Abstract
Objective
To identify urine biomarkers predictive of acute kidney injury (AKI) in infants admitted to level 2 and 3 neonatal intensive care units with birth weight >2000 g and 5-minute Apgar score ≤7.
Study design
A nested case-control study was performed comparing 8 candidate urine AKI biomarkers in infants with AKI (defined as a rise in serum creatinine of at least 0.3 mg/dL or a serum creatinine elevation ≥1.7 mg/dL persisting for 3 days) and 24 infants from the described cohort without AKI. Urine was analyzed for neutrophil gelatinase–associated lipocalin, osteopontin, cystatin C, albumin, β2 microglobulin, epithelial growth factor, uromodulin (UMOD), and kidney injury molecule 1.
Results
Compared with the infants without AKI, those with AKI had higher levels of urine cystatin C (1123 pg/mL [95% CI, 272-4635 pg/mL] vs 90 pg/mL [95% CI, 39-205 pg/mL]; P < .004; area under the receiver operating characteristic curve [AUC] = 0.82), lower levels of UMOD (11.0 pg/mL [95% CI, 5.7-21.4 pg/mL] vs 26.2 pg/mL [95% CI, 17.4-39.4 pg/mL]; P < .03; AUC = 0.77), and lower levels of epithelial growth factor (6.7 pg/mL [95% CI, 4.0-11.3 pg/mL] vs 17.4 pg/mL [95% CI, 12.7-23.8 pg/mL; P = .003; AUC = 0.82). Although the differences were not statistically significant, levels of urine neutrophil–associated gelatinase lipocalin, kidney injury molecule 1, and osteopontin trended higher in infants with AKI.
Conclusion
Urinary biomarkers can predict AKI in neonates admitted to level 2 and 3 neonatal intensive care units.
The reported incidence of acute kidney injury (AKI) in neonates with a 5-minute Apgar score <7 ranges from 47% to 61%.1-3 AKI has been shown to be an independent predictor of mortality in critically ill neonates,4,5 children,6,7 and adults8-12 even after controlling for comorbidities, interventions, and demographic data. AKI not only impairs fluid and electrolyte homeostasis, but also may hamper systemic inflammatory autoregulation.13 The injured kidneys may play a key role in the systemic derangement present during multiorgan failure. Given that a rise in serum creatinine (SCr) level usually does not occur until several days after renal injury,14 the search for early AKI biomarkers has assumed a prominent role in advancement of AKI research.
SCr-based definitions are used to diagnose AKI.15,16 SCr-based definitions are not ideal, however, because (1) SCr measures function, not injury; (2) SCr may not change until 25%-50% of kidney function has already been lost; (3) SCr overestimates renal function due to tubular secretion of creatinine at lower glomerular filtration rates; (4) SCr varies by muscle mass, hydration status, sex, and age, and its measurement can be affected by endogenous substances; (5) SCr cannot distinguish between prerenal azotemia (a transient, reversible decrease in glomerular filtration rate), and bona fide kidney injury; (6) SCr is not specific to different types of AKI (nephrotoxic, sepsis-associated, or hypoxic/ischemic); and (7) SCr cannot be used to assess kidney function while a patient is receiving dialysis.17 Other problems with using SCr as a measure of AKI specific to neonates include the fact that SCr in the first few days of life reflects maternal kidney function, and there is a very wide distribution of normal SCr values that change over time, depending on the level of prematurity.18,19
Urine AKI biomarkers have shown to be predictive of AKI and mortality in children undergoing cardiopulmonary bypass20-25 and in critically ill preterm infants.26 To date, only limited evaluations of biomarkers have been performed in neonates. To investigate the utility of urine AKI biomarkers in neonates, we evaluated 8 previously identified candidate urinary biomarkers: neutrophil gelatinase–associated lipocalin (NGAL), osteopontin (OPN), cystatin C (Cys C), albumin, β2 microglobulin (B2mG), epithelial growth factor (EGF), uromodulin (UMOD), and kidney injury molecule 1 (KIM-1). We explored the individual and combined ability of these biomarkers to predict AKI. Because urinary proteins are reabsorbed poorly in the most preterm infants,27-29 we performed regression analysis for gestational age to ensure that elevation of biomarkers were not due solely to immature tubular function.
