Abstract
Inquiry-based laboratory instruction has been shown to actively engage students in the content and skills being taught. These courses are further intended to teach students not only what is known, but also the process by which investigators come to know it. We sought to take this approach one step further and incorporate novel research questions into an inquiry-based laboratory model early in the undergraduate course of study. In this research-based introductory laboratory course, first-year students acquired basic lab skills not just for their own sake, but rather within the context of a research question of a member of the faculty. Student projects investigated potential neuroanatomical changes in animal models of dyslexia and aging and included measurements of neuron numbers and levels and distribution of neuronal proteins. Students played an active role in designing and implementing an experimental plan, explored data analysis techniques, and reflected on the results that they obtained in scholarly forms such as research papers and a departmental poster session. Student feedback on this approach has been extremely positive, and the data collected were research quality preliminary data that are being actively pursued for further study. Based on our encouraging experiences, we conclude that designing an introductory course around novel research, including some assessments modeled after scholarly practices, provides motivation and excitement for the students, instills good scientific habits, and can potentially benefit departmental research.
Keywords: inquiry-based instruction, research-based instruction, neuroanatomy, immunohistochemistry, microscopy, thalamus, auditory cortex, nature of science
Student-driven, inquiry-based science laboratory curricula have received increasing support as an instructional model in order to increase student interest and engagement with course material as well as to increase literacy in the nature of the scientific process and sense of responsibility for the success of their learning (Luckie et al., 2004; Frantz et al., 2006; Weaver et al., 2008). In this model students play an integral role in directing the activities in the lab by asking questions and designing experiments. Often, although the students direct the design of experiments, they are revisiting well-studied scientific questions and typically know that they are doing so (Weaver et al., 2008). Compounding this is the trove of scientific information of variable depth and reliability that is readily available through online sources. Students should evaluate this information before using it, but they may not have the skills to do so. One of the skills needed is a good understanding of how that information has come to be through the process of scientific experimentation. Without this experience in the design and execution of experiments, the ability of students to critically evaluate information is limited.
A variation on the inquiry-based approach involves students participating in novel research projects as part of their undergraduate laboratory courses (NAS Bio 2010), an approach that has received bolstered support with the 2011 AAAS/NSF Vision and Change in Undergraduate Biology Education document. Thus far, these have ranged from brief activities within a general lab course (Birkett, 2009) to partial semester research-based laboratory classes (Weaver et. al., 2006; Weaver et al., 2008) to full- semester courses (Luckie et al., 2004; Frantz et al., 2006; current study). These courses follow the steps for incorporating inquiry put forth by Concannon and Brown (2008). The students are provided with the background knowledge and skills required to formulate questions, design a novel experiment or set of experiments, carry out the experiments under the supervision of the instructional staff for the course, and analyze and reflect on their results. This model, which carries some risks (results are unknown), has been well-received by students, promotes student engagement, and promotes confidence in laboratory science, experiments and research (Weaver et al., 2008; Birkett, 2009). In the biological sciences, these classes are typically reserved for middle or upper division courses, but the largest impact in terms of student engagement, the development of scientific thinking, and the potential for extensive research experiences during college will come from research experiences beginning in the first year.
Introductory biology students at Purdue University are required to complete a one-semester laboratory course that is independent from their introductory biology lecture courses. This course is designed to provide a rigorous treatment of the laboratory skills that will allow students to be successful in their future laboratory courses within the Biology major. While the students are instructed on important laboratory skills, they are not exposed to their application within an actual experimental context. This separation of the skills from their applied use in experimental science is not in line with the current recommendations for undergraduate education which calls for an engagement of students in the process of research as one of six core competencies (Vision and Change, 2011).
In an effort to introduce students to the nature of science early in their college careers, we have sought to increase student excitement and engagement in biology by exposing them to the culture of discovery that incorporates novel research projects into their introductory biology laboratory experience. As part of an NSF-funded project, we have been adapting the CASPiE (Center for Authentic Science Practice in Education) model (Weaver et al., 2006; Weaver et al. 2008) for science laboratories pioneered in the Department of Chemistry at Purdue University to our life sciences curriculum (Bio-CASPiE). In this model, instructional staff work with research faculty to identify novel research projects to use as the basis for teaching students not only the fundamentals of laboratory work (i.e., basic lab skills), but also skills used by scientists as researchers.
In the Fall 2010 offering of Bio-CASPiE, student research projects were driven by research questions about neuroanatomical changes in auditory forebrain structures in an animal model of dyslexia and an animal model of aging. Both of these research models are attractive because they have broad, multifaceted possibilities for research. In addition, the projects have clear “big-picture” problems that the students are working towards rather than perceiving that they are pursuing more esoteric basic research. The auditory thalamus, or medial geniculate body (MGB), is the main sensory input to the auditory cortex, and the MGB can be subdivided further into multiple subdivisions that project to primary or non-primary auditory cortex (Winer, 1992). Despite its pivotal position in the auditory pathway, the role of the MGB in controlling the form and content of neural representations to auditory cortex is often overlooked. Because of this, the MGB and auditory cortex together are fertile ground for the research questions discussed in the next two paragraphs.
Human dyslexics have reading difficulties, often coupled with auditory and other sensory deficits (Fitch et al., 1994; Goswami et al., 2002; Shaywitz and Shaywitz, 2005). Auditory and reading deficits observed in people with dyslexia have been correlated with anatomical abnormalities in the auditory thalamus and cortex (Galaburda et al., 1985, 1994; Livingstone et al., 1991; Escabi et al., 2007). Specifically, the brains of dyslexics often contain cortical malformations known as microgyria that are correlated with a decrease in the number of large cells in the auditory thalamus (Galaburda et al., 1994; de Vasconcelos Hage et al., 2006). Similar cortical malformations can be induced experimentally in rats by placing a freezing probe on the skull overlying somatosensory cortex in early postnatal rats (Fitch et al., 1994; Herman et al., 1997; Rosen et al., 2006; Escabi et al., 2007). Microgyric rats exhibit anatomical abnormalities in the auditory thalamus and auditory processing deficits similar to dyslexic humans (Herman et al., 1997; Clark et al., 2000; Peiffer et al., 2002; 2004). Therefore, microgyric rats serve as an excellent experimental model to determine the neuronal differences in the auditory thalamus and auditory cortex that contribute to the observed deficits in auditory processing. There remains a major knowledge gap regarding the cellular alterations of neurons in the auditory pathway that would underlie the behavioral deficits.
