Abstract
For many years atherosclerosis was believed to be the passive accumulation of cholesterol in vessel walls. Today the picture is more complex, as immune processes occur in atherogenesis. Considerable attention is focused on the particular role of adaptive immune responses orchestrated by T cell subsets. Since the role of Th1/Th2 balance and Th1 cell domination in atherogenesis is already known, the involvement of regulatory T lymphocytes and recently described Th17 cells raises new concerns. On one hand, each of these cells may specifically drive responses of vascular wall tissues and immune cells; however, they are subject to the control of a plethora of tissue- and pathogen-derived agents. Due to ineffective tissue regeneration, remodeling of the vascular wall occurs. The understanding of the immune regulatory network gives perspectives of innovative immunomodulatory therapies of atherosclerosis and the prevention of its complications, such as coronary artery disease.
Keywords: atherosclerosis, regulatory T cells, Th17 cells, endothelium, immunoregulation
Atherosclerotic plaque shows a predominant pattern of Th1 lymphocyte-driven cellular responses. This profile is believed to develop as the consequence of atherogenic antigen presentation by dendritic cells; 10% of T cells found in the plaque may recognize oxidized LDL via MHC class II molecules [1–3]. As interferon-γ (IFN-γ), essentially produced by Th1 cells, promotes macrophage and endothelial activation followed, firstly, by the synthesis of chemokines, cytokines, radicals and proteases, and, secondly, by the up-regulation of adhesion molecule expression, it is identified to be the major pro-atherogenic cytokine. Additionally, it inhibits cell proliferation, collagen production and cholesterol efflux.
The biological significance of IFN-γ and T helper 1 (Th1) cells in atherogenesis has been shown in mice models. In LDL receptor and apolipoprotein E (ApoE) deficient mice, the lack of either IFN-γ or IFN-γ receptors attenuated atherosclerosis [4, 5]. Moreover, injection of recombinant IFN-γ to ApoE deficient mice caused the development of larger plaques as well as the higher accumulation of fatty deposits and macrophages within the arterial wall [6]. On the other hand, low density lipoprotein (LDL) receptor deficient rodents lacking T-bet (the transcription factor of Th1 cells), which do not produce Th1 lymphocytes, had fewer atherosclerotic lesions in the descending aorta [7]. Of note, IFN-γ may also destabilize plaques due to the reduction of collagen synthesis, increased production of proteins that degrade extracellular matrix, as well as prevention of smooth muscle proliferation, leading to the formation of rupture-prone plaques [8].
The excess of Th1-type immune processes in atherogenesis may be attributed to Th1 cells’ functional domination over Th2 cells, which produce interleukin-5 (IL-5) and interleukin-4 (IL-4), being in charge of humoral response development. Indeed, IL-5 has been proved to possess anti-atherosclerotic properties, as, firstly, it induced synthesis of atheroprotective antibodies by B1 cells and, secondly, the engraftment of bone marrow from rodents lacking IL-5 to LDL receptor deficient mice led to the enhancement of atherosclerotic plaque formation [9, 10].
Taking the above-mentioned data into consideration, one may expect that Th2 cells would exhibit protective properties in atherosclerosis. However, IL-4 has been shown to be rather a pro-atherogenic factor, as it may destabilize plaques due to the reduction of collagen synthesis, stimulation of proteases, production of elastases, and the enhancement of smooth muscle cell apoptosis [11]. Moreover, LDL receptor deficient mice lacking IL-4 show less advanced atherosclerosis and a smaller total plaque area [12, 13]. These data indicate that the involvement of Th2 cells in atherogenesis is poorly understood and requires more studies.
Protective role of regulatory T cells
Since the improper balance between Th1 and Th2 responses does not fully explain the immune dysregulation in atherogenesis, the key role of regulatory T cell (Treg) insufficiency leading to the breaking of the immune tolerance to atherogenic self antigens is at present being widely explored. Tregs, a subset of T helper cells, described originally by Sakaguchi, are produced in the thymus, from where they migrate to the peripheral tissues and regulate immune responses through interleukin-10 (IL-10) and transforming growth factor β (TGF-β) release, as well as via cytotoxic T-lymphocyte antigen 4 (CTLA-4) surface molecule expression. Their physiological role is to sustain the immune tolerance to antigens and to inhibit the inflammatory processes through the suppression of effector T lymphocytes (Teff) and memory cell proliferation, the reduction of antibody synthesis, as well as dendritic cell maturation, activity and survival.
