Abstract
Despite being highly effective for newly diagnosed chronic myeloid leukemia (CML), imatinib not only is inactive against quiescent CML stem cells, but also has limited activity against blast crisis (BC) CML. The relative activity of Bcr-Abl and the expression levels of antiapoptotic proteins in proliferating and quiescent CD34+ BC CML progenitor cells and the effects of targeting antiapoptotic proteins in these cells are unknown. Here we report higher levels of p-CrkL in quiescent than in proliferating CD34+ progenitor cells and comparable expression levels of Bcl-2, Bcl-xL, Mcl-1, and XIAP in the two populations in BC CML. Inhibition of Bcl-2/Bcl-xL by ABT-737 in cells from patients with tyrosine kinase inhibitor (TKI)-resistant BC CML promoted apoptosis in quiescent CD34+ progenitor cells with an efficacy similar to that in proliferating cells. Combination of ABT-737 with imatinib (which decreases Mcl-1 levels) or triptolide (which decreases Mcl-1 and XIAP) synergistically induced death of both proliferating and quiescent CD34+ progenitor cells obtained from TKI-resistant BC CML patients. These results suggest that antiapoptotic proteins are critical targets in BC CML and that activation of apoptosis signaling can eliminate both proliferating and quiescent CD34+ progenitor cells in BC CML, independent of response to TKIs.
Keywords: apoptosis, Bcl-2/Bcl-xL, Mcl-1, XIAP, quiescent, CML, progenitors
Introduction
Imatinib, a Bcr-Abl tyrosine kinase inhibitor (TKI), is a highly successful front-line therapy for newly diagnosed patients with chronic myeloid leukemia (CML). Development of resistance/intolerance in a subset of patients is a problem for this therapy, but it can be largely overcome with more potent second generation TKIs. Limited activity in blast crisis (BC) CML and the insensitivity of quiescent CD34+ CML progenitor cells to TKIs have restricted the potential of TKIs in curing CML. Alternative strategies are needed to overcome these challenges.
Our knowledge of CML stem cells is largely obtained from CML in chronic phase (CP). These cells, originated from hematopoietic stem cells by the acquisition of the BCR-ABL fusion gene confer self-renew and maintain the indolent CP disease. Most CML progenitor cells have a higher proliferative capacity than normal progenitor cells1 and are sensitive to TKIs, but a subpopulation is quiescent. Although quiescent cells have Bcr-Abl signaling activity,2;3 they are insensitive to TKIs.2–4 The persistence of these quiescent cells is the main reason that the majority of patients relapsed upon drug discontinuation, even when they had undetectable BCR-ABL transcript levels during therapy. In BC CML, in addition to those derived from hematopoietic stem cells, committed granulocyte-macrophage progenitors have gained an abnormal self-renewal capacity by activation of β-catenin.5 The coexistence of these progenitors, which possess stem cell characteristics, is responsible for the rapid expansion of the advanced disease. Therefore, effective therapies for BC CML rely on eliminating both CML stem cells and the rapid growing blast cells. While BCR-ABL mRNA has been found6;7 and Bcr-Abl tyrosine kinase is active3 in quiescent CD34+ progenitor cells in CP CML, whether it is expressed in quiescent CD34+ progenitor cells in BC CML and whether it translates to Bcr-Abl protein and activates Bcr-Abl tyrosine kinase signaling are unknown.
Bcl-2 family proteins are the key regulators of the intrinsic apoptotic pathway, whereas XIAP inhibits caspases-9, -3, and -7 and suppresses both intrinsic and extrinsic pathways. BCR-ABL-expressing cells have been shown to have high levels of antiapoptotic proteins Bcl-xL and Mcl-1 and low levels of proapoptotic protein Bim. Inhibition of Bcr-Abl tyrosine kinase leads to decreased Mcl-1 and increased Bim levels.8–10 ABT-737, a selective inhibitor of Bcl-2, Bcl-xL, and Bcl-w, has shown potent antileukemic activity, including against AML progenitor and stem cells.11 Inhibition of Bcl-2/Bcl-xL by ABT-737 was recently shown by us to augment imatinib-induced apoptosis in CML cells.12 The second-generation Bcr-Abl TKI bafetinib, in combination with ABT-737, enhances apoptosis, even in bafetinib-resistant cells with BCR-ABL point mutations (except the T315I mutation).13 Bcl-2 antisense oligonucleotide (ASO) was also found to be active against imatinib-resistant BCR-ABL-positive cells.14 We have reported that survivin is regulated by Bcr-Abl tyrosine kinase and therefore highly expressed in CML. Targeting survivin with ASO bypasses imatinib resistance and induces apoptosis in BC CML.15 Collectively, targeting antiapoptotic proteins maybe an effective strategy in BC and TKI resistant CML. In addition, we found that XIAP is highly expressed and that triptolide, an antitumor agent isolated from a Chinese herb, decreases XIAP, Mcl-1, and Bcr-Abl levels and promotes cell death, independent of cellular response to imatinib in BC CML cells, including quiescent CD34+ progenitor cells16 suggesting that inhibition of antiapoptotic proteins may also have the potential to eliminate CD34+ progenitor cells, both proliferating and quiescent, in BC CML. Although upregulation of antiapoptotic Bcl-2 genes in CD34+ CML progenitor cells has been associated with CML blastic transformantion,17 the expression levels of antiapoptotic proteins in quiescent CD34+ BC CML progenitor cells and the effects of targeting antiapoptotic proteins on viabilities of these cells are unknown.