Methods
We conducted a nested case-control study to evaluate the ability of 8 urine biomarkers to predict AKI. Nested case-control studies are particularly advantageous for studies of biologic precursors of disease, such as those described in this study.30
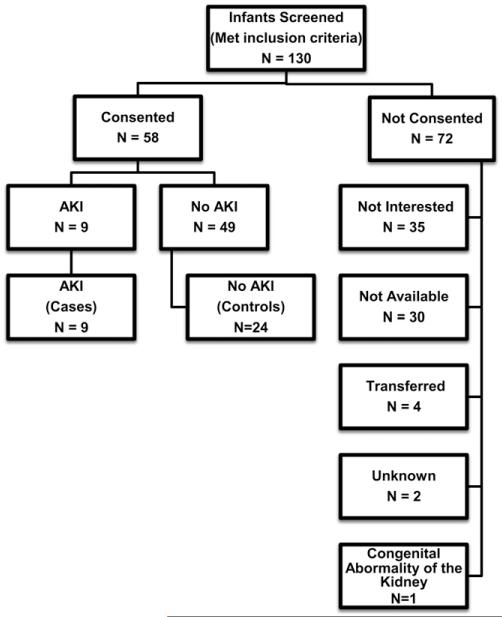
Subjects were newborns in the regional neonatal intensive care unit of the University of Alabama at Birmingham between January 2010 and March 2011. The inclusion criteria for eligibility were birth weight >2000 g, gestational age >34 weeks, 5-minute Apgar score ≤7, and parental informed consent. Of the 130 infants who met the inclusion criteria, 1 infant was excluded for congenital anomaly of the kidney, parents of 71 infants did not consent to participate (35 not interested, 30 not available, 4 infants were transferred to another unit, 2 unknown), and parents of 58 infants consented. Nine of these infants met the criteria for AKI. To serve as controls, we chose 24 infants with ample SCr samples to allow confirmation of negative AKI status (Figure 1; available at www.jpeds.com). The University of Alabama at Birmingham’s Institutional Review Board approved the study.
Figure 1.
Flow diagram depicting the patients consenting for the study, those excluded because of findings of congenital renal anomaly, those with AKI, and those without AKI.
AKI was defined as an acute rise in SCr of at least 0.3 mg/dL within 48 hours (stage 1 of the AKI Network definition16) or a persistent rise in SCr to ≥1.7 mg/dL for 3 days after birth. Controls comprised infants who met the inclusion criteria and had at least 2 blood samples to confirm negative AKI status. SCr values were measured by the Jaffe reaction and obtained during the first 4 days of life. The average number of SCr measurements, infant demographic data, infant interventions, and maternal characteristics in those with AKI and without AKI are presented in Table I.
Table I.
Demographic data for infants with AKI and those without AKI
| No AKI (n = 24) | AKI (n = 9) | P value | |
|---|---|---|---|
| Infant characteristics | |||
| Male sex, n (%) | 9 (38) | 8 (89) | <.01 |
| Race, n (%) | .50 | ||
| Black | 13 (54) | 4 (44) | |
| White | 9 (38) | 5 (56) | |
| Hispanic | 2 (8) | 0 (0) | |
| Gestational age, weeks, mean ± SD |
35 ± 3 | 37 ± 3 | .10 |
| Birth weight, g, mean ± SD | 2421 ± 631 | 3425 ± 863 | <.001 |
| Length, cm, mean ± SD | 46.4 ± 3.6 | 49.2 ± 5.0 | .87 |
| Head circumference, cm, mean ± SD |
31.5 ± 2.1 | 33.4 ± 1.8 | <.05 |
| Survival, n (%) | 24 (100) | 7 (77) | .07 |
| Number of urine collections, mean ± SD |
2 (1-3) | 2 (1-3) | NS |
| 1-minute Apgar, median (IQR) | 2 (1-6) | 1 (0-5) | .10 |
| 5-minute Apgar, median (IQR) | 7 (4-7) | 5 (0-7) | .06 |
| Cord PH, mean ± SD | 7.14 ± 0.15 | 6.97 ± 0.21 | <.01 |
| Infant interventions | |||
| Delivery room bag, n (%) | 7 (29) | 3 (33) | .56 |
| Delivery room oxygen, n (%) | 23 (95) | 8 (88) | .47 |
| Delivery room pressors, n (%) | 20 (83) | 7 (77) | .53 |
| Delivery room intubation, n (%) | 4 (16) | 5 (55) | <.05 |
| Surfactant, n (%) | 2 (8) | 2 (22) | .30 |
| Phenobarbitol, n (%) | 4 (16) | 1 (11) | <.01 |
| Pressors during hospital, n (%) | 5 (20) | 1 (11) | <.01 |
| Aminoglycoside, n (%) | 21 (88) | 7 (78) | .60 |
| Standard ventilator, n (%) | 18 (75) | 6 (67) | .70 |
| Maternal characteristics | |||
| Age, mean ± SD | 25.6 ± 6.0 | 23.2 ± 4.8 | .30 |
| Hypertension, n (%) | 6 (25) | 1 (11) | .36 |
| Preeclampsia, n (%) | 8 (33) | 0 (0) | .07 |
| Smoking, n (%) | 6 (25) | 2 (22) | .62 |
| Antibiotic, n (%) | 16 (66) | 6 (66) | .65 |
| Diabetes mellitus, n (%) | 5 (20) | 3 (33) | .37 |
| Chorioamnionitis, n (%) | 3 (12) | 0 (0) | .37 |
| Steroids, n (%) | 7 (29) | 2 (22) | .52 |
| Prenatal care, n (%) | 23 (95) | 8 (88) | .47 |
Urine specimens were collected during the first 4 days of life using cotton balls placed at the perineum. Not all infants had urine collected on all days. The average number of urine samples obtained in cases was 2 (range, 1-3), and the average for controls was 2 (range, 2-3). Urine was extracted, centrifuged for 10 minutes, and then frozen at −80°C until evaluation. Urine biomarker analysis was performed at Core A of the National Institutes of Health’s P30 O’Brien Core Center for AKI research (www.obrienaki.org).