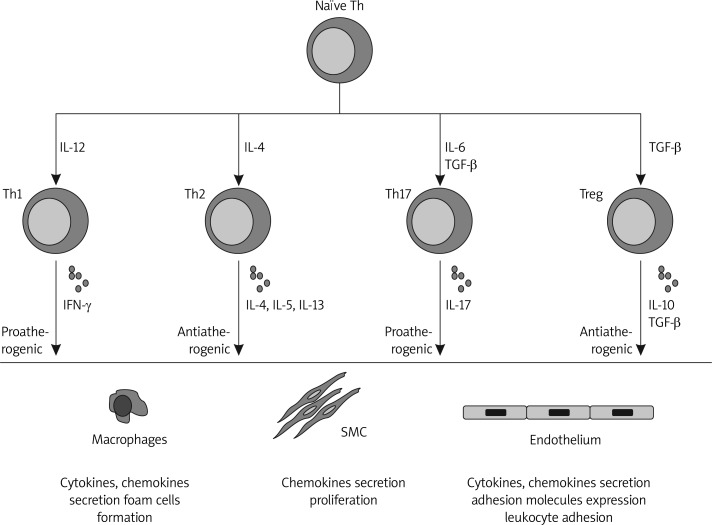
Figure 1.
A schematic presentation of current knowledge of T cell subsets’ potential effects on atherogenesis
The protective role of Tregs in atherogenesis has been evidenced in both rodents and humans. In spleen, lymph nodes and thymus of atherosclerotic mice, one may find low numbers of Tregs. Moreover, in vitro induction of CD4+ cells from these mice with oxidized LDL (oxLDL) causes a decreased Treg percentage [14]. Similarly, the administration of anti-CD25 antibodies to ApoE-deficient mice led to an increase of the number and size of atherosclerotic lesions more strongly infiltrated by macrophages and T cells, with a concomitant decrease of the CD4 + CD25+ Treg count [15].
Interleukin-10 alone, credited to be released by Tregs, has been shown to protect against the development of early stages of plaque. In the aorta of ApoE-deficient mice not capable of producing IL-10, more fatty deposits and larger plaques were found, whereas in mice over-expressing IL-10 fewer fatty deposits and more stable plaques were observed [1]. Correspondingly, in mice with TGF-β receptor disorders, more advanced atherosclerosis is observed. Additionally, the administration of neutralizing anti-TGF-β antibodies to ApoE-deficient mice led to the development of more severe atherosclerosis. It is assumed that IL-10 may exert anti-atherosclerotic properties through the decrease of IFN-γ and interleukin-12 (IL-12) synthesis, reduction of apoptosis as well as the decrease of proteases enzymatic activity degrading intercellular matrix. Tumor growth factor-β promotes the stabilization of the plaque through the increase of synthesis of collagen and inhibitors of matrix metalloproteinases (MMPs), shown to be elevated in patients with metabolic syndrome [16], as well as proliferation of smooth muscle cells. Additionally, it was shown to decrease macrophage infiltration, and to inhibit foam cell formation and lymphocyte activation. Therefore, as a Treg-derived cytokine, TGF-β is also believed to protect against atherosclerosis [1].
Several recent papers support the significance of regulatory T cells in atherogenesis and coronary artery disease development in humans. Firstly, presence of Treg cells has been observed during all stages of plaque development in the intima and adventitia [17]. Secondly, a reverse correlation between Treg cells and high-density lipoprotein levels was also noted. Thirdly, their relevance has been evidenced in patients with coronary artery disease; however, these results are contrasting. In patients with acute coronary syndrome as well as ST-elevated myocardial infarction a lower number of Tregs in peripheral blood was found as compared to subjects with stable coronary artery disease [18–20]. This phenomenon might be explained by immunosuppressive T-cell subset compartmentalization within the acutely ischemic myocardium to limit the ongoing inflammation associated with this condition. On the other hand, in non-STEMI (ST-elevation myocardial infarction) acute coronary syndrome patients, decreased Treg-cell levels and in STEMI subjects increased Treg-cell levels compared with controls and patients with CSA were noted. However, Treg-cell levels were elevated in both groups after 55 days, thus supporting the hypothesis of a compensatory role for Treg cells due to either the myocardial damage or the return to a steady state balance between regulator- and effector-cell activity [21]. Of note, induction of CD4+ cells from patients with acute coronary syndrome with oxLDL caused a decrease of number of Treg cells unable to suppress the effector T cells [18]. To sum up, the increase in Treg-cell levels, as well as IL-10 observed only in STEMI patients, might represent the hallmark of a counter-regulatory response aimed at limiting the cardiovascular damage or alternatively a pro-inflammatory state preceding the acute coronary event.
