Abstract
An increased level of low-density lipoprotein (LDL) is a very well established risk factor of coronary artery disease (CAD). Unoxidized LDL is an inert transport vehicle of cholesterol and other lipids in the body and is thought to be atherogenic. Recently it has been appreciated that oxidized products of LDL are responsible for plaque formation properties previously attributed to the intact particle. The goal of this article is to review the recent understanding of the LDL oxidation pathway. The role of oxidized products and key enzymes (lipoprotein-associated phospholipase A2 and carboxyl ester lipase) are also extensively discussed in the context of clinical conditions.
Keywords: carboxyl ester lipase, lipoprotein-associated phospholipase A2, atherosclerosis, lipoproteins
Introduction
It is widely accepted that increased levels of low-density lipoprotein (LDL), triglycerides (TGs) and total cholesterol (TC) are associated with atherosclerosis [1]. Low-density lipoprotein is a vehicle of cholesterol and other lipids in the body. It seems not to be atherogenic until it becomes oxidized (ox-LDL) in the arterial wall. Subsequently ox-LDL, known as a small dense LDL (sd-LDL), activates the cascade of local inflammation. It promotes cytokine release, chemotaxis and extravasation of leucocytes as well as the activation of deeper parts of the artery wall. Upon phagocytosis of ox-LDL monocytes transform into macrophages and later into foam cells with a lipid core. This is the beginning of atherosclerosis and plaque formation [2–5]. The plaque can rupture or erode, which clinically will manifest as stable angina or myocardial infarction respectively.
Therefore, the goal for primary and secondary prevention of coronary artery disease (CAD) is the reduction of plasma levels of LDL, TG and TC, which leads to decreased levels of atherogenic ox-LDL particles [1].
Is the low-density lipoprotein particle really harmful?
The fraction of large, buoyant LDL is not a direct inducer of inflammatory changes in the endothelium. This role is assigned to the small dense LDL derived from peroxidation of lipids contained in the structure of LDL [2–5]. Similarly, other atherogenic contributors are lipoproteins (Lps) that contain apolipoprotein B-100 (apoB) (very low density lipoproteins – VLDL, and intermediate density lipoproteins – IDL) or apoB-48 (chylomicrons and chylomicron remnants) in their structure. However, the LDL fraction is most vulnerable to oxidative modifications due to its long half-life.
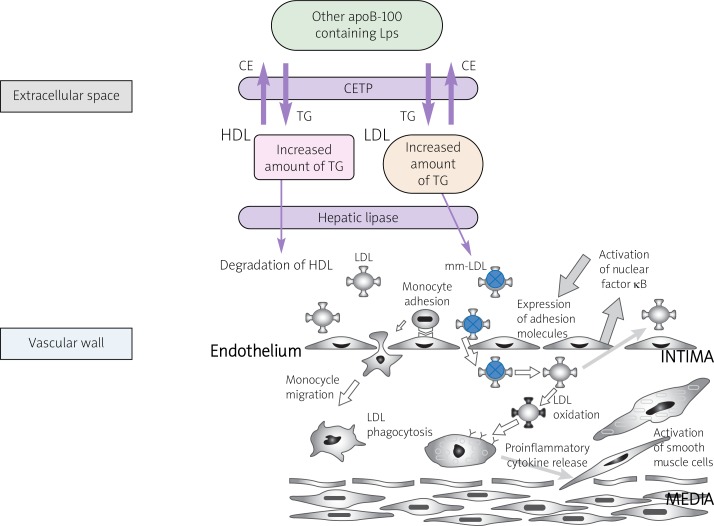
The oxidation process may also occur whenever there is an accumulation of specific lipid fractions in LDL. Shifting the balance between cholesterol esters (CEs) and TGs into a TG dominant ratio in both high-density lipoprotein (HDL) and LDL promotes the activation of hepatic lipase (HL) (Figure 1). While HL induces the degradation of triglycerides in HDL, which explains low levels of this lipoprotein, e.g. in patients with diabetes, HL converts LDL into a small, atherogenic molecule (sd-LDL) [6]. This atherogenic molecule activates nuclear factor kappa B, which is responsible for the stimulation of endothelium cells and consequently the initiation of inflammation in the vessel wall [7–9] (Figure 1). Additionally, sd-LDL binds to the scavenger receptor (SR)-B1 on the surface of macrophages, causing them to release inflammatory cytokines and specific enzymes such as carboxyl ester lipase (CEL) or lipoprotein-associated phospholipase A2 (LP-PLA2). These enzymes are indirectly involved in atherosclerosis. High levels of non-oxidized LDL are not absorbed by macrophages, due to the reduced number of LDL receptors on their surface. However, the threat posed by elevated levels of plasma Lps might be caused by their greater susceptibility to oxidation [10] (Figure 2).
Figure 1.
