and medical factors. One of the few consistent results is that centers with more PD patients have better outcomes (2,5-9). However, few studies have looked at the importance of social factors, such as education, employment, insurance, and marital status.
In the present study, we investigated the associations of technique failure with demographic, medical, and social and health-care-related factors in a nationally representative cohort of incident PD patients. We also examined whether any of these putative associations varied with time.
METHODS
DATA SOURCE AND STUDY POPULATION
We used the US Renal Data System Dialysis Morbidity and Mortality Study Wave 2, a prospective study of adult (18 years of age or older) patients who started dialysis in 1996 - 1997 (1). A random sample of 25% of American dialysis units were surveyed and asked to enroll all incident PD patients and a 20% random sample of incident HD patients. Baseline information, including dialysis modality, was collected at the study start date, defined as 60 days after the first regular dialysis treatment. If a patient was not stabilized on a modality by day 60, the study start date could be postponed up to day 70. Dialysis facility personnel completed the medical questionnaire by abstracting data from facility records, medical records, billing records, dialysis logs, patient rosters, and patients themselves if information was not otherwise available. Dialysis patients completed a questionnaire on the preparation for, and impact of, end-stage renal disease on their life, including medical care before regular dialysis and choosing treatment for their kidney failure.
After restricting the Wave 2 cohort to adult patients who were on PD at their study start date and whose first dialysis service date was validated as being between 1996 and 1997 in the updated Wave 2 dataset, the cohort included 1836 patients (Figure 1). To validate the reported dialysis modality, we included only patients who had evidence in the treatment history file supporting use of continuous ambulatory PD (CAPD), continuous cycler-assisted PD (CCPD), or other PD before day 71 of dialysis. Of the 1614 patients who met that condition, 25 were excluded for missing a study start date, 1 died before the study start date, and 1 underwent transplantation before the study start date. Our final study cohort therefore included 1587 adult incident PD patients.
Figure 1.
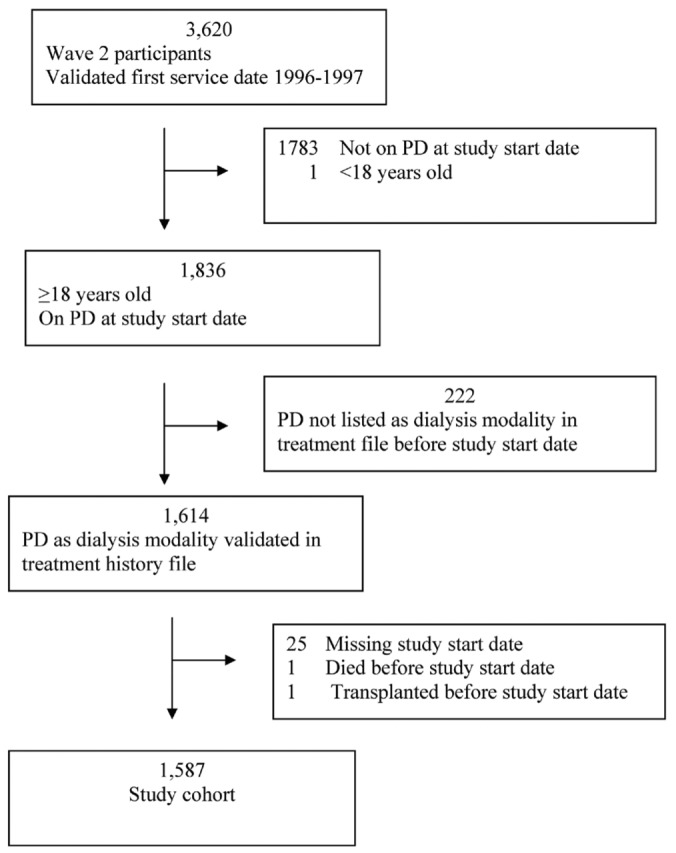
— Study population selection. The study cohort was drawn from the US Renal Data System Dialysis Morbidity and Mortality Study Wave 2. PD = peritoneal dialysis.
OUTCOME
We defined technique failure as any transfer from PD to HD that lasted for 30 days or more. To test the sensitivity of our defined outcome, we conducted additional analyses restricting “failure” to transfers to HD for at least 1 and 7 days. Some patients switched modalities multiple times; only the first event was analyzed.
VARIABLES
Demographic, medical, social, and pre-dialysis health care factors were analyzed as potential correlates of technique failure; these factors were chosen a priori as potentially clinically relevant determinants. Demographic factors included sex, age at study start date, race (white, black, or other), and Hispanic ethnicity. Comorbidities included smoking status (nonsmokers vs never-smokers), coronary artery disease, congestive heart failure, peripheral vascular disease, cerebrovascular disease, and diabetes mellitus. Clinical factors included dialysis location (home training or in-center vs home), type of dialysis (cycler or combined vs CAPD only), type of catheter (double-cuff vs single-cuff), albumin, body mass index (in kilograms per square meter: <18.5, 25 - 30, or >30 vs 18.5 - 25), and systolic blood pressure [SBP (in millimeters of mercury: <120, 140 - 160, or >160, vs 120 - 140)]. Social factors included marital status (single or widowed/separated/divorced vs married), living alone (not living alone or living in an institution/nursing home/homeless vs living alone), education level (high school graduate, some college, college graduate, or missing information vs <12 years of education), employment status (part time, homemaker, retired, unemployed, disabled, or other vs full time), and Medicaid status (any vs none). Pre-dialysis factors included timing of referral to a nephrologist (3 months or less before dialysis initiation vs more than 3 months) and the person who took the lead in choosing the method of treatment for kidney failure [medical team or equal vs patient (the survey asked “Which of the following best describes the process of choosing your method of treatment” and patients chose either one of “The medical team took the lead in selecting my treatment,” “The medical team and I contributed equally to selecting my treatment,” “I took the lead in selecting my treatment.”)] Information was drawn primarily from baseline data collection at the study start date; data missing from that source were abstracted from the medical evidence report form when possible.
STATISTICAL ANALYSIS
Cox proportional hazards regression was used to model time from the date of enrollment in Dialysis Morbidity and Mortality Study Wave 2 to technique failure. Patients were censored for the earliest of death, kidney transplantation, loss to follow-up, recovery of renal function, or completion of 3 years’ follow-up. Departures from proportional hazards were examined using interaction terms with time and, if present, corrected by the inclusion of time-dependent covariates.