Although not fully described yet, the protective role of regulatory T cells in atherogenesis seems to be unambiguous.
Th17 cells may aggravate atherosclerosis
Of T helper lymphocyte subsets, Th17 cells releasing interleukin-17 (IL-17) have been since not long ago considered to play a negative role in atherogenesis. Experimental studies evidenced that ApoE-deficient mice had higher IL-17 concentrations in the aortic wall, spleen, and mesenteric and peripheral lymph nodes [22]. Moreover, the administration of soluble IL-17 receptor to ApoE-deficient mice led to a decrease of atherosclerotic lesions in the aorta. Treatment of murine aorta with IL-17 caused higher monocyte infiltration. Additionally, the IL-17A/IL-17RA axis was shown to increase aortic arch inflammation during atherogenesis through the induction of aortic chemokines, and the acceleration of neutrophil and monocyte recruitment to this site [23]. Other results indicate that IL-17 deficiency in mice reduced vascular inflammation and atherosclerosis. Expression of inflammatory cytokines (monocyte chemotactic protein-1 (MCP-1), interleukin-1β (IL-1β), interleukin-6 (IL-6), IFN-γ, and interleukin-12 (IL-12) p40 and scavenger receptors in the plaques in Western Diet-fed apoE(–/–)IL-17(–/–) mice was inhibited [24]. In contrast, it was found that in human arteries and aortic T lymphocytes, IL-17 induced endothelium to produce pro-inflammatory cytokines.
The significance of both IL-17 and Th17 lymphocytes in atherogenesis was also proved in humans. In coronary artery disease and myocardial infarction patients, higher levels of IL-17, IFN-γ and Th17 cells and fewer Treg cells were noted [25, 26]. According to some authors, IL-17 may promote atherogenesis, when Th1 cells are less active. Of note, Th17 cells may play a relevant role in patients with carotid artery plaques. Th17 cells, Th17-related cytokines (IL-17, IL-6, IL-23, and TNF-α), and RORγt mRNA levels were found to be higher in patients with stable plaques, and the highest in subjects with unstable plaques, as compared to non-plaque individuals [27]. The role of Th17 cells in atherogenesis is still unclear and requires further studies.
Immune regulation in atherogenesis may be determined by vascular wall tissues and bacterial agents
As the consequence of cholesterol accumulation, cell death and increased extracellular matrix turnover, endogenous damage-associated molecular patterns (DAMPs) are generated by vascular wall tissue and plaque, which further promotes atherosclerosis. Often they act through Toll-like receptors (TLRs), serving as an alert of tissue injury for the innate immune system. Of them, heat shock proteins hsp60 and hsp70 and high-mobility group protein B1 (HMGB-1) have been shown to be particularly involved in atherogenesis and coronary artery disease [28, 29]. They strongly affect endothelium mainly through Toll-like receptor 2 (TLR2) and TLR4 signaling, thus leading to the increased synthesis of a plethora of pro-inflammatory mediators, which aggravate local inflammation and regulate negative adaptive immune responses [30].
Interestingly, although hsp60, which is a human mitochondrial chaperone displaying extra-mitochondrial and extracellular functions, is typically cytoprotective, a number of stress conditions determine its conversion to a potentially toxic molecule for cells and tissues [31]. E.g. hsp60-stimulated adipocytes secrete TNF-α, IL-6 and IL-8, cytokines widely involved in atherogenesis and responsible for activation of innate immune mechanisms [32]. As it has been shown to be implicated in the initiation and/or progression of cardiovascular diseases, it may also potentially serve as a biomarker with applications for diagnosis, assessing prognosis and response to treatment, as well as for preventing and treating such disorders.
Table I.