Lipoproteins metabolism due to increased TGs exchange (according to [11], modyfied by Igor Morski)
CE – cholesterol esters, CETP – cholesterol esters transporting protein, HDL – high density lipoprotein, LDL – low density lipoprotein, LPS – lipoproteins, mm-LDL – minimaly oxidized LDL, TG – triglicerydes
Figure 2.
Various mechanisms of LDL oxidation
apoB100 – apolipoprotein B100, FFA – free fatty acids, LDL – low density lipoprotein, LDLR – low density lipoprotein receptor, oxLDL – oxidized LDL
Low-density lipoprotein oxidative modification process
On average, LDL is composed of 1600 CE molecules, 170 TGs, 600 molecules of free cholesterol (FC), and 1 apoB-100 molecule, and is surrounded by a single layer of 700 phospholipids (the main phospholipids involved being sphingomyelin and lysolecithin). Half of LDL fatty acids are polyunsaturated fatty acids (PUFA) and these are protected from free radical attack by α-tocopherol, a small amount of γ-tocopherol, carotenoids, cryptoxanthin and ubiquinone-10 [11].
The oxidation of LDL is a multi-step process both dependent (carried out by lipoxygenase and by reactive oxygen species [ROS]) and independent of metal ions (carried out by myeloperoxidase) [10, 11].
This enzymatic cascade (Figure 2) may take place in endothelial cells [10–13], vascular smooth muscle cells, granulocytes, or less frequently in plasma (plasma lipoprotein lipids are well protected from oxidation by the antioxidant defenses).
Experimental studies have documented LDL oxidation in both in vitro as well as in vivo conditions. In vitro, the process is mainly dependent on metal ions (e.g. Cu2+ and Fe2+), whereas in vivo, it is dependent on active neutrophils, using myeloperoxidase-H2O2 complex [14] to produce acetyl β-hydroxyphenyl aldehyde (a major product of L-tyrosine oxidation) [10, 15, 16].
During the oxidation of LDL, the fatty acids are oxidized and active aldehydes are formed. As a result, malonyldialdehyde or oxidized polyunsaturated fatty acids esterify phosphatidylcholine or cholesterol [10, 17, 18] and interact with apoB-100, which causes its fragmentation [10, 17, 18]. At the root of the oxidative fragmentation of apoB-100 is probably the neutralization of positively charged ɛ-amino groups of lysine [10, 17, 18]. The intact lysines in apoB-100 are responsible for the interaction of LDL molecule with the LDL receptor (LDLR). Derivatization change of as much as 5% of lysines can cause a decrease in LDL degradation due to weakened affinity to the LDLR. On the other hand, the oxidatively modified apoB-100s are ligands for SR of macrophages [10, 19]. The crucial role of oxidation of apoB-100 in this scavenging process is exemplified in experiments where the fragmentation of apoB-100 using specific enzymes did not cause the absorption of modified LDL molecules by macrophages [20].
What are the clinical implications of an increased risk of lipoprotein oxidation?
An in vivo increased risk of metal ion-dependent oxidation may accompany hemochromatosis and Wilson's disease, whereas the risk of oxidation conditioned by myeloperoxidase activity is increased in diseases that cause the activation of local inflammation [10, 11]. It may explain more CAD cases among these groups of patients [10, 11]. The tendency for lipid peroxidation is also observed in several clinical syndromes, e.g. chronic fatigue syndrome in women [21]. However, in common clinical conditions, a strong and multidirectional inducer of lipid oxidation is hyperglycemia and insulin resistance [10, 22].
Lipoprotein oxidation in conditions of impaired glucose tolerance and diabetes
In vivo, glucose is an extremely strong reducing agent, and persistent hyperglycemia can induce the formation of ROS, such as in the sorbitol cycle. The ROS formed by glucose is produced by competition between the overactive aldose reductase with glutathione reductase for the substrate, nicotinamide (NAD) [22]. In addition, disturbances of carbohydrate metabolism stimulate lipoxygenase, which catalyzes the metal ion-dependent mechanism of oxidation and in fact the formation of ox-LDL. Hyperglycemia causes the glycation of LDL (glyco-LDL) by the covalent attachment of glucose to lysine residues in apoB-100, as well as glycation of apoA1 in HDL [10]. Nevertheless, oxidized LDL may be generated during glycation, but oxidized LDL is not necessarily glycated [10].
In patients with chronic hyperglycemia, excessive production of ROS, ox-LDL, glyco-LDL and sd-LDL, and a changed profile of synthesized lipids (increased TG and VLDL) [11] might be responsible for the earlier occurrence of atherosclerosis than in people with normal glucose levels.
It turns out that the leukocytes besides the oxidized LDL can equally absorb the modified glyco-LDL, VLDL, IDL and chylomicrons [25] overproduced in the course of atherogenic dyslipidemia, which is characteristic of type 2 diabetes. Nevertheless, since VLDL has a higher content of 18: 1 than 18: 2 fatty acids and a lower activity of Lp-PLA2, it appears to be more resistant to metal ion-dependent oxidation in plasma [11, 25].