We conducted unadjusted and adjusted analyses. For multivariate analyses, two models were fit: The first model included all variables except for pre-dialysis factors (early referral to a nephrologist and the person taking the lead in choosing the method of treatment for kidney failure), because pre-dialysis data for those variables were missing for more than one third of the patients. The second model included patients in whom the pre-dialysis factors were reported, but only hazard ratios for pre-dialysis factors were reported for this model. Only subjects without any missing information for the study variables were included in the analysis; missing data was not imputed. All statistical tests were two-tailed. Values of p < 0.05 were considered statistically significant; adjustments were not made for multiple comparisons.
All analyses were performed using SAS Enterprise Guide (version 4.3: SAS Institute, Cary, NC, USA).
RESULTS
PATIENT CHARACTERISTICS
The average age of the study participants was 56 years, and most were male (54%) and white (69%, Table 1). Almost one quarter were black (22%), and 9% were of Hispanic ethnicity. Several comorbidities were common: half the patients had diabetes, and more than one third had coronary artery disease or heart failure. Table 1 shows additional clinical details. Nearly all patients were performing PD at home at the study start date. Two thirds used CAPD rather than CCPD. Most had double-cuff catheters, although 11% had single-cuff catheters.
TABLE 1.
Baseline Characteristics of Study Participants
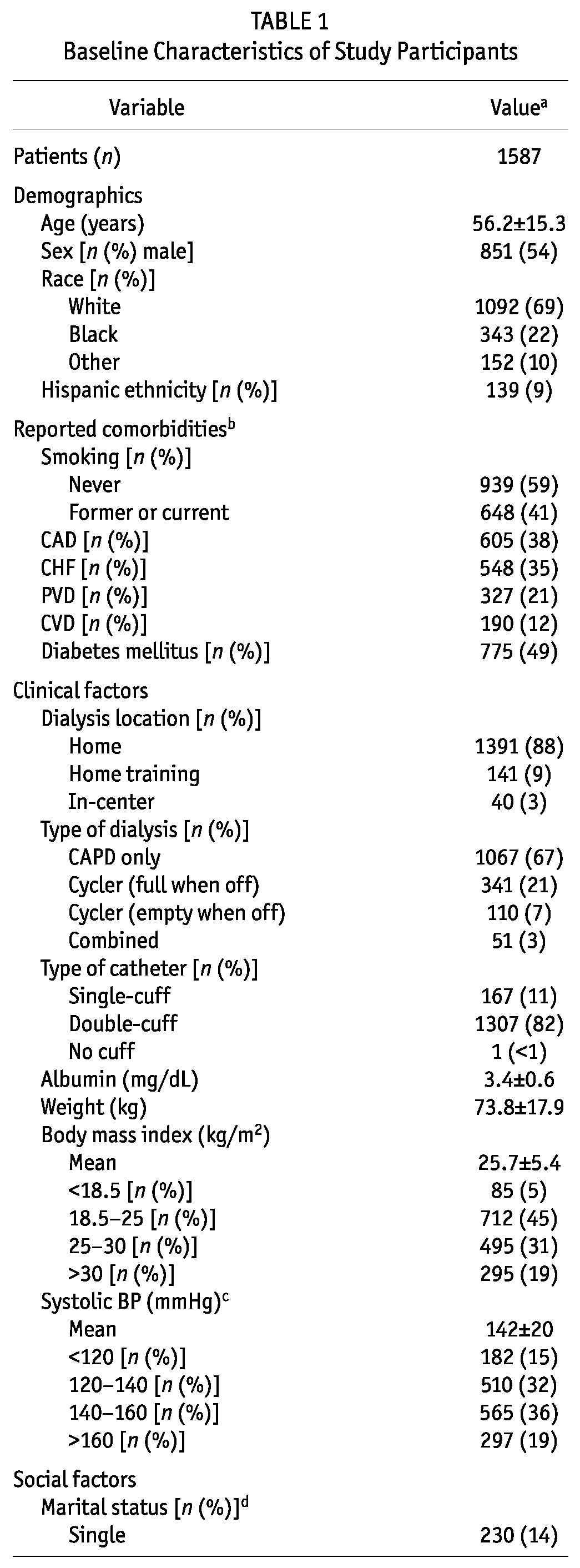
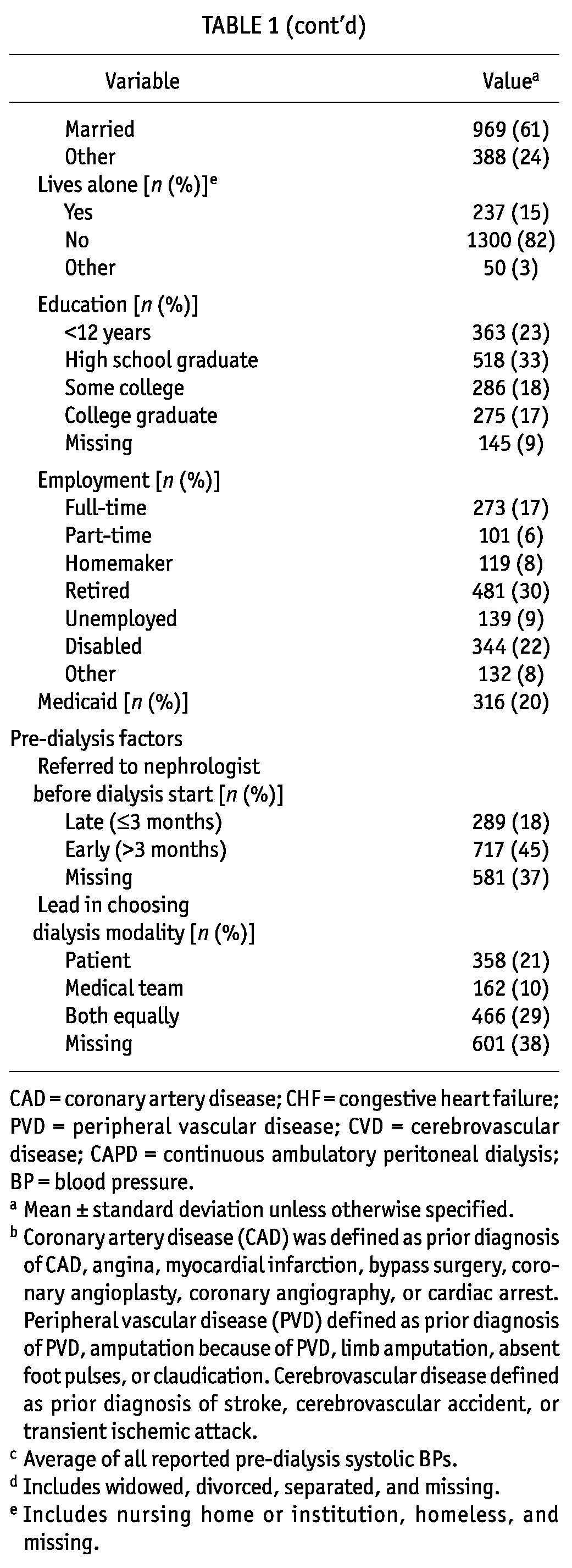
Most incident PD patients were married (61%); 14% were single; and the remaining 25% included patients who were widowed, divorced, or separated, or who had missing marital status information. Most patients (82%) reported living with others. Two thirds had completed high school, but only 17% were full-time employees at the time of the study. Part-time work was reported by 6%. One fifth were receiving Medicaid. Almost half had received more than 3 months of nephrology care before the start of dialysis; at least half took the lead or were equally involved in choosing their dialysis modality.