In this study, we measured the activity of Bcr-Abl signaling and the expression levels of Bcl-xL, Bcl-2, Mcl-1, and XIAP mRNAs in proliferating and quiescent CD34+ progenitor cells from BC CML patients and determined the response of these cells to apoptosis activation. We found that p-CrkL levels are higher in quiescent than in proliferating CD34+ BC CML progenitors and comparable mRNA levels of Bcl-2, Bcl-xL, Mcl-1, and XIAP are expressed in quiescent and proliferating CD34+ BC CML progenitor cells and that ABT-737, combined with imatinib or triptolide, synergistically induced cell death in all CD34+ progenitor cells regardless their proliferating status from patients with TKI-resistant BC CML.
Materials and Methods
Cell culture, treatment, and viability assay
KBM5,18 an imatinib-sensitive BC CML cell line, and KBM5STI571,19 an imatinib-resistant KBM5 subline harboring a T315I mutation in the BCR-ABL gene, were cultured in Iscove’s Modified Dulbecco’s Medium supplemented with 10% heat-inactivated fetal calf serum (FCS), 2 mM L-glutamine, 100 U/mL penicillin, and 100 µg/mL streptomycin. KBM5 and KBM5STI571 cells (0.2×106/mL) were treated with imatinib (LC Laboratories, Woburn, MA, USA), ABT-737 (synthesized at The University of Texas MD Anderson Cancer Center, Houston, TX, USA based on the published structure), or both for 48 hours. For ABT-737 treatment of Mcl-1 or XIAP knockdown KBM5 and KBM5STI571 cells, exponentially growing cells were electroporated as described previously15;20 with Mcl-1 ASO or XIAP ASO (ISIS Pharmaceuticals, Carlsbad, CA, USA) according to the manufacturer’s instructions (Lonza, Cologne, Germany) for 4 hours and then treated with ABT-737 for additional 44 hours. Apoptosis was estimated by flow cytometry measurements of phosphatidylserine externalization with annexin V-Cy5 (BD Biosciences, San Diego, CA, USA) using a BD FACSArray Bioanalyzer. Membrane integrity was assessed simultaneously by 7-amino-actinomycin D (7-AAD) exclusion in the annexin V-stained cells.
Western blot analysis
Mcl-1, CrkL, phospho(p)-CrkL, and XIAP protein levels were determined by western blot analysis, as described previously.15;20 XIAP antibody was purchased from BD-Transduction Laboratory (BD Biosciences), CrkL, p-CrkL, and Mcl-1 antibodies from Cell Signaling Technology (Danvers, MA, USA). Signals were detected using Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA), and quantitated using Odyssey Software version 3.0 (LI-COR Biosciences). β-Actin was used as a loading control.
Patient sample preparation
Bone marrow (BM) or peripheral blood (PB) samples from CML patients and control BM samples from normal donors were acquired under informed consent, according to institutional guidelines and in concordance with the declaration of Helsinki. Mononuclear cells from these samples were purified by Ficoll-Hypaque (Sigma-Aldrich, St. Louis, MO, USA) density-gradient centrifugation.