In summary, hyperglycemia leads to LDL oxidation and glycation, which can easily initiate extravasation of monocytes into the subendothelial space. It is established that impaired fasting glucose is not associated with increased cardiovascular risk, but there is still little known about its influence on lipoprotein oxidation.
Insulin resistance and development of metabolic syndrome
The effect of susceptibility to oxidation is intensified by the development of insulin resistance. It is well known that overweight and obesity lead to insulin resistance and metabolic syndrome. The excess of carbohydrates causes the secretion of large amounts of pancreatic insulin. This hormone enhances the expression of glucose receptors in adipose tissue. In addition, by inhibiting hormone-sensitive lipases, it promotes the lipogenesis pathway. Lipogenesis, however, requires the presence of active glycerol (glycerol-3-phosphate), which is produced in adipose tissue during glycolysis, due to the lack of glycerol kinase [23]. Therefore, in patients with a genetic predisposition to inadequate lipolysis (hormone-sensitive lipase disorder) in adipose tissue, there is no possibility of using free fatty acids (FFA) as an energy source for cells and they require a supplementary energy source [23]. Consequently, these patients have increased carbohydrate requirements. The supply of carbohydrates to their system causes a significant release of insulin, which increases the internalization of glucose and FFA to adipose tissue. As a result, TGs form and are deposited in fat cells. Over time, this mechanism is responsible for the development of metabolic syndrome, which arises due to an imbalance between the Lp fractions and lipases. In these conditions the effect of susceptibility to oxidation is intensified.
Supposedly, decreased carbohydrates and increased fat consumption should be recommended for dietary prevention of metabolic syndrome among patients with adequate function of lipoprotein lipase, hepatic lipase and cholesteryl ester transfer protein
Ironically, protection against lipid oxidation in obese patients might be provided by consumption of fatty products which do not cause insulin secretion, and thereby can be directly used as an energy source [23]. Dietary fat excess increases the chylomicron fraction, which is degraded in the plasma by lipoprotein lipase to FFA, which is transported to adipose tissue. However, they cannot be esterified there, due to the lack of glycerol-3-phosphate, which is created only during glycolysis. Therefore, the FFAs are transported back to the liver. The clinical outcome of these processes is an increase in HDL fraction in plasma [23, 24]. As a result of the increased plasma levels of FFA, its synthesis in the liver is inhibited, which explains the decrease in total cholesterol. The FFAs in the absence of insulin do not actually accumulate in adipose tissue. This process requires adequate function of lipoprotein lipase, hepatic lipase as well as cholesteryl ester transfer protein (CETP), because of their crucial role in lipoprotein catabolism.
However, an excess of both carbohydrates and fats is the most dangerous, because under the influence of insulin the adipose tissue vacuolation process will be significantly increased [23, 24]. Thus, hypothetically assuming the existence of identical terms of in vivo oxidation, in those patients whose energy demands are covered by carbohydrates, their bodies are much more susceptible to obesity as well as to increased oxidation of lipids (contained in Lps), compared to those who live on high-fat diets.
Prevention of lipoprotein oxidation, and catabolism of oxidized lipids contained in lipoproteins
Antioxidants and antioxidant role of high-density lipoprotein
Nitric oxide is a potent antioxidant. However, it is rapidly inactivated by superoxide anion to form peroxynitrite (ONOO–), a potent oxidant and peroxynitrous acid (ONOOH), which subsequently is decomposed to nitric oxide radical and hydroxyl radical. These radicals react with unsaturated fatty acids and cause lipid peroxidation. LDL oxidation in endothelial cells takes place when NO bioavailability is low. Inhibition of LDL oxidation is associated with elevated NO release. Shifting the balance (between NO and superoxide) into superoxide production may increase LDL peroxidation, due to the decreasing anti-atherogenic action of NO and providing pro-atherogenic action of peroxynitrite [10].
On the other hand, NO synthesis could be inhibited by impairment of asymmetric dimethylarginine (ADMA) catabolism in oxidative stress conditions. Increased plasma levels of ADMA are proposed to be a novel risk factor for endothelium dysfunction as well as for cardiovascular events [26]. The antioxidant properties of glutathione, n-acetyl cysteine [27], β carotene, ascorbic acid, vitamin A, vitamin E (α-tocopherol) and plant polyphenols were observed to correlate with reduced risk of atherosclerosis in a large number of experimental studies [28]. However, controlled clinical trials have so far failed to demonstrate a positive effect of vitamin A, C or E (α-tocopherol) supplementation for cardiovascular risk [29]. What is more, in some cases the phenomenon of pro-oxidant properties of antioxidants, with increased risk of LDL oxidation and atherosclerosis, was observed. The mechanism of this process is poorly explained and is still being investigated.
HDL also acts as a homeostatic factor for lipids which are susceptible to oxidation, such as apoB-100-rich molecules (LP-B). The antioxidant potential of HDL is conditioned by the availability of enzymatic and structural proteins such as paraoxonase and acyltransferase lecithin, including cholesterol, phospholipase A2 (PLA2) and protein apoA1 [10, 30–32].