A similar knowledge gap exists for understanding cellular level changes in the aging auditory system. Auditory deficits are present in a growing population of millions of elderly listeners in the USA alone (NIDCD). Deficits are most evident during situations in which there are competing sounds or when the cues to discriminate sounds are weak (Schneider et al., 2005). Much of the research focus has been on understanding cochlear degeneration, but the mechanisms and consequences of age on central auditory structures are not nearly as well understood despite numerous anatomical changes (Caspary et al., 1990; Tadros et al., 2007). Earlier studies have revealed relatively subtle changes in the neural responses of aged animals that do not seem to capture the extent of behavioral difficulties (Shaddock Palombi et al., 2001; Walton et al., 2002). At the anatomical level, one consistent finding across central auditory regions is that markers of inhibitory GABAergic activity decline with age, either when measuring the enzyme for GABA synthesis (Ling et al., 2005) or GABA itself (Caspary et al., 1990). In fact, in the cerebral cortex, this age-related decrease has been shown to be selective for auditory cortex while adjacent parietal cortex showed no such decline (Ling et al., 2005). Therefore, we will be evaluating age-related changes in neural activity in the context of decreased GABAergic inhibition, though other mechanisms will be considered if they fit the data better and more broadly. By determining how MGB responses have been altered by aging, it will be possible to isolate subcortical changes from cortical changes. Moreover, in terms of evaluating a reduction in GABAA receptors in central auditory nuclei as an underlying cause of functional deficits, the MGB has a GABAergic input pattern that is unique among sensory systems (Peruzzi et al., 1997; Bartlett and Smith, 1999), but the distribution and functionality of those receptors is unknown for the aged or microgyric rat MGB.
MATERIALS AND METHODS
Student populations
Thirteen first semester freshman (nine women and four men) enrolled in the research-based introductory biology laboratory took part in the activities and research. Students self-selected into this class, but needed to indicate their intentions of majoring in biology by being concurrently enrolled in the introductory Biology lecture course and the first semester of introductory inorganic Chemistry. The laboratory class met once a week for four hours for the duration of the semester (15 weeks).
Introductory biology students who were not taking the research-based, Bio-CASPiE course formed the comparison population for pre- and post-semester attitudinal surveys.
All data from students have been collected and analyzed in accordance with protocols approved by the Institutional Review Board at Purdue University.
Individual and team assessment
Students were grouped into teams of 3–4 students on the first day of class by the instructor based on responses to online personality and learning styles inventories (Myers-Briggs [http://www.humanmetrics.com/cgi-win/JTypes2.asp] and VARK [http://www.vark-learn.com/english/index.asp]). An effort was made to group together students of varying personalities and learning styles. The students worked with their team throughout the duration of the semester. To facilitate good teamwork and to identify potential problems within groups, the students performed a self and peer evaluation within their teams three times during the semester. This allowed the students to thoughtfully reflect on their performance within the team as well as their teammates’ performances. Students gave themselves a score of 1–4 in the categories of: preparation, technical proficiency, and teamwork (see supplemental material). Each person received a score out of 12 points which was the average of all of scores given for each evaluation.
Assessment by the instructor of student performance in the class took two forms: standard classroom assessments and assignments modeled after scholarly activity. Standard classroom assessments included weekly laboratory notebook checks, weekly quizzes on background information for the week, and participation in the lab activities. Scholarly activity-based assessments included three guided discussions of primary and secondary research literature, three lab reports written in scientific paper format, a research proposal worksheet, a chalk talk research report of preliminary data, and a public poster presentation at the end of the semester (Figure 1) that was attended by Biology faculty and graduate students. Students were evaluated individually on all of the assessments except the research proposal, chalk talk, and poster design and presentation.
Figure 1.
Scholarly activity-based assessments. Similar colors indicate how a general scholarly activity was realized as specific class activities.
Organization of the semester
The semester was roughly divided into thirds with the first five weeks being devoted to skills and knowledge building exercises and activities, the middle seven weeks being devoted to the independent research projects, and the last three weeks being devoted to data analysis and presentation.
During the skills building weeks, students worked individually and with their teams to acclimate to working in a lab and to develop the necessary lab skill set and background knowledge to proceed into the research portion of the semester. Each week the students were provided with a laboratory manual, written by the instructor, which included background information relevant to the topic of the course and with specific details for the experiments and procedures being used that week.
On the first day of class students performed sheep brain dissections to become familiar with basic neuroanatomy, with emphasis on the location of the auditory brain structures (auditory thalamus and auditory cortex) that they would be analyzing in their projects. In addition, students started working with bright field microscopy to learn the basics of operation and function. The students practiced viewing and making observations of prepared slides of brain tissue, using an eyepiece graticule and stage micrometer to measure neuronal soma diameters, etc. The second week of class moved forward to the basics of image analysis using ImageJ software (http://rsbweb.nih.gov/ij/) (gridding and random selection of analysis fields across several brain sections, manual and automated cell counts, measuring neuronal soma diameters, and optical density measurements). The data generated from neuronal cell counts and measurement was used to teach the students about basic descriptive and inferential statistics. During weeks 3–5, students built towards being able to understand and perform immunohistochemical experiments. Students learned how to properly use a micropipetter and to perform dilutions with distilled water and food coloring. A blood typing activity with simulated blood (Wards Scientific) provided the students with an opportunity to visually observe antibody-antigen interactions and to design and test hypotheses about these interactions with a quick and reliable system. Finally, students performed a Nissl stain and immunohistochemical stain of rat brain sections (VGLuT2 and calbindin staining, see methods below).
With the background knowledge and skills acquired in the first part of the semester, student teams were asked to formulate hypotheses and design an experiment to investigate the effects of either aging or an induced cortical malformation (microgyria) on neuron number and/or the distribution of some neuronal proteins in the rat auditory thalamus or primary auditory cortex. Students were provided with previously sectioned rat brain tissue mounted onto microscope slides and were to use combined Nissl staining and immunohistochemistry in their study. They had access to the following antibodies: GAD 65/67, VGluT2, calbindin, GABAA receptor (α 1 subunit). Students were given six weeks to perform their staining twice and analyze the results. Students were kept blind to the identity of the tissue (young vs. old or operated vs. sham) until all analyses were complete. Drawing upon their experience during the skills building weeks and with continued guidance from the instructors, students performed all of the tissue processing and data analysis themselves
In addition to the development of laboratory techniques and the planning and execution of experiments, students were given several opportunities to organize, analyze, and summarize their results and present them in written and oral formats.