Tracking and isolating proliferating and quiescent CD34+ CML progenitor cells
Mononuclear BM or PB cells (5×106/mL) from CML patients were labeled with 1 µM 5-(and 6-) carboxy-fluorescein diacetate succinimidyl ester (CFSE) as described elsewhere.21 They were then co-cultured in RPMI-1640/10%FCS medium with human mesenchymal stromal cells (hMSCs) or with MS-5 cells, a murine MSC cell line known to support primitive human progenitor cells.22–24 Cell proliferation was monitored using flow cytometric measurement of cellular CFSE fluorescence intensity, which halved with each cell division, and compared with that of Colcemid-treated (100 ng/mL) cells. At the end of culture (4 to 12 days), CD34+ cells were labeled with anti-CD34 antibody (CD34-APC). Quiescent CD34+ CML progenitor cells, defined as those in a region of CFSE fluorescence of Colcemid-treated nonproliferating control cells (CD34+CFSEbright) and proliferating CD34+ CML progenitor cells, defined as those with a fluorescent intensity less than that of nonproliferating control cells (CD34+CFSEdim) were isolated by FACS sorting in the propidium iodide (PI)-negative populations (FACSAria II Cell Sorter, BD Biosciences).
Determination of p-CrkL by laser scanning cytometry
FACS-sorted cells (2×104 per slide) were cytospun onto glass slides and fixed in 2% formaldehyde/PBS for 15 minutes at ambient temperature. The slides were then washed with PBS and soaked in ice-cold methanol for 10 minutes at −20°C. After being rinsed with PBS, the slides were blocked with DAKO-Cytomation protein block buffer (DAKO Corporation, Carpinteria, CA, USA) and incubated overnight with anti-p-CrkL (Tyr207) antibody (1:100) (Cell Signaling Technology) at 4°C in a humidified chamber. The slides were then washed with PBS/0.1% Tween and incubated with FITC-conjugated goat anti-rabbit IgG (whole molecule) (Sigma-Aldrich) (1:200) for 1 hour at ambient temperature. The slides were counterstained with PI (0.5 µg/mL). Autofluorescence was determined and subtracted in cells from the same sample processed as described above, without the anti-p-CrkL antibody. FITC fluorescence was measured by laser scanning cytometry (LSC) (CompuCyte Corporation, Westwood, MA, USA). The 488-nm line of the argon laser was used for excitation and emission was measured with a 530/30 nm bandpass filter. PI fluorescence was excited using the argon 488-nm line and measured by a 625/28 nm bandpass filter. We analyzed 800 to 2000 cells on each slide.
Real-time RT-PCR
Real-time PCR was performed as previously described.16 The forward/reverse primers for Bcl-2 were 5’- AACTGTACGGCCCCAGCAT-3’/5’-GGGCCAAACTGAGCAGAGTCT-3’, Bcl-xL 5’-TGACCTGACATCCCAGCTCC-3’/5’-GTCTACGCTTTCCACGCACA-3’, Mcl-1 5'-GAGGCTGGGATGGGTTTGT-3'/5'-AAAGCCAGCAGCACATTCCT-3', XIAP 5'-CCCAAATTGCAGATTTATCAACG-3'/5'-TGCATGTGTCTCAGATGGCC-3', and 18S 5’- TTTTCGGAACTGAGGCCATG-3’/5’-CTTGGCAAATGCTTTCGCTC-3’. 18S RNA was used as an internal control. The abundance of each transcript relative to that of 18S was calculated using the 2−ΔCt method, where ΔCt is the mean Ct of the transcript of interest minus the mean Ct of the transcript for 18S.
Treatment and viability assay of quiescent and proliferating CD34+ CML progenitor cells
CFSE-stained cells from CML patients were treated with ABT-737, imatinib, triptolide (Alexis Biochemicals, San Diego, CA, USA), ABT-737 and imatinib, or ABT-737 and triptolide for 24 hours and then stained with CD34-PE antibody and annexin V-Cy5. Assay was performed using a BD LSR-II flow cytometer, and apoptosis of quiescent CD34+ CML progenitor cells was defined as annexin V positivity in the CD34+CFSEbright population, whereas apoptosis of proliferating CD34+ CML progenitor cells was defined as annexin V positivity in the CD34+CFSEdim population. To eliminate variations from spontaneous apoptosis in CML patient samples, apoptosis was expressed as specific apoptosis:
Similarly, CFSE stained BM cells from normal controls were treated with ABT-737, imatinib, or both and cell viability in quiescent and proliferating CD34+ progenitor cells was determined.
Statistical analysis
Experiments using cell lines were repeated 3 times and results were expressed as means±SD. For studies with patient or normal control samples, the results were expressed as the mean±SEM. Statistical significance was set at p<0.05, where applicable, using paired Student’s t-test. The combination index (CI), determined using the Chou-Talalay method25 and Calcusyn software, was expressed as the mean of the CI values obtained at ED50, ED75, and ED90. A CI of <1 was considered synergistic, a CI of 1 additive, and a CI of >1 antagonistic.
