Abstract
♦ Background: Data on obesity as a risk factor for peritonitis and catheter infections among peritoneal dialysis (PD) patients are limited. Furthermore, little is known about the microbiology of PD-related infections among patients with a high body mass index (BMI).
♦ Methods: Using a cohort that included all adult patients residing in the province of Manitoba who received PD during the period 1997 - 2007, we studied the relationship between BMI and PD-related infections. After categorizing patients into quartiles of BMI, a multivariate Cox regression model was used to determine the independent relationship between BMI and peritonitis or exit-site infection (ESI). We also studied whether increasing BMI was associated with a propensity to infections with particular organisms.
♦ Results: Among 990 PD patients, 938 (95%) had accurate BMI data available. Those 938 patients experienced 1338 peritonitis episodes and 1194 exit-site infections. In unadjusted analyses, patients in the highest BMI quartile (median: 33.5; interquartile range: 31.9 - 36.4) had an increased risk of peritonitis overall, and also an increased risk of peritonitis with gram-positive organisms and coagulase-negative Staphylococcus (CNS). After multivariate adjustment for age, sex, diabetes, cause of renal disease, Aboriginal race, PD modality, and S. aureus nasal carriage, the relationship between overall peritonitis risk and BMI disappeared, but the increased risk of CNS peritonitis among patients in the highest BMI quartile persisted (hazard ratio: 1.80; 95% confidence interval: 1.06 to 3.06; p = 0.03). There was no increased risk of ESI among patients in the highest BMI quartile on univariate analysis or after multivariate adjustment.
♦ Conclusions: Among Canadian PD patients, obesity was not associated with an increased risk of peritonitis overall, but may be associated with a higher risk of CNS peritonitis.
Key words: Body mass index, exit-site infection, obesity, peritonitis
Body mass index (BMI) is frequently taken into account when selecting a dialysis modality. One concern with regard to peritoneal dialysis (PD) is the possibility that obesity may increase the risk of mechanical complications such as leaks and hernias because of increased intra-abdominal pressure (1). An additional concern is the impact of obesity on PD-related infectious complications—a subject about which data are limited.
The two largest observational studies to have assessed the relationship between BMI and PD peritonitis both come from ANZDATA, the Australian and New Zealand Dialysis and Transplant Registry (2,3). In those studies, obesity was associated with an increased risk of peritonitis, with one study reporting a hazard ratio (HR) of 1.08 per 5 kg/m2 increase in BMI (3), and the other reporting a HR of 1.29 in patients with a BMI of 30 or more (2). The microbiology of peritonitis was not reported in those studies.
Using a large cohort of Canadian PD patients, our primary objective was to study the relationship between obesity and PD-related infection, including peritonitis and exit-site infection (ESI). Our secondary objective was to determine whether high BMI among PD patients is associated with a propensity for infection with particular organisms.
METHODS
PATIENTS
The study cohort consisted of all adult patients (≥18 years of age) residing in the province of Manitoba, Canada, who received PD during the period 1997 - 2007 (n = 990) and for whom BMI data were available (n = 938, 95%). Manitoba has a population of approximately 1.2 million people, and 20% of the prevalent end-stage renal disease population utilize PD. The study used PD-MAN, the peritoneal dialysis database of Manitoba, whose development and validation have been described elsewhere (4). The study protocol was approved by the St. Boniface General Hospital Research Ethics Board.
PERITONITIS AND ESI
The diagnosis of peritonitis and ESI was made by the treating physician in accordance with standard International Society for Peritoneal Dialysis definitions. Organisms causing peritonitis and ESI were categorized as follows: gram-positive, gram-negative, culture-negative, and fungal, with subsets for coagulase-negative Staphylococcus (CNS), S. aureus, and Streptococcus species. During the period of the study, the protocol in the PD unit was to treat S. aureus nasal carriers with intranasal mupirocin and oral rifampin for 5 days every 2 months until 3 negative nasal swabs for S. aureus were obtained.
COVARIATES
Demographic data available for the current study included age, sex, race, cause of end-stage renal disease, diabetes status, PD modality [continuous ambulatory (CAPD) vs. automated (APD)], S. aureus nasal carriage, and BMI. Body mass index was determined based on a single measure recorded at the time of PD initiation.
