Abstract
Introduction
The appearance of post-operative cognitive dysfunction as a result of open heart surgery has been proven by several studies. Focal and/or sporadic neuron damage emerging in the central nervous system may not only appear as cognitive dysfunction, but might strongly influence features of physiological tremor.
Material and methods
We investigated 110 patients (age: 34-73 years; 76 male, 34 female; 51 coronary artery bypass grafting (CABG), 25 valve replacement, 25 combined open heart surgery, 9 off-pump CABG) before surgery and after open-heart surgery on the 3rd to 5th post-operative day. The assessment of the physiological tremor analysis was performed with our newly developed equipment based on the Analog Devices ADXL 320 JPC integrated accelerometer chip. Recordings were stored on a PC and spectral analysis was performed by fast Fourier transformation (FFT). We compared power integrals in the 1-4 Hz, 4-8 Hz and 8-12 Hz frequency ranges and these were statistically assessed by the Wilcoxon rank correlation test.
Results
We found significant changes in the power spectrum of physiological tremor. The spectrum in the 8-12 Hz range (neuronal oscillation) decreased and a shift was recognised to the lower spectrum (p < 0.01). The magnitude of the shift was not significantly higher for females than for males (p < 0.157). We found no significant difference between the shift and the cross-clamp or perfusion time (p < 0.6450).
Conclusions
The assessment of physiological tremor by means of our novel, feasible method may provide a deeper insight into the mechanism of central nervous system damage associated with open heart surgery.
Keywords: physiological tremor, cognitive dysfunction, open heart surgery
Introduction
More than one million coronary artery bypass graft (CABG) surgical operations are performed world-wide every year [1]. The risk factors of coronary artery disease are hypertension, diabetes mellitus, smoking and hypercholesterolaemia [2–4]. As a result of introducing the first cardio-pulmonary bypass machine in the 1950s, coronary bypass surgery, valve replacement and heart transplantation became widely available [5, 6]. Due to rapid developments in the fields of general and cardiac surgery and anaesthetics in the second half of the 20th century, morbidity and mortality declined enormously. However, brain damage after heart surgery has shown a growing tendency – based on the experience of the last 20-30 years [7–11]. In the literature, operations other than heart surgery might also cause cognitive impairment in the nervous system, but the damage occurring after heart operations is in a relatively extreme form [12–14]. The spectrum of cerebral injury might appear in the form of a major stroke (an incidence of 5.2% was reported by Shaw et al. while Breuer et al. found 4.8%) [15, 16]. However, milder cognitive decline was also significant (Newman and associates report 53% cognitive dysfunction from the 261 patients who underwent CABG surgery) [7, 17, 18]. In the past decade the incidence of neuro-cognitive dysfunction was 50-80% by the 8th post-operative day, declining to 15-30% by the end of the following year [19–21]. Post-operative cognitive deficit is characterised by a deterioration of memory and social integration, by the impairment of orientation, attention and motor speed and a much delayed reaction time. The quality of life of these patients is negatively affected after the operation, not to mention the difficulty of the social re-integration of elderly people [22, 23].
Reasons for brain injury related to cardiac surgery are: a) macro-embolism (occlusion of arteries with a diameter of 200 µm or more: air embolism, thrombus from atherosclerotic plaque); b) micro-embolism (smaller arterioles and capillaries: air bubbles, fat particles, platelet aggregates); c) hypo-perfusion (systemic or local hypo-perfusion); d) metabolic dysfunction of the central nervous system; e) systemic inflammatory response; f) changes in rheological parameters of the blood [24–29].
There are numerous possibilities to diagnose damage to the central nervous system. Ischaemic strokes are usually visible by scanning – computed tomography (CT), magnetic resonance imaging (MRI). With the help of the new method known as diffusion-weighted MRI, the whole ischaemic zone can be defined, including small infarcts [30]. Various neuro-psychological tests provide great help when assessing the neuro-cognitive deficit and they give a more general view of the changes in memory, attention and reaction time than do biochemical tests and scanning. Their disadvantage is that they may overload the patients, since they are time-consuming. Electro-physiological examinations (EEG, event-related potentials) are also effective in revealing post-operative brain damage and they do not exhaust the patients, although an EEG laboratory background is required [31, 32].
