Previously, we reported results of a peritoneal dialysis (PD) regimen with lower content of glucose and glucose degradation products (GDPs) in new continuous ambulatory PD (CAPD) patients (1-4). In the same study, plasma markers of endothelial function and inflammation such as soluble vascular cell adhesion molecule 1 (sVCAM-1), soluble intercellular adhesion molecule 1 (sICAM-1), soluble E-selectin (sE-selectin), von Willebrand factor (vWf), and high-sensitivity C-reactive protein (hsCRP) were determined. In this short communication, we present the results of those markers during the application of the new PD regimen so as to estimate its cardiovascular effects.
METHODS
A detailed description of the study design was previously reported (1). In brief, we conducted an open, randomized, multicenter crossover study in 74 new CAPD patients, comparing NEPP [2 Physioneal, 1 Nutrineal, and 1 Extraneal dwell (all fluids: Baxter Healthcare BV, Utrecht, Netherlands)] with standard therapy [4 Dianeal dwells (Baxter Healthcare BV)]. During the PD teaching period and the first 6 weeks stabilizing on PD, patients were treated with their allocated PD regimen, after which membrane characteristics were obtained. Thereafter, patients were treated for 24 weeks, and then they switched to the other regimen. The appropriate medical ethics committees approved the study, and written informed consent was obtained from all patients.
Concentrations of the markers of endothelial function were assessed in plasma samples that had been deep-frozen (-70°C). Levels of vW antigen were estimated in duplicate by ELISA using polyclonal antibodies from Dako (Glostrup, Denmark) (5) and are reported as a percentage of a reference pool. Duplicate measurements of sVCAM-1, sICAM-1 and sE-selectin were measured using commercially available ELISA kits (Diaclone, Besançon, France). Median values (ranges) in healthy, nonobese, nonsmoking volunteers were 908 ng/mL (538 - 1286 ng/mL) for sVCAM-1, 483 ng/mL (98 - 647 ng/mL) for sICAM-1, and 82 ng/mL (46 - 83 ng/mL) for sE-selectin as determined in our laboratory (n = 20). Measurements of hsCRP were obtained using a highly sensitive in-house ELISA (6). Methodologic considerations pertaining to other variables, such as glucose degradation products and AGEs, were reported previously (4).
The study was conducted in a period during which icodextrin batches might have been contaminated with peptidoglycans. Retrospectively, in collaboration with Baxter, who could retain the badge numbers for fluids used by our patients, we have been able to analyze whether patients used possibly contaminated bags in their treatment and, if so, for how long.
Data were analyzed using the generalized estimating equations (GEE) longitudinal data analysis technique in Stata 7 and 11 for Windows (StataCorp LP, College Station, TX, USA), as indicated in previous reports.
RESULTS
Of the 74 patients randomized (39 NEPP→standard; 35 standard→NEPP), 50 (24 NEPP→standard, 26 standard→NEPP) completed the entire 54-week study period. Patient characteristics, reasons for withdrawal, and side effects were published previously (4).
At the start of PD treatment (0 - 6 weeks), statistically significant increases in plasma sICAM-1 and vWf were observed. In contrast, changes in sE-selectin, sVCAM-1, and hsCRP during that period were not statistically significant (p = 0.08 and higher). We observed no statistically significant differences between the two regimens.
During the subsequent study period (6 - 54 weeks), levels of vWf and hsCRP were both higher during application of the NEPP regimen (p < 0.0001 and p = 0.02 respectively, Figures 1 and 2); sE-selectin, sVCAM-1 (Figure 3), and sICAM-1 did not differ between the groups. However, the increase in hsCRP associated with NEPP occurred in the group treated with NEPP during the first period, and it had disappeared by the end of that period. The same increase did not occur in the standard→NEPP group after the switch to NEPP from the standard regimen (Figure 2). In the statistical models, the mean increase in vWf attributable to NEPP was 8.4% (explained variance in the model: 0.65). The increase in hsCRP values with NEPP was 23%. However, in the hsCRP models, the total explained variance was very low (0.07), indicating that hsCRP was determined mainly by factors not included in the multivariable analysis. The hsCRP change was probably already occurring before the crossover, indicating that unidentified factors other than NEPP are stronger determinants of hsCRP.
Figure 1.