Tissue processing and image acquisition
All animals were handled and used in accordance with Purdue Animal Use and Care Committee (PACUC) guidelines. Students worked with rat brain tissue that had been harvested and prepared for sectioning in the Bartlett laboratory. Briefly, the rats were euthanized with Euthasol and perfused transcardially with phosphate buffered saline (PBS) followed by 4% paraformaldehyde. The brains were removed and cryoprotected with 30% sucrose in PBS. Brains were blocked into hemispheres, and the spinal cord and olfactory bulbs were removed. Brains were then embedded in OCT embedding medium (Ted Pella). The auditory thalamus and primary auditory cortex were frozen sectioned by the instructors at 30 microns, mounted directly onto slides (Superfrost Plus microscope slides, Fisher Scientific), and stored frozen until needed (Park and Cunningham, 2007).
Students performed Nissl staining and most steps for immunohistochemistry during the course of one laboratory period. Students prepared all of the reagents used for the Nissl stain. Nissl staining was performed in EasyDip™ slide staining system (Electron Microscopy Sciences) and followed standard procedure with demyelination, staining, destaining, and dehydration steps (Paul et al.,1997; Cold Spring Harbor protocols).
Immunohistochemical staining consisted of a primary antibody with the ABC detection method with a DAB (diaminobenzidine) substrate (Vector Laboratories) following similar procedures to those used for free floating brain sections (Graziano et al., 2008). Students prepared all reagents and determined and performed all dilutions necessary for each step along the process on their laboratory day. In a previous laboratory period, each student group had prepared a 10X PBS stock solution that was diluted to 1X for use in the immunostaining procedure (final concentrations in mM: 154 NaCl, 6.6 KH2PO4, 1 NaH2PO4·H2O, pH 7.4). On the day preceding the laboratory period, the instructional staff initiated the staining process by blocking the tissue (5% normal goat serum in PBS + 0.25% TritonX-100) and incubating the sections in the appropriate dilution of primary antibody (overnight at 20 degrees C) in slide processing chambers (Ted Pella). Primary antibodies and their dilutions in blocking solution were: Calbindin 1:500 (mouse, Sigma), GAD65/67 1:1000 (rabbit, Millipore), VGluT2 1:500 (guinea pig, Millipore), GABAA receptor α 1 subunit 1:1000 (rabbit, Millipore). The primary antibody was omitted from the blocking solution on one slide for each group and served as a negative control for antibody specificity.
The students picked up the staining process the next day with washing off the primary antibody. A biotinylated secondary antibody against the appropriate species was used (1:200 dilution; Vector Laboratories). Following incubation in secondary antibody and washes in PBS, the ABC method was used to visualize the antibody localization (Vectastain Elite ABC kit, Vector Laboratories) with DAB (diaminobenzidine) as the substrate (Vector DAB substrate kit, Vector Laboratories) for the peroxidase enzyme which produced a brown reaction product. Students empirically determined the appropriate reaction times for this step (7–10 minutes for the tissue processed directly on slides). The stained tissue was taken through a dehydration series by the instructional staff. Both Nissl stained and antibody-labeled tissue were coverslipped using Permount mounting medium (Fisher Scientific).
DAB was chosen as the peroxidase substrate for visualization because we wanted to be able to easily compare our results with those published by others in the field. Using DAB as the chromogen as used in the manner described here (with amplification) has limitations for quantitative measurements of antigen numbers (Matkowskyj et al., 2003). However, we are interested in relative optical densities and our stained structures had average pixel intensities of <100 on an 8 bit scale (0 is black and 255 is white) which we feel is still in the linear range (See supplemental Figure 1 for an example histogram of pixel intensity distribution). In addition, DAB is a suspected carcinogen. Students were instructed on the proper handling of the DAB and the instructors removed all DAB waste and neutralized it (3% potassium permanganate and 2% sodium carbonate) before disposal. All work with DAB was carried out with the use of gloves and the slides were processed on top of disposable underpads (Med Vet International) to prevent contamination of bench surfaces.
Images to be used for analysis were captured by the instructor and students using a compound microscope and digital camera (AMscope Clinic Vet Laboratory Trinocular Microscope 40X-2000X, Model T490B and 8 MegaPixel USB 2.0 Microscope Color Digital Camera, Model MD1800). Digital images were analyzed by the students using ImageJ software and included use of the ‘analyze particles’, ‘cell counter’, ‘histogram’ (gray values of selected area for optical density) and ‘measure and label’ tools and plugins (Ferreira and Rasband, 2010). Students were instructed on how to adjust the brightness and contrast and thresholds of their images, as needed.
Peer-Led Team Learning (PLTL) workshops
Students in the class were divided into two groups that met with a student peer leader for five workshops during the semester. The workshops allowed the students to explore topics and concepts relevant to the activity of research in a setting that was not graded. The peer leaders were upperclassmen Biology majors chosen based on their knowledge of the basic research topic of the class and their involvement in the previous offering of a Bio-CASPiE lab (Spring 2010, microbiology). The material covered in the workshops was meant to complement and extend concepts and activities covered in the course. The topics were: how to keep a laboratory notebook, how to write a scientific paper, how to read a research article, and two workshops on ethical conduct in science. Each workshop required minimal outside preparation from the students and consisted of activities that students worked on in pairs and as a whole group (6–7 students). The workshops were all adapted from workshops designed and implemented in CASPiE labs in the chemistry department (Varma-Nelson et al., in preparation).
Evaluation of the effectiveness of the course
Anonymous responses to the online institutional end of the semester student evaluations were gathered to determine student satisfaction with the course and evaluate student feedback in the free response area within the evaluation.
In addition, an attitudinal survey consisting of 37, Likert type items regarding student experiences in their previous Biology laboratory classes was administered to Bio-CASPiE and non-Bio-CASPiE students before their participation in their respective biology laboratory classes. A similar survey consisting of the same items was administered to the students at the end of their fall 2010 biology laboratory experiences. Response categories for the items on the attitudinal survey were: 6 = strongly agree, 5 = agree, 4 = barely agree, 3 = barely disagree, 2 = disagree, 1 = strongly disagree. Thirty-nine students responded to the pre-survey and 32 students responded to the post-survey. However, there were only 12 successfully matched pre-post pairs (four Bio-CASPiE and eight non-Bio-CASPiE). Due to the small sample size, ordinal structure of response categories, and non-normal distribution of the data, a non-parametric statistical method was employed to examine differences between the two groups. Specifically, Mann-Whitney U analyses were conducted to evaluate group differences in the median scores on the pre- and post-participation survey items.