These proteins inhibit not only the oxidation of lipids in LDL, but also the expression of adhesion molecules in the stimulated vascular endothelium [33].
Role of lipoprotein-associated phospholipase A2
The formation of sd-LDL is closely related to the role of lipoprotein-associated phospholipase A2 [5]. lipoprotein-associated phospholipase A2 is an enzyme which interacts with apoB of LDL. Initially PLA2 was suspected of anti-atherosclerosis activity due to the degradation of platelet activating factor [34, 35]. Currently, epidemiological studies suggest that increased activity and quantity of PLA2 is an independent risk factor for cardiovascular disorders. Like PLA2, Lp-PLA2 is an esterase which catalyzes the breakdown of the ester bond in the SN2 position of TGs and phospholipids [36]. Most likely, the atherogenic properties of Lp-PLA2 are not directly related to the hydrolysis of oxidized phospholipids, which is a desirable phenomenon, but to the products of this reaction, among which are lysophosphatidylcholine (lyso-PC), other polar phospholipids (PAF-like) and oxidized, non-esterified free fatty acids (NEFA) [37, 38]. These compounds, being similar to platelet activating factor (PAF), bind with its receptors on leukocytes. Moreover, they intensify the targeting of leukocytes and secretion of pro-inflammatory mediators in areas of atherosclerotic plaque formation, and reduce the ‘susceptibility’ of the vascular wall [5]. In addition, lysophosphatidylcholine induces the expression of intercellular adhesion molecule 1 (ICAM-1) in the vascular wall and CD8 and CD11 on the surface of neutrophils and intensifies the mitogen-activated protein kinase pathway dependent proliferation of vascular smooth muscle cells [39, 40]. Moreover, the Lp-PLA2 reaction products are presumably cytotoxic for macrophages; hence, they indirectly foster formation of the lipid core of the atherosclerotic plaque [5, 41]. The activity and intracellular pool of Lp-PLA2 are dependent on the amount of LDL as well as the type and availability of substrates of the reaction it catalyses. The increased availability of reaction substrates results in the increased activity of Lp-PLA2. In addition, the activity of Lp-PLA2 may be genetically determined. Analysis of the gene in the Japanese population revealed several polymorphisms, some of which are associated with low Lp-PLA2 activity, and consequently a lower incidence of cardiovascular diseases [42–45]. However, while the role of Lp-PLA2 in the process of atherosclerosis is to some extent documented, the role of another important enzyme, CEL, is hypothetical and controversial [46].
Carboxyl ester lipase degrades oxidized lipids in lipoproteins
Carboxyl ester lipase is a pancreatic lipase which digests the ester bond of dietary waxes and TGs in the SN2 position. A peculiar feature of CEL is that it is synthesized in the mammary gland, hepatocytes, vascular endothelium and macrophages [2, 46]; in particular, its expression in the latter 2 cell types suggests that CEL may be involved in vascular homeostasis [2].
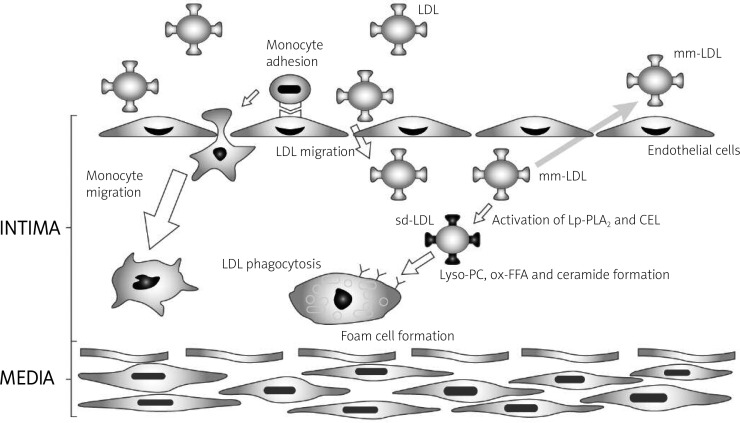
Carboxyl ester lipase is responsible for the hydrolysis and absorption of CEs, TGs, and fat-soluble vitamins. Although its role in the digestion of dietary lipids in adults is secondary, it can be important when pancreatic phospholipases and pancreatic lipase (PL) are deficient. Carboxyl ester lipase probably does not affect the absorption of free cholesterol from the gastrointestinal tract [47, 48]; instead, it possesses both lysophospholipase and ceramidase properties. In enterocytes, in the absence of hydrolysis of dietary ceramides (in CEL deficiency), Lps are formed with a diameter corresponding to VLDL rather than chylomicrons, and chylomicrons have pro-atherosclerotic properties [49]. The similarity in the enzymatic properties of CEL in the gastrointestinal tract to those of phospholipase, ceramidase and lipase, which are capable of digesting the ester bond in the SN2 position, suggests that CEL may have similar functions in macrophages, plasma and the vascular wall. It is hypothesized that the similarities of CEL and Lp-PLA2 functions not only are restricted to the gastrointestinal tract, but also play a similar role in the metabolism of plasma Lps, and thus in the initiation of the arteriosclerosis process (Figure 3).