Our aim was to evaluate a novel, clinically feasible, non-invasive method which provides enough information after open heart surgery about the changes in the neuro-cognitive system and physiological tremor.
Physiological tremor
Tremor is a rhythmic sinusoid movement which cannot be influenced by will. A special type of tremor is physiological tremor. Everyone has physiological tremor, but usually it cannot be seen and can only be measured and analysed by sensitive methods.
Physiological tremor consists of two main oscillations. The first type is the 8-12 Hz neuronal oscillation, which arises from the central nervous system. The second is peripheral oscillation, which controls the body posture against the Earth's gravity.
Two major components of physiological tremor [33–35]:
- Peripheral oscillation:
- rigidity and elasticity of the arm,
- spinal reflexes.
- Central oscillation:
- the oscillation and synchronisation of motor-neurons at 8-12 Hz,
- the most important structures of the central nervous system which influence the oscillation of motor neurons: cortex, thalamus, inferior olive, globus pallidus, cerebellum, spinal column.
The central oscillation probably arises from the thalamic nuclei, which have a strong connection to the cerebral cortex, although the exact role of this oscillation is yet to be determined. The irregular oscillation measurable in a wide frequency band controls the stability of the extended hand in gravitational space. Persisting lower frequency regulation is consistent with – and can be explained in terms of – the linear negative feedback theory (servo-control theory). Stretch reflex times and frequency depend on the time constant of the regulating loop as well as body mass. The relative distribution of the respective frequency bands (1-4 Hz and 4-8 Hz) is conditional on the burden and the altered regulatory dynamics which it provokes. The frequency band of physiological tremor is virtually independent (spectrum energy) of the frequency of the regulating loop, which determines mechanical features of the body (length, mass, posture), and of the regulatory features of the reflex circle. It originates from the oscillation synchronised with the neuronal regulation of the central nervous system.
Material and methods
Instrumentation
The vast majority of mechanical transducers used to measure the physical features of physiological tremor are sensors registering acceleration. Using their electronic signals, we are able to measure the instantaneous movement and velocity of the limb in tremor, as well as the amplitude of its acceleration.
The tremor measurement was performed by our equipment based on the Analog Devices ADXL 320 JPC integrated accelerometer chip, and the digitised record was stored on a PC. The weight used for the purposes of our measurements is 0.1 g and measures the acceleration of the movement in 2D. As the patient turns the measurement tip from the vertical Y plane in direction X, we register the amplitude of the acceleration based on the cosine of the angle of the tip when turned. Signals emitted by our sensors are captured by a DC amplifier, with a frequency band ranging from 0 to 1000 Hz (–3 dB). The baseline sensitivity of the device is 1 V/g, and can be adjusted in 4 steps between 0.01 V/g and 5 V/g.
The equipment, together with a PC, is placed on a stainless steel trolley enabling bed-side measurement.
Patients and data acquisition
We analysed 110 patients (age: 30-74 years; 76 male and 34 female; 51 on-pump, 9 off-pump CABG, 25 valve replacement, 25 combined surgery) preoperatively and on the 3rd, 4th, or 5th post-operative day. All patients were informed and gave written consent to the examinations. The study was approved by the Local Ethical Committee. Caffeine and alcohol consumption was not allowed for the patients 6 h before the examination.
We used commercial stereo headphones on the patients’ ears and blinkers over their eyes to exclude disturbing factors from the environment. The hand unit with the accelerometer was held in the patients’ dominant hand and they were asked to raise and hold their hands at a 45-degree angle for 30 s, during which time the measurement of the physiological tremor was registered.