RESULTS
Direct application of basic skills and acquisition of research-related skills
Similar to laboratory courses at other large universities, the traditional introductory biology laboratory course offered to freshmen at Purdue University focuses on the acquisition of a skill set that will be useful to students as they progress through their coursework as Biology majors. The research-based approach to introductory biology laboratories aims to provide students with the same necessary skills, but with an immediate application of the skills to a research project that the students are engaged in. These skills include the proper use of a balance, making molar and percent solutions, proper micropipetting technique, performing dilutions, using a pH meter, using a compound microscope and performing descriptive and inferential statistics. The learning of these skills occurs during the skills-building portion of the semester before the students embark on their research projects.
Because of the research theme of this laboratory course, students in the Bio-CASPiE lab acquire some research-related skills that are not explicitly taught in the traditional laboratory course (Table 1).
Table 1.
Research-related skills acquired by students in introductory biology laboratory courses
| Research-related skills | Introductory Biology laboratory course | |
|---|---|---|
| Traditional lab | Bio-CASPiE lab | |
| Keeping a lab notebook | X | X |
| “Design and refine” experimental design and data analysis | X | |
| Writing lab reports in scientific paper format | X | |
| Reading primary and secondary literature | X | |
| Chalk talk of preliminary results | X | |
| Poster design and presentation | X | |
| Descriptive and inferential statistics | X | X |
| Teamwork | X | |
Student-directed experiments and analysis
One of the primary goals of this research-based approach to introductory biology laboratory courses is to increase student engagement in the coursework. In an effort to facilitate this, throughout the semester, students were given the opportunity to design several aspects of their experiments and data analysis, discovering the limitations of their design and being able to refine their approach during the semester. The first opportunity for this “design and refine” approach came with the data analysis for their first lab report. They had been given basic instruction and guidance for making neuron counts and measuring neuron soma diameters from Nissl stained brain sections from the instructors, but each team was responsible for coming up with the specific methods that were to be used by every member of their group. Each team member was responsible for making measurements that would be pooled by the group to form their data set to report on. In the lab report the students were asked to identify potential limitations of their analysis and propose ideas for improving their approach. Two common themes emerged from these reflections: 1) imperfect standardization of analysis techniques across all team members (criteria for distinguishing neurons from glia in Nissl stains and uniform measuring of least and greatest neuron diameters) and 2) small sample size of neurons counted/area and number of brain sections analyzed. Based on these reflections, the students were asked to reconvene with their teammates and refine their data analysis procedure to create a standardized, reproducible technique that could be employed. They were then asked to re-write their lab reports using the new analysis procedure that they designed.
The second area where students were responsible for the direction of their work in the class came during the independent experiment section of the course. Student teams completed an experiment proposal worksheet written by the instructor (supplemental material) to assist them in the experimental design process. The worksheet consisted of the following elements: 1) Relevant background information, 2) Statement of their research question, 3) Rationale and hypothesis to be tested, and 4) the basic experimental protocol to test the hypothesis (what will be quantified, the test conditions, controls, number of repetitions, and the type of statistical analysis to be used). Each team met with the instructor to discuss their proposal and to converge onto a set of experiments that could be finished within the rest of the semester. There were four teams in the class and the projects topics were: 1) Neuron numbers in the medial geniculate body in young and aged rats, 2) Distribution of GABAA α1 receptor subunit in the auditory cortex of young and aged rats, 3) Distribution of VGLuT2 and GAD65/67 in the medial geniculate body of young and aged rats, and 4) Distribution of calbindin in the medial geniculate body and primary auditory cortex in microgyric rats.
Products from the research projects
Research teams worked with instructors in the course to design and carry out analysis of Nissl and immunostained sections of rat auditory brain structures. The types of quantitation included neuron cell body counts and/or optical density measurements of antibody labeling in their experimental and control rate brain tissue. These data formed the basis for two lab reports, a chalk talk on their preliminary findings (supplementary material), and the final poster at the end of the semester. Through these assignments students could explore various means of organizing their data for visual presentation (tables, bar graphs, images of stained brain tissue). Examples of student-generated figures from each of the projects are shown in Figures 3–6.
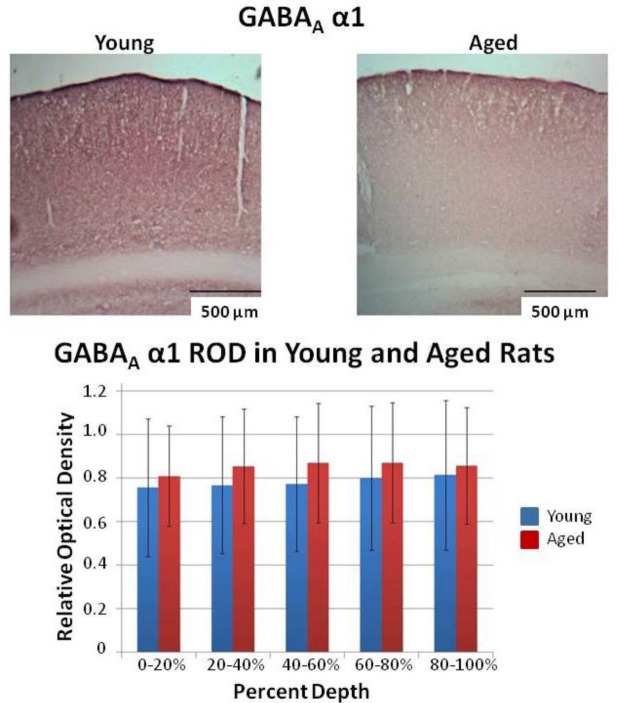
Figure 3.
Example of student quantitation and presentation of immunohistochemical data. Example images from immunostaining of auditory cortex with the GABAA α1 subunit. The bar graph is a summary of average ± SD of the relative optical density of sections of cortex measured as % depth from the cortical surface to the deep white matter. There was a significant difference between young and old animals across all depths (p<0.05, Mann-Whitney test).
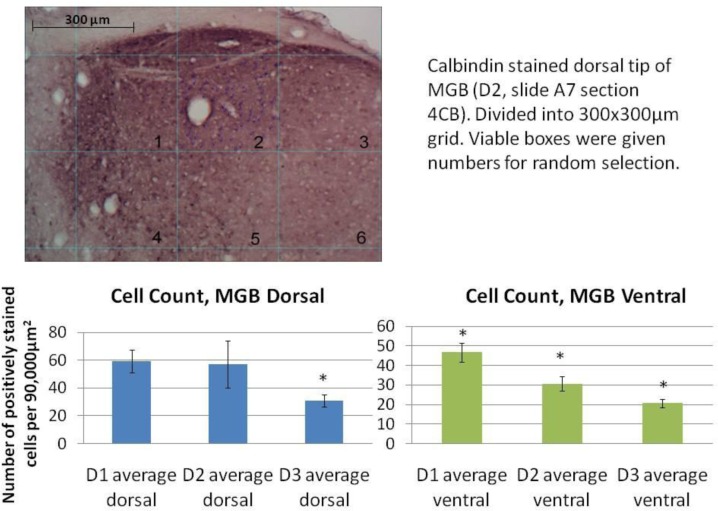
Figure 6.