Results
Bcr-Abl tyrosine kinase signaling pathway is active in quiescent CD34+ BC CML progenitor cells
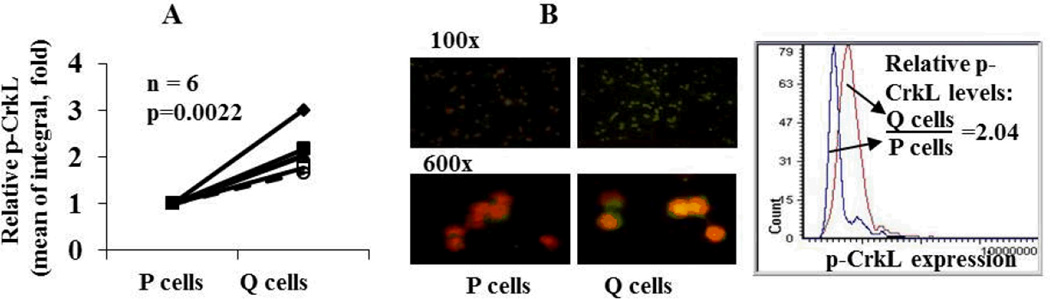
To determine the status of the Bcr-Abl tyrosine kinase signaling pathway in quiescent CD34+ BC CML progenitor cells, p-CrkL levels in quiescent and proliferating CD34+ cells obtained from 6 BC CML patients were measured using LSC. In all 6 samples, p-CrkL was expressed and the levels were significantly higher in quiescent CD34+ progenitor cells than in their proliferating counterparts (p=0.0022, Figure 1A), suggesting that as in CP CML, quiescent CD34+ progenitor cells in BC CML express Bcr-Abl protein and are highly active in Bcr-Abl tyrosine kinase signaling. A representative p-CrkL image and quantification is shown in Figure 1B.
Figure 1.
Quiescent (Q) CD34+ CML progenitor cells have significantly higher p-CrkL levels than their proliferating (P) counterparts in BC CML. A. p-CrkL levels in P and Q CD34+ progenitor cells from BC CML patients as determined by LSC (n=6). B. A representative p-CrkL image (p-CrkL in green; PI in red) and quantification in P and Q CD34+ CML progenitor cells.
Quiescent and proliferating CD34+ BC CML progenitor cells express similar mRNA levels of antiapoptotic proteins
To investigate if activation of apoptotic signaling is a feasible strategy for eliminating both proliferating and quiescent CD34+ BC CML progenitor cells, we first determined the expression levels of the antiapoptotic Bcl-xL, Bcl-2, Mcl-1, and XIAP mRNAs in quiescent CD34+ CML progenitor cells by real-time RT-PCR and compared them with those in their proliferating counterparts. Among the nine samples analyzed, one was from a newly diagnosed CML patient who had accelerated phase (AP) CML and later did not respond to TKI treatment, and all others were from patients with BC CML, who were resistant to or relapsed after imatinib and/or other TKIs treatment. Interestingly, Bcl-xL, Bcl-2, Mcl-1, and XIAP mRNAs were found in both proliferating and quiescent CD34+ cells, with no significant differences in expression levels. P values for Bcl-xL, Bcl-2, Mcl-1, and XIAP were 0.66, 0.48, 0.28, and 0.43, respectively (n=9). Levels of Mcl-1 mRNAs tend to be higher in quiescent than in proliferating cells (data not shown), but they are not statistically significant. The results showed that, similar to proliferating CD34+ BC CML cells, antiapoptotic proteins are also expressed in quiescent CD34+ BC CML progenitor cells.