Simple reaction time (sRT1) and physiological tremor measurement
The patient heard random 1000 Hz sound waves and he or she had to turn them off as quickly as possible by pressing the button on the hand unit. After 30 s the patient was allowed to rest his or her arm on the bed. The number of target signal cycles can be set to 32, 64 or 128.
Choice reaction time (cRT1) and physiological tremor measurement
In 250 Hz random sound waves, generated by the Odd-ball paradigm, we hide 1000 Hz sound waves randomly. The cRT measurement requires 4-5 times more standard signals of 250 Hz than target signals of 1000 Hz. The patient had to react only to the 1000 Hz signals. After 30 s the patient was allowed to rest his or her arm. Results are displayed and stored in the same way as with the sRT measurement.
The digitised record was stored on a PC as a TXT file. Spectral analysis was performed by fast Fourier transformation on the tremor function, and we compared power integrals in the 1-4 Hz, 4-8 Hz, 8-12 Hz frequency ranges, after which we calculated the ratio of the low and high frequency ranges:
LowsRT1 = P1-4 Hz/(P4-8 Hz + P8-12 Hz) frequency ranges before cardiac surgery at sRT measurement.
LowsRT2 = P1-4 Hz/(P4-8 Hz + P8-12 Hz) frequency ranges after cardiac surgery at sRT measurement.
LowcRT1 = P1-4 Hz/(P4-8 Hz + P8-12 Hz) frequency ranges before cardiac surgery at cRT measurement.
LowcRT2 = P1-4 Hz/(P4-8 Hz + P8-12 Hz) frequency ranges after cardiac surgery at cRT measurement.
Statistical analysis
The Wilcoxon rank correlation test – a non-parametric rank test – was used to compare the power integrals. This test was performed for the quotients of the low and high frequency ranges by sRT tremor and cRT tremor. A value of p below 0.05 was considered statistically significant.
Results
The mean cross-clamp time during the operation was 51.5 ±2.8 min (mean ± SEM), and the average time of the cardiopulmonary bypass (CPB) procedure was 60.3 ±3.1 min in the investigated population of patients (not including the off-pump CABG group). Different types of open heart surgery which were completed in this study and the number of patients are summarised in Table I.
Table I.
Characteristics of study patients who underwent open heart surgery
| Open heart surgery | Men | Women | Σ |
|---|---|---|---|
| A | 5 (60.2 ±3.6) | 4 (58.7 ±2.1) | 9 (59.6 ±2.2) |
| B | 39 (58.0 ±1.1) | 12 (55.3 ±2.1) | 51 (57.4 ±1.0) |
| C | 11 (54.3 ±3.9) | 14 (53.7 ±2.9) | 25 (54.0 ±2.3) |
| D | 21 (59.9 ±1.9) | 4 (53.8 ±5.7) | 25 (58.8 ±1.9) |
| S | 76 (58.2 ±1.0) | 34 (54.7 ±1.6) | 110 (57.1 ±0.8) |
A – group: off-pump CABG, B – group: on-pump CABG with extracorporeal circulation, C – group: isolated valve replacement (AVR or MVR), D – group: combined surgery (AVR + MVR, or MVR + CABG), AVR – aortic valve replacement, MVR – mitral valve replacement
Spectral analysis was performed by fast Fourier transformation on the 110 × 4 tremor functions. All measurements were performed by the patients themselves within 15 min in a separate, quiet room without exhausting themselves. From the measured results, we excluded data points between 12 Hz and 30 Hz, since the results in this range were beyond the main objective of our study.