The Meso Scale Discovery Human Kidney Injury Panel-5 Prototype 7-Plex Assay Kit (catalog no. N75CA-1; Meso Scale Discovery, Gaithersburg, Maryland) was used to measure OPN, Cys C, NGAL, albumin, B2mG, EGF, and UMOD concentrations in 91 human urine samples. Good reproducibility of standard duplicates was obtained, with an average signal confidence of variability (CV) of 6.5%. For 24 samples measured in duplicate, good reproducibility was observed, with an average signal CV of 6.1% and an average calculated concentration CV of 7.04%. The Human Kidney Injury Panel-5 7-Plex assay has picogram per milliliter sensitivity and covers a broad concentration range, from low pg/mL up to 200 000 pg/mL. Urine samples diluted 1:100 yielded good values for Cys C, NGAL, OPN, EGF, and UMOD. At a 1:100 dilution, several albumin and B2mG values were above the curve fit range.
The Meso Scale Discovery Human KIM-1 Assay Kit (catalog no. N45ZA-1; Meso Scale Discovery, Gaithersburg, Maryland) was used to estimate KIM-1 concentrations in 91 human urine samples. Excellent reproducibility of standard duplicates was obtained, with an average signal CV of 2.5%. For 24 samples measured in duplicate, good reproducibility was seen, with an average signal CV of 3.5% and an average calculated concentration CV of 6.2%. The human KIM-1 assay covers a broad concentration range, from <7 pg/mL up to >100 000 pg/mL, allowing for measurement of samples that differ in concentration by >1000-fold. A 1:50 sample dilution yielded good values of KIM-1, with all values within the range of detection.
Descriptive statistical analyses were performed to determine differences between groups. The Shapiro-Wilk test and normal probability plot were used to test for normality of data. Normally distributed continuous variables were compared using the Student t test or Fisher exact test when appropriate. Nonnormal distributed continuous variables were analyzed using the Mann-Whitney U test. For all descriptive statistics, statistical significance was defined as an α value of 0.05.
To evaluate differences in urine biomarkers as predictors of AKI, values were converted to natural log to achieve normal distribution. Mixed regression model analysis with one random intercept included per child was performed to calculate the differences in geometric mean for each biomarker in children with AKI and those without AKI. This statistical approach was used to incorporate multiple time points, controlling for individual patients.
To control for gestational age as a potential confounder, multiple logistic regression analysis was performed, with gestational age and AKI forced into the model. For the regression analysis, biomarkers were converted to natural log to achieve normal distribution. The formula (exp(β) − 1) * 100% from the regression coefficients was used to express the percent change. SAS version 9.2 (SAS Institute, Cary, North Carolina) was used for all statistical analyses. A 2-sided α value of 0.05 was used to verify statistical significance.
Results
Baseline Characteristics
Nine infants were identified as having AKI. Twenty-four infants without AKI served as a control group. Table I presents differences in infant characteristics, infant interventions, and maternal characteristics between infants with AKI and controls without AKI. The infants with AKI were more likely to be male and to have higher birth weight, larger head circumference, and lower cord blood pH. They received more phenobarbital and pressure support and were more likely to be intubated in the delivery room. No statistically significant differences in demographic variables were seen between the infants with AKI and those without AKI, although no mothers of infants with AKI had preeclampsia, compared with 8 of 24 mother (33%) of infants without AKI (P = .07). Two of the 9 infants (78%) with AKI died, compared with no infants without AKI (P = .07). There was no evident difference in the number of urine samples collected between the groups.