Inhibition of Bcl-2/Bcl-xL by ABT-737 induces apoptosis in both proliferating and quiescent CD34+ BC CML progenitor cells that is further enhanced by imatinib
To determine the effects of activating apoptosis signaling on the viability of both quiescent and proliferating CD34+ BC CML progenitor cells and determine whether inhibition of Bcl-2/Bcl-xL enhances imatinib’s proapoptotic activity, we treated cells from CML patients (n=8, 7 in BC and 1 in AP) with imatinib, ABT-737, or both in vitro and measured apoptosis induction in proliferating CD34+ progenitor cells and quiescent CD34+ progenitor cells. All but one (who was later treated with various TKIs and did not respond) of these patients had been treated with and had not clinically responded to or had relapsed after treatment with TKIs at the time of sampling. As expected, imatinib had little effect on the viability of proliferating and quiescent CD34+ CML cells in vitro (Figure 2A). ABT-737 alone induced apoptosis not only in proliferating CD34+ CML progenitor cells but also in quiescent CD34+ CML progenitor cells (Figure 2A). Interestingly, when imatinib and ABT-737 were combined, the effects were greatly enhanced (Figure 2A): ABT-737 and imatinib synergistically induced apoptosis in proliferating CD34+ (CI=0.38±0.07) and, most importantly, also in quiescent CD34+ BC CML progenitor cell compartments (CI=0.77±0.17) (Figure 2A, n=8). This effect was also seen in the sample from a BC CML patient with T315I mutation (CIs’ for proliferating and quiescent cells were 0.27±0.09 and 0.30±0.14, respectively for this patient). The apoptosis induction was significantly higher in ABT-737 and imatinib treated cells than in ABT-737 alone treated cells in most doses in proliferating CD34+ CML cells and some doses in quiescent CD34+ CML cells (P < 0.05, * in Figure 2A). We next treated CD34+ cells from normal BM samples (n = 4) with ABT-737, imatinib, or both and determined apoptosis induction in proliferating and quiescent cells. We found that the treatments induced cell death in normal cells but to a significantly lower degree than in CML cells: P values of CML vs. normal BM controls for ABT-737 alone are 0.01 and 0.02 and for ABT-737 and imatinib are <0.001 and 0.01 for proliferating and quiescent CD34+ cells, respectively (Figure 2A).
Figure 2.
Combination of ABT-737 and imatinib synergistically induces apoptosis in proliferating (P) and quiescent (Q) CD34+ progenitor cells from BC CML patients via imatinib-mediated Mcl-1 inhibition. A. Cells from BC CML patients (n=8) and normal BM controls (n=4) were treated with ABT-737, imatinib, or both for 24 hours. Cell death was determined in proliferating CD34+ progenitor cells and quiescent CD34+ progenitor cells. * denotes significant difference between ABT-737 and ABT-737 + imatinib. B. KBM5 and KBM5STI571 cells were treated with ABT-737, imatinib, or both and cell death was determined at 48 hours. C. KBM5 and KBM5STI571 cells were treated with imatinib and protein levels were determined at 48 hours. D. KBM5 and KBM5STI571 cells were treated with Mcl-1 ASO for 4 hours then with ABT-737 and cell death was determined 44 hours after ABT-737 treatment. h, hour; IM, imatinib; ABT, ABT-737; NBM, normal BM.
To understand the mechanism of this synergy, we treated KBM5 and KBM5STI571 cells with imatinib, ABT-737, or both. As expected, ABT-737 or imatinib as single agents induced apoptosis in KBM5 cells, while ABT-737 induced apoptosis, but imatinib had no effect on viability in KBM5STI571 cells (Figure 2B). However, the combination of the two agents was highly synergistic in both KBM5 and KBM5STI571 cells with CI=0.58±0.08 for KBM5 cells and CI=0.028±0.018 for KBM5STI571 cells at 48 hours (Figure 2B). Mcl-1 is a known resistant factor for ABT-737 and is regulated by the Bcr-Abl signaling. We found that imatinib treatment inhibited Bcr-Abl as measured by reduction in p-CrkL levels and decreased Mcl-1 protein levels in KBM5 cells (Figure 2C). Interestingly, we found that imatinib also inhibited CrkL phosphorylation and decreased Mcl-1 protein level in KBM5STI571 cells, although to a much lesser degree (Figure 2C). To demonstrate that decrease in Mcl-1 by TKIs contributes to potentiating ABT-737-induced cell death in CML cells, we treated KBM5 and KBM5STI571 cells with Mcl-1 ASO. Indeed, when Mcl-1 was knocked down at less than 30% (equivalent to its reduction by 1 µM imatinib in KBM5 cells and by 5 µM imatinib in KBM5STI571 cells), ABT-737 induced cell death was enhanced in both in KBM5 and KBM5STI571 cells (Figure 2D).
Combination of ABT-737 and triptolide synergistically induces cell death in both proliferating and quiescent CD34+ BC CML progenitor cells
XIAP suppresses both intrinsic and extrinsic apoptotic pathways. Mcl-1 is a major resistance factor of ABT-737.11 We hypothesized that simultaneous inhibition of Bcl-2/Bcl-xL, Mcl-1, and XIAP would remove four major apoptosis blockers and could maximize apoptosis activation. To test this, we treated CML cells obtained from patients with TKI-resistant CML (n=7, 6 in BC and 1 in AP) with ABT-737 and triptolide, taking advantage of triptolide’s ability to decrease both, XIAP and Mcl-1.16;26 As shown in Figure 3A, triptolide or ABT-737 alone induced apoptosis in all CD34+ BC CML progenitor cells. The combination of ABT-737 and triptolide resulted in significantly increased cell death in proliferating and quiescent CD34+ BC CML cells, with CI values of 0.69±0.13 and 0.47±0.04, respectively, suggesting a high degree of synergism. To support the notion that the synergistic effect of the ABT-737/triptolide combination is at least in part via triptolide-mediated decreases of Mcl-1 and XIAP, we treated KBM5 and KBM5STI571 cells with Mcl-1 or XIAP ASO and found that ABT-737 induced cell death was enhanced by either Mcl-1 (Figure 2D) or XIAP inhibition (Figure 3B) in both KBM5 and KBM5STI571 cells.