UNADJUSTED ANALYSES
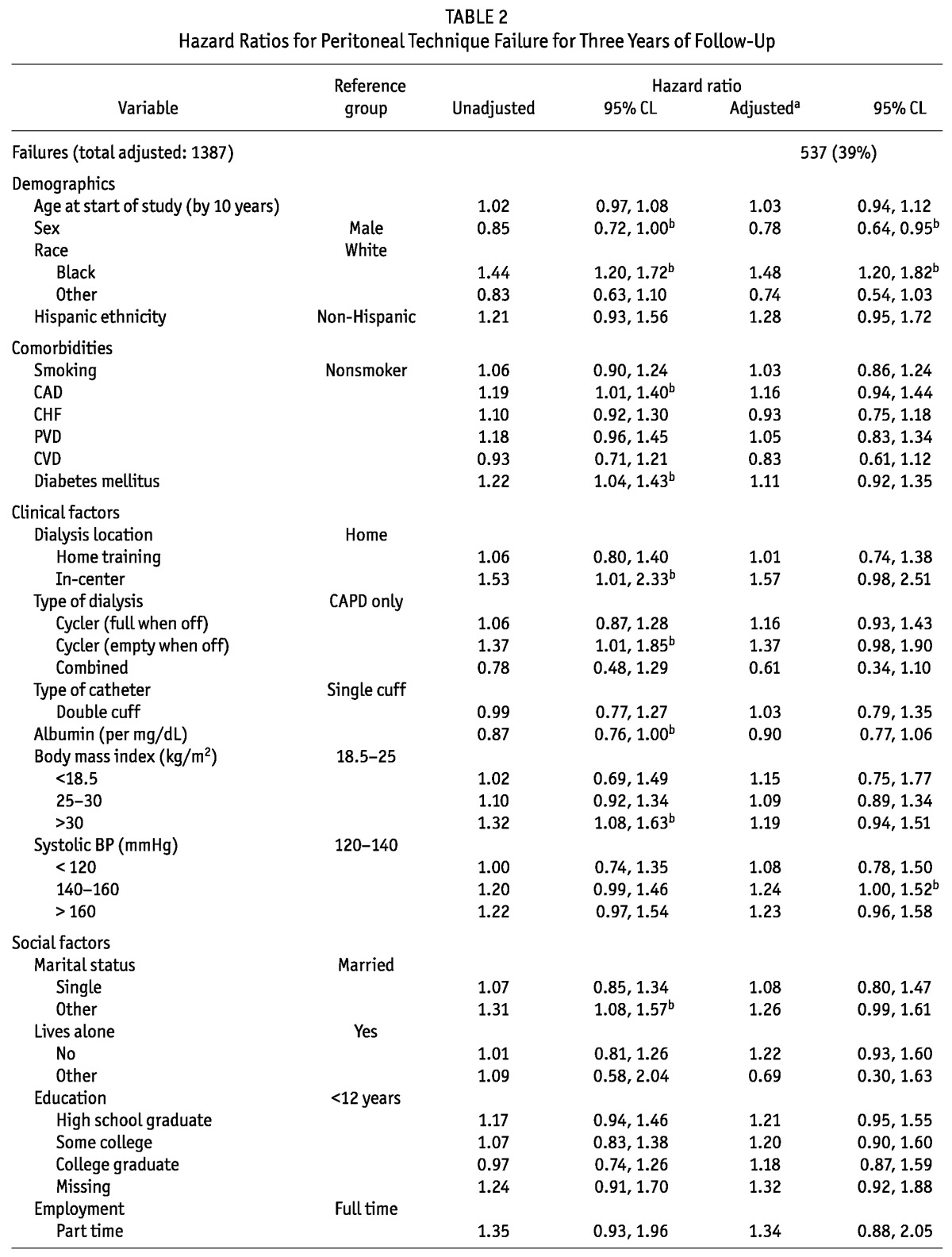
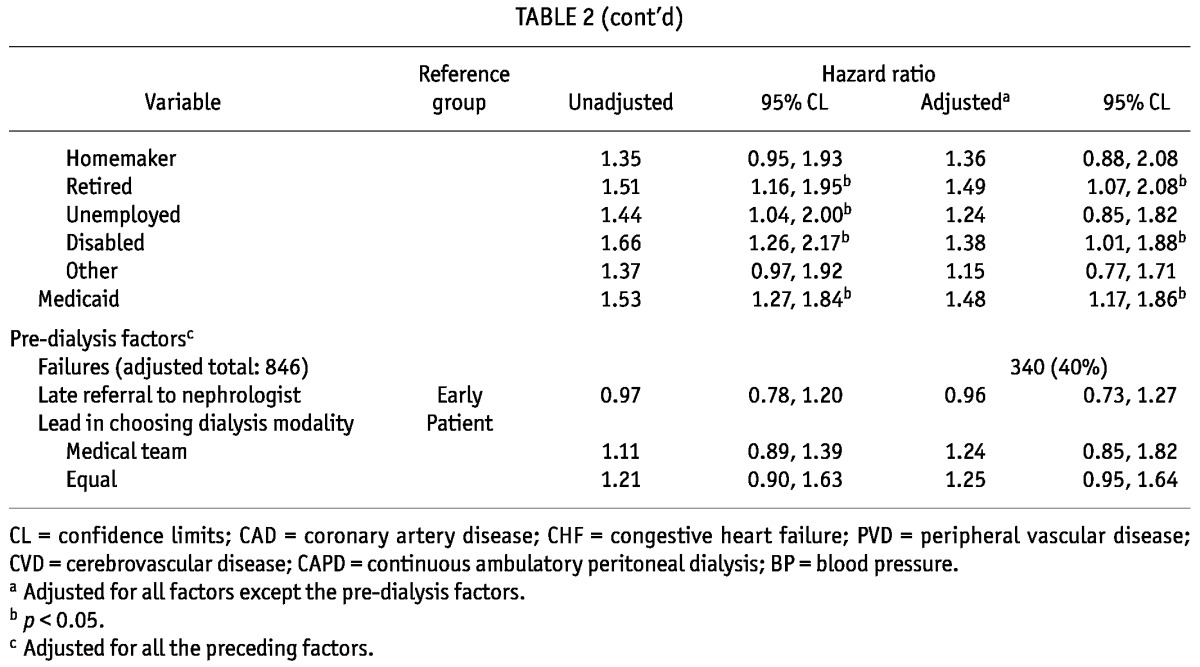
Median technique survival time was 2.7 years, and 39% of patients had experienced at least one 30-day switch to HD by 3 years (Figure 2, Table 2). In unadjusted analyses, female sex was associated with less technique failure (Table 2). Black race (vs white race), coronary artery disease, and diabetes were all significantly associated with increased hazards for technique failure. Compared with patients dialyzing independently at home, patients dialyzing in-center also experienced higher rates of technique failure. Compared with patients on CAPD, users of cyclers had a higher rate of technique failure, but only if they had no fills when being off-cycler. Each 1 mg/dL increase in albumin was associated with a 13% decrease in the hazard of technique failure. Conversely, compared with patients having a normal body mass index (18.5 - 25 kg/m2), patients with the highest body mass index (>30 kg/m2) had a 32% increased hazard of technique failure. Although being single (compared with being married) was not associated with technique failure, patients who were widowed, divorced, or separated were 31% more likely to fail. Compared with full-time workers, patients who were retired, unemployed, and disabled had at least a 44% increase in their rate of technique failure. Medicaid was significantly associated with a 53% higher hazard of technique failure.