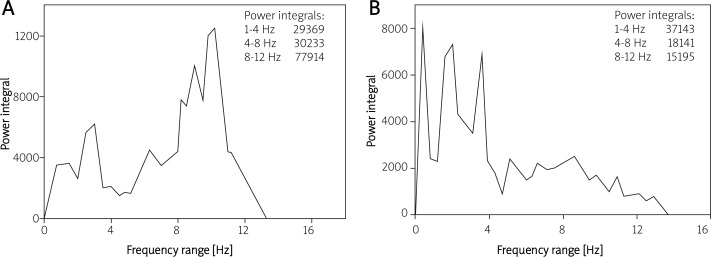
Figures 1 and 2 show the changes in the power spectrum of physiological tremor in patients who underwent open heart surgery. Figure 1 demonstrates the frequency range of sRT tremor of a 65-year-old patient before (panel A) and after (panel B) aortic valve replacement. At baseline this patient had a dominant peak in the 8-12 Hz range (neuronal oscillation). In contrast, after open heart surgery this spectrum decreased and shifted due to the ischaemic brain damage caused by the operation.
Figure 1.
Frequency range of sRT tremor of a 65-year-old patient before (A) and after (B) aortic valve replacement. This patient had a dominant peak at the 8-12 Hz range (neuronal oscillation) before open heart surgery, and this spectrum decreased due to ischaemic brain damage caused by surgery
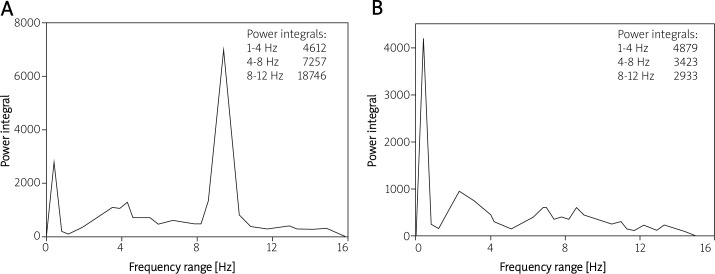
Figure 2.
Frequency range of cRT tremor which was recorded in a 47-year-old patient before (A) and after (B) combined heart surgery. The spectrum of central oscillation has almost disappeared after open heart surgery (B)
In Figure 2 we demonstrate the frequency range of cRT tremor in a 47-year-old patient before (Figure 2A) and after (Figure 2B) combined open heart surgery. This figure clearly demonstrates that the spectrum of central oscillation decreased dramatically following open heart surgery.
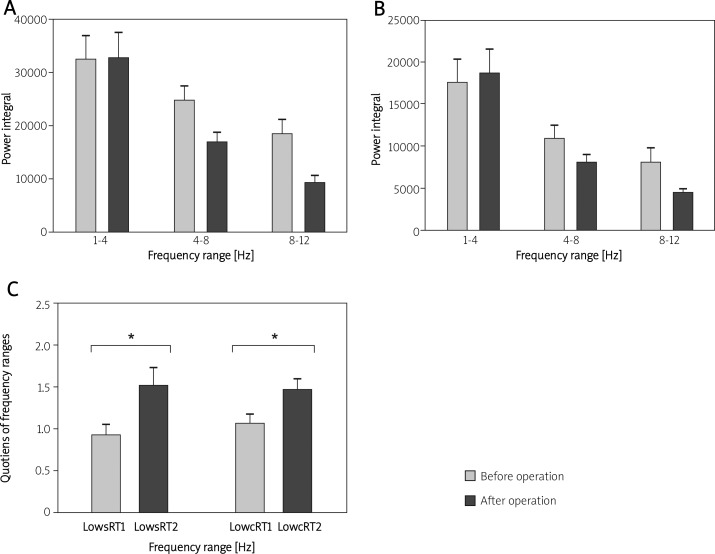
The shifts and the quotients of the power integrals in every range are presented in Figure 3. The decrease of neuronal oscillation is caused by the injury to the central nervous system during cardiopulmonary bypass surgery.
Figure 3.
A and B – each frequency range of sRT and cRT tremor before and after heart surgery. C – the ratio of sRT and cRT tremor before and after open heart surgery. Both patients exhibited a significant increase of shift after surgery due to intra-operative brain injury
The spectrum at the 8-12 Hz range (neuronal oscillation) decreased during both lowsRT and lowcRT measurements and a shift was recognised to the lower spectrum (low sRT1: 1.11 ±0.145 vs. low sRT2: 1.74 ±0.22, p < 0.008; low cRT1: 1.28 ±0.13 vs. lowcRT2: 1.76 ±0.16, p < 0.006).