Damage-associated molecular patterns (DAMPs) and pathogens involved in atherogenesis
| DAMPs | Pathogens |
|---|---|
| Biglycan | Chlamydia pneumoniae |
| Fibrinogen | Helicobacter pylori |
| FNEDA | Porphyromonas gingivalis |
| Heparan sulfate | Streptococcus sanguis |
| HMGB1 | Streptococcus viridans |
| HSPs | Mycoplasma pneumonia |
| SA100A8/A9 | Haemophilus influenzae |
| Tenascin-C | Cytomegalovirus |
| Neutrophil elastase | Herpes simplex virus |
| Versican | Ebstein-Barr virus |
Apart from tissue-derived agents, the association of chronic bacterial infections, such as Porphyromonas gingivalis, Helicobacter pylori and Chlamydia pneumoniae, with atherosclerosis and the development of its cardiovascular complications has been evidenced in epidemiological studies [33–36]. Experimental research supports the influence of these bacteria on adaptive immune responses, as splenocytes from P. gingivalis-infected mice showed significantly higher proliferation and immunoglobulin G (IgG) production in response to P. gingivalis. Furthermore, the selective up-regulation of MMP3, intercellular adhesion molecule 1 (ICAM-1) and chemokine (C-X-C motif) ligand 7 was observed, suggesting that oral infection with P. gingivalis may induce alterations in systemic cytokine production which are supposed to be fundamental in the development not only of periodontitis but also of atherosclerosis [37]. Interestingly, humans with periodontitis show higher concentrations of circulating hsp60, which inversely correlates with HDL, and positively with triglycerides and small, dense LDL serum levels – a subclass strongly associated with cardiovascular risk, with a significant role beyond traditional risk factors, mentioned in the statement of the European panel of experts [38, 39].
Helicobacter spp. replication in the coronary arterial wall was also shown to be associated with atherosclerotic plaque formation. Although H. pylori is suggested to affect the development of ischemic heart disease through changes in the lipid profiles, endothelial cell colonization, increased coagulation and platelet aggregation, its effect on immune adaptive responses, including induction of molecular mimicry mechanisms and the promotion of low-grade systemic inflammation, is seriously considered [40]. In coronary artery disease patients, seropositivity for H. pylori was shown to enhance serum total cholesterol and LDL concentrations [41]. Helicobacter pylori seropositive patients are at a higher risk for coronary atherosclerosis even regardless of traditional cardiovascular risk factors. Interestingly, H. pylori eradication improves endothelial dysfunction [42].
As the mechanism of gastrointestinal microorganisms’ effect on vascular endothelium in atherogenesis is attempted to be explained by the autoantibody production and inflammatory environment generation by pathogen-associated molecular patterns (PAMPs), Ch. pneumoniae, found to reside in human coronary artery plaques, has been proved to directly induce synthesis of MCP-1, IL-8 and sICAM-1 by endothelial cells, thus leading to atheroprogression [43]. Interestingly, Ch. pneumoniae phospholipase D was shown to be able to drive the expression of interleukin-23 (IL-23), interleukin-6 (IL-6), IL-1β, TGF-β, and chemokine (C-C motif) ligand 20 (CCL-20) by monocytes. This intracellular pathogen elicited Th17 mechanisms playing an emerging role in atherogenesis, suggesting its potential for regulatory adaptive immune responses stimulation [44].
One should underline that the feature of tissue- and bacteria-derived factors mentioned above for Th1- and Th17-type responses promotion may exert a huge impact on lesion growth and atherogenesis progression [45].
Immune processes may affect local tissues and drive remodeling of vascular wall
Persistent inflammation of the vascular wall driven by cholesterol accretion induces release of fibrogenic mediators that cause neointimal hyperplasia as well as smooth muscle cell (SMC) and fibroblast proliferation, leading to medial thickening and adventitial fibrosis, and overall remodeling of the vascular wall. Of pro-inflammatory mediators released by injured endothelium and SMCs as well as activated immune cells, several exert a huge impact on neointimal formation [46, 47]. Fractalkine and MCP-1 drive neointimal SMC expansion, which exposed to sheer stress produce monocyte colony stimulating factor (M-CSF) and IFN-γ, thus inducing differentiation of monocytes into macrophages as well as Th1 cell development.
It is believed that damaged endothelium is not able to restore its proper structure and functions. However, regeneration seems to occur, including recruitment of circulating endothelial progenitor cells (EPCs) from the bone marrow leading to vascular wall re-endothelialization. Endothelial progenitor cells, released from the bone marrow by plaque- and immune cell-derived stimuli, migrate into the circulation and, being capable of differentiating into mature endothelial cells, re-institute and regenerate the damaged intima. Indeed, some data show the association of cardiovascular disease with lower numbers of circulating EPCs [48, 49]. They have been evidenced to delay the development of an atherosclerotic plaque. Moreover, reduced EPC numbers correlate with the impairment of endothelial function.
Regeneration of intima in atherosclerosis might be not only insufficient, but also improper. It has been shown that smooth muscle cells, firstly, migrate from the media to the intima, secondly, they change their phenotype towards the fibroblast-like one and, finally, they proliferate and remain there. Chemokines and cytokines, such as MCP-1, fractalkine, and RANTES released by injured endothelium and hyperproliferative vascular smooth muscle cells may aggravate local inflammation by attracting monocytes and lymphocytes as well as inducing smooth muscle cell proliferation, but at the same time they recruit smooth muscle progenitor cells (SPCs) from the bone marrow by releasing stromal cell-derived factor-1 (SDF-1) [50, 51].