STATISTICAL ANALYSIS
Patients were divided into quartiles of BMI for the purpose of the analyses, with the lowest BMI quartile assigned as the referent. Continuous variables are reported as mean ± standard deviation, or median with interquartile range. One-way analysis of variance was used for the comparison of continuous variables between BMI quartiles. Categorical variables are reported as percentages and were compared between BMI quartiles using the chi-square test.
To examine for associations between BMI and PD-related infections, time-to-first-event analyses were used. Univariate and multivariate Cox proportional hazards models adjusting for age, sex, diabetes, cause of renal disease, Aboriginal race, CAPD versus APD, and S. aureus nasal carriage were used to determine the relationship between obesity and peritonitis and between obesity and ESI. The absence of colinearity between BMI, diabetes, and Aboriginal race was verified before their entry into the model. In addition to the time-to-event modeling, a negative binomial model was used as a sensitivity analysis to determine univariate and multivariate rate ratios for the relationship between obesity and peritonitis and between obesity and ESI. Statistical significance was defined as a p value less than 0.05. All statistical analyses were performed using the PASW software application (version 17.0: PASW Statistics, Chicago, IL, USA).
RESULTS
Of the 938 patients treated with PD in the province of Manitoba between 1997 and 2007 for whom accurate BMI data were available, median time on PD was 611 days (interquartile range: 235 - 1133 days). Table 1 presents the demographic characteristics of the overall cohort and of the patients in each BMI quartile. The mean age of the cohort was 59.5 years, with a slight male predominance. Most of the patients were white (65%), followed by Aboriginal (24%). Diabetes was present in 49% of patients.
TABLE 1.
Patient Demographics
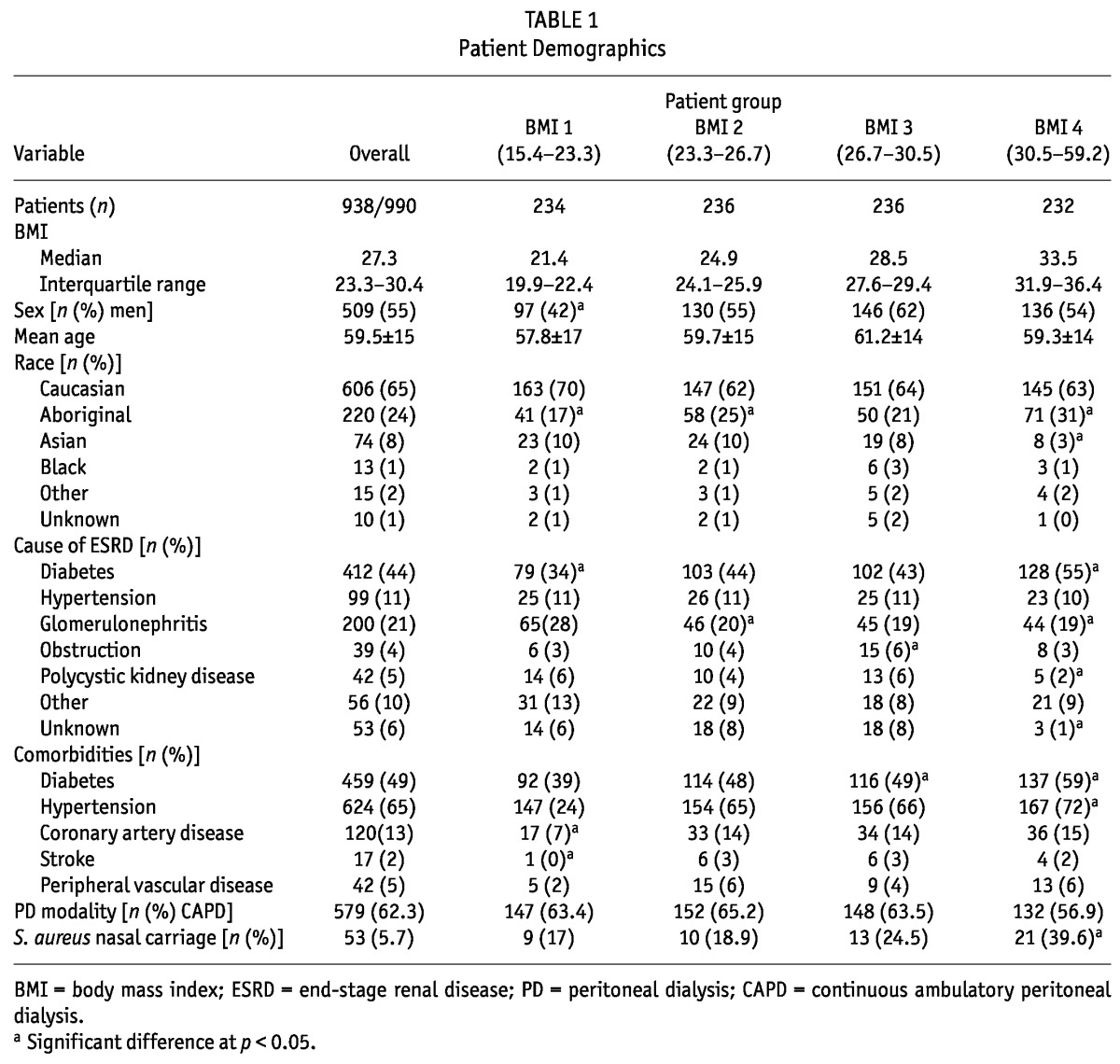
The 938 patients experienced 1338 peritonitis episodes and 1194 ESIs, corresponding to an overall peritonitis rate of 0.64 episodes per patient-year and an ESI rate of 0.61 episodes per patient-year. At least 1 peritonitis episode occurred in 51% of the patients, and at least 1 ESI in 47%. Table 2 shows the distribution of organisms causing peritonitis and ESI, and the infection rates for each organism category by BMI quartile.