Figure 2.
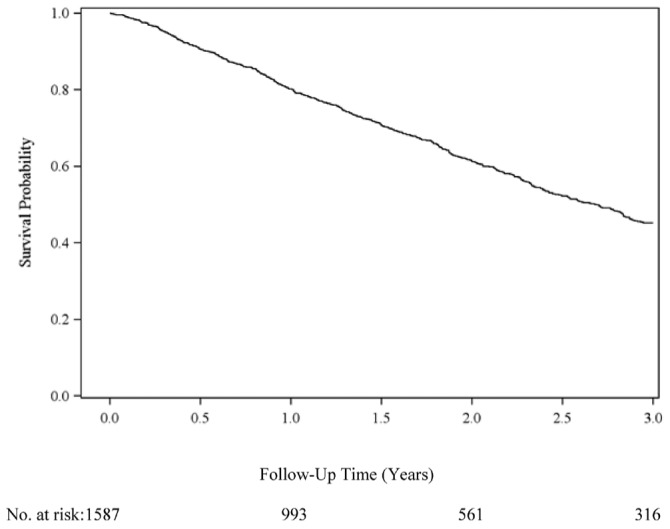
— Technique survival in patients initiating peritoneal dialysis. The Kaplan-Meier actuarial technique survival at 1, 2, and 3 years was 80.2%, 61.2%, and 45.2% respectively. Median survival was 2.7 years. Failure was defined as a switch from peritoneal dialysis to hemodialysis lasting 30 days or more.
TABLE 2.
Hazard Ratios for Peritoneal Technique Failure for Three Years of Follow-Up
MULTIVARIATE ANALYSIS
After multivariate adjustment, female sex remained associated with a 22% lower hazard of technique failure, and black race continued to be independently associated with a 48% increased hazard of technique failure (Table 2). No comorbidity was associated with technique failure in the adjusted analysis. Systolic blood pressure was the only clinical factor that was significantly associated with technique failure: compared with patients having a SBP of 120 - 140 mmHg, those with a SBP of 140 - 160 mmHg had a 24% increased failure rate. Among social factors, level of employment remained a significant correlate of technique failure: retired and disabled patients both had increased rates of technique failure. Finally, Medicaid remained a determinant of technique failure, associated with an almost 50% higher rate of failure.
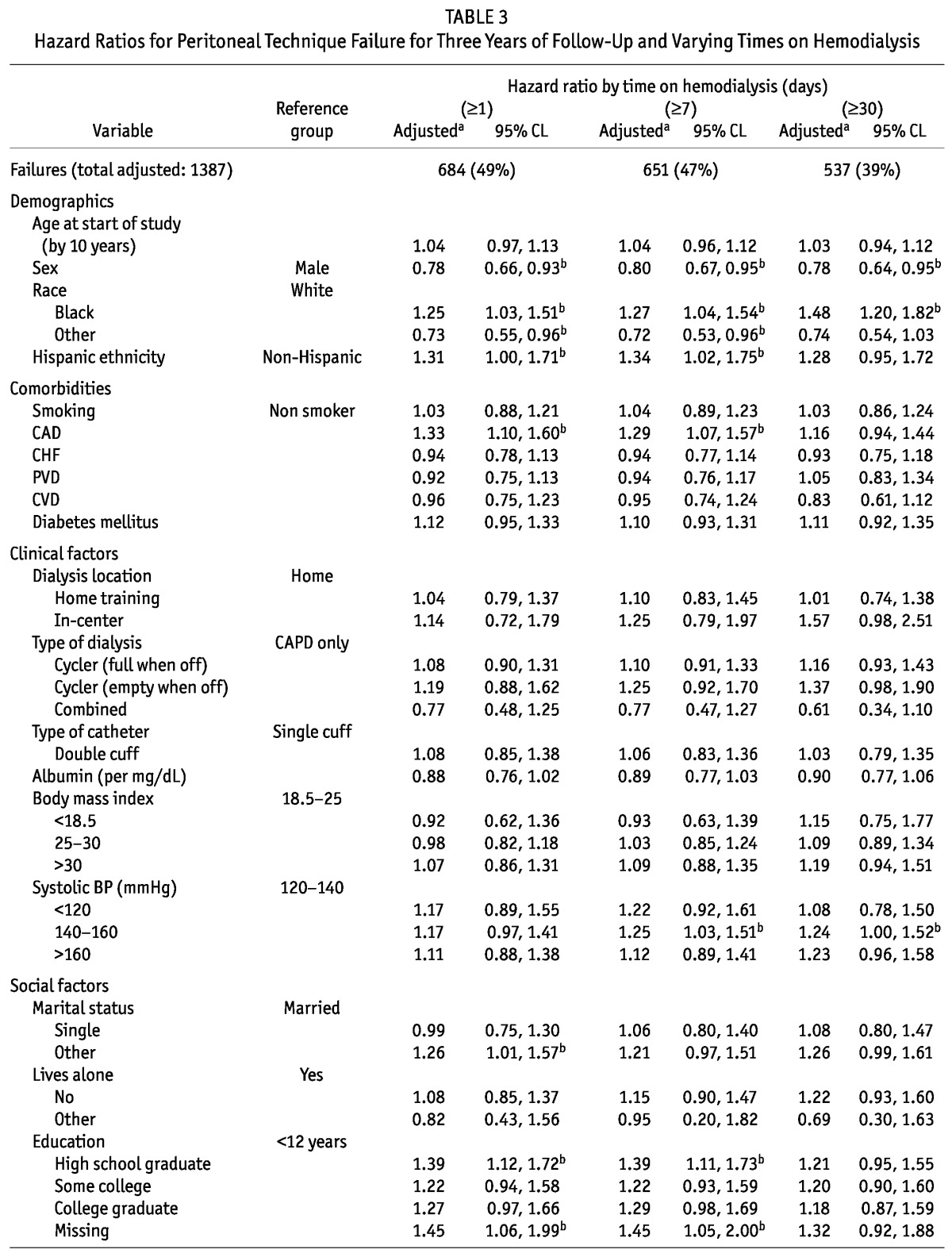
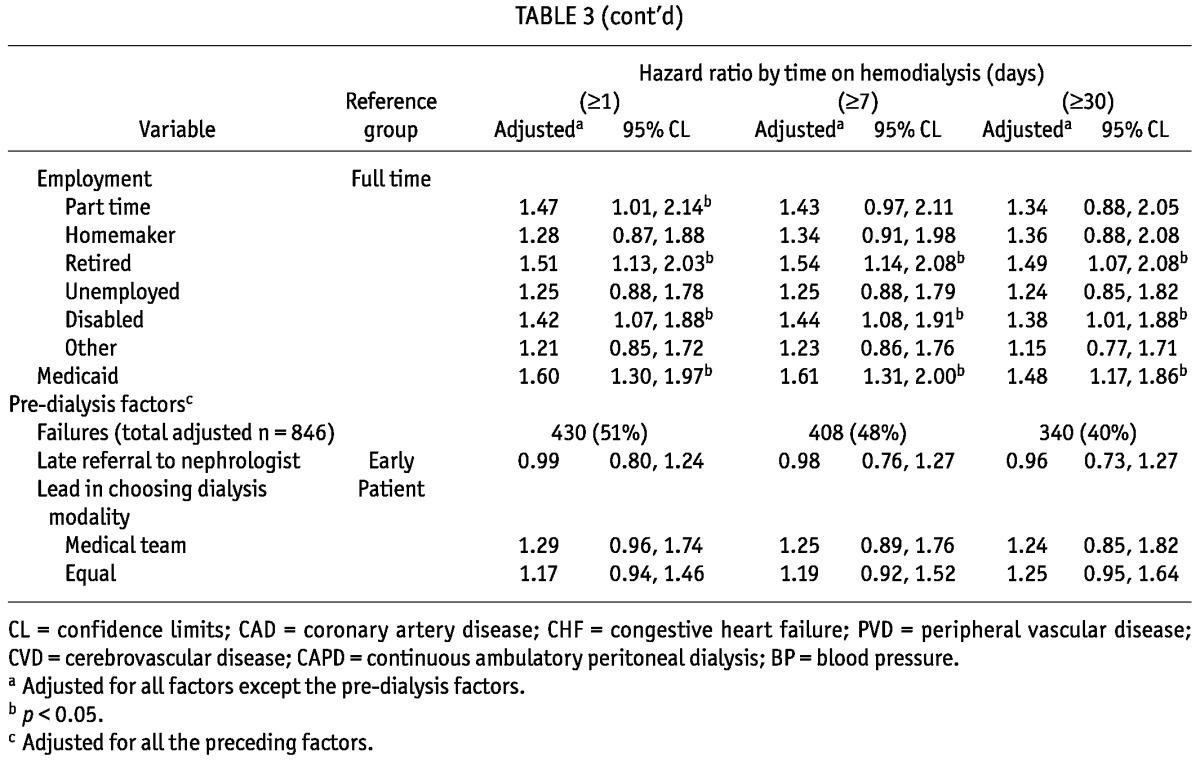
We found no significant departures from the assumption of proportional hazards; thus, no time stratification was necessary. We also conducted sensitivity analyses on the outcome of interest, varying the number of days on HD required to constitute a modality switch (Table 3). In the primary analysis, in which the outcome was defined as switching to HD for at least 30 days, 537 patients (39%) experienced technique failure. Those numbers increased to 651 (47%) and 684 (49%) when technique failure was defined as switching to HD for at least 7 days and 1 day respectively. The primary findings were remarkably robust to the specific outcome definition used. The only variable that lost statistical significance with a shorter minimum time on HD was SBP; the point estimates of hazard ratio (HR) were similar, though.