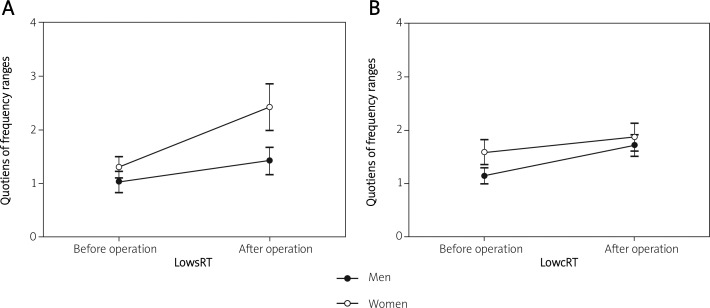
Figure 4 shows the ratio of the frequency ranges by males and females at lowsRT (Figure 4A) and lowcRT (Figure 4B) before and after cardiac surgery. Both men and women show an increase of the ratio after the heart operation. As a result, the inter-subject time effect between male and female patients was not significant (lowsRT: p < 0.157, lowcRT: p < 0.362).
Figure 4.
A and B – the ratio of the frequency ranges for male and female patients before and after open heart surgery. Both men and women show an increase of shift (lowsRT, lowcRT) after the heart operation. As a result, the intersubject time effect between males and females was not significant (lowsRT: p < 0.157, lowcRT: p < 0.362)
We also investigated the correlation between physiological tremor and cross-clamp time of open heart surgery. We did not find a significant correlation between the shift and the cross-clamp time (lowsRT: r = 0.06, p < 0.53; lowcRT: r = 0.10, p < 0.31). This could be explained as a consequence of the multifactorial aetiology of brain injury during surgery and the inhomogeneous, multimorbid patient population at baseline.
Discussion
Brain injury after open heart surgery has been a well-known phenomenon since the first successful application of the cardio-pulmonary bypass machine in 1952 [3], and can be a devastating complication. The reasons for brain damage associated with CPB are multifactorial: macro- and micro-embolism, hypoperfusion due to extracorporeal circulation and systemic inflammatory response [21, 22]. The different types of damage can affect the appearance of the physiological tremor. Physiological tremor acts as an indicator of oscillomotoric regulation, involving a significant area of the cortex through the motor control system.
The measurement of physiological tremor is fast and simple and can be performed in a hospital ward within 10-15 min without exhausting a patient who has undergone open heart surgery. This simple and feasible method can, therefore, be used in routine medical practice. During measurement, we do not need an EEG laboratory background. We plan to complete our findings with neuropsychological tests in order to help in the evaluation of a patient's postoperative quality of life. The disadvantage of these tests is that they are time-consuming. Neither biochemical examinations nor imaging techniques (CT, MRI) are routinely used for these purposes. The disadvantage of biochemical markers (protein S100, neuron-specific enolase) is that they are not specific as to damage to the brain and do not indicate the localisation of the injury [36, 37].
In our method the frequency range of physiological tremor changed both at sRT tremor (the patient had to react only to the 1000 Hz signal) and at cRT tremor (the patient had to choose between 250 Hz and 1000 Hz signals). The central oscillation decreased and a shift can be recognised to the lower spectrum. The decrease of 8-12 Hz oscillation is caused by ischaemic brain damage during the operation. According to this, the central oscillation arises from the thalamic nuclei and is influenced by the cortex, cerebellum, spinal column and the inferior olive. Since the exact role of each structure of the central nervous system in generating central oscillation is not perfectly clear, we have no information on the location of the cerebral lesion.
The magnitude of the ratio was elevated for both females and males, but we did not find significant differences between the two groups, either during lowsRT or during lowcRT. This could be because the main causes of brain injury during open heart surgery (macroembolism, microembolism, hypoperfusion and systemic inflammatory response) occurred in both genders in a similar manner.