Figure 3.
The role of Lp-PLA2 and CEL in atherosclerotic palque formation (according to [11], modyfied by Igor Morski)
CEL – carboxyl ester lipase, LDL – low density lipoprotein, mm-LDL – minimaly oxidized low density lipoprotein, Lp-PLA2 – lipoprotein phospholipase A2, Lyso-PC – lisophosphatydylocholine, ox-FFA – oxidised free fatty acids, sd-LDL – small dense low density lipoprotein
Effect of carboxyl ester lipase on plasma lipoprotein metabolism and its putative role in the process of atherosclerosis
Atherogenic properties of carboxyl ester lipase
Carboxyl ester lipase may participate in the process of atherogenesis by affecting the metabolism of plasma Lps. A deficiency of CEL in the intestine promotes the synthesis of Lps of smaller diameter than the chylomicrons; chylomicrons have anti-atherosclerotic properties [2, 50].
Increased plasma activity of CEL in vivo correlates with high levels of LDL, and other Lps which contain apoB (Lp-B) [51]. An increase in Lp-B fraction is accompanied by a change in FC location: from the molecule core to its superficial layers. Increases in Lp-B are also associated with increases in the free cholesterol-to-cholesterol ester ratio (FC/CE), which is not documented in the case of HDL [52]. This effect might be related to CEL's affinity to apoB, but not to apoA1 [53]. A change in the FC/CE ratio of Lp-B slows the degradation of Lp-B, which is likely to explain their elevated levels in plasma [53]. The phenomenon of delayed plasma clearance of VLDL and LDL can be caused by the conformational change of apoB-100 in these molecules (through the accumulation of greater amounts of FC), following which the affinity of hepatic lipase to these Lps is decreased [53]. It is also suggested that CEL in the normolipidemic population accelerates the conversion of LDL into sd-LDL and that its plasma concentrations correlate positively with levels of LDL [2].
Anti-atherogenic properties of carboxyl ester lipase
The results from experimental studies showed that CEL decreases concentrations of lyso-PC in sd-LDL and consequently decreases its uptake by macrophages [53]. Moreover, CEL increases the uptake of CE, which is transported by HDL into liver cells; this increased uptake is partially independent of the SR B-1 scavenger receptor, thus accelerating the reverse transport of cholesterol and decreasing its tissue accumulation [54]. Increased activity of CEL in macrophages favors the hydrolysis of CEs, encouraging the release of free cholesterol outside the cell; such processes might be genetically conditioned or accompany increased reverse cholesterol transport (HDL fraction increases). In the case of low levels of HDL, as well as in genetically determined low activity of CEL, CEs are less hydrolyzed and therefore deposited in macrophages. A similar scenario occurs during increased FC accumulation inside macrophages: it leads to its increased esterification, which, combined with low levels of HDL (and decreased reverse cholesterol transport), may lead to cell necrosis. While the cholesterol in foam cells and LDL exists mainly in the form of CE, in advanced atherosclerosis an important component of the plaque is the free cholesterol crystallizing on its surface, formed by hydrolysis of CE in the extracellular space. Studies in a transgenic mouse model of myocardial infarction without the CEL gene lend support to this phenomenon [2].
In advanced atherosclerosis increased expression of CEL in macrophages promotes the accumulation of CEs, since it preferentially hydrolyzes pro-atherosclerotic ceramide and lysophosphatidylcholine. The existing data on this process are conflicting; both protective and pro-atherosclerotic properties of CEL have been reported [2].
Taken together, the function of CEL in Lp metabolism and atherosclerotic plaque formation is unclear. The inconsistencies reported on the role and function of CEL might be influenced by the fact that there is considerable inter-population and interspecies polymorphism of CEL, resulting in considerable variability of structure and function of this enzyme [2].
Summary
The oxidation of lipids in plasma Lps, especially LDL, seems to be the most important contributing factor to this disease. Lipoprotein peroxidation products, mostly in the arterial wall, activate lipases Lp-PLA2 and CEL to degrade oxy-lipids. Function and activity of these lipases are genetically determined.
The conditions with higher levels of Lps correspond to a greater probability of oxidation. On the other hand, high plasma levels of unoxidized LDL have low or even zero affinity to macrophages. Thus, high amounts of LDL are not dangerous, but reducing its level is a clinically proven way to prevent its oxidation and to influence vascular homeostasis. Of course, it should be noted that oxidized lipoproteins are a common but not the sole initiator of an inflammation cascade in the vascular wall.
It seems that an imbalance between the antioxidant plasma properties (e.g. NO, HDL, etc) and susceptibility to oxidation of Lps containing apoB-100 are important for the process of atherogenesis. Therefore, the guidelines of European and American cardiac societies recognize low levels of HDL and high levels of LDL in plasma as risk factors for coronary heart disease [1, 55, 56].