TABLE 3.
Hazard Ratios for Peritoneal Technique Failure for Three Years of Follow-Up and Varying Times on Hemodialysis
DISCUSSION
We used a nationally representative sample of patients initiating renal replacement therapy on PD to study determinants of modality failure. We were able to examine social factors, which were recorded in unique detail in this special study, and for which previous evidence was sparse (10,11). While adjusting for several other baseline factors, we found associations between several socio-demographic indicators and the hazard of PD modality failure.
We found a strong protective association of full-time employment compared with retirement or disability, for which the hazards of PD failure were substantially higher (38% - 49%). Clearly, patients who are able to maintain full-time jobs are more independent and have more stable environments that are conducive to technique survival. They are probably in better health as well (partly not measurable in datasets such as the one used here), which would put them at decreased risk of complications such as infection that lead to technique failure. On the other hand, disabled patients are, by definition, in such poor health that they cannot be employed. We suspect that many retired patients may also be too disabled to work, but label themselves as retired (rather than disabled) because that label is the conventional one for unemployed people in their age group (mean age: 70 ± 8 years). Notably, the only other study that had previously examined the association between employment status and PD failure did not observe an association (11). However, the population in that study numbered only 262, limiting the power to detect a significant difference.
Medicaid status, another indicator of low socioeconomic status, was also a strong determinant of modality failure. We could not find any other study examining the role of insurance type and technique survival. However, Medicaid status has been associated with generally poorer access to quality care in the dialysis population, which is closely tied to worse outcomes (12-15).