Figure 3.
Combination of ABT-737 and triptolide synergistically induces apoptosis in proliferating and quiescent CD34+ progenitor cells from BC CML patients in part via triptolide-mediated decrease of XIAP. A. Cells from BC CML patients (n=7) were treated with ABT-737, triptolide, or both for 24 hours. Cell death was determined in proliferating CD34+ CML progenitor cellsand quiescent CD34+ CML progenitor cells. B. KBM5 and KBM5STI571 cells were treated with XIAP ASO for 4 hours and then with ABT-737. Cell death was determined 44 hours after ABT-737 treatment. Tript, triptolide; ABT, ABT-737.
Discussion
In this study, we demonstrated that the Bcr-Abl signaling pathway is active in the quiescent CD34+ cell compartment in BC CML, that mRNAs encoding antiapoptotic Bcl-2 proteins and XIAP are comparably expressed in quiescent and proliferating CD34+ BC CML progenitor cells, and that activation of apoptosis signaling by inhibition of Bcl-2/Bcl-xL with ABT-737 promotes apoptosis and the apoptosis induction was further enhanced by imatinib via Mcl-1 inhibition and by triptolide in part via reduction of XIAP/Mcl-1 protein levels in proliferating and quiescent CD34+ progenitor BC CML cells, independent of response to TKIs. Hence, these agents may allow eliminating not only the proliferating but also quiescent CD34+ BC CML progenitor cells, both are insensitive to TKIs in BC CML.
We found higher levels of p-CrkL in quiescent than in proliferating CD34+ BC CML progenitor cells reflecting higher Bcr-Abl tyrosine kinase activity in quiescent CD34+ cells than in their proliferating counterparts. Several antiapoptotic proteins have been reported to be regulated by Bcr-Abl signaling. However, we found that mRNAs of antiapoptotic proteins are expressed in quiescent CD34+ BC CML cells, but their levels are not significantly higher than those in their proliferation counterparts. Although we cannot rule out the possibility that protein levels of Bcl-2/Bcl-xL, Mcl-1 or XIAP are higher in quiescent, as compared to proliferating CD34+ BC CML cells, the finding that both cell populations are sensitive to the inhibitors of antiapoptotic proteins supports activation of apoptosis signaling as a strategy to eliminate TKI resistant/refractory CD34+ BC CML progenitor cells.
Interestingly, synergistic apoptosis induction was observed with ABT-737 and imatinib in all CD34+ cell compartments, even in samples from patients who did not respond or were insensitive to TKI-based therapies. We demonstrated that this synergy, at least in part, results from imatinib-mediated Mcl-1 inhibition, even in resistant cells. It needs to be pointed out that many mechanisms contribute to TKI resistance and that cells resistant to TKIs at cell levels may not be completely resistant to TKIs at molecular levels. Minimal inhibition of Bcr-Abl by TKIs leading to slight reduction of Mcl-1 is likely sufficient to synergize ABT-737 even in TKI resistant CML cells. This is supported by our results showing that knockdown less than 30% Mcl-1 protein level sensitized both KBM5 and KBM5STI571 cells to ABT-737. Importantly, we showed that ABT-737 and imatinib combination is significantly more toxic to CML cells than normal control cells. TKIs are clinically proven drugs for CML, and Navitoclax (ABT-263), an orally available derivative of ABT-737, is under phase I/II clinical evaluationsas a single agent for relapsed or refractory chronic lymphocytic leukemia, relapsed or refractory lymphoid malignancies, and advanced small cell lung cancer. This combination strategy holds promise for expedited translation into clinical use for BC CML.