Example of student quantitation and presentation of calbindin-positive neuronal counts in the MGB in microgyric and sham-operated rats. The top image shows calbindin-stained MGB in a control rat gridded for cell count analysis. It is accompanied by the student-written figure legend. At the bottom is the neuron count quantitation in experimental (D1 and D3) and control (D2) rats. The D3 rat had a verified cortical malformation and impaired auditory function. The D1 rat did not show differences from control rats in auditory function and no cortical malformations were observed. Calbindin-positive neuron counts were significantly different in the dorsal MGB between experimental (D3) and animals with normal auditory function (D1 and D2 (p< 0.01, t test). All three animals had significantly different numbers of calbindin-positive neurons in the ventral MGB (p<0.05, t test).
The individual lab reports and team chalk talk helped students organize their data, evaluate it, and provided check-points for feedback from the instructor. At the end of the semester, each team organized their project and its data into a poster that was presented to the public.
Evaluation of the effectiveness of the research-based introductory biology laboratory model
Course evaluations of students in the CASPiE course and attitudinal pre/post surveys of CASPiE and non-CASPiE students formed the basis of evaluation of the CASPiE model of introductory biology laboratory courses. Standard course evaluations completed by the CASPiE students at the end of the semester indicate that the students felt that the course was an excellent learning experience (Table 2).
Table 2.
Student responses to institutional course evaluations. [E] Excellent=5 [G] Good=4 [F] Fair=3 [P] Poor=2 [VP] Very Poor
| Question | Response (mean ± SD) |
|---|---|
| Overall, I would rate this course as: | 4.9 ± 0.29 |
| As a learning experience, this course was: | 4.7 ± 0.45 |
| The amount that I learned in this course in proportion to the amount of work that I put into it was: | 4.5 ± 0.66 |
| As a learning experience the PLTL workshops were: | 3.9 ± 0.79 |
| The guided discussions of research papers were: | 4.4 ± 0.48 |
| As a learning experience the poster presentations were: | 4.7 ± 0.46 |
| Number of respondents | 11 of 13 |
Student feedback in the free response portion of the course evaluations was generally very positive and included the following comments: “Overall, the class was very well organized. I didn’t feel overwhelmed with information, but was still able to start the semester with no research experience and end the semester having completed a research project that was interesting and that I can be proud of. I also liked starting the first day of class by dissecting sheep brains.” and “Excellent class and excellent professor that put A LOT of time and effort into the course. I wish I would have worked harder myself throughout the semester so that I would have got even more from it.” Most constructive criticisms of the course were related to specific activities in it and not the model of the class itself. One student remarked, referring to the first day of class: “In the future, especially for freshmen taking this class I think it would be really nice just to get to talk before the first class, because it was a very intimidating experience.”
Introductory biology students enrolled in the Bio-CASPiE lab and students enrolled in the traditional, skills- based lab were asked to complete an online survey at the beginning and the end of the semester to evaluate their attitudes about their experiences in their most recent laboratory courses. The pre-semester survey probed their experiences in laboratory courses prior to that semester and the post-semester survey probed their experiences in the laboratory courses that they were enrolled in during the fall of 2010 using a Likert scale for responses. Only data from students who completed the pre and the post semester surveys (matched pairs) were included in the analysis of the data. There were no significant differences in the responses on the pre-semester survey between CASPiE and non-CASPiE students, suggesting that the two populations were not significantly different with respect to their past laboratory course experiences (data not shown). However, there was a significant difference between CASPiE and non-CASPiE students for several response categories on the post semester survey (Table 3).
Table 3.
Group differences in post-participation survey responses.
| Bio-CASPiE participants | Bio-CASPiE non-participants | ||||
|---|---|---|---|---|---|
| Items | Mean | S.D. | Mean | S.D. | U test |
| 1. I gained a better understanding of the process of scientific research. | 6.00 | 0.00 | 4.62 | 1.27 | 21.00* |
| 2. The lab experiences were very similar to real research. | 6.00 | 0.00 | 4.27 | 1.34 | 15.00* |
| 3. The lab experiences made me realize I could do science research in a real science lab (for instance, at a college or with a pharmaceutical company). | 6.00 | 0.00 | 4.38 | 1.33 | 15.00* |
| 4. Lab experiments presented real science to students, similar to what scientists do in real research labs. | 6.00 | 0.00 | 4.23 | 1.42 | 12.00* |
| 5. I better understood the ideas of biology, in general, as a result of completing the experiments. | 5.83 | 0.41 | 4.40 | 1.47 | 25.50* |
| 6. I believe I could accurately explain a biology experiment from the course to other student. | 6.00 | 0.00 | 4.84 | 1.18 | 24.00* |
| 7. The lab experience made me more interested in biology. | 5.83 | 0.41 | 4.40 | 1.66 | 31.50* |
| 8. The lab experience made me more interested in science. | 6.00 | 0.00 | 4.48 | 1.64 | 24.00* |
| 9. Finding answers to real research questions motivated me to do well in the biology lab. | 5.83 | 0.41 | 4.32 | 1.55 | 22.50* |
| 10. Even if I don’t end up working in a science related job, the laboratory experience in the most recent biology course I took will still benefit me. | 5.83 | 0.41 | 4.56 | 1.39 | 23.50* |
| 11. The concepts covered in the laboratory were relevant to the real world. | 6.00 | 0.00 | 4.56 | 1.45 | 21.00* |
U test = Mann-Whitney U Statistics;
denotes p < 0.05
DISCUSSION
Student engagement in research
Undergraduate research experiences have increasingly become among the minimum necessary requirements for admission into graduate schools (PhD programs as well as medical and veterinary school). However, these experiences are varied depending on the expectations of both the research mentor and the student. In a review of faculty-mentored research experiences, Wilson et al. (2011), identified different categories of experiences that depend on the perceived goal of the experience as viewed by the research faculty: 1) retention or selection 2) research exposure 3) advanced learning or de facto streaming 4) developing scientific research skills 5) thinking like a researcher and 6) entry into the research culture. These categories lie along a continuum with respect to the role of the student in the research project and process ranging from largely passive (1 and 2) to a more active participant (4–6). As a result of these varied goals, the degree of active involvement of the students in the design and execution of the experiments and enjoyment of the experiences and learning is also highly variable (Howitt et al., 2010).