The relationship between sex and PD failure, independent of social factors, add to the conflicting data in this field. We found female sex to be associated with a lower rate of technique failure—a finding that was insensitive to the definition of outcome and the length of follow-up. Most studies on technique failure have not found any difference by sex (2,10,16,17). However, a study of 12 932 PD patients in Australia and New Zealand did find a 13% increased risk of death-censored technique failure for men, consistent with our results (18). We speculate that men may be more dependent on others to maintain their care, leaving them more vulnerable to modality failure. Unfortunately, data on the use of a caregiver were not captured in our dataset.
We also found a strong correlation between black race and modality failure. That finding mirrors those of multiple other US cohort studies (11,19-21). In both the United States and Canada, black patients are known to experience higher rates of peritonitis than white patients do (19,22-26). Given that peritonitis is one of the leading causes of technique failure, a higher peritonitis rate in black patients may explain the higher rate of technique failure in that population (27). Kim et al. examined outcomes of black patients after technique failure and found no difference in mortality between those who switched to HD and those who remained on PD (23,28). Thus, the increased rate of technique failure in black patients should not discourage the use of PD in this racial group. In fact, underutilization of PD in this group may be contributing to their higher rate of failure, because centers that serve communities with high proportions of black patients may be less experienced in the modality and therefore be delivering less optimal PD care (29). Providing increased patient education about PD to this minority group might increase use of and, thus, familiarity with PD, which could in turn reduce the rate of technique failure (30).
Taken together, the socio-demographic factors that are correlated with technique failure suggest that increased social and financial support play a critical role in maintaining patients on PD. Providing reimbursement for assisted PD, in which a home assistant helps with PD, could lower the rate of technique failure. Many of these patients may depend on family members for the required assistance. If, however, that assistance interferes with the family member’s ability to find employment, patients are left vulnerable to failure when their caregivers are forced to work instead of assisting, a common scenario for patients with low economic status. Assisted PD could offset that concern through two mechanisms:
provision of home assistants, freeing family members to work outside the home; and
payment to family members for providing in-home support, giving them a financial incentive to continue in that role.
Assisted PD programs in Europe and Canada have proved to be cost effective when compared with in-center HD (31-33). In the United States, the cost to Medicare when a patient switches from PD to HD for at least 60 days exceeds $20 000 (3). Home assistants could be paid less than $20 000 per year, translating into a net savings (30). Analysis of a community-based homecare assistance program in Toronto, Canada, offers some insight into the effect of assisted PD (33). Of 27 PD patients, 22 used assisted PD. Of those 22 patients, 2 (9%) started with self-care and later needed assistance, and 5 (23%) graduated from assisted PD to self-care. Based on that Canadian experience, provision of subsidized assisted PD has the potential to decrease technique failure in a cost-effective manner and should be seriously considered in the United States.
In this analysis, we also examined two pre-dialysis factors: timing of the first nephrology encounter, and the person who made the decision to choose PD as the initial modality. Interestingly, neither factor was associated with technique failure. Later referral to a nephrologist has been associated with poor outcomes in the dialysis population, including a lower rate of timely placement of vascular access, decreased referral for transplantation, and increased mortality (34-36). Chidambaram et al. recently published a Canadian study of 5162 incident PD patients from 1995 - 2005 and also found no association between pre-dialysis nephrology care and technique failure (10), but in a study of older US patients initiating dialysis, later nephrology care was independently associated with a 47% increased risk of modality failure (37). However, the latter study followed PD patients from the first day of treatment, and the excess failure rate was clearly concentrated during the first month of follow-up. Because the present study followed patients who were on PD at approximately 60 days after the start of renal replacement therapy, we did not have the opportunity to confirm or refute the results from that earlier study.
We are unaware of any other studies examining the relationship between patient autonomy in modality choice and technique failure. Studies have shown that patients enjoy a better quality of life when they choose the modality themselves (38); however, in the present analysis, choice did not appear to influence technique survival rates. The result is limited by the imprecise and subjective definition of autonomy, though. Patients answered the question “Which of the following best describes the process of choosing your method of treatment” based on their own perception of the process. For example, a patient may have indicated that the medical team took the lead if that patient had medical contraindications to HD and was offered only one modality; in such a case, there would truly be no role for patient autonomy. Contrast that scenario with one in which a patient may have deferred the decision to the medical team because of insufficient patient education about the different modalities. In the latter case, poor communication between the team and the patient may have led to the perception of no autonomy. Still, despite those limitations, we felt that this factor was important to analyze because it had not previously been examined in relation to modality failure.
The only clinical factor that was significantly correlated with technique failure was SBP in the range 140 - 160 mmHg compared with SBP in the range 120 - 140 mmHg (HR: 1.24; 95% CI: 1.00 to 1.52). Patients with a SBP above 160 mmHg had a similarly high HR, but it did not reach significance (HR: 1.23; 95% CI: 0.96 to 1.58). Systolic blood pressure correlates with volume overload (39). Volume overload can be a consequence of ultrafiltration failure (a common cause of technique failure) or simply a marker of a less-experienced PD provider who has not optimized the PD prescription (40). If a PD patient is hypertensive, practitioners should consider changing a patient’s PD prescription to maximize fluid control, because such action could potentially prevent an unnecessary switch to HD.
Notably, the rate of modality failure in the present study was higher than the rates in other contemporary cohorts [5-year technique survival: 29.8% vs 55 - 70% (27)]. Most of the comparator studies were single-center cohorts or multicenter cohorts from a few centers, all of which had particular expertise in treating PD patients. Only two studies had more than 1500 patients in the cohort. Technique survival is known to be higher in centers with more PD patients, and so it is likely that these single-center studies had higher rates of technique survival because of their experience (2,7). In contrast, our study was derived from a true random sample and included patients from PD centers of various sizes.
Only 7% of US prevalent dialysis patients in 2009 used PD, which is much lower than usage in other countries, including Australia (21%), Canada (18%), Hong Kong (78%), the Netherlands (18%), and the United Kingdom (15%) (1). Greater experience with PD improves technique survival in both the United States and abroad (2,7). Thus, breaking the systematic barriers to PD use in the United States, as outlined by Golper et al., could be one of the most effective interventions in improving technique survival (30). As noted earlier, barrier-breaking would include providing subsidized assisted PD and increased modality education to patients. It would also involve increased physician education in PD; changes to certain governmental regulations, including reimbursement schemes; and alteration of certain philosophies and business practices of dialysis providers—for instance, lifting restrictions on the use of icodextrin-based PD solution.