Biomarker Differences in Infants with AKI and Infants without AKI
Values of biomarkers in infants with AKI and those without AKI are compared in Table II and Figure 2. Infants with AKI had higher urine Cys C levels (1123 pg/mL [95% CI, 272-4635 pg/mL] vs 90 pg/mL [95% CI, 39-205 pg/mL]; P < .004; area under the receiver operating characteristic curve [AUC] = 0.82), lower UMOD levels (11.0 pg/mL [95% CI, 5.7-21.4 pg/mL] vs 26.2 pg/mL [95% CI, 17.4-39.4 pg/mL]; P < .03; AUC = 0.23), and lower EGF levels (6.7 mg/mL [95% CI, 4.0-11.3 mg/mL] vs 17.4 pg/mL [95% CI, 12.7-23.8 pg/mL]; P = .003; AUC = 0.18).
Table II.
Variation of candidate urine AKI biomarker levels in infants with AKI and those without AKI
| No AKI (n = 24) | AKI (n = 9) | P value | AUC | |
|---|---|---|---|---|
| NGAL, ng/mL | 2.3 (1.3-4.2) | 5.5 (2.1-14.7) | .12 | 0.68 |
| UMOD, ng/mL | 26.2 (17.4-39.4) | 11 (5.7-21.4) | .03 | 0.23 |
| KIM-1, pg/mL | 1.2 (0.7-2.0) | 2.1 (0.9-4.8) | .25 | 0.63 |
| Cys C, pg/mL | 89.6 (39.3-204.5) | 1123 (272.4-4635) | .004 | 0.82 |
| OPN, ng/mL | 2.3 (1.3-4.2) | 3.0 (1.2-7.7) | .64 | 0.64 |
| B2mG, ng/mL | 10 (4.8-21.3) | 7.7 (2.3-26.3) | .70 | 0.56 |
| EGF, pg/mL | 17.4 (12.7-23.8) | 6.7 (4.0-11.3) | .003 | 0.18 |
| Albumin, ng/mL | 75.1 (54.6-109.3) | 111 (60.1-205) | .27 | 0.72 |
Data are geometric mean (95% CI) for all urine measurements.
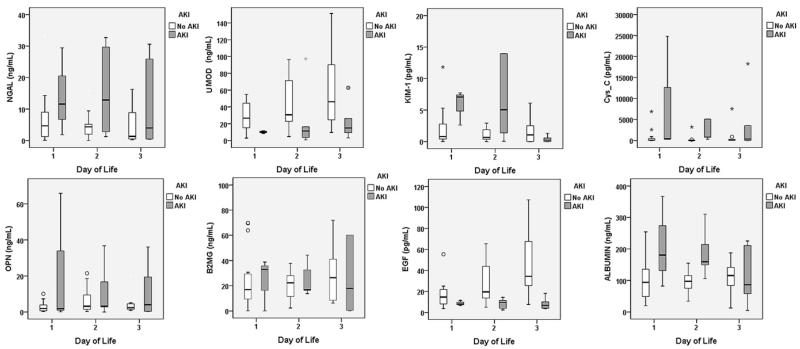
Figure 2.
Urine values of NGAL, UMOD, KIM-1, Cys C, OPN, B2mG, EGF, and albumin in infants with AKI (gray bars) and infants without AKI (white bars) during the first 3 days of life.
Figure 2 depicts urine biomarker values for infants with AKI and those without AKI during the first 3 days of life. In addition to the differences in Cys C, EGF, and UMOD, there appear to be differences in NGAL, KIM-1, OPN, and albumin on different days, although the possibility that those differences might have occurred simply due to chance (ie, P > .05) cannot be ruled out.
Likely because of poor renal tubular development, some urine biomarkers are higher in very preterm infants, even those without AKI.27-29 Therefore, we performed a regression analysis to control for this potential confounder. After controlling for gestational age, significant between-group differences in urine biomarkers persisted. Compared with infants without AKI, those with AKI had 1771% higher Cys C levels (P < .0001), 62% lower UMOD levels (P = .02), and 64% lower EGF levels (P = .002) (Table III; available at www.jpeds.com).
Table III.
Mixed model regression to predict log of urine biomarkers
| Biomarker/ parameter |
Point estimate, percent change |
95% CI for percent change |
P value |
|---|---|---|---|
| Cys C* | |||
| Gestational age | −20.5% per week | (−37.6% to 1.3%) | .06 |
| AKI present | 1771.3% | (265.1% to 9491.9%) | .001 |
| UMOD† | |||
| Gestational age | 5.7% per week | (−6.7% to 20.0%) | .37 |
| AKI present | −62.1% | (−83.3% to −14.0%) | .02 |
| EGF‡ | |||
| Gestational age | 5.5% per week | (−3.9% to 15.7%) | .24 |
| AKI present | −64.8% | (−81% to −35%) | .002 |
A mixed linear model with a random intercept per subject for log biomarker was used. The formula (exp(β) − 1) * 100% from the regression coefficients was used to express the percent change. Gestational age and AKI were forced into all models.
log (Cys-C) = gestational age + AKI.
log (UMOD) = gestational age + AKI.
log (EGF) = gestational age + AKI.