Surprisingly, we found no significant correlation between the detail and the cross-clamp time or the perfusion time. The possible reason may be the inhomogeneous patient population, the multifactorial aetiology of brain damage associated with open heart surgery and the different areas of the ischaemic lesions of the central nervous system which influence physiological tremor.
Our aim is to continue and extend these measurements for a longer period of time after heart surgery. It is well known that 60% of neuro-cognitive dysfunction appears about 1 week after heart surgery and declines to 25-30% after 1 year [16–18]. This phenomenon emphasises the importance of evaluating physiological tremor within 1 year of surgery. After this first year, these effects become harder to ascertain and differentiate from the symptoms of general atherosclerosis. Atherosclerosis is a generalised vascular disease, playing an important role in the deterioration of memory and in neuro-cognitive decline in the elderly. We are currently unable to cure atherosclerosis and can only slow its progress by means of a complex treatment which has to be started in time. We need, therefore, to be mindful of these effects when evaluating the results of our present clinical study.
In conclusion, there is a strong need for more research to understand the neuro-cognitive deficit after open heart surgery in order to develop preventive techniques and, in consequence, to diminish possible neurological damage and support the rapid recovery of patients. Brain injury has a great impact on the rehabilitation of patients and their future possibilities and roles at work and in society.
We are planning to undertake large, wide-scale, multicentre clinical research, to analyse the changes in physiological tremor with the help of neuro-psychological tests (and maybe MRI) in order to evaluate neurological damage in patients after open heart surgery. In the future, our aim is to develop novel, simple, clinically feasible methods to recognise early cognitive impairment in the central nervous system and to widen neuro-protective rehabilitation which can improve the quality of life.
Acknowledgments
Attila Cziraki and Sandor Szabados equally contributed. This study was supported by the Hungarian National Research Foundation (OTKA) grant no. TO43403.
References
- 1.Ahonen J, Salmenperä M. Brain injury after adult cardiac surgery. Acta Anaesthesiol Scand. 2004;48:4–19. doi: 10.1111/j.1399-6576.2004.00275.x. [DOI] [PubMed] [Google Scholar]
- 2.Bach RG. Acute coronary syndromes in high risk groups: patients with diabetes, the elderly, and women. Arch Med Sci. 2010;6(Suppl. 1A):S89–103. [Google Scholar]
- 3.Olsen MH, Sehestedt T, Hansen TW, et al. New cardiovascular risk markers in the general population and in hypertension. Do they improve risk prediction and influence treatment? Arch Med Sci. 2009;5(Suppl. 2A):S236–42. [Google Scholar]
- 4.Bielecka-Dąbrowa A, Michalska M, Rysz J, Banach M. Arterial hypertension in patients with coronary artery disease treated with coronary artery bypass surgery. Arch Med Sci. 2009;5(Suppl. 2A):S378–92. [Google Scholar]
- 5.Ajtay Z, Kellényi L, Hejjel L, et al. Simple and choice reaction time elongates following extracorporeal circulation. A potential method for the assessment of acute neuro-cognitive deficit. Med Sci Monit. 2009;15:CR470–6. [PubMed] [Google Scholar]
- 6.Gibbon JH. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37:171–85. [PubMed] [Google Scholar]