Summing up, in atherosclerotic patients the mechanisms described above seem to be insufficient to regenerate the intima properly, thus leading to the sustention of persistent inflammation.
Immunomodulation in the perspective of atherosclerosis treatment and prevention
The knowledge of immune processes and tissue interactions with both innate and adaptive immune system elements occurring in atherogenesis justifies the question about the perspectives of the development of novel therapies of atherosclerosis based on immunomodulation. Of classic drugs, statins already have been shown to exert their effect also through the reduction of synthesis of pro-inflammatory cytokines, such as tumor necrosis factor-1 α (TNF-α), IL-6, MCP-1 and IL-1β by immune cells and endothelium, and the decrease of serum levels of interleukin-2 (IL-2) and C-reactive protein (CRP), thus preventing migration of macrophages and T-cells through the endothelium to inflamed tissues and reducing the macrophage content inside atherosclerotic lesions [52]. Of note, statins have also been proved to switch Th1 to Th2-type response and induce T regulatory cells in atherogenesis [52].
Apart from immunomodulatory properties, statins have been shown to strongly affect endothelial progenitor cells. In vitro, statins reduce their apoptosis induced by oxidative stress [53]. Additionally, as experiments on animal models revealed, statins may promote mobilization of EPC from bone marrow and improve neovascularization after experimental myocardial infarction [54]. Moreover, the results of a clinical study showed atorvastatin treatment of patients with stable coronary artery disease to exhibit an approximately 1.5-fold increase in EPCs [53]. All these data suggest that statins may affect re-endothelialization.
One should bear in mind that targeting of immunoregulatory mechanisms of atherogenesis may bring the development of novel therapies or even the prevention of atherosclerosis. Modifying the activity of Th cell subsets, such as Th1, Th2, Tregs and/or Th17 lymphocytes, may offer an innovative therapeutic strategy of atherosclerosis treatment in order to tip the balance towards an anti-inflammatory T cell response. At present, two possibilities based on Tregs appear: transfer of T regulatory cells and vaccination in order to induce immune tolerance toward atherogenic antigens. Interestingly, it has been shown that the adoptive transfer of Tr1 cells leads to the decrease of atherosclerotic lesions, down-regulation of arterial wall infiltration by macrophages, inhibition of Th1 responses and increased IL-10 synthesis [55]. Furthermore, the administration of oxLDL in a mouse model of atherosclerosis led to slowed disease progression with concomitant enhancement of CD4 + CD25 + FoxP3+ cell number and higher production of TGF-β in mesenteric lymph nodes as well as higher expression of foxp3, CD25 and CTLA-4 – markers of Treg cells. This indicates that oxLDL leads to tolerance induction, thus decreasing the progression of atherosclerosis [56]. Similarly, the administration of ApoB100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice [57].
In conclusion, this immune regulatory responses networking with vascular wall tissues and their agents as well as exogenous pathogens are believed to fundamentally affect the development, progression and clinical complications of atherosclerotic disease. Precise description of their associations may bring very valuable outcomes in terms of atherogenesis treatment and prevention.