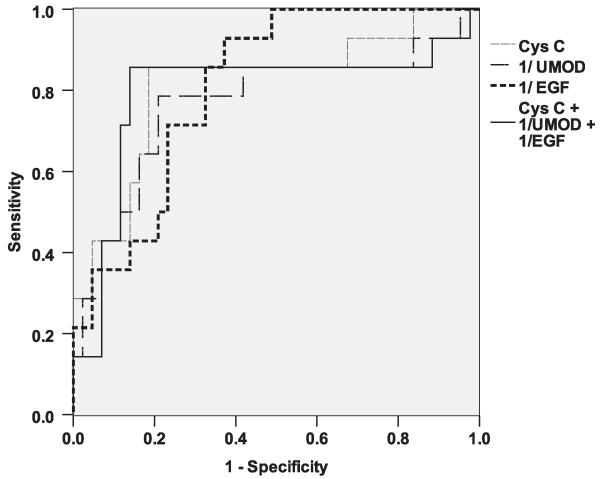
Biomarkers with an AUC value of 0.5 are not better than a coin flip in predicting the disease of interest. Those with an AUC value close to 1 have excellent positive prediction, whereas those with an AUC value close to 0 have excellent negative prediction. The AUC for Cys C was 0.82. The AUCs for EGF and UMOD to predict AKI were 0.18 and 0.23, respectively, and their ability to predict no AKI (the reciprocal of EGF and UMOD) provided AUCs of 0.82 and 0.77, respectively. To examine how a combination of the 3 biomarkers could improve the ability to predict AKI, we took the reciprocal of EGF and UMOD values and combined them with Cys C. The model using all 3 biomarkers was not significantly different (AUC = 0.81) from the models using Cys C or EGF alone (Figure 3).
Figure 3.
Biomarkers with an AUC value of 0.5 are no better than a coin flip in predicting the disease of interest. Those with an AUC value close to 1 have excellent positive predictive ability, whereas those with an AUC value close to 0 have excellent negative predictive ability. The reciprocal of a value will change a negative predictive value to a positive predictive value. This Figure shows the AUCs for Cys C, EGF, UMOD, and a combination of these 3 biomarkers. The model using all 3 biomarkers was not significantly different (AUC = 0.81) from the model with Cys C alone.
Discussion
In this study, we evaluated the association between 8 urine biomarkers and AKI in neonates without cardiac disease or very low birth weight. We found that Cys C, EGF, and UMOD levels measured during the first days of life were associated with AKI in this cohort. In addition, the biomarkers NGAL, OPN, albumin, and KIM-1 had AUC values of 0.68, 0.64, 0.72, and 0.63, respectively. The graphical data (Figure 2) indicate differences at different days of life, which suggests their potential as biomarkers of AKI.
Cys C is normally filtered freely and completely reabsorbed and catabolized within the proximal tubule.31 Urine Cys C levels increase with structural and functional renal tubular impairment independent of glomerular filtration rate.30 Urine Cys C is highly predictive of AKI in children23 and adults32 who undergo cardiopulmonary bypass surgery and kidney transplantation.33 We recently reported that very low birth weight infants with AKI have higher maximum urine Cys C concentrations during the first week of life than those without AKI, independent of gestational age (AUC = 0.73). The present study provides additional support for urine Cys C as a biomarker of AKI in neonates.
EGF is a reparative growth factor in rats,34 and a few studies have explored its role in humans. In 1996, Tsong et al35 reported that children with AKI had lower EGF levels in the initial and recovery stages of AKI compared with children without AKI. In 1997, Chen et al36 reported lower urine EGF levels in infants with severe perinatal asphyxia compared with controls, with the lowest EGF levels in those with severe asphyxia. In 2010, Kwon et al37 reported that increased urinary EGF level predicted renal functional recovery, with adult patients with AKI with the lowest EGF levels more likely to have either slow or no recovery of kidney function after AKI. Our data on EGF are consistent with those previous findings. Further evaluation of EGF’s role in repair and as a urine AKI biomarker is needed.
UMOD (also known as Tamm-Horsfall protein) is a glycoprotein found in casts formed during AKI. Recently, UMOD has been shown to have a protective role in AKI via down-regulation of inflammation in the outer medulla.38 Among adult recipients of deceased donor transplant, those with acute tubular injury have lower UMOD levels within the tubules and higher UMOD-positive casts.39 Our finding of decreased UMOD levels in patients with AKI should encourage further research on UMOD’s role in AKI and as a candidate biomarker.