Our study has several limitations. We did not account for the number of PD patients treated at each center, which is known to influence the risk of technique failure (2,5-9). Although we were interested in studying data on residual renal function and ultrafiltration as candidate predictors [factors that have been shown to be determinants of failure (11,41-43)], the relevant data were missing for a large proportion of the patients in our cohort. Another drawback of our cohort is that it is drawn from an earlier era. Icodextrin, which has been associated with less technique failure, was not yet widely used (44). Also, rates of technique failure have since declined, likely because of lower infection rates (2,16,45). As mentioned earlier, the rate of PD use in the United States is substantially lower than that seen in other countries, which limits the generalizability of our study to other countries. Finally, detection of significant covariates was the result of a discovery process, and our findings need to be validated in an independent dataset.
CONCLUSIONS
We found that several social factors were associated with risk of modality failure in patients initiating renal replacement therapy using PD. Our findings point to the importance of social and financial support in maintaining PD. That information may help guide physicians in their recommendations for dialysis modality, especially in light of recent changes to the reimbursement of dialysis in the United States, which incentivizes providers to direct patients toward PD. More broadly, it suggests that future studies need to determine if increases in social and financial support—for example, through assisted PD programs—can alter the rate of technique failure.
DISCLOSURES
The authors have no financial conflicts of interest to disclose.
Acknowledgments
JIS is a 2010 American Kidney Fund-Amgen Clinical Scientist in Nephrology fellow. We gratefully acknowledge Dr. Alice Whittemore’s comments on earlier drafts of this manuscript. The data reported here were supplied by the US Renal Data System. The interpretation and reporting of those data are the responsibility of the authors and in no way should be seen as official policy or an interpretation of the US Renal Data System. The study was conducted under a data use agreement between the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and Dr. Winkelmayer. The manuscript was reviewed by an officer of the NIDDK and approved for publication.
References
- 1. United States, Department of Health and Human Services, Public Health Service, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, U.S. Renal Data System (USRDS). USRDS 2011 annual data report: atlas of chronic kidney disease and end-stage renal disease in the United States. Bethesda, MD: USRDS; 2011. [Google Scholar]
- 2. Mujais S, Story K. Peritoneal dialysis in the US: evaluation of outcomes in contemporary cohorts. Kidney Int Suppl 2006; (103):S21–6 [DOI] [PubMed] [Google Scholar]
- 3. Shih YC, Guo A, Just PM, Mujais S. Impact of initial dialysis modality and modality switches on medicare expenditures of end-stage renal disease patients. Kidney Int 2005; 68:319–29 [DOI] [PubMed] [Google Scholar]
- 4. Chaudhary K, Sangha H, Khanna R. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol 2011; 6:447–56 [DOI] [PubMed] [Google Scholar]
- 5. Schaubel DE, Blake PG, Fenton SS. Effect of renal center characteristics on mortality and technique failure on peritoneal dialysis. Kidney Int 2001; 60:1517–24 [DOI] [PubMed] [Google Scholar]
- 6. Plantinga LC, Fink NE, Finkelstein FO, Powe NR, Jaar BG. Association of peritoneal dialysis clinic size with clinical outcomes. Perit Dial Int 2009; 29:285–91 [PMC free article] [PubMed] [Google Scholar]
- 7. Huisman RM, Nieuwenhuizen MG, Th de Charro F. Patient-related and centre-related factors influencing technique survival of peritoneal dialysis in the Netherlands. Nephrol Dial Transplant 2002; 17:1655–60 [DOI] [PubMed] [Google Scholar]
- 8. Afolalu B, Troidle L, Osayimwen O, Bhargava J, Kitsen J, Finkelstein FO. Technique failure and center size in a large cohort of peritoneal dialysis patients in a defined geographic area. Perit Dial Int 2009; 29:292–6 [PubMed] [Google Scholar]
- 9. Tangri N, Ansell D, Naimark D. Determining factors that predict technique survival on peritoneal dialysis: application of regression and artificial neural network methods. Nephron Clin Pract 2011; 118:c93–100 [DOI] [PubMed] [Google Scholar]
- 10. Chidambaram M, Bargman JM, Quinn RR, Austin PC, Hux JE, Laupacis A. Patient and physician predictors of peritoneal dialysis technique failure: a population-based, retrospective cohort study. Perit Dial Int 2011; 31:565–73 [DOI] [PubMed] [Google Scholar]
- 11. Jaar BG, Plantinga LC, Crews DC, Fink NE, Hebah N, Coresh J, et al. Timing, causes, predictors and prognosis of switching from peritoneal dialysis to hemodialysis: a prospective study. BMC Nephrol 2009; 10:3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ward MM. Medical insurance, socioeconomic status, and age of onset of endstage renal disease in patients with lupus nephritis. J Rheumatol 2007; 34:2024–7 [PubMed] [Google Scholar]
- 13. Ward MM. Laboratory abnormalities at the onset of treatment of end-stage renal disease: are there racial or socioeconomic disparities in care? Arch Intern Med 2007; 167:1083–91 [DOI] [PubMed] [Google Scholar]
- 14. Holley JL, DeVore CC. Why all prescribed medications are not taken: results from a survey of chronic dialysis patients. Adv Perit Dial 2006; 22:162–6 [PubMed] [Google Scholar]
- 15. Gill JS, Hussain S, Rose C, Hariharan S, Tonelli M. Access to kidney transplantation among patients insured by the United States Department of Veterans Affairs. J Am Soc Nephrol 2007; 18:2592–9 [DOI] [PubMed] [Google Scholar]
- 16. Han SH, Lee JE, Kim DK, Moon SJ, Kim HW, Chang JH, et al. Long-term clinical outcomes of peritoneal dialysis patients: single center experience from Korea. Perit Dial Int 2008; 28(Suppl 3):S21–6 [PubMed] [Google Scholar]
- 17. Guo A, Mujais S. Patient and technique survival on peritoneal dialysis in the United States: evaluation in large incident cohorts. Kidney Int Suppl 2003; (88):S3–12 [DOI] [PubMed] [Google Scholar]