References
- 1.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–12. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 2.Stemme S, Faber B, Holm J, Wiklund O, Witztum JL, Hansson GK. T lymphocytes from human atherosclerotic plaques recognize oxidized low density lipoprotein. Proc Natl Acad Sci U S A. 1995;92:3893–7. doi: 10.1073/pnas.92.9.3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansson GK, Holm J, Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989;135:169–75. [PMC free article] [PubMed] [Google Scholar]
- 4.Buono C, Come CE, Stavrakis G, Maguire GF, Connelly PW, Lichtman AH. Influence of interferon-gamma on the extent and phenotype of diet-induced atherosclerosis in the LDLR-deficient mouse. Arterioscler Thromb Vasc Biol. 2003;23:454–60. doi: 10.1161/01.ATV.0000059419.11002.6E. [DOI] [PubMed] [Google Scholar]
- 5.Whitman SC, Ravisankar P, Daugherty A. IFN-gamma deficiency exerts gender-specific effects on atherogenesis in apolipoprotein E-/- mice. J Interferon Cytokine Res. 2002;22:661–70. doi: 10.1089/10799900260100141. [DOI] [PubMed] [Google Scholar]
- 6.Whitman SC, Ravisankar P, Elam H, Daugherty A. Exogenous interferon-gamma enhances atherosclerosis in apolipoprotein E-/- mice. Am J Pathol. 2000;157:1819–24. doi: 10.1016/s0002-9440(10)64820-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci U S A. 2005;102:1596–601. doi: 10.1073/pnas.0409015102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLaren JE, Ramji DP. Interferon gamma: a master regulator of atherosclerosis. Cytokine Growth Factor Rev. 2009;20:125–35. doi: 10.1016/j.cytogfr.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Binder CJ, Hartvigsen K, Chang MK, et al. IL-5 links adaptive and natural immunity specific for epitopes of oxidized LDL and protects from atherosclerosis. J Clin Invest. 2004;114:427–37. doi: 10.1172/JCI20479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horkko S, Bird DA, Miller E, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest. 1999;103:117–28. doi: 10.1172/JCI4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leskinen MJ, Kovanen PT, Lindstedt KA. Regulation of smooth muscle cell growth, function and death in vitro by activated mast cells: a potential mechanism for the weakening and rupture of atherosclerotic plaques. Biochem Pharmacol. 2003;66:1493–8. doi: 10.1016/s0006-2952(03)00503-3. [DOI] [PubMed] [Google Scholar]
- 12.Davenport P, Tipping PG. The role of interleukin-4 and interleukin-12 in the progression of atherosclerosis in apolipoprotein E-deficient mice. Am J Pathol. 2003;163:1117–25. doi: 10.1016/S0002-9440(10)63471-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.King VL, Szilvassy SJ, Daugherty A. Interleukin-4 deficiency decreases atherosclerotic lesion formation in a site-specific manner in female LDL receptor-/- mice. Arterioscler Thromb Vasc Biol. 2002;22:456–61. doi: 10.1161/hq0302.104905. [DOI] [PubMed] [Google Scholar]
- 14.Mor A, Planer D, Luboshits G, et al. Role of naturally occurring CD4+ CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:893–900. doi: 10.1161/01.ATV.0000259365.31469.89. [DOI] [PubMed] [Google Scholar]
- 15.Ait-Oufella H, Salomon BL, Potteaux S, et al. Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med. 2006;12:178–80. doi: 10.1038/nm1343. [DOI] [PubMed] [Google Scholar]
- 16.Mieczkowska J, Mosiewicz J, Barud W, Kwasniewski W. Changes in the activity of connective tissue matrix enzymes in the metabolic syndrome. Arch Med Sci. 2011;7:634–41. doi: 10.5114/aoms.2011.24133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Boer OJ, van der Meer JJ, Teeling P, van der Loos CM, van der Wal AC. Low numbers of FOXP3 positive regulatory T cells are present in all developmental stages of human atherosclerotic lesions. PLoS One. 2007;2:e779. doi: 10.1371/journal.pone.0000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of CD4(+)CD25(+) regulatory T cells in patients with acute coronary syndromes. Eur Heart J. 2006;27:2530–7. doi: 10.1093/eurheartj/ehl222. [DOI] [PubMed] [Google Scholar]
- 19.Kofler S, Sisic Z, Shvets N, Lohse P, Weis M. Expression of circulatory dendritic cells and regulatory T-cells in patients with different subsets of coronary artery disease. J Cardiovasc Pharmacol. 2011;57:542–9. doi: 10.1097/FJC.0b013e3182124c53. [DOI] [PubMed] [Google Scholar]