Several previous studies have shown that urinary concentrations of some biomarkers are dependent on gestational age in children without AKI.27-29 This might be secondary to the inability of immature tubules to reabsorb these proteins in underdeveloped kidneys. Controlling for this important confounder is necessary to ensure that the associations between urine biomarkers and AKI are not simply a reflection of tubular maturation. When gestational age was incorporated into this analysis, the association between biomarkers and AKI persisted, suggesting that these biomarkers did not fluctuate in our cohort of near-term and term infants.
A strength of this study is its evaluation of 8 urinary biomarkers individually and jointly. The study has some limitations, however. Because of the small sample size and because daily urine samples were not available for all individuals, the precise day on which these biomarkers are most useful cannot be determined. Similarly, our analysis cannot determine whether these biomarkers can detect AKI before changes in SCr occur. Our analysis also cannot discriminate among different etiologies of AKI (eg, sepsis-associated AKI, nephrotoxic AKI, hypoxic-ischemic AKI). Graphical data suggest that with larger studies, Cys C, EGF, UMOD, NGAL, KIM-1, OPN, and albumin may be able to identify AKI sooner than Scr. Future studies in larger cohorts are needed to determine which biomarkers at which time points can best predict different etiologies of neonatal AKI.
Another important limitation of this study is the possibility that other confounders besides gestational age could explain the association between AKI and these biomarkers. Because of limitations of sample size, we were not able to control for other potential confounders, such as inflammatory changes with multiorgan disease, maternal and infant genetic variations, and others. Our selection criteria led to a heterogeneous population of sick newborns. Apgar scores are not definitive surrogates for asphyxia or disease severity, and gestational age can confound Apgar scores. Studies with larger populations will control for these limitations.
SCr-based definitions have significant shortcomings when defining AKI, mainly because SCr provides an estimate of function, not injury. Thus, the current SCr-based gold standard definition of AKI is likely not 100% accurate in detecting AKI, and some control infants might have had actual renal injury, and some infants with elevated SCr might not have had kidney damage. Future definitions will need to incorporate injury markers such as those described here, using hard clinical endpoints such as mortality, renal support requirement, or other surrogate outcomes. In addition, these biomarkers need to be studied in therapeutic/preventive trials before they can be incorporated into standard clinical care.
Acknowledgments
We thank Amy Logue, RN and the many nurses in the Nephrology and Neonatology Divisions at the University of Alabama at Birmingham for assisting with the collection of laboratory samples.
Supported by a The Normal Siegel Scholar Young Investigator Award from the American Society of Nephrology. (to D.A.), the Kaul Pediatric Research Institute, and a pilot and feasibility grant from the National Institutes of Health–sponsored O’Brien Center for Acute Kidney Injury research (www.obrienaki.org). D.A. serves as consultant and is on the speaker’s bureau for Gambro Renal Products.
Glossary
- AKI
Acute kidney injury
- AUC
Area under the receiver operating characteristic curve
- B2mG
β2 microglobulin
- Cys C
Cystatin C
- CV
Confidence of variability
- EGF
Epithelial growth factor
- KIM-1
Kidney injury molecule 1
- NGAL
Neutrophil gelatinase–associated lipocalin
- OPN
Osteopontin
- SCr
Serum creatinine
- UMOD
Uromodulin
Footnotes
The other authors declare no conflicts of interest.
References
- 1.Aggarwal A, Kumar P, Chowdhary G, Majumdar S, Narang A. Evaluation of renal functions in asphyxiated newborns. J Trop Pediatr. 2005;51:295–9. doi: 10.1093/tropej/fmi017. [DOI] [PubMed] [Google Scholar]
- 2.Gupta BD, Sharma P, Bagla J, Parakh M, Soni JP. Renal failure in asphyxiated neonates. Indian Pediatr. 2005;42:928–34. [PubMed] [Google Scholar]
- 3.Karlowicz MG, Adelman RD. Nonoliguric and oliguric acute renal failure in asphyxiated term neonates. Pediatr Nephrol. 1995;9:718–22. doi: 10.1007/BF00868721. [DOI] [PubMed] [Google Scholar]