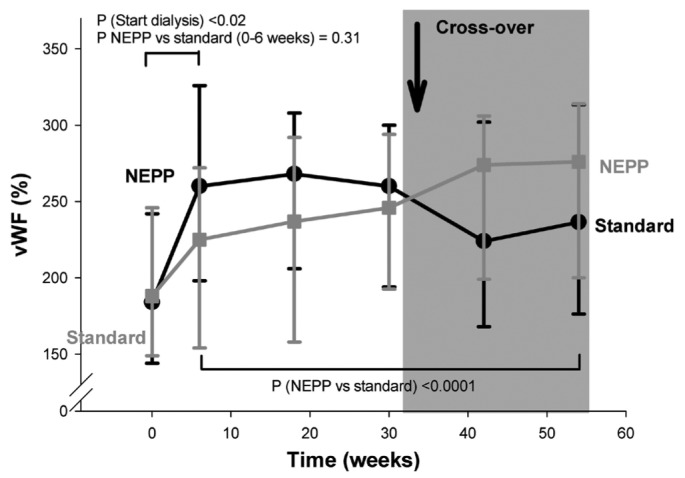
— Change of von Willebrand factor (vWF) during dialysis with a NEPP regimen (1 Nutrineal, 1 Extraneal, and 2 Physioneal dwells) and with a standard regimen (4 Dianeal dwells). All solutions from Baxter Healthcare BV (Utrecht, Netherlands). Data are given as medians, with 25th and 75th percentiles.
Figure 2.
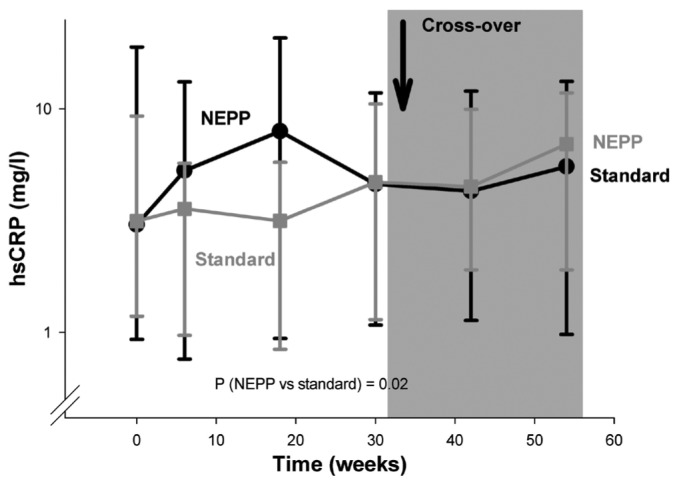
— Change of high-sensitivity C-reactive protein (hsCRP) with a NEPP regimen (1 Nutrineal, 1 Extraneal, and 2 Physioneal dwells) and with a standard regimen (4 Dianeal dwells). All solutions from Baxter Healthcare BV (Utrecht, Netherlands). Data are given as medians, with 25th and 75th percentiles.
Figure 3.
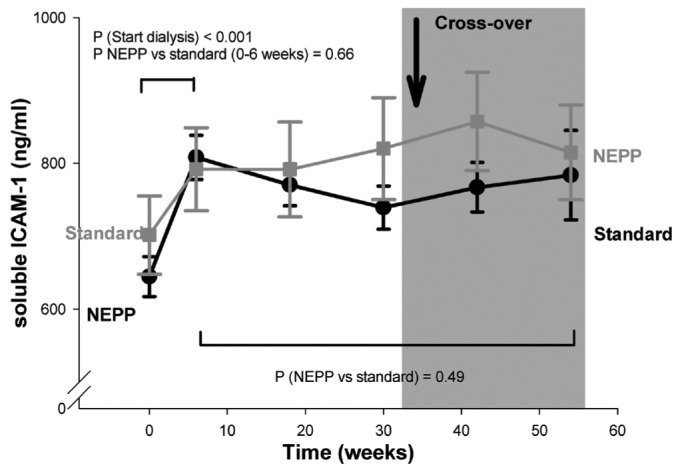
— Soluble intercellular adhesion molecule 1 (ICAM-1) with a NEPP regimen (1 Nutrineal, 1 Extraneal, and 2 Physioneal dwells) and with a standard regimen (4 Dianeal dwells). All solutions from Baxter Healthcare BV (Utrecht, Netherlands). Data are given as means with standard error.