- 20.Sardella G, De Luca L, Francavilla V, et al. Frequency of naturally-occurring regulatory T cells is reduced in patients with ST-segment elevation myocardial infarction. Thromb Res. 2007;120:631–4. doi: 10.1016/j.thromres.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 21.Ammirati E, Cianflone D, Banfi M, et al. Circulating CD4 + CD25hiCD127lo regulatory T-cell levels do not reflect the extent or severity of carotid and coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2010;30:1832–41. doi: 10.1161/ATVBAHA.110.206813. [DOI] [PubMed] [Google Scholar]
- 22.Smith E, Prasad KM, Butcher M, et al. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–55. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butcher MJ, Gjurich BN, Phillips T, Galkina EV. The IL-17A/ IL-17RA axis plays a proatherogenic role via the regulation of aortic myeloid cell recruitment. Circ Res. 2012;110:675–87. doi: 10.1161/CIRCRESAHA.111.261784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Usui F, Kimura H, Ohshiro T, et al. Interleukin-17 deficiency reduced vascular inflammation and development of atherosclerosis in Western diet-induced apoE-deficient mice. Biochem Biophys Res Commun. 2012;420:72–7. doi: 10.1016/j.bbrc.2012.02.117. [DOI] [PubMed] [Google Scholar]
- 25.Eid RE, Rao DA, Zhou J, et al. Interleukin-17 and interferon-gamma are produced concomitantly by human coronary artery-infiltrating T cells and act synergistically on vascular smooth muscle cells. Circulation. 2009;119:1424–32. doi: 10.1161/CIRCULATIONAHA.108.827618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao Z, Wu Y, Cheng M, et al. Activation of Th17/Th1 and Th1, but not Th17, is associated with the acute cardiac event in patients with acute coronary syndrome. Atherosclerosis. 2011;217:518–24. doi: 10.1016/j.atherosclerosis.2011.03.043. [DOI] [PubMed] [Google Scholar]
- 27.Liu ZD, Wang L, Lu FH, et al. Increased Th17 cell frequency concomitant with decreased Foxp3+ Treg cell frequency in the peripheral circulation of patients with carotid artery plaques. Inflamm Res. 2012;61:1155–65. doi: 10.1007/s00011-012-0510-2. [DOI] [PubMed] [Google Scholar]
- 28.Yang K, Li D, Luo M, Hu Y. Generation of HSP60-specific regulatory T cell and effect on atherosclerosis. Cell Immunol. 2006;243:90–5. doi: 10.1016/j.cellimm.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 29.Bielecka-Dabrowa A, Barylski M, Mikhailidis DP, Rysz J, Banach M. HSP 70 and atherosclerosis: protector or activator? Expert Opin Ther Targets. 2009;13:307–17. doi: 10.1517/14728220902725149. [DOI] [PubMed] [Google Scholar]
- 30.den Dekker WK, Cheng C, Pasterkamp G, Duckers HJ. Toll like receptor 4 in atherosclerosis and plaque destabilization. Atherosclerosis. 2010;209:314–20. doi: 10.1016/j.atherosclerosis.2009.09.075. [DOI] [PubMed] [Google Scholar]
- 31.Rizzo M, Macario AJ, de Macario EC, et al. Heat shock protein-60 and risk for cardiovascular disease. Curr Pharm Des. 2011;17:3662–8. doi: 10.2174/138161211798220981. [DOI] [PubMed] [Google Scholar]
- 32.Marker T, Sell H, Zillessen P, et al. Heat shock protein 60 as a mediator of adipose tissue inflammation and insulin resistance. Diabetes. 2012;61:615–25. doi: 10.2337/db10-1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ayada K, Yokota K, Kobayashi K, Shoenfeld Y, Matsuura E, Oguma K. Chronic infections and atherosclerosis. Clin Rev Allergy Immunol. 2009;37:44–8. doi: 10.1007/s12016-008-8097-7. [DOI] [PubMed] [Google Scholar]
- 34.Jitsuiki K, Yamane K, Nakajima M, et al. Association of Chlamydia pneumoniae infection and carotid intima-media wall thickness in Japanese Americans. Circ J. 2006;70:815–9. doi: 10.1253/circj.70.815. [DOI] [PubMed] [Google Scholar]
- 35.Tasaki N, Nakajima M, Yamamoto H, et al. Influence of Chlamydia pneumoniae infection on aortic stiffness in healthy young men. Atherosclerosis. 2003;171:117–22. doi: 10.1016/j.atherosclerosis.2003.08.004. [DOI] [PubMed] [Google Scholar]
- 36.Taniguchi A, Nishimura F, Murayama Y, et al. Porphyromonas gingivalis infection is associated with carotid atherosclerosis in non-obese Japanese type 2 diabetic patients. Metabolism. 2003;52:142–5. doi: 10.1053/meta.2003.50001. [DOI] [PubMed] [Google Scholar]
- 37.Miyauchi S, Maekawa T, Aoki Y, et al. Oral infection with Porphyromonas gingivalis and systemic cytokine profile in C57BL/6.KOR-ApoE(shl) mice. J Periodontal Res. 2012;47:402–8. doi: 10.1111/j.1600-0765.2011.01441.x. [DOI] [PubMed] [Google Scholar]