- 4.Askenazi DJ, Griffin R, McGwin G, Carlo W, Ambalavanan N. Acute kidney injury is independently associated with mortality in very low birthweight infants: a matched case-control analysis. Pediatr Nephrol. 2009;24:991–7. doi: 10.1007/s00467-009-1133-x. [DOI] [PubMed] [Google Scholar]
- 5.Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D. Acute kidney injury reduces survival in very low birth weight infant. Pediatr Res. 2010;69:354–8. doi: 10.1203/PDR.0b013e31820b95ca. [DOI] [PubMed] [Google Scholar]
- 6.Akcan-Arikan A, Zappitelli M, Loftis LL, Washburn KK, Jefferson LS, Goldstein SL. Modified RIFLE criteria in critically ill children with acute kidney injury. Kidney Int. 2007;71:1028–35. doi: 10.1038/sj.ki.5002231. [DOI] [PubMed] [Google Scholar]
- 7.Zappitelli M, Parikh CR, Akcan-Arikan A, Washburn KK, Moffett BS, Goldstein SL. Ascertainment and epidemiology of acute kidney injury varies with definition interpretation. Clin J Am Soc Nephrol. 2008;3:948–54. doi: 10.2215/CJN.05431207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int. 2008;73:538–46. doi: 10.1038/sj.ki.5002743. [DOI] [PubMed] [Google Scholar]
- 9.Cuhaci B. More data on epidemiology and outcome of acute kidney injury with AKIN criteria: benefits of standardized definitions, AKIN and RIFLE classifications. Crit Care Med. 2009;37:2659–61. doi: 10.1097/CCM.0b013e3181ad76c2. [DOI] [PubMed] [Google Scholar]
- 10.Uchino S. Outcome prediction for patients with acute kidney injury. Nephron Clin Pract. 2008;109:c217–23. doi: 10.1159/000142931. [DOI] [PubMed] [Google Scholar]
- 11.Macedo E, Castro I, Yu L, Abdulkader RR, Vieira JM., Jr. Impact of mild acute kidney injury (AKI) on outcome after open repair of aortic aneurysms. Ren Fail. 2008;30:287–96. doi: 10.1080/08860220701857522. [DOI] [PubMed] [Google Scholar]
- 12.Bagshaw SM, George C, Bellomo R. Changes in the incidence and outcome for early acute kidney injury in a cohort of Australian intensive care units. Crit Care. 2007;11:R68. doi: 10.1186/cc5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elapavaluru S, Kellum JA. Why do patients die of acute kidney injury? Acta Clin Belg Suppl. 2007;2:326–31. doi: 10.1179/acb.2007.074. [DOI] [PubMed] [Google Scholar]
- 14.Jo SK, Rosner MH, Okusa MD. Pharmacologic treatment of acute kidney injury: why drugs haven’t worked and what is on the horizon. Clin J Am Soc Nephrol. 2007;2:356–65. doi: 10.2215/CJN.03280906. [DOI] [PubMed] [Google Scholar]
- 15.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network (AKIN): report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoste EA, Kellum JA. RIFLE criteria provide robust assessment of kidney dysfunction and correlate with hospital mortality. Crit Care Med. 2006;34:2016–7. doi: 10.1097/01.CCM.0000219374.43963.B5. [DOI] [PubMed] [Google Scholar]
- 17.Askenazi DJ, Ambalavanan N, Goldstein SL. Acute kidney injury in critically ill newborns: what do we know? What do we need to learn? Pediatr Nephrol. 2009;24:265–74. doi: 10.1007/s00467-008-1060-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallini F, Maggio L, Romagnoli C, Marrocco G, Tortorolo G. Progression of renal function in preterm neonates with gestational age < or = 32 weeks. Pediatr Nephrol. 2000;15:119–24. doi: 10.1007/s004670000356. [DOI] [PubMed] [Google Scholar]
- 19.Brion LP, Fleischman AR, McCarton C, Schwartz GJ. A simple estimate of glomerular filtration rate in low birth weight infants during the first year of life: noninvasive assessment of body composition and growth. J Pediatr. 1986;109:698–707. doi: 10.1016/s0022-3476(86)80245-1. [DOI] [PubMed] [Google Scholar]
- 20.Parikh CR, Devarajan P, Zappitelli M, Sint K, Thiessen-Philbrook H, Li S, et al. Postoperative biomarkers predict acute kidney injury and poor outcomes after adult cardiac surgery. J Am Soc Nephrol. 2011;22:1748–57. doi: 10.1681/ASN.2010121302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haase M, Devarajan P, Haase-Fielitz A, Bellomo R, Cruz DN, Wagener G, et al. The outcome of neutrophil gelatinase–associated lipocalin-positive subclinical acute kidney injury: a multicenter pooled analysis of prospective studies. J Am Coll Cardiol. 2011;57:1752–61. doi: 10.1016/j.jacc.2010.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krawczeski CD, Woo JG, Wang Y, Bennett MR, Ma Q, Devarajan P. Neutrophil gelatinase–associated lipocalin concentrations predict development of acute kidney injury in neonates and children after cardiopulmonary bypass. J Pediatr. 2011;158:1009–15. doi: 10.1016/j.jpeds.2010.12.057. [DOI] [PubMed] [Google Scholar]