The advantage of the Bio-CASPiE laboratories is that students are provided with methodical and consistent guidance by the instructors while still giving the students the freedom to direct some aspects of their experiments and data analysis. This structured experience with novel research has thus far been a positive experience for the students, instructors and research faculty (see below). This is a departure from most of their experiences prior to the class where labs have consisted of exercises with known outcomes and can be unsettling to some students. Students are encouraged to embrace the unknown as an exciting challenge. A further goal of the present study is to present a developed and assessed course so that faculty are also encouraged to embrace the unknown in their courses as an exciting challenge rather than a daunting hurdle.
Research quality data
The students in the neuroanatomy Bio-CASPiE course were able to generate good quality preliminary data for the collaborating research faculty along several different lines of inquiry within the scope of the general research questions developed for the class. Some of the brains used in the undergraduate research were from animals whose electrophysiological responses to sound were recorded (Parthasarathy et al., 2010). Many of these data will be followed up in the Bartlett lab, with summer research students (directed by Dr. Gardner), and in the next offering of the neuroanatomy course in the fall of 2011. In addition, we are including some of the CASPiE student data in an abstract for the upcoming Society for Neuroscience meeting, including the students in the trip to the meeting and presentation of the data.
As is the nature of any research program, the projects done by students in all of the Bio-CASPiE classes will evolve as data are generated and new hypotheses are formulated. We have already begun to experience the evolving nature of the projects and progress made by the teams of undergraduate researchers this spring with the second offering of our microbiology Bio-CASPiE course. The data gathered by students in the spring of 2010 has been pursued in the lab of the researcher (LN Csonka) and by the new crop of CASPiE students. The current students have been inspired by the fact that they are further analyzing and contributing to projects initiated by their fellow Biology majors in the previous class!
Limitations and future directions
Our initial experiences have been very positive and the students involved have expressed their enthusiasm for the course in course evaluations and indicated that the course had a positive impact on their attitudes surrounding laboratory science. However, there are limitations of the approach we have taken with respect to its broad adoption in undergraduate curricula. This approach is time-intensive in its development and implementation during the semester and requires dedicated instructional staff. The staff needs to familiarize themselves with the research topics and techniques as well as consult with the collaborating research faculty before and during the semester. In this sense, it helps to have TAs whose research is similar to that being used in the course. Encouraging research faculty and their postdoctoral fellows, graduate students, or experienced undergraduates to be directly involved in delivering the class would alleviate this. This would provide an excellent teaching experience for the postdoctoral students and/or graduate students because they would have a greater connection to the work done in the class and this would likely translate into their investment in quality instruction. Repeating the course with similar topics to those previously offered allows the instructional staff to improve on past courses, see the projects evolve, and reduce the time and effort needed to start a brand new topic.
We have chosen ambitious projects for our students to become involved in, but feel that scaled-back versions would be highly valuable experiences for the students and reduce the cost of the lab course/student. For example, an approach similar to the one used by Birkett (2009) in which students had access to previously-stained brain tissue could be used. This would decrease the cost of the course as the expensive reagents, like antibodies and tissue would be incurred by the research faculty within the normal course of their research program. Students could perform some staining of other cell types or a small amount of brain tissue, to get the practical experience to connect them to the research. In many cases, there may be data that a faculty member has fully or partially collected but has not had the time to complete the data collection and analysis.
We have thus far only had the opportunity to evaluate the near-term impact that taking this research-based course has had on student learning and attitudes about laboratory science. We are currently following the alumni of these classes to determine what lasting impact this experience has had on their attitudes about laboratory science, the likelihood of seeking out other undergraduate research experiences, their retention within the biology major, their performance in courses within the biology major, and their career goals and plans.
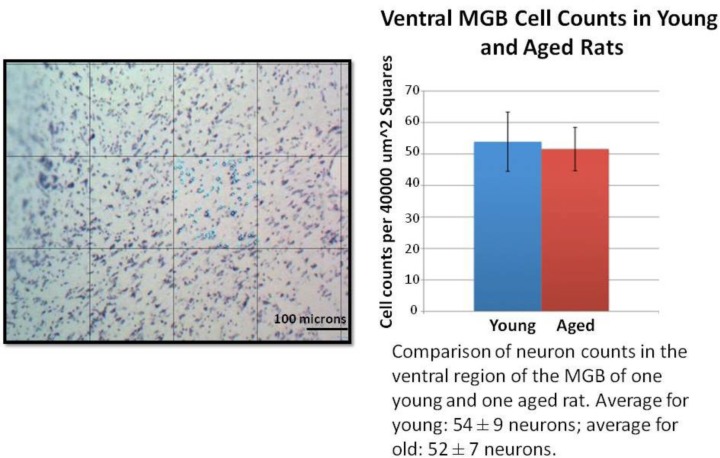
Figure 4.
Example of student quantitation and presentation of neuron counting data in the MGB in young and aged animals. On the left is an example image of Nissl stained ventral MGB with a 200x200 micron grid superimposed on it. The students randomly selected a grid square to analyze and repeated this for multiple sections of tissue/animal. An average neuron count/area was calculated for each animal. The graph on the right is a summary of average neuron counts/ area in young and aged animals (mean ± SD, n=1 animal in each age group) with the student-written figure legend. There was no significant difference between young and aged animals (p>0.05, t test).
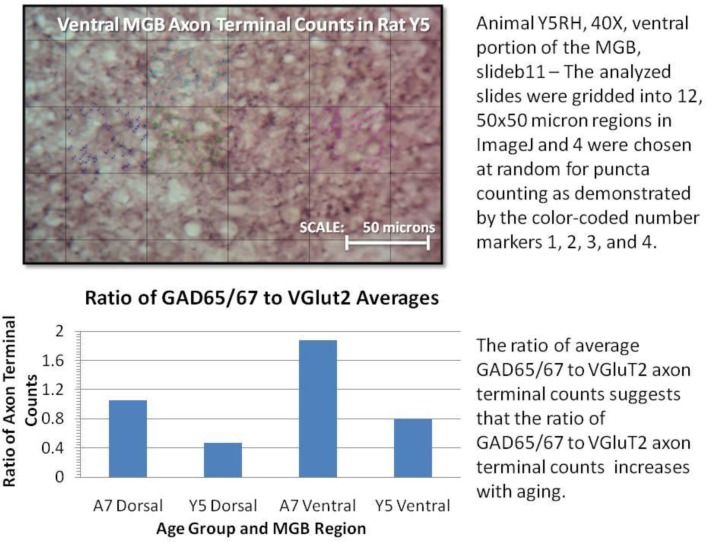
Figure 5.