- 7.Newman MF, Kirchner LJ, Phillips-Bute B, et al. Longitudinal assessment of neuro-cognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;6:369–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 8.Zimpfer D, Czerny M, Vogt F, et al. Neuro-cognitive deficit following coronary artery bypass grafting: a prospective study of surgical patients and nonsurgical controls. Ann Thorac Surg. 2004;78:513–9. doi: 10.1016/j.athoracsur.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Lillehei CW. Historical development of cardio-pulmonary bypass. In: Gravlee GB, Davis RF, Utley JR, editors. Cardio-pulmonary bypass: principles and practice. Baltimore: Williams & Wilkins; 1993. pp. 1–26. [Google Scholar]
- 10.Taylor KM. Cardiac surgery and the brain: an introduction. In: Smith PL, Taylor KM, editors. Cardiac surgery and the brain. London: Edward Arnold; 1993. pp. 1–14. [Google Scholar]
- 11.Jones EL, Weintraub WS, Craver JM, Guyton RA, Cohen CL. Coronary bypass surgery: is the operation different today? J Thorac Cardiovasc Surg. 1991;101:108–15. [PubMed] [Google Scholar]
- 12.Jarvinen O, Saarinen T, Julkunen J, Laurikka J, Huhtala H, Tarkka M. Improved health-related quality of life after coronary artery bypass grafting is unrelated to use of cardio-pulmonary bypass. World J Surg. 2004;28:1030–5. doi: 10.1007/s00268-004-7486-1. [DOI] [PubMed] [Google Scholar]
- 13.Diegeler A, Hirsch R, Schneider F, et al. Neuro-monitoring and neuro-cognitive outcome in off-pump versus conventional coronary bypass operations. Ann Thorac Surg. 2000;69:1162–6. doi: 10.1016/s0003-4975(99)01574-x. [DOI] [PubMed] [Google Scholar]
- 14.Vingerhoets G, Van Nooten G, Vermassen F, et al. Short-term and long-term neuropsychological consequences of cardiac surgery with extra-corporeal circulation. Eur J Cardiothorac Surg. 1997;11:424–31. doi: 10.1016/s1010-7940(96)01031-7. [DOI] [PubMed] [Google Scholar]
- 15.Shaw PJ, Bates D, Cartlidge NE, Heaviside D, Julian DG, Shaw DA. Early neurological complications of coronary artery bypass surgery. Br Med J. 1985;291:1384–7. doi: 10.1136/bmj.291.6506.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Breuer AC, Furlan AJ, Hanson MR, et al. Neurologic complications of open heart surgery. Cleve Clin Q. 1981;48:205–6. doi: 10.3949/ccjm.48.1.205. [DOI] [PubMed] [Google Scholar]
- 17.Taylor KM. Brain damage during cardio-pulmonary bypass. Ann Thorac Surg. 1998;65(4 Suppl):S20–6. doi: 10.1016/s0003-4975(98)00072-1. [DOI] [PubMed] [Google Scholar]
- 18.Zimpfer D, Kilo J, Czerny M, et al. Neuro-cognitive deficit following aortic valve replacement with biological/mechanical prosthesis. Eur J Cardiothorac Surg. 2003;23:544–51. doi: 10.1016/s1010-7940(02)00843-6. [DOI] [PubMed] [Google Scholar]
- 19.Taylor KM. Central nervous system effects of cardio-pulmonary bypass. Ann Thorac Surg. 1998;66:S20–4. doi: 10.1016/s0003-4975(98)00970-9. [DOI] [PubMed] [Google Scholar]
- 20.Smith PL. The cerebral consequences of coronary artery bypass surgery. Ann R Coll Surg Engl. 1988;70:212–6. [PMC free article] [PubMed] [Google Scholar]
- 21.Borowicz LM, Goldsborough MA, Selnes OA, McKhann GM. Neuropsychologic change after cardiac surgery: a critical review. J Cardiothorac Vasc Anesth. 1996;10:105–12. doi: 10.1016/s1053-0770(96)80185-6. [DOI] [PubMed] [Google Scholar]
- 22.Gao L, Taha R, Gauvin D, Othmen LB, Wang Y, Blaise G. Post-operative cognitive dysfunction after cardiac surgery. Chest. 2005;128:3664–70. doi: 10.1378/chest.128.5.3664. [DOI] [PubMed] [Google Scholar]