- 18. Lim WH, Dogra GK, McDonald SP, Brown FG, Johnson DW. Compared with younger peritoneal dialysis patients, elderly patients have similar peritonitis-free survival and lower risk of technique failure, but higher risk of peritonitis-related mortality. Perit Dial Int 2011; 31:663–71 [DOI] [PubMed] [Google Scholar]
- 19. Firanek CA, Vonesh EF, Korbet SM. Patient and technique survival among an urban population of peritoneal dialysis patients: an 8-year experience. Am J Kidney Dis 1991; 18:91–6 [Erratum in: Am J Kidney Dis 1992; 19:93] [DOI] [PubMed] [Google Scholar]
- 20. Korbet SM, Shih D, Cline KN, Vonesh EF. Racial differences in survival in an urban peritoneal dialysis program. Am J Kidney Dis 1999; 34:713–20 [DOI] [PubMed] [Google Scholar]
- 21. Tanna MM, Vonesh EF, Korbet SM. Patient survival among incident peritoneal dialysis and hemodialysis patients in an urban setting. Am J Kidney Dis 2000; 36:1175–82 [DOI] [PubMed] [Google Scholar]
- 22. Farias MG, Soucie JM, McClellan W, Mitch WE. Race and the risk of peritonitis: an analysis of factors associated with the initial episode. Kidney Int 1994; 46:1392–6 [DOI] [PubMed] [Google Scholar]
- 23. Golper TA, Brier ME, Bunke M, Schreiber MJ, Bartlett DK, Hamilton RW, et al. Risk factors for peritonitis in long-term peritoneal dialysis: the Network 9 peritonitis and catheter survival studies. Academic Subcommittee of the Steering Committee of the Network 9 Peritonitis and Catheter Survival Studies. Am J Kidney Dis 1996; 28:428–36 [DOI] [PubMed] [Google Scholar]
- 24. Holley JL, Bernardini J, Piraino B. A comparison of peritoneal dialysis-related infections in black and white patients. Perit Dial Int 1993; 13:45–9 [PubMed] [Google Scholar]
- 25. Oo TN, Roberts TL, Collins AJ. A comparison of peritonitis rates from the United States Renal Data System database: CAPD versus continuous cycling peritoneal dialysis patients. Am J Kidney Dis 2005; 45:372–80 [DOI] [PubMed] [Google Scholar]
- 26. Nessim SJ, Bargman JM, Austin PC, Nisenbaum R, Jassal SV. Predictors of peritonitis in patients on peritoneal dialysis: results of a large, prospective Canadian database. Clin J Am Soc Nephrol 2009; 4:1195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Davies SJ, Phillips L, Griffiths AM, Russell LH, Naish PF, Russell GI. What really happens to people on long-term peritoneal dialysis? Kidney Int 1998; 54:2207–17 [DOI] [PubMed] [Google Scholar]
- 28. Kim GC, Vonesh EF, Korbet SM. The effect of technique failure on outcome in black patients on continuous ambulatory peritoneal dialysis. Perit Dial Int 2002; 22:53–9 [PubMed] [Google Scholar]
- 29. Walker DR, Inglese GW, Sloand JA, Just PM. Dialysis facility and patient characteristics associated with utilization of home dialysis. Clin J Am Soc Nephrol 2010; 5:1649–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Golper TA, Saxena AB, Piraino B, Teitelbaum I, Burkart J, Finkelstein FO, et al. Systematic barriers to the effective delivery of home dialysis in the United States: a report from the Public Policy/Advocacy Committee of the North American Chapter of the International Society for Peritoneal Dialysis. Am J Kidney Dis 2011; 58:879–85 [DOI] [PubMed] [Google Scholar]
- 31. Brown EA, Dratwa M, Povlsen JV. Assisted peritoneal dialysis—an evolving dialysis modality. Nephrol Dial Transplant 2007; 22:3091–2 [DOI] [PubMed] [Google Scholar]
- 32. Lobbedez T, Moldovan R, Lecame M, Hurault de Ligny B, El Haggan W, Ryckelynck JP. Assisted peritoneal dialysis. Experience in a French renal department. Perit Dial Int 2006; 26:671–6 [PubMed] [Google Scholar]
- 33. Oliver MJ, Quinn RR, Richardson EP, Kiss AJ, Lamping DL, Manns BJ. Home care assistance and the utilization of peritoneal dialysis. Kidney Int 2007; 71:673–8 [DOI] [PubMed] [Google Scholar]
- 34. Avorn J, Winkelmayer WC, Bohn RL, Levin R, Glynn RJ, Levy E, et al. Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol 2002; 55:711–16 [DOI] [PubMed] [Google Scholar]
- 35. Winkelmayer WC, Mehta J, Chandraker A, Owen WF, Jr, Avorn J. Predialysis nephrologist care and access to kidney transplantation in the United States. Am J Transplant 2007; 7:872–9 [DOI] [PubMed] [Google Scholar]
- 36. Winkelmayer WC, Owen WF, Jr, Levin R, Avorn J. A propensity analysis of late versus early nephrologist referral and mortality on dialysis. J Am Soc Nephrol 2003; 14:486–92 [DOI] [PubMed] [Google Scholar]
- 37. Winkelmayer WC, Glynn RJ, Levin R, Owen W, Jr, Avorn J. Late referral and modality choice in end-stage renal disease. Kidney Int 2001; 60:1547–54 [DOI] [PubMed] [Google Scholar]
- 38. Szabo E, Moody H, Hamilton T, Ang C, Kovithavongs C, Kjellstrand C. Choice of treatment improves quality of life. A study on patients undergoing dialysis. Arch Intern Med 1997; 157:1352–6 [PubMed] [Google Scholar]
- 39. Ortega LM, Materson BJ. Hypertension in peritoneal dialysis patients: epidemiology, pathogenesis, and treatment. J Am Soc Hypertens 2011; 5:128–36 [DOI] [PubMed] [Google Scholar]
- 40. Chaudhary K. Peritoneal dialysis drop-out: causes and prevention strategies. Int J Nephrol 2011; 2011:434608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kolesnyk I, Dekker FW, Boeschoten EW, Krediet RT. Time-dependent reasons for peritoneal dialysis technique failure and mortality. Perit Dial Int 2010; 30:170–7 [DOI] [PubMed] [Google Scholar]
- 42. Jager KJ, Merkus MP, Dekker FW, Boeschoten EW, Tijssen JG, Stevens P, et al. Mortality and technique failure in patients starting chronic peritoneal dialysis: results of the Netherlands Cooperative Study on the Adequacy of Dialysis. NECOSAD Study Group. Kidney Int 1999; 55:1476–85 [DOI] [PubMed] [Google Scholar]
- 43. Genestier S, Hedelin G, Schaffer P, Faller B. Prognostic factors in CAPD patients: a retrospective study of a 10-year period. Nephrol Dial Transplant 1995; 10:1905–11 [PubMed] [Google Scholar]
- 44. Kuriyama R, Tranæus A, Ikegami T. Icodextrin reduces mortality and the drop-out rate in Japanese peritoneal dialysis patients. Adv Perit Dial 2006; 22:108–10 [PubMed] [Google Scholar]
- 45. Mehrotra R, Chiu YW, Kalantar-Zadeh K, Vonesh E. The outcomes of continuous ambulatory and automated peritoneal dialysis are similar. Kidney Int 2009; 76:97–107 [DOI] [PubMed] [Google Scholar]