In additional multivariable analyses, we tried to unravel whether estimated intraperitoneal glucose and GDP loads were associated with levels of vWf, hsCRP, and sVCAM-1. The vWf levels were associated with neither the intraperitoneal glucose nor the GDP load (p > 0.68). Higher glucose loads were associated with higher levels of hsCRP (p = 0.008); GDP loads were not associated with hsCRP. The association between NEPP and higher levels of hsCRP was not fully explained by glucose load, indicating that this particular association also depends on characteristics other than the NEPP regimen. We observed weak but statistically significant associations between sVCAM-1 on the one hand and 3-deoxyglucosone, glyoxal, and methylglyoxal on the other (p values between 0.002 and 0.07).
Because of the design of the study, statistical models cannot unravel the effects of the various characteristics of the NEPP regimen, such as icodextrin, amino acids, and the alternating use of the fluids. Because Nε-(carboxymethyl)lysine (CML) increases after the start of icodextrin use (7), we analyzed the contribution of CML to hsCRP and vWf levels. Levels of hsCRP were not associated with CML. Higher vWf levels were associated with higher CML levels (p = 0.047). The association between NEPP and higher vWf levels were not explained by CML. After adjusting for hsCRP levels, the higher vWf levels during NEPP remained statistically significant (p = 0.002).
A small number of patients in both study groups were treated for a limited period with icodextrin solutions contaminated with peptidoglycans. The addition of a dichotomous variable representing the presence of possibly contaminated icodextrin did not change the relationship between NEPP and plasma levels of the various endothelial function markers, indicating that changes observed were not a consequence of the constituents contaminating the icodextrin.
DISCUSSION
The present study shows, first, that initiating PD—regardless of the assigned regimen—causes an increase in plasma markers of endothelial function. Second, during NEPP treatment (a regimen low in glucose and GDPs), we observed higher plasma levels of vWf and, in a weaker fashion, higher plasma levels of hsCRP. Although a few patients in both study groups were treated for a limited period with icodextrin solutions contaminated with peptidoglycans, we could, through statistical analyses, exclude the possibility that that the findings of the present study were a result of the contamination, but must be explained by differences in the dialysis regimens studied.
The increase in markers of endothelial function in the first 6 weeks might be caused by several mechanisms—for example, increased intraperitoneal pressure, resulting in stretch of intraperitoneal capillaries; the presence of PD solution in the peritoneal cavity; toxicity of absorbed constituents of PD fluids, such as glucose and GDPs; changes in fluid status; and medication changes. The participants in the present study were not sufficient in number to permit the contributions of those factors to be evaluated in a multivariable analysis.
During the treatment period (6 - 54 weeks), plasma levels of vWf and hsCRP were higher during application of NEPP than during standard therapy, but levels of the other endothelial factors did not vary. Our statistical analysis could not reveal which constituent or characteristic of NEPP, apart from glucose and GDPs, was responsible for those elevations. Two possible characteristics may therefore be responsible for our observations. First, icodextrin was previously shown to increase CML (7). Second, alternating high glucose with non-glucose-containing fluids might cause the observed results because, compared with a constant high concentration of glucose, intermittently high levels have been reported to induce more endothelial activation and apoptosis in endothelial cells (8,9).
At the present moment, it is difficult to estimate the clinical relevance of the observed increases. In several nonrenal patient groups, high levels of hsCRP and markers of endothelial activation were associated with a significantly higher risk for cardiovascular morbidity and mortality (10,11). However, at study baseline, markers of endothelial activation were already high compared with those in populations for whom the relationship between those markers and cardiovascular risk have been established. In prevalent PD patients, vWF appeared not to be associated with cardiovascular disease (12); however, the clinical relevance of the increase in vWF in the incident patients of the present study remains to be elucidated. Nevertheless, our hypothesis that NEPP improves endothelial function because of the lower load of GDPs must be rejected. The design of the present study cannot untangle the characteristic of the NEPP regimen that is responsible for the disappointing results. For such an analysis, studies using a single type of PD fluid are needed. Furthermore, it could be that any advantages of NEPP would have become clearer if our follow-up period had been longer and if the design of the study had not included a crossover.