- 23.Rothenhäusler HB, Grieser B, Nollert G, Reichart B, Schelling G, Kapfhammer HP. Psychiatric and psychosocial outcome of cardiac surgery with cardio-pulmonary bypass: a prospective 12-month follow-up study. Gen Hosp Psychiatry. 2005;27:18–28. doi: 10.1016/j.genhosppsych.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 24.Merkle F, Boettcher W, Schulz F, et al. Reduction of microemboli count in the priming fluid of cardio-pulmonary bypass circuits. J. Extra Corpor Technol. 2003;35:133–8. [PubMed] [Google Scholar]
- 25.Cook DJ, Huston J, 3rd, Trenerry MR, Brown RD, Jr, Zehr KJ, Sundt TM., 3rd Post-cardiac surgical cognitive impairment in the aged using diffusion-weighted magnetic resonance imaging. Ann Thorac Surg. 2007;83:1389–95. doi: 10.1016/j.athoracsur.2006.11.089. [DOI] [PubMed] [Google Scholar]
- 26.Arrowsmith JE, Grocott HP, Newman MF. Neurologic risk assessment, monitoring and outcome in cardiac surgery. Cardiothorac Vasc Anesth. 1999;13:736–43. doi: 10.1016/s1053-0770(99)90132-5. [DOI] [PubMed] [Google Scholar]
- 27.Jones RH, Hannan EL, Hammermeister KE, et al. Identification of preoperative variables needed for risk adjustment of short-term mortality after coronary artery bypass graft surgery: the Working Group Panel on the Cooperative CABG Database Project. J Am Coll Cardiol. 1996;28:1478–87. doi: 10.1016/s0735-1097(96)00359-2. [DOI] [PubMed] [Google Scholar]
- 28.Abildstrom H, Christiansen M, Siersma VD, et al. ISPOCD2 Investigators. Apolipoprotein E genotype and cognitive dysfunction after non-cardiac surgery. Anesthesiology. 2004;101:855–61. doi: 10.1097/00000542-200410000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Hsiung GY, Sadovnick AD, Feldman H. Apolipoprotein E €4 genotype as a risk factor for cognitive decline and dementia: data from the Canadian Study of Health and Ageing. Can Med Assoc J. 2004;171:863–7. doi: 10.1503/cmaj.1031789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wityk RJ, Goldsborough MA, Hillis A, et al. Diffusion- and perfusion-weighted brain magnetic resonance imaging in patients with neurologic complications after cardiac surgery. Arch Neurol. 2001;58:571–6. doi: 10.1001/archneur.58.4.571. [DOI] [PubMed] [Google Scholar]
- 31.Liu YQ, Zhang LL, Zhao XH, Liu ZL, Mei ZH. Relationship between P300 and intelligence quotient in severe head injury patients. Fa Yi Xue Za Zhi. 2007;23:108–9. [PubMed] [Google Scholar]
- 32.Rodriguez RA. Human auditory evoked potentials in the assessment of brain function during major cardiovascular surgery. Semin Cardiothorac Vasc Anesth. 2004;8:85–99. doi: 10.1177/108925320400800203. [DOI] [PubMed] [Google Scholar]
- 33.Deuschl G, Krack P, Lauk M, Timmer J. Clinical neurophysiology of tremor. J Clin Neurophysiol. 1996;13:110–21. doi: 10.1097/00004691-199603000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Elble RJ. Central mechanisms of tremor. J Clin Neurophysiology. 1996;13:133–44. doi: 10.1097/00004691-199603000-00004. [DOI] [PubMed] [Google Scholar]
- 35.Smaga S. Tremor. Review. Am Fam Physician. 2003;68:1545–52. [PubMed] [Google Scholar]
- 36.Anderson R, Hansson LO, Dijlai-Merzoug R, Settergren G. High serum S100beta levels for trauma patients without head injuries. Neurosurgery. 2001;48:1255–8. doi: 10.1097/00006123-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 37.Soinne L, Roine RO. Blood tests for cognitive decline? Acta Anaesthesiol Scand. 1999;43:491–3. doi: 10.1034/j.1399-6576.1999.430501.x. [DOI] [PubMed] [Google Scholar]