- 38.Rizzo M, Cappello F, Marfil R, et al. Heat-shock protein 60 kDa and atherogenic dyslipidemia in patients with untreated mild periodontitis: a pilot study. Cell Stress Chaperones. 2012;17:399–407. doi: 10.1007/s12192-011-0315-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mikhailidis DP, Elisaf M, Rizzo M, et al. European panel on low density lipoprotein (LDL) subclasses": a statement on the pathophysiology, atherogenicity and clinical significance of LDL subclasses. Curr Vasc Pharmacol. 2011;9:533–71. doi: 10.2174/157016111796642661. [DOI] [PubMed] [Google Scholar]
- 40.Vizzardi E, Bonadei I, Piovanelli B, et al. Helicobacter pylori and ischemic heart disease. Panminerva Med. 2011;53:193–202. [PubMed] [Google Scholar]
- 41.Izadi M, Fazel M, Sharubandi SH, et al. Helicobacter species in the atherosclerotic plaques of patients with coronary artery disease. Cardiovasc Pathol. 2012;21:307–11. doi: 10.1016/j.carpath.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 42.Blum A, Tamir S, Mualem K, Ben-Shushan RS, Keinan-Boker L, Paritsky M. Endothelial dysfunction is reversible in Helicobacter pylori-positive subjects. Am J Med. 2011;124:1171–4. doi: 10.1016/j.amjmed.2011.08.015. [DOI] [PubMed] [Google Scholar]
- 43.Takaoka N, Campbell LA, Lee A, Rosenfeld ME, Kuo CC. Chlamydia pneumoniae infection increases adherence of mouse macrophages to mouse endothelial cells in vitro and to aortas ex vivo. Infect Immun. 2008;76:510–4. doi: 10.1128/IAI.01267-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Benagiano M, Munari F, Ciervo A, et al. Chlamydophila pneumoniae phospholipase D (CpPLD) drives Th17 inflammation in human atherosclerosis. Proc Natl Acad Sci U S A. 2012;109:1222–7. doi: 10.1073/pnas.1111833109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lundberg AM, Hansson GK. Innate immune signals in atherosclerosis. Clin Immunol. 2010;134:5–24. doi: 10.1016/j.clim.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 46.Schober A. Chemokines in vascular dysfunction and remodeling. Arterioscler Thromb Vasc Biol. 2008;28:1950–9. doi: 10.1161/ATVBAHA.107.161224. [DOI] [PubMed] [Google Scholar]
- 47.von Hundelshausen P, Weber KS, Huo Y, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–7. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 48.Eizawa T, Ikeda U, Murakami Y, et al. Decrease in circulating endothelial progenitor cells in patients with stable coronary artery disease. Heart. 2004;90:685–6. doi: 10.1136/hrt.2002.008144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 50.Sata M, Saiura A, Kunisato A, et al. Hematopoietic stem cells differentiate into vascular cells that participate in the pathogenesis of atherosclerosis. Nat Med. 2002;8:403–9. doi: 10.1038/nm0402-403. [DOI] [PubMed] [Google Scholar]
- 51.Schober A, Karshovska E, Zernecke A, Weber C. SDF-1alpha-mediated tissue repair by stem cells: a promising tool in cardiovascular medicine? Trends Cardiovasc Med. 2006;16:103–8. doi: 10.1016/j.tcm.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 52.Chalubinski M, Broncel M. Influence of statins on effector and regulatory immune mechanisms and their potential clinical relevance in treating autoimmune disorders. Med Sci Monit. 2010;16:RA245–51. [PubMed] [Google Scholar]
- 53.Vasa M, Fichtlscherer S, Adler K, et al. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–90. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 54.Landmesser U, Engberding N, Bahlmann FH, et al. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–9. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 55.Mallat Z, Gojova A, Brun V, et al. Induction of a regulatory T cell type 1 response reduces the development of atherosclerosis in apolipoprotein E-knockout mice. Circulation. 2003;108:1232–7. doi: 10.1161/01.CIR.0000089083.61317.A1. [DOI] [PubMed] [Google Scholar]
- 56.van Puijvelde GH, Hauer AD, de Vos P, et al. Induction of oral tolerance to oxidized low-density lipoprotein ameliorates atherosclerosis. Circulation. 2006;114:1968–76. doi: 10.1161/CIRCULATIONAHA.106.615609. [DOI] [PubMed] [Google Scholar]
- 57.Hermansson A, Johansson DK, Ketelhuth DF, Andersson J, Zhou X, Hansson GK. Immunotherapy with tolerogenic apolipoprotein B-100-loaded dendritic cells attenuates atherosclerosis in hypercholesterolemic mice. Circulation. 2011;123:1083–91. doi: 10.1161/CIRCULATIONAHA.110.973222. [DOI] [PubMed] [Google Scholar]