- 23.Krawczeski CD, Vandevoorde RG, Kathman T, Bennett MR, Woo JG, Wang Y, et al. Serum cystatin C is an early predictive biomarker of acute kidney injury after pediatric cardiopulmonary bypass. Clin J Am Soc Nephrol 2010. 5:1552–7. doi: 10.2215/CJN.02040310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramesh G, Krawczeski CD, Woo JG, Wang Y, Devarajan P. Urinary netrin-1 is an early predictive biomarker of acute kidney injury after cardiac surgery. Clin J Am Soc Nephrol. 2010;5:395–401. doi: 10.2215/CJN.05140709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, et al. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care. 2009;13:R104. doi: 10.1186/cc7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Askenazi DJ, Montesanti A, Hunley H, Koralkar R, Pawar P, Shuaib F, et al. Urine biomarkers predict acute kidney injury and mortality in very low birth weight infants. J Pediatr. 2011;159:907–12. doi: 10.1016/j.jpeds.2011.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lavery AP, Meinzen-Derr JK, Anderson E, Ma Q, Bennett MR, Devarajan P, et al. Urinary NGAL in premature infants. Pediatr Res. 2008;64:423–8. doi: 10.1203/PDR.0b013e318181b3b2. [DOI] [PubMed] [Google Scholar]
- 28.Huynh TK, Bateman DA, Parravicini E, Lorenz JM, Nemerofsky SL, Sise ME, et al. Reference values of urinary neutrophil gelatinase–associated lipocalin in very low birth weight infants. Pediatr Res. 2009;66:528–32. doi: 10.1203/PDR.0b013e3181baa3dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Askenazi D, Koralkar R, Levitan EB, Goldstein SL, Devarajan P, Khandrika S, et al. Baseline values of candidate urine acute kidney injury (AKI) biomarkers vary by gestational age in premature infants. Pediatr Res. 2011;70:302–6. doi: 10.1203/PDR.0b013e3182275164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herget-Rosenthal S, van Wijk JA, Brocker-Preuss M, Bokenkamp A. Increased urinary cystatin C reflects structural and functional renal tubular impairment independent of glomerular filtration rate. Clin Biochem. 2007;40:946–51. doi: 10.1016/j.clinbiochem.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 31.Nejat M, Pickering JW, Walker RJ, Westhuyzen J, Shaw GM, Frampton CM, et al. Urinary cystatin C is diagnostic of acute kidney injury and sepsis, and predicts mortality in the intensive care unit. Crit Care. 2010;14:R85. doi: 10.1186/cc9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Koyner JL, Bennett MR, Worcester EM, Ma Q, Raman J, Jeevanandam V, et al. Urinary cystatin C as an early biomarker of acute kidney injury following adult cardiothoracic surgery. Kidney Int. 2008;74:1059–69. doi: 10.1038/ki.2008.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hall IE, Koyner JL, Doshi MD, Marcus RJ, Parikh CR. Urine cystatin C as a biomarker of proximal tubular function immediately after kidney transplantation. Am J Nephrol. 2011;33:407–13. doi: 10.1159/000326753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Go AS, Parikh CR, Ikizler TA, Coca S, Siew ED, Chinchilli VM, et al. The Assessment, Serial Evaluation, and Subsequent Sequelae of Acute Kidney Injury (ASSESS-AKI) Study: design and methods. BMC Nephrol. 2010;11:22. doi: 10.1186/1471-2369-11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsau YK, Sheu JN, Chen CH, Teng RJ, Chen HC. Decreased urinary epidermal growth factor in children with acute renal failure: epidermal growth factor/creatinine ratio not a reliable parameter for urinary epidermal growth factor excretion. Pediatr Res. 1996;39:20–4. doi: 10.1203/00006450-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Chen L, Liu W. Effect of asphyxia on urinary epidermal growth factor levels in newborns. J Tongji Med Univ. 1997;17:144–6. doi: 10.1007/BF02888289. [DOI] [PubMed] [Google Scholar]
- 37.Kwon O, Ahn K, Zhang B, Lockwood T, Dhamija R, Anderson D, et al. Simultaneous monitoring of multiple urinary cytokines may predict renal and patient outcome in ischemic AKI. Ren Fail. 2010;32:699–708. doi: 10.3109/0886022X.2010.486496. [DOI] [PubMed] [Google Scholar]
- 38.El-Achkar TM, Wu XR, Rauchman M, McCracken R, Kiefer S, Dagher PC. Tamm-Horsfall protein protects the kidney from ischemic injury by decreasing inflammation and altering TLR4 expression. Am J Physiol Renal Physiol. 2008;295:F534–44. doi: 10.1152/ajprenal.00083.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nadasdy T, Laszik Z, Blick KE, Johnson DL, Burst-Singer K, Nast C, et al. Human acute tubular necrosis: a lectin and immunohistochemical study. Hum Pathol. 1995;26:230–9. doi: 10.1016/0046-8177(95)90042-x. [DOI] [PubMed] [Google Scholar]