TABLE 2.
Organisms Causing Exit-Site Infection and Peritonitis
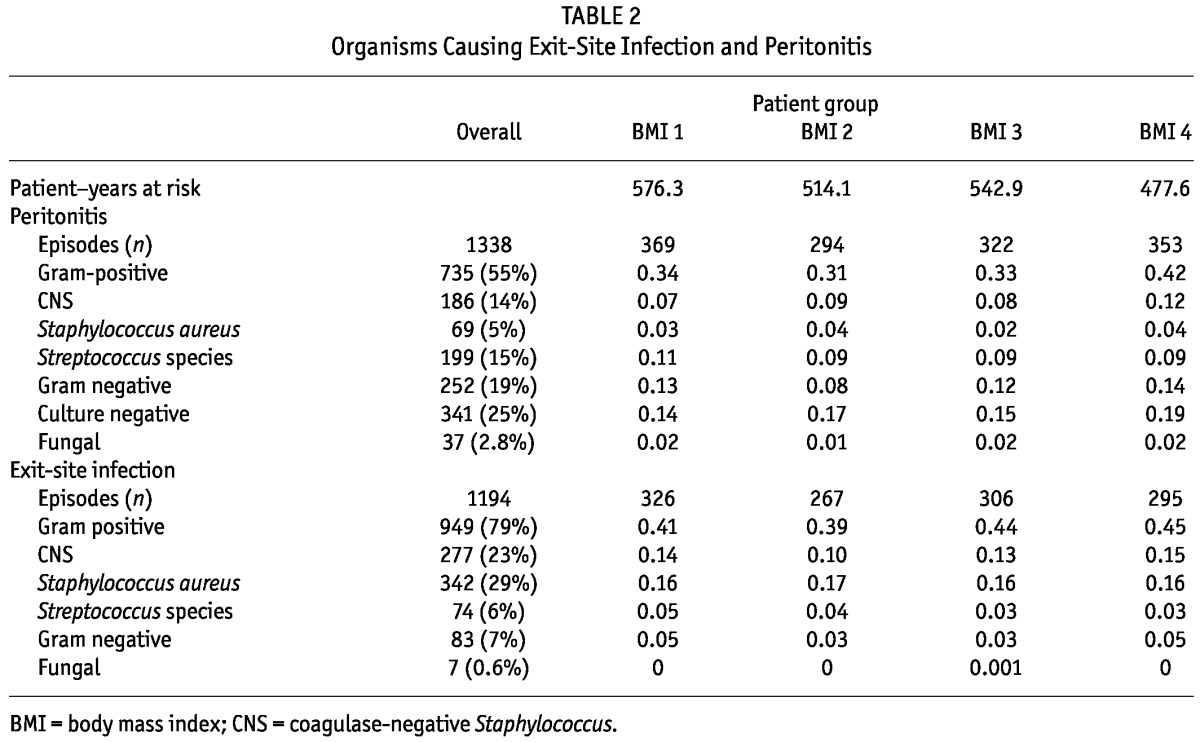
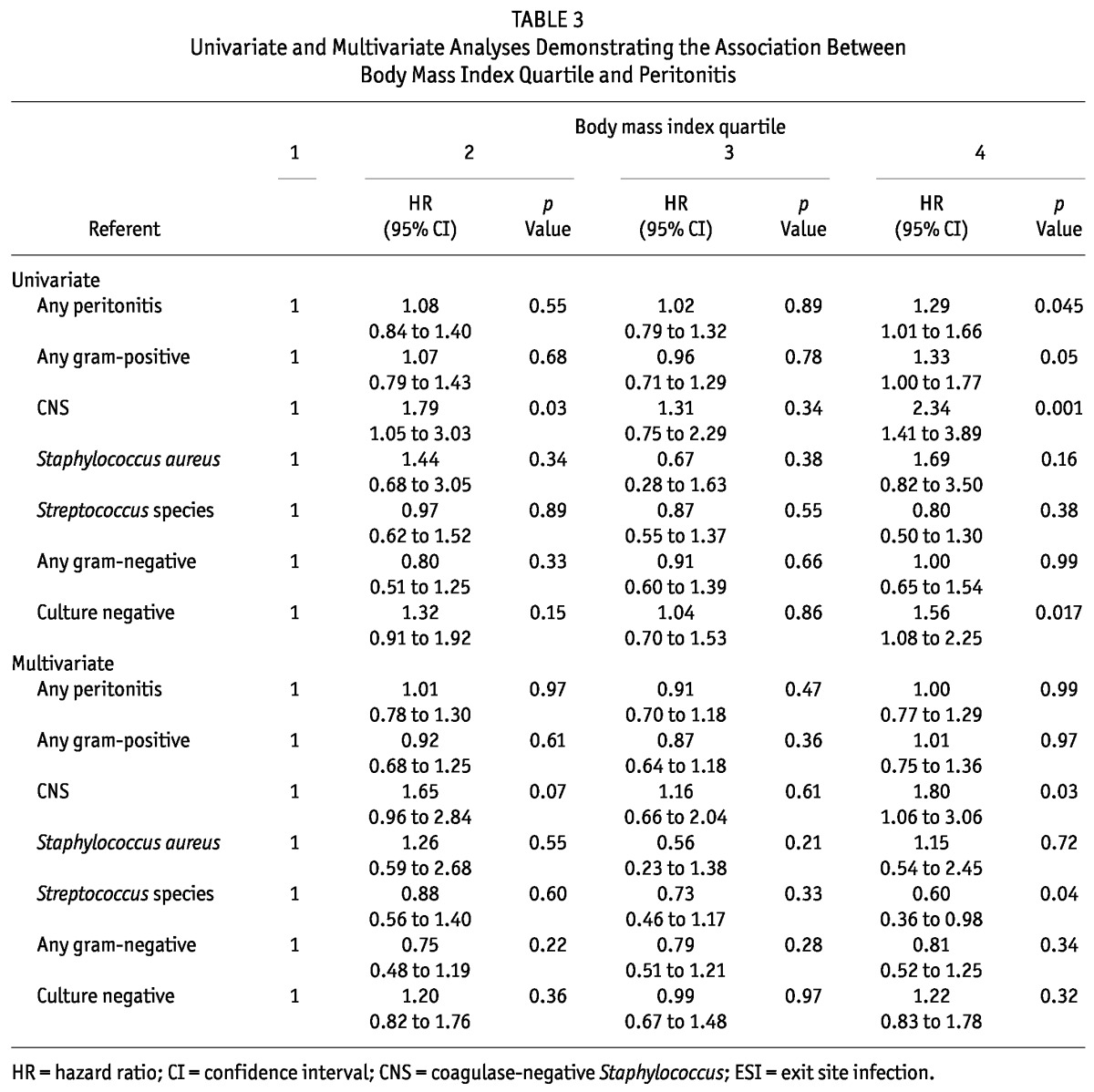
On unadjusted analysis, patients in the highest BMI quartile (median: 33.5; interquartile range: 31.9 - 36.4), relative to those in the lowest BMI quartile (median: 21.4; interquartile range: 19.9 - 22.4), had an increased risk of peritonitis overall [HR: 1.29; 95% confidence interval (CI): 1.01 to 1.66; p = 0.045) and an increased risk of peritonitis with gram-positive organisms (HR: 1.33; 95% CI: 1.00 to 1.77; p = 0.05) and with CNS (HR: 2.34; 95% CI: 1.41 to 3.89; p = 0.001). There was also an increased risk of culture-negative peritonitis in the highest BMI quartile (HR: 1.56; 95% CI: 1.08 to 2.25; p = 0.02). After multivariate adjustment, only the increased risk of CNS peritonitis persisted among patients in the highest BMI quartile (HR: 1.80; 95% CI: 1.06 to 3.06; p = 0.03). Patients in the highest BMI quartile had a reduced risk of Streptococcus peritonitis (HR: 0.60; 95% CI: 0.36 to 0.98; p = 0.04). Table 3 shows the results of the univariate and multivariate analyses for peritonitis.