Example of student quantitation and presentation of VGluT2 and GAD 65/67 axon terminals with student-written figure legends. Students counted puncta from randomly-selected grid squares across multiple tissue sections to get average axon terminal densities. A ratio of the terminal densities was calculated as a means to assess the balance of excitatory and inhibitory inputs to neurons in the MGB in young and aged rats.
Footnotes
This work was supported by funding from the National Science Foundation (CCLI/TUES Grant Number 104399), National Organization for Hearing Research (to E.L.B.) and the International Dyslexia Association (to E.L.B). The authors thank teaching assistants/interns Stephen Chabot, Samuel Conover, Stephanie Karozos, and Annamarie Bustion and the students in Biology 19500 (Fall 2010) for their hard work and feedback on this class. In addition, many thanks to the staff at the DLRC and to Dennis Minchella for his support.
Supplemental Materials
Plan for Independent Experiment- Worksheet (30pts): This worksheet is to be completed by your group. It is designed to help you through the experimental design process by forcing you to be very deliberate in the way you approach designing the experiment to test your hypothesis. I will read this and we will discuss it in lab.
We acknowledge equal effort toward this assignment and approve of its contents:
______________ ______________ ______________
Chalk talk on preliminary findings :: (20 pts)
This presentation is meant to give you an opportunity to organize and interpret your preliminary findings and communicate them to the class. You should put together a brief Powerpoint presentation (5–6 slides) to do this. I want you to pay particular attention to describing what your stain labels and what that shows and also are there any promising trends in the data (see instructions in the text below). Please refer to the Guide to Figures and Graphs handout to help you decide on the appropriate way to best display your data and consult with me for any inferential/test statistics you could perform.
You will be assessed according to the following rubric where:
| Metric | Outcomes | Score | |||
| Presentation (3 pts each) |
√ □ - □ x □ o □ Rationale and hypothesis |
√ □ - □ x □ o □ Basic experimental approach |
√ □ - □ x □ o □ Description of the figure(s) |
√ □ - □ x □ o □ Conclusion and future direction |
/12 |
| Figure(s) (1.5 pt each) |
√ □ - □ x □ o □ Appropriate display |
√ □ - □ x □ o □ Labeled axes and title |
√ □ - □ x □ o □ Descriptive and inferential/test statistics |
√ □ - □ x □ o □ Visually accessible |
/6 |
| Oral Communication (1 pts each) |
√ □ - □ x □ o □ Appropriate detail |
√ □ - □ x □ o □ Clarity |
/2 | ||
| Total | /20 |
√ = 100% v = 75% - = 50% o = 0%
Relevant background and rationale- state what information you know, have learned, or gathered regarding the model you wish to use (aging or dyslexia (physical model)), the brain region (inferior colliculus, medial geniculate body, and/or auditory cortex), and parameters that you can measure (cell size, density, axons, synapses, etc) that leads you to your question. You should cite papers you/we have read.
Question - The question should logically flow from the background information you present.
Hypothesis - your take-a-stand statement about how things will be based on your observations and reading.
Experimental protocol – you need to talk about what histological method(s) that you are going to use and why. You have Nissl stain and immunohistochemistry as options (refer to the table in lab 5 for antibodies to choose from). There are several types of controls to consider. There are whole animal level controls, how you will actually do the staining, and immunohistochemical controls (negative and positive), for example. Be specific about what you are actually going to measure (cell number, size, axon terminal sizes, etc.)
(8pts) Relevant background information and rationale
(2pts) Question
(2pts) Rationale and hypothesis
-
(18pts) Experimental protocol - PLEASE BE VERY SPECIFIC ABOUT YOUR EXPERIMENTAL CONDITIONS AND WHAT YOU ARE GOING TO MEASURE!!!
Experiment/procedure to test your hypothesis (please describe this very carefully with the manipulation, how many animals, how sections/cells, etc.)
control condition/comparison condition
what is to be quantified and descriptive statistics (inferential statistics, if appropriate) (be very specific in what you are going to measure and what are you going to specifically compare)
-
Presentation
-
Rationale and hypothesis (1 slide for bullet point rationale and 1 slide for hypothesis)
-
The rationale for performing the experiment
State any previous observations you have made in lab that led you to ask the question you are asking
-
provide background information that leads to your hypothesis
review papers we discussed
other ideas?
-
-
Basic experimental approach (1 slide of bullet points)
A very brief overview of what was manipulated and measured in the experiment
Describe what your stain labels and what that might tell you
-
Description of figure (s) (1 slide or more, depending on data presentation)
A walk-through of the figure saying what is plotted as a function of what and a statement of the conclusion
-
Future direction (1 slide of bullet points)
-
What would you do next?
repeat the same experiment?
change?
-
-
-
Figure(s)
-
Appropriate display – REMEMBER: A well-designed graph is better than a table with numbers!
Use of a bar graph, scatter plot, or line graph appropriate to the data
-
Labeled axes and title
Descriptive title for the graph that summarizes the main finding and axes that are clearly labeled with units
-
Descriptive and inferential/test statistics
Plots of averages with standard deviations (descriptive statistics) are preferred to single observation data points. If appropriate, control data should be plotted on the same graph and any results of test statistics (t test) should be noted on the figure (ex. An asterisk over data points that are statistically different from control values).
-
Visually accessible
Is the main finding clear by eye to the observer
-
-
Oral Communication
Appropriate detail
-
Clarity
Information communicated clearly
Pixel intensity distribution for VGluT2 staining. Example of a pixel intensity distribution from VGluT2 immunostaining in the MGB (200x200 micron area). This staining is the darkest staining we observed in the class.