Acknowledgments
Special thanks to Igor Morski for design of figures.
References
- 1.Graham I. European guidelines on cardiovascular disease prevention in clinical practice: executive summary. Atherosclerosis. 2007;194:1–45. doi: 10.1016/j.atherosclerosis.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Hui DY, Howles PN. Carboxyl ester lipase: structure-function relationship and physiological role in lipoprotein metabolism and atherosclerosis. J Lipid Res. 2002;43:2017–30. doi: 10.1194/jlr.r200013-jlr200. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg D, Witztum JL. Oxidized low-density lipoprotein and atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:2311. doi: 10.1161/ATVBAHA.108.179697. [DOI] [PubMed] [Google Scholar]
- 4.Steinberg D. The LDL modification hypothesis of atherogenesis: an update. J Lipid Res. 2009;50:S376–80. doi: 10.1194/jlr.R800087-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:923–31. doi: 10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 6.Hirayama S, Miida T. Small dense LDL: an emerging risk factor for cardiovascular disease. Clin Chim Acta. 2012;24:414, 215–24. doi: 10.1016/j.cca.2012.09.010. [DOI] [PubMed] [Google Scholar]
- 7.Baker RG, Hayden MS, Ghosh S. NF-kappaB, inflammation, and metabolic disease. Cell Metabolism. 2011;13:11–22. doi: 10.1016/j.cmet.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pung YF, Chilian WM. Corruption of coronary collateral growth in metabolic syndrome: role of oxidative stress. World J Cardiol. 2010;2:421–7. doi: 10.4330/wjc.v2.i12.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins T, Cybulsky M. NF-kppa B: pivotal mediator or innocenty bystender inatherogenesis. J Clin Invest. 2001;107:255–64. doi: 10.1172/JCI10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mertens A, Hobvoet P. Oxidized and HDL: antagonists in atherothrombosis. FASEB J. 2001;15:2073–84. doi: 10.1096/fj.01-0273rev. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida H, Kisugi R. Mechanisms of LDL oxidation. Clinica Chimica Acta. 2010;411:23–24. doi: 10.1016/j.cca.2010.08.038. 1875-82. [DOI] [PubMed] [Google Scholar]
- 12.Andrews AM, Jaron D, Buerk DG, Kirby PL, Barbee KA. Direct, real-time measurement of shear stress-induced nitric oxide produced from endothelial cells in vitro. Nitric Oxide. 2010;23:335–42. doi: 10.1016/j.niox.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsimikas S. Oxidized low-density lipoprotein biomarkers in atherosclerosis. Curr Atheroscler Rep. 2006;8:55–61. doi: 10.1007/s11883-006-0065-1. [DOI] [PubMed] [Google Scholar]
- 14.Misra MK, Sarwat M, Bhakuni P, Tuteja R, Tuteja N. Oxidative stress and ischemic myocardial syndromes. Med Sci Monit. 2009;15:RA209–19. [PubMed] [Google Scholar]
- 15.Holvoet P, Lee DH, Steffes M, Gross M, Jacobs DR., Jr Association between circulating oxidized low-density lipoprotein and incidence of the metabolic syndrome. JAMA. 2008;299:2287–93. doi: 10.1001/jama.299.19.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holvoet P, Vanhaecke J, Janssens S, Van de Werf F, Collen D. Oxidized LDL and malondialdehyde-modified LDL in patients with acute coronary syndromes and stable coronary artery disease. Circulation. 1998;98:1487–94. doi: 10.1161/01.cir.98.15.1487. [DOI] [PubMed] [Google Scholar]
- 17.Steinbrecher UP. Oxidation of human low densitylipoprotein results in derivatization of lysine residues of apolipoprotein B by lipid peroxide decomposition products. J Biol Chem. 1987;262:3603–8. [PubMed] [Google Scholar]
- 18.Itabe H. Oxidative modification of LDL: its pathological role in atherosclerosis clinic. Rev Allerg Immunol. 2009;37:4–11. doi: 10.1007/s12016-008-8095-9. [DOI] [PubMed] [Google Scholar]
- 19.Adachi H, Tsujimoto M. Endothelial scavenger receptors. Progress in Lipid Research. 2006;45:379–404. doi: 10.1016/j.plipres.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 20.Brown MS, Basu SK, Falck JR, Ho YK, Goldstein JL. The scavenger cell pathway for lipoprotein degradation: specificity of the binding site that mediates the uptake of negatively-charged LDL by macrophages. J Supramol Struct. 1980;13:67–81. doi: 10.1002/jss.400130107. [DOI] [PubMed] [Google Scholar]
- 21.Brkic S, Tomic S, Maric D, Novakov Mikic A, Turkulov V. Lipid peroxidation is elevated in female patients with chronic fatigue syndrome. Med Sci Monit. 2010;16:CR628–32. [PubMed] [Google Scholar]