TABLE 3.
Univariate and Multivariate Analyses Demonstrating the Association Between Body Mass Index Quartile and Peritonitis
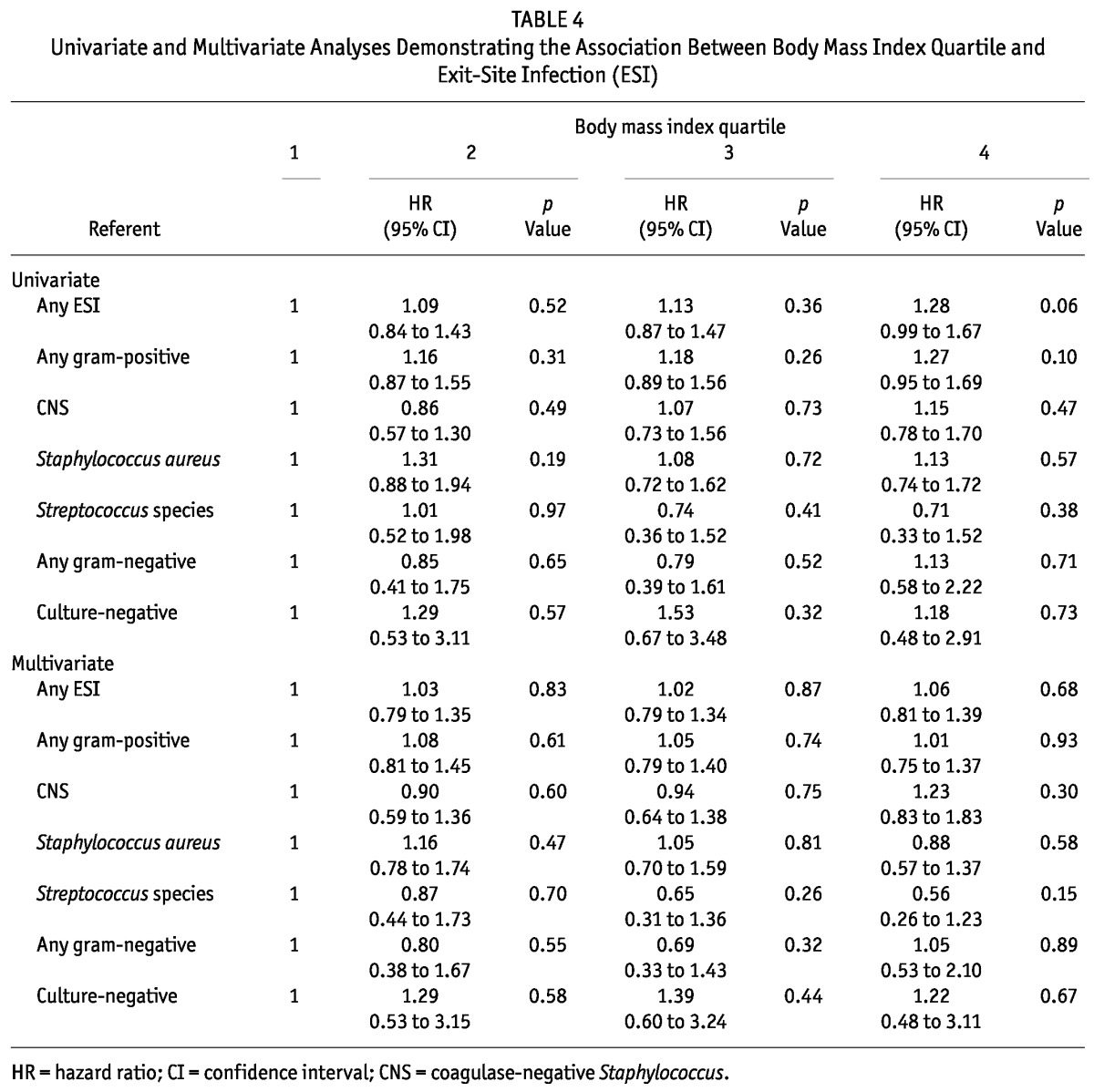
With regard to the relationship between obesity and ESI, there was a trend, on unadjusted analysis, toward an increased risk of ESI among patients in the highest BMI quartile (HR: 1.28; 95% CI: 0.99 to 1.67; p = 0.06). However, no significant associations between BMI and ESI were evident after multivariate adjustment. Table 4 shows the results of the univariate and multivariate analyses for ESI. Nasal carriage of S. aureus was a significant independent predictor of S. aureus ESI (HR: 2.01; 95% CI: 1.17 to 3.46; p = 0.01), but not S. aureus peritonitis.
TABLE 4.
Univariate and Multivariate Analyses Demonstrating the Association Between Body Mass Index Quartile and Exit-Site Infection (ESI)
When the same analyses were performed using a negative binomial model rather than a Cox proportional hazards model, the univariate rate ratio for CNS peritonitis in the highest BMI quartile was 1.63 (95% CI: 1.02 to 2.59; p = 0.04); after multivariate adjustment, it was 1.58 (95% CI: 0.96 to 2.60; p = 0.07).
DISCUSSION
In this large observational study, on unadjusted analysis, patients in the highest BMI quartile (BMI > 30.5) had an increased risk of peritonitis overall and of gram-positive and CNS peritonitis, and showed a trend toward an increased risk of ESI relative to patients in the lowest BMI quartile. After multivariate adjustment, only the increased risk of CNS peritonitis persisted among patients in the highest BMI quartile.
The earliest study to have looked at the relationship between body weight and PD-related infections was published in 1991 by Piraino et al. (5). In that study, no increased risk of peritonitis or catheter infection was observed among the 55 of 228 PD patients who were classified as overweight. However, a trend toward higher peritonitis rates was noted as the ratio of actual to ideal body weight increased, and catheter loss because of infection was more frequent among patients in the overweight category. Subsequently, Afthentopoulos et al. demonstrated