CONCLUSIONS
The start of PD resulted in an increase in plasma levels of markers of inflammation and endothelial activation. Higher plasma levels of vWf and hsCRP were associated with treatment using NEPP than with treatment using a standard regimen. It is unclear whether these data indicate that that the NEPP regimen has unfavorable effects on markers of endothelial function and inflammation. It does reject our hypothesis that NEPP can improve endothelial function. Further prospective studies should reveal whether elevations in these markers are consequences of one particular constituent of NEPP or the alternation of different PD fluids, and whether other modifications of the PD regimen might contribute to an improvement of endothelial function and a reduction in cardiovascular risk.
DISCLOSURES
The authors have no financial conflicts of interest to declare.
Acknowledgments
This work was supported by an unrestricted grant from Baxter International Inc. and the Dutch Kidney Foundation.
References
- 1. le Poole CY, van Ittersum FJ, Weijmer MC, Valentijn RM, ter Wee PM. Clinical effects of a peritoneal dialysis regimen low in glucose in new peritoneal dialysis patients: a randomized crossover study. Adv Perit Dial 2004; 20:170–6 [PubMed] [Google Scholar]
- 2. le Poole CY, Welten AG, Weijmer MC, Valentijn RM, van Ittersum FJ, ter Wee PM. Initiating CAPD with a regimen low in glucose and glucose degradation products, with icodextrin and amino acids (NEPP) is safe and efficacious. Perit Dial Int 2005; 25(Suppl 3):S64–8 [PubMed] [Google Scholar]
- 3. le Poole CY, Welten AA, ter Wee PM, Paauw N, Djorai AN, Valentijn RM, et al. A peritoneal dialysis regimen low in glucose and glucose degradation products results in increased cancer antigen 125 and peritoneal activation. Perit Dial Int 2012; 32:305–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. le Poole CY, van Ittersum FJ, Valentijn RM, Teerlink T, Lindholm B, ter Wee PM, et al. “NEPP” peritoneal dialysis regimen has beneficial effects on plasma CEL and 3-DG, but not pentosidine, CML, and MGO. Perit Dial Int 2012; 32:45–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ingerslev J. A sensitive ELISA for von Willebrand factor (vWf:Ag). Scand J Clin Lab Invest 1987; 47:143–9 [PubMed] [Google Scholar]
- 6. Schalkwijk CG, Poland DC, van Dijk W, Kok A, Emeis JJ, Drager AM, et al. Plasma concentration of C-reactive protein is increased in type I diabetic patients without clinical macroangiopathy and correlates with markers of endothelial dysfunction: evidence for chronic inflammation. Diabetologia 1999; 42:351–7 [DOI] [PubMed] [Google Scholar]
- 7. Konings CJ, Schalkwijk CG, van der Sande FM, Leunissen KM, Kooman JP. Influence of icodextrin on plasma and dialysate levels of Nε-(carboxymethyl)lysine and Nε-(carboxyethyl)lysine. Perit Dial Int 2005; 25:591–5 [PubMed] [Google Scholar]
- 8. Jonasson P, Bagge U, Wieslander A, Braide M. Heat-sterilized PD fluid blocks leukocyte adhesion and increases flow velocity in rat peritoneal venules. Perit Dial Int 1996; 16(Suppl 1):S137–40 [PubMed] [Google Scholar]
- 9. Risso A, Mercuri F, Quagliaro L, Damante G, Ceriello A. Intermittent high glucose enhances apoptosis in human umbilical vein endothelial cells in culture. Am J Physiol Endocrinol Metab 2001; 281:E924–30 [DOI] [PubMed] [Google Scholar]
- 10. Schram MT, Stam F, de Jongh RT, Vries G, van Dijk RA, Serne EH, et al. The effect of calcium dobesilate on vascular endothelial function, blood pressure, and markers of oxidation in obese male smokers: a placebo-controlled randomised clinical trial. Atherosclerosis 2003; 170:59–72 [DOI] [PubMed] [Google Scholar]
- 11. Ridker PM, Hennekens CH, Roitman-Johnson B, Stampfer MJ, Allen J. Plasma concentration of soluble intercellular adhesion molecule 1 and risks of future myocardial infarction in apparently healthy men. Lancet 1998; 351:88–92 [DOI] [PubMed] [Google Scholar]
- 12. Pequeriaux NC, Fijnheer R, Gemen EF, Barendrecht AD, Dekker FW, Krediet RT, et al. Plasma concentration of von Willebrand factor predicts mortality in patients on chronic renal replacement therapy. Nephrol Dial Transplant 2012; 27:2452–7 [DOI] [PubMed] [Google Scholar]