Bcl-2, Bcl-xL, Mcl-1, and XIAP are four main antiapoptotic proteins. ABT-737 is a selective Bcl-2/Bcl-xL antagonist that can induce cell death in all BC CML cell compartments. However, ABT-737 does not antagonize Mcl-1 and has no effect on XIAP. Currently, selective small molecule inhibitors are not available for these two proteins. Triptolide has been found to decrease Mcl-1 and XIAP levels in both CML and AML cells.16;26 Although not specific, this ability, combined with Bcl-2/Bcl-xL inhibition by ABT-737, antagonizes all four potent apoptosis inhibitors and thus contributes to synergistic induction of cell death in BC CML cells. This is further supported by our results showing that knockdown of either XIAP or Mcl-1 enhances ABT-737 induced cell death. However, other mechanisms of triptolide activity could of course contribute to the observed effect. Various derivatives of triptolide are under preclinical and clinical development. A phase I clinical trial with a water-soluble derivative of triptolide in solid tumors is presently ongoing and importantly, a clinical Phase I trial in France has determined the maximal tolerated dose for triptolide derivative F6008 and reported complete remissions in relapsed/refractory AML patients.27
In conclusion, our results demonstrate that activation of apoptosis signaling is a novel and effective strategy to eliminate CD34+ progenitor cells, both proliferating and quiescent, in BC CML, independent of response to TKIs; this approach may allow overcoming a major limitation of TKI-based therapies.
Acknowledgements
We thank Deanna A. Alexander, Janis E. Smith, and Ann M. Sutton for helping with the manuscript.
Supported in part by grants from the US Department of Defense to B.Z.C and from the National Institutes of Health (P01 CA49639, CA55164, and CA16672) to M.A and by the Paul and Mary Haas Chair in Genetics to M.A.
Footnotes
Hyperlink to the published article: http://www.nature.com/leu/journal/v26/n4/pdf/leu2011287a.pdf
Conflict of interest: Authors declare no competing financial interests in relation to this work.
Reference List
- 1.Eaves C, Cashman J, Eaves A. Defective regulation of leukemic hematopoiesis in chronic myeloid leukemia. Leuk Res. 1998;22:1085–1096. doi: 10.1016/s0145-2126(98)00113-1. [DOI] [PubMed] [Google Scholar]
- 2.Copland M, Hamilton A, Elrick LJ, et al. Dasatinib (BMS-354825) targets an earlier progenitor population than imatinib in primary CML but does not eliminate the quiescent fraction. Blood. 2006;107:4532–4539. doi: 10.1182/blood-2005-07-2947. [DOI] [PubMed] [Google Scholar]
- 3.Corbin AS, Agarwal A, Loriaux M, et al. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J.Clin.Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graham SM, Jorgensen HG, Allan E, et al. Primitive, quiescent, Philadelphia-positive stem cells from patients with chronic myeloid leukemia are insensitive to STI571 in vitro. Blood. 2002;99:319–325. doi: 10.1182/blood.v99.1.319. [DOI] [PubMed] [Google Scholar]
- 5.Jamieson CH, Ailles LE, Dylla SJ, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N.Engl.J.Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 6.Holtz MS, Forman SJ, Bhatia R. Nonproliferating CML CD34+ progenitors are resistant to apoptosis induced by a wide range of proapoptotic stimuli. Leukemia. 2005;19:1034–1041. doi: 10.1038/sj.leu.2403724. [DOI] [PubMed] [Google Scholar]
- 7.Holyoake T, Jiang X, Eaves C, Eaves A. Isolation of a highly quiescent subpopulation of primitive leukemic cells in chronic myeloid leukemia. Blood. 1999;94:2056–2064. [PubMed] [Google Scholar]
- 8.Amarante-Mendes GP, Naekyung KC, Liu L, et al. Bcr-Abl exerts its antiapoptotic effect against diverse apoptotic stimuli through blockage of mitochondrial release of cytochrome C and activation of caspase-3. Blood. 1998;91:1700–1705. [PubMed] [Google Scholar]
- 9.Aichberger KJ, Mayerhofer M, Krauth MT, et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1 antisense oligonucleotides. Blood. 2005;105:3303–3311. doi: 10.1182/blood-2004-02-0749. [DOI] [PubMed] [Google Scholar]
- 10.Aichberger KJ, Mayerhofer M, Krauth MT, et al. Low-level expression of proapoptotic Bcl-2-interacting mediator in leukemic cells in patients with chronic myeloid leukemia: role of BCR/ABL, characterization of underlying signaling pathways, and reexpression by novel pharmacologic compounds. Cancer Res. 2005;65:9436–9444. doi: 10.1158/0008-5472.CAN-05-0972. [DOI] [PubMed] [Google Scholar]