similar peritonitis rates among 242 patients classified based on a weight either greater or less than 80 kg (6). Since then, the ANZDATA cohort has provided additional data on the risk of PD-related infection with increasing BMI, obesity having been observed, after multivariate adjustment, to be associated with a shorter time to first peritonitis (2,3). Since the early study by Piraino et al. (5), neither the relationship between BMI and ESI nor the microbiology of PD-related infection across various BMI levels has been investigated further.
The main finding of our study is an apparently increased risk of CNS peritonitis among patients in the highest BMI quartile. Although the incidence of obesity is higher among patients with diabetes and patients of Aboriginal race [both factors being known to increase the peritonitis risk (3,4,7,8)], the increase in CNS peritonitis among obese patients was independent of those variables. Peritonitis attributable to CNS occurs most commonly because of intraluminal introduction of organisms at the time of catheter connection, but it may also occur because of periluminal migration of organisms from the exit site along the catheter tunnel into the peritoneal cavity.
One plausible reason for greater exit-site colonization and subsequent peritonitis among obese patients could be increased perspiration leading to a moist exit site if the catheter sits within an abdominal fold. In addition, obese patients may have decreased visualization of the exit site, which could affect their ability to readily identify early signs of ESI. If greater exit-site colonization, with subsequent periluminal entry of organisms, were the major mechanism of the increased risk of CNS peritonitis among obese PD patients, an increase in S. aureus ESI and peritonitis might also be expected, given that this organism commonly enters by periluminal migration along the catheter tunnel. There was, however, no increase in S. aureus ESI or peritonitis among patients in the highest BMI quartile. Furthermore, among patients in the highest BMI quartile, there was only 1 episode of CNS ESI followed by CNS peritonitis in the subsequent 3 months.