- 22.Obrosova IG. Increased sorbitol pathway activity generates oxidative stress in tissue sites for diabetic complications. Antioxidants Redox Signal. 2005;7:1543–52. doi: 10.1089/ars.2005.7.1543. [DOI] [PubMed] [Google Scholar]
- 23.Karpe F, an GD. Adipose tissue function in the insulin-resistance syndrome. Biochem Soc Trans. 2005;33:1045–48. doi: 10.1042/BST0331045. [DOI] [PubMed] [Google Scholar]
- 24.Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206–18. doi: 10.1016/j.physbeh.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 25.Lee C, Sigari F, Segrado T, et al. All apoB-containing lipoproteins induce monocyte chemotaxis and adhesion when minimally modified: modulation of lipoprotein bioactivity by platelet-activating factor acetylhydrolase. Arterioscler Thromb Vasc Biol. 1999;19:1437–46. doi: 10.1161/01.atv.19.6.1437. [DOI] [PubMed] [Google Scholar]
- 26.De Gennaro C, Bianchi VM, Pascale V, et al. Asymmetric dimethylarginine (ADMA): an endogenous inhibitor of nitric oxide synthase and a novel cardiovascular risk molecule. Med Sci Monit. 2009;15:RA91–101. [PubMed] [Google Scholar]
- 27.Sung HJ, Kim J, Kim Y, Jang SW, Ko J. N-acetyl cysteine suppresses the foam cell formation that is induced by oxidized low density lipoprotein via regulation of gene expression. Mol Biol Rep. 2012;39:3001–7. doi: 10.1007/s11033-011-1062-1. [DOI] [PubMed] [Google Scholar]
- 28.Setorki M, Asgary S, Eidi A, Rohani AH. Effects of acute verjuice consumption with a high-cholesterol diet on some biochemical risk factors of atherosclerosis in rabbits. Med Sci Monit. 2010;16:BR124–30. [PubMed] [Google Scholar]
- 29.Hasnain B, Mooradian A. Recent trials of antioxidant therapy: what should we be telling our patients? Cleve Clin J Med. 2004;71:327–34. doi: 10.3949/ccjm.71.4.327. [DOI] [PubMed] [Google Scholar]
- 30.Jayakumari N, Thejaseebai G. High prevalence of low serum paraoxonase1 in subjects with coronary artery disease. J Clin Biochem Nutr. 2009;45:278–84. doi: 10.3164/jcbn.08-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alwaili K, Awan Z, Alshahrani A, Genest J. High-density lipoproteins and cardiovascular disease: 2010 update. Expert Rev Cardiovasc Ther. 2010;8:413–23. doi: 10.1586/erc.10.4. [DOI] [PubMed] [Google Scholar]
- 32.McPherson PA, Young IS, McEneny J. A dual role for lecithin:cholesterol acyltransferase (EC 2.3.1.43) in lipoprotein oxidation. Free Radic Biol Med. 2007;43:1484–93. doi: 10.1016/j.freeradbiomed.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 33.Murphy AJ, Woollard KJ, Hoang A, et al. High-density lipoprotein reduces the human monocyte inflammatory response. Arterioscler Thromb Vasc Biol. 2008;28:2071–7. doi: 10.1161/ATVBAHA.108.168690. [DOI] [PubMed] [Google Scholar]
- 34.Stafforini DM, McIntyre TM, Zimmerman GA, Prescott SM. Platelet activating factor acetylhydrolases. J Biol Chem. 1997;272:17895–8. doi: 10.1074/jbc.272.29.17895. [DOI] [PubMed] [Google Scholar]
- 35.Watson AD, Navab M, Hama SY, et al. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest. 1995;95:774–82. doi: 10.1172/JCI117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Min JH, Jain MK, Wilder C, et al. Membrane-bound plasma platelet activating factor acetylhydrolase acts on substrate in the aqueous phase. Biochemistry. 1999;38:12935–42. doi: 10.1021/bi991149u. [DOI] [PubMed] [Google Scholar]
- 37.Lundberg AM, Hansson GK. Innate immune signals in atherosclerosis. Clin Immunol. 2010;134:5–24. doi: 10.1016/j.clim.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 38.Jayaraman H, Gantz DL, Gursky O. Effects of phospholipase A2 and its products on structural stability of human LDL: relevance to formation of LDL-derived lipid droplets. J Lipid Res. 2011;52:549–57. doi: 10.1194/jlr.M012567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wong WT, Ng CH, Tsang SY, Huang Y, Chen ZY. Relative contribution of individual oxidized components in ox-LDL to inhibition on endothelium-dependent relaxation in rat aorta. Nutr Metabolism Cardiovasc Dis. 2011;21:157–64. doi: 10.1016/j.numecd.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 40.Markakis KP, Koropouli MK, Grammenou-Savvoglou S, et al. Implication of lipoprotein associated phospholipase A2 activity in oxLDL uptake by macrophages. J Lipid Res. 2010;51:2191–201. doi: 10.1194/jlr.M003558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reddy KJ, Singh M, Bangit JR, Batsell RR. The role of lipoprotein-associated phospholipase A2 on cardiovascular disease risk assessment and plaque rupture: a clinical review. J Clin Lipidology. 2009;4:85–93. doi: 10.1016/j.jacl.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Ishihara M, Iwasaki T, Nagano M, et al. Functional impairment of two novel mutations detected in lipoprotein-associated phospholipase A2 (Lp-PLA2) deficiency patients. J Hum Genet. 2004;49:302–7. doi: 10.1007/s10038-004-0151-6. [DOI] [PubMed] [Google Scholar]