- 11.Konopleva M, Contractor R, Tsao T, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Kuroda J, Kimura S, Andreeff M, et al. ABT-737 is a useful component of combinatory chemotherapies for chronic myeloid leukaemias with diverse drug-resistance mechanisms. Br J Haematol. 2007;140:181–190. doi: 10.1111/j.1365-2141.2007.06899.x. [DOI] [PubMed] [Google Scholar]
- 13.Kuroda J, Kimura S, Strasser A, et al. Apoptosis-based dual molecular targeting by INNO-406, a second-generation Bcr-Abl inhibitor, and ABT-737, an inhibitor of antiapoptotic Bcl-2 proteins, against Bcr-Abl-positive leukemia. Cell Death Differ. 2007;14:1667–1677. doi: 10.1038/sj.cdd.4402168. [DOI] [PubMed] [Google Scholar]
- 14.Tauchi T, Sumi M, Nakajima A, et al. BCL-2 antisense oligonucleotide genasense is active against imatinib-resistant BCR-ABL-positive cells. Clin Cancer Res. 2003;9:4267–4273. [PubMed] [Google Scholar]
- 15.Carter BZ, Mak D, Schober WD, et al. Regulation of survivin expression through bcr-abl/MAPK cascade: targeting survivin overcomes Imatinib resistance and increases Imatinib sensitivity in Imatinib responsive CML cells. Blood. 2006;107:1555–1563. doi: 10.1182/blood-2004-12-4704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mak DH, Schober WD, Chen W, et al. Triptolide induces cell death independent of cellular responses to imatinib in blast crisis chronic myelogenous leukemia cells including quiescent CD34+ primitive progenitor cells. Mol.Cancer Ther. 2009;8:2509–2516. doi: 10.1158/1535-7163.MCT-09-0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Radich JP, Dai H, Mao M, et al. Gene expression changes associated with progression and response in chronic myeloid leukemia. Proc.Natl.Acad.Sci.U.S.A. 2006;103:2794–2799. doi: 10.1073/pnas.0510423103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Beran M, Pisa P, O'Brien S, et al. Biological properties and growth in SCID mice of a new myelogenous leukemia cell line (KBM-5) derived from chronic myelogenous leukemia cells in the blastic phase. Cancer Res. 1993;53:3603–3610. [PubMed] [Google Scholar]
- 19.Ricci C, Scappini B, Divoky V, et al. Mutation in the ATP-binding pocket of the ABL kinase domain in an STI571-resistant BCR/ABL-positive cell line. Cancer Res. 2002;62:5995–5998. [PubMed] [Google Scholar]
- 20.Carter BZ, Mak DH, Shi Y, et al. Regulation and targeting of Eg5, a mitotic motor protein in blast crisis CML: overcoming imatinib resistance. Cell Cycle. 2006;5:2223–2229. doi: 10.4161/cc.5.19.3255. [DOI] [PubMed] [Google Scholar]
- 21.Holtz MS, Slovak ML, Zhang F, et al. Imatinib mesylate (STI571) inhibits growth of primitive malignant progenitors in chronic myelogenous leukemia through reversal of abnormally increased proliferation. Blood. 2002;99:3792–3800. doi: 10.1182/blood.v99.10.3792. [DOI] [PubMed] [Google Scholar]
- 22.Itoh K, Tezuka H, Sakoda H, et al. Reproducible establishment of hemopoietic supportive stromal cell lines from murine bone marrow. Exp Hematol. 1989;17:145–153. [PubMed] [Google Scholar]
- 23.Issaad C, Croisille L, Katz A, Vainchenker W, Coulombel L. A murine stromal cell line allows the proliferation of very primitive human CD34++/CD38- progenitor cells in long-term cultures and semisolid assays. Blood. 1993;81:2916–2924. [PubMed] [Google Scholar]
- 24.Konopleva M, Konoplev S, Hu W, et al. Stroma cells prevent apoptosis of AML cells by upregulation of anti-apoptotic proteins. Leukemia. 2002;16:1713–1724. doi: 10.1038/sj.leu.2402608. [DOI] [PubMed] [Google Scholar]
- 25.Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. 27-55. [DOI] [PubMed] [Google Scholar]
- 26.Carter BZ, Mak DH, Schober WD, et al. Triptolide induces caspase-dependent cell death mediated via the mitochondrial pathway in leukemic cells. Blood. 2006;108:630–637. doi: 10.1182/blood-2005-09-3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harousseau JLH, Dombret HD, Pigneux AP, Michallet MM, Brandely MB. Phase I study of F60008, a triptolide derivative, in patients with refractory or relapsing acute leukemias [abstract] 13th Congress of the European Hematology Association (EHA) 2008 [Google Scholar]