A more plausible explanation is that the relationship between obesity and CNS peritonitis is associative rather than causative, in which case obesity may simply be a marker of other comorbidities or poor socioeconomic status that could augment the risk of touch contamination leading to intraluminal introduction of organisms and subsequent CNS peritonitis.
Understanding whether obesity directly increases the risk of PD-related infection is important because, if such is the case, then changing the location of the exit site to a presternal location might be of benefit (9-11). If, however, obesity is simply a marker of peritonitis risk, then the factors associated with obesity that are contributing to the development of CNS peritonitis should instead be targeted. The lack of impact of BMI on the risk of ESI suggests that the latter possibility is more likely.
Data regarding the effect of body size on PD outcomes are conflicting, with some studies reporting a deleterious effect of obesity on technique failure (12), and others showing a favorable or neutral relationship (6,13-15). Interestingly, despite our finding of a possible increased risk of CNS peritonitis, an analysis of the same patient cohort showed that technique survival and patient survival in the highest BMI quartile were not adversely affected (unpublished data). The lack of impact of an increased risk of CNS peritonitis on outcomes among obese patients in this cohort may relate to the fact that CNS peritonitis typically follows a relatively indolent course, with most infections resolving without catheter removal (16-18).
The lower risk of Streptococcus peritonitis seen among patients in the highest BMI quartile is somewhat unexpected. The most common type of Streptococcus to cause peritonitis is S. viridans, which typically originates from the gastrointestinal tract. While it is plausible that obese PD patients could have reduced translocation of organisms across the bowel wall, the lack of an impact of BMI on enteric gram-negative peritonitis suggests against that possibility. Given that there was no signal to suggest an association between Streptococcus peritonitis and BMI in the univariate analysis, it is possible that this finding represents a type 1 statistical error because of multiple comparisons.
Our study is the largest Canadian study on the relationship between BMI and PD-related infection. Nevertheless, several limitations mitigate the strength of our conclusions. First, we must acknowledge the possibility of type 1 error because of multiple statistical comparisons. As a result, we can conclude only that CNS peritonitis may be associated with high BMI. Future studies will be needed to confirm or refute that finding. Second, BMI was recorded only at the time of PD initiation, and therefore we could not account for changes in BMI that occurred over time on PD. One might argue, however, that the predictive value of a baseline BMI measure with regard to infection risk is at least as important as that obtained using a time-averaged BMI over the duration of follow-up. Third, because of the observational nature of the study, we cannot rule out the possibility of residual confounding because of variables such as socio-economic status, access to adequate housing, and other determinants of health that might have affected the reported associations. Fourth, the proportion of culture-negative peritonitis was relatively high, possibly limiting our ability to identify organism-specific associations. Finally, only S. aureus nasal carriers received prophylaxis during the years that the study was ongoing, and that prophylaxis was intermittent, depending on nasal carriage status. That factor, and others, may have contributed to the high frequency of ESI and peritonitis relative to most contemporary cohorts. As a result, although our results are informative, we cannot determine with certainty whether continuous use of exit-site prophylaxis in all patients would have altered our findings.
CONCLUSIONS
We found that obesity may be associated with an increased risk of CNS peritonitis, suggesting that particular attention should be paid to patient training and exit-site care in this patient population. However, based on the lack of association between obesity and overall peritonitis or ESI risk and based on the relatively indolent course of most CNS infections, our findings suggest that obesity should not be a contraindication to selecting PD on the basis of infection risk.
DISCLOSURES
SJN has received speaker honoraria from Baxter Healthcare and Merck Frosst Canada. PK, CR, MV, and MMS have no financial conflicts of interest to declare. All authors contributed significantly and approved the manuscript.
Acknowledgments
The authors thank Dolores Friesen, Loretta Eng, Amanda Eng, and the Manitoba Renal Program. This research reported here was funded by a grant from the Renal Research and Development Committee.