- 43.Koshy B, Miyashita A, Jean P, et al. Genetic deficiency of plasma lipoprotein-associated phospholipase A2 (PLA2G7 V297F Null Mutation) and risk of Alzheimer's disease in Japan. J Alzheimer's Dis. 2010;21:775–80. doi: 10.3233/JAD-2010-100513. [DOI] [PubMed] [Google Scholar]
- 44.Thompson AR, Drenos F, Hafez H, Humphries SE. Candidate gene association studies in abdominal aortic aneurysm disease: a review and meta-analysis. Eur J Vasc Endovasc Surg. 2008;35:19–30. doi: 10.1016/j.ejvs.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 45.Mallat Z, Lambeau G, Tedgui A. Lipoprotein-associated and secreted phospholipases A2 in cardiovascular disease. Roles as biological effectors and biomarkers. Circulation. 2010;122:2183–200. doi: 10.1161/CIRCULATIONAHA.110.936393. [DOI] [PubMed] [Google Scholar]
- 46.Holmes RS, Cox LA. Comparative structures and evolution of vertebrate carboxyl ester lipase (CEL) genes and proteins with a major role in reverse cholesterol transport. Cholesterol. 2011;2011:781643. doi: 10.1155/2011/781643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li F, Hui DY. Synthesis and secretion of the pancreatic-type carboxyl ester lipase by human endothelial cells. Biochem J. 1998;329:675–9. doi: 10.1042/bj3290675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howles PN, Carter CP, Hui DY. Dietary free and esterified cholesterol absorption in cholesterol esterase (bile salt-stimulated lipase) gene-targeted mice. J Biol Chem. 1996;271:7196–202. doi: 10.1074/jbc.271.12.7196. [DOI] [PubMed] [Google Scholar]
- 49.Weng W, Li L, van Bennekum AM, et al. Intestinal absorption of dietary cholesteryl ester is decreased but retinyl ester absorption is normal in carboxyl ester lipase knockout mice. Biochemistry. 1999;38:4143–9. doi: 10.1021/bi981679a. [DOI] [PubMed] [Google Scholar]
- 50.Kirby RJ, Zheng S, Tso P, Howles PN, Hui DY. Bile salt-stimulated carboxyl ester lipase influences lipoprotein assembly and secretion in intestine. A process mediated via ceramide hydrolysis. J Biol Chem. 2002;277:4104–9. doi: 10.1074/jbc.M107549200. [DOI] [PubMed] [Google Scholar]
- 51.Brodt-Eppley J, White P, Jenkins S, Hui DY. Plasma cholesterol esterase level is a determinant for an atherogenic lipoprotein profile in normolipidemic human subjects. Biochim Biophys Acta. 1995;1272:69–72. doi: 10.1016/0925-4439(95)00083-g. [DOI] [PubMed] [Google Scholar]
- 52.Shamir R, Johnson WJ, Morlock-Fitzpatrick K, et al. Pancrea tic carboxyl ester lipase: a circulating enzyme that modifies normal and oxidized lipoproteins in vitro. J Clin Invest. 1996;97:1696–704. doi: 10.1172/JCI118596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li L, Weng W, Harrison EH, Fisher EA. Plasma carboxyl ester lipase activity modulates apolipoprotein B-containing lipoprotein metabolism in a transgenic mouse model. Metabol Clin Exp. 2008;57:1361–8. doi: 10.1016/j.metabol.2008.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Camarota LM, Chapman JM, Hui DY, Howles PN. Carboxyl ester lipase cofractionates with scavenger receptor BI in hepatocyte lipid rafts and enhances selective uptake and hydrolysis of cholesteryl esters from HDL3. J Biol Chem. 2004;279:27599–606. doi: 10.1074/jbc.M402946200. [DOI] [PubMed] [Google Scholar]
- 55.Reiner Z, Catapano A, De Backer G, et al. ESC/EAS Guidelines for the management of dyslipidemias. Eur Heart J. 2011;32:1769–818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 56.Rizzo M, Barylski M, Rizvi AA, Montalto G, Mikhailidis DP, Banach M. Combined dyslipidemia: should the focus be LDL cholesterol or atherogenic dyslipidemia? Curr Pharm Des. 2012 Dec 26; doi: 10.2174/13816128113199990324. Epub ahead of print. [DOI] [PubMed] [Google Scholar]