References
- 1. Del Peso G, Bajo MA, Costero O, Hevia C, Gil F, Díaz C, et al. Risk factors for abdominal wall complications in peritoneal dialysis patients. Perit Dial Int 2003; 23:249–54 [PubMed] [Google Scholar]
- 2. McDonald SP, Collins JF, Rumpsfeld M, Johnson DW. Obesity is a risk factor for peritonitis in the Australian and New Zealand peritoneal dialysis patient populations. Perit Dial Int 2004; 24:340–6 [PubMed] [Google Scholar]
- 3. Lim WH, Johnson DW, McDonald SP. Higher rate and earlier peritonitis in Aboriginal patients compared to non-Aboriginal patients with end-stage renal failure maintained on peritoneal dialysis in Australia: analysis of ANZDATA. Nephrology (Carlton) 2005; 10:192–7 [DOI] [PubMed] [Google Scholar]
- 4. Sood MM, Komenda P, Sood AR, Reslerova M, Verrelli M, Sathianathan C, et al. Adverse outcomes among Aboriginal patients receiving peritoneal dialysis. CMAJ 2010; 182:1433–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Piraino B, Bernardini J, Centa PK, Johnston JR, Sorkin MI. The effect of body weight on CAPD related infections and catheter loss. Perit Dial Int 1991; 11:64–8 [PubMed] [Google Scholar]
- 6. Afthentopoulos IE, Oreopoulos DG. Is CAPD an effective treatment for ESRD patients with a weight over 80 kg? Clin Nephrol 1997; 47:389–93 [PubMed] [Google Scholar]
- 7. Oo TN, Roberts TL, Collins AJ. A comparison of peritonitis rates from the United States Renal Data System database: CAPD versus continuous cycling peritoneal dialysis patients. Am J Kidney Dis 2005; 45:372–80 [DOI] [PubMed] [Google Scholar]
- 8. Chow KM, Szeto CC, Leung CB, Kwan BC, Law MC, Li PK. A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit Dial Int 2005; 25:374–9 [PubMed] [Google Scholar]
- 9. Warchol S, Ziolkowska H, Roszkowska-Blaim M. Exit-site infection in children on peritoneal dialysis: comparison of two types of peritoneal catheters. Perit Dial Int 2003; 23:169–73 [PubMed] [Google Scholar]
- 10. Twardowski ZJ, Prowant BF, Nichols WK, Nolph KD, Khanna R. Six-year experience with swan neck presternal peritoneal dialysis catheter. Perit Dial Int 1998; 18:598–602 [PubMed] [Google Scholar]
- 11. Crabtree JH, Burchette RJ. Comparative analysis of two-piece extended peritoneal dialysis catheters with remote exit-site locations and conventional abdominal catheters. Perit Dial Int 2010; 30:46–55 [DOI] [PubMed] [Google Scholar]
- 12. McDonald SP, Collins JF, Johnson DW. Obesity is associated with worse peritoneal dialysis outcomes in the Australia and New Zealand patient populations. J Am Soc Nephrol 2003; 14:2894–901 [DOI] [PubMed] [Google Scholar]
- 13. Johnson DW, Herzig KA, Purdie DM, Chang W, Brown AM, Rigby RJ, et al. Is obesity a favorable prognostic factor in peritoneal dialysis patients? Perit Dial Int 2000; 20:715–21 [PubMed] [Google Scholar]
- 14. Fried L, Bernardini J, Piraino B. Neither size nor weight predicts survival in peritoneal dialysis patients. Perit Dial Int 1996; 16:357–61 [PubMed] [Google Scholar]
- 15. Aslam N, Bernardini J, Fried L, Piraino B. Large body mass index does not predict short-term survival in peritoneal dialysis patients. Perit Dial Int 2002; 22:191–6 [PubMed] [Google Scholar]
- 16. Mujais S. Microbiology and outcomes of peritonitis in North America. Kidney Int Suppl 2006; (103):S55–62 [DOI] [PubMed] [Google Scholar]
- 17. Szeto CC, Kwan BC, Chow KM, Lau MF, Law MC, Chung KY, et al. Coagulase negative staphylococcal peritonitis in peritoneal dialysis patients: review of 232 consecutive cases. Clin J Am Soc Nephrol 2008; 3:91–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Coagulase-negative staphylococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 936 cases. Nephrol Dial Transplant 2010; 25:3386–92 [DOI] [PubMed] [Google Scholar]