REFERENCES
- Bartlett EL, Smith PH. Anatomic, intrinsic, and synaptic properties of dorsal and ventral division neurons in rat medial geniculate body. J Neurophysiol. 1999;81:1999–2016. doi: 10.1152/jn.1999.81.5.1999. [DOI] [PubMed] [Google Scholar]
- Birkett MA. Every cell counts: An inquiry-based approach to address a novel research question in an undergraduate neuroscience lab. J Undergrad Neurosci Ed. 2009;7:A53–A64. [PMC free article] [PubMed] [Google Scholar]
- Caspary DM, Raza A, Lawhorn Armour BA, Pippin J, Arnerić SP. Immunocytochemical and neurochemical evidence for age-related loss of GABA in the inferior colliculus: implications for neural presbycusis. J Neurosci. 1990;10:2363–2372. doi: 10.1523/JNEUROSCI.10-07-02363.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark MG, Rosen GD, Tallal P, Fitch RH. Impaired processing of complex auditory stimuli in rats with induced cerebrocortical microgyria: An animal model of developmental language disabilities. J Cogn Neurosci. 2000;12:828–839. doi: 10.1162/089892900562435. [DOI] [PubMed] [Google Scholar]
- Committee on Undergraduate Biology Education to Prepare Research Scientists for the 21st Century. Bio2010: Transforming Undergraduate Education for Future Research Biologists. Washington, DC: The National Academies Press; 2010. [PubMed] [Google Scholar]
- Concannon JP, Brown PL. Transforming osmosis labs to address standards for inquiry. Science Activities. 2008;45:23–25. [Google Scholar]
- de Vasconcelos Hage SR, Cendes F, Montenegro MA, Abramides DV, Guimarães CA, Guerreiro MM. Specific language impairment: linguistic and neurobiological aspects. Arq Neuropsiquiatr. 2006;64:173–180. doi: 10.1590/s0004-282x2006000200001. [DOI] [PubMed] [Google Scholar]
- Escabí MA, Higgins NC, Galaburda AM, Rosen GD, Read HL. Early cortical damage in rat somatosensory cortex alters acoustic feature representation in primary auditory cortex. Neuroscience. 2007;150:970–983. doi: 10.1016/j.neuroscience.2007.07.054. [DOI] [PubMed] [Google Scholar]
- Ferreira TA, Rasband W. The ImageJ user guide, version 1.43. 2010.
- Fitch RH, Tallal P, Brown CP, Galaburda AM, Rosen GD. Induced microgyria and auditory temporal processing in rats: a model for language impairment? Cereb Cortex. 1994;4:260–270. doi: 10.1093/cercor/4.3.260. [DOI] [PubMed] [Google Scholar]
- Frantz KJ, DeHaan RL, Demetrikopoulos MK, Carruth LL. Routes to research for novice undergraduate neuroscientists. Cell Bio Educ. 2006;5:175–187. doi: 10.1187/cbe.05-09-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galaburda AM, Sherman GF, Rosen GD, Aboitiz F, Geschwind N. Developmental dyslexia: four consecutive patients with cortical anomalies. Ann Neurol. 1985;18:222–233. doi: 10.1002/ana.410180210. [DOI] [PubMed] [Google Scholar]
- Galaburda AM, Menard MT, Rosen GD. Evidence for aberrant auditory anatomy in developmental dyslexia. Proc Natl Acad Sci U S A. 1994;91:8010–8013. doi: 10.1073/pnas.91.17.8010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goswami U, Thomson J, Richardson U, Stainthorp R, Hughes D, Rosen S, Scott SK. Amplitude envelope onsets and developmental dyslexia: A new hypothesis. Proc Natl Acad Sci U S A. 2002;99:10911–10916. doi: 10.1073/pnas.122368599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziano A, Liu XB, Murray KD, Jones EG. Vesicular glutamate transporters define two sets of glutamatergic afferents to the somatosensory thalamus and two thalamocortical projections in the mouse. J Comp Neurol. 2008;507:1258–1276. doi: 10.1002/cne.21592. [DOI] [PubMed] [Google Scholar]
- Herman AE, Galaburda AM, Fitch RH, Carter AR, Rosen GD. Cerebral microgyria, thalamic cell size and auditory temporal processing in male and female rats. Cereb Cortex. 1997;7:453–464. doi: 10.1093/cercor/7.5.453. [DOI] [PubMed] [Google Scholar]
- Howitt SM, Wilson AN, Wilson KF, Roberts P. ‘Please remember we are not all brilliant’: undergraduates’ experiences of an elite, research-intensive degree at a research-intensive university. Higher Education Research & Development. 2010;29:405–420. [Google Scholar]
- Ling LL, Hughes LF, Caspary DM. Age-related loss of the GABA synthetic enzyme glutamic acid decarboxylase in rat primary auditory cortex. Neuroscience. 2005;132:1103–1113. doi: 10.1016/j.neuroscience.2004.12.043. [DOI] [PubMed] [Google Scholar]
- Livingstone MS, Rosen GD, Drislane FW, Galaburda AM. Physiological and anatomical evidence for a magnocellular defect in developmental dyslexia. Pro natl Acad Sci U S A. 1991;88:7943–7947. doi: 10.1073/pnas.88.18.7943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckie DB, Maleszewski JJ, Loznak SD, Krha M. Infusion of collaborative inquiry throughout a biology curriculum increases student learning: a four-year study of “Teams and Streams”. Adv Physiol Educ. 2004;28:199–209. doi: 10.1152/advan.00025.2004. [DOI] [PubMed] [Google Scholar]
- Matkowskyj KA, Cox R, Jensen RT, Benya RV. Quantitative immunohistochemistry by measuring cumulative signal strength accurately measures receptor number. J Histochem Cytochem. 2003;51:205–214. doi: 10.1177/002215540305100209. [DOI] [PubMed] [Google Scholar]
- Park JJ, Cunningham MG. Thin sectioning of slice preparations for immunohistochemistry. J Vis Exp. 2007;3:194. doi: 10.3791/194. Epub 2007 Apr 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parthasarathy A, Cunningham PA, Bartlett EL. Age-related differences in auditory processing as assessed by amplitudemodulation following responses in quiet and in noise. Front Aging Neurosci. 2010;2:152. doi: 10.3389/fnagi.2010.00152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul CA, Beltz B, Berger-Sweeney J. The Nissl stain: A stain for cell bodies in brain sections. In: Paul, et al., editors. Adapted from Discovering neurons: The experimental basis of neuroscience. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 1997. [Google Scholar]
- Peiffer AM, McClure MM, Threlkeld SW, Rosen GD, Fitch RH. Severity of focal microgyria and associated rapid auditory processing deficits. Neuroreport. 2004;15:1923–1926. doi: 10.1097/00001756-200408260-00018. [DOI] [PubMed] [Google Scholar]
- Peiffer AM, Rosen GD, Fitch RH. Rapid auditory processing and MGN morphology in microgyric rats reared in varied acoustic environments. Brain Res Dev Brain Res. 2002;138:187–193. doi: 10.1016/s0165-3806(02)00472-8. [DOI] [PubMed] [Google Scholar]
- Peruzzi D, Bartlett E, Smith PH, Oliver DL. A monosynaptic GABAergic input from the inferior colliculus to the medial geniculate body in rat. J Neurosci. 1997;17:3766–3777. doi: 10.1523/JNEUROSCI.17-10-03766.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen GD, Mesples B, Hendriks M, Galaburda AM. Histometric changes and cell death in the thalamus after neonatal neocortical injury in the rat. Neuroscience. 2006;141:875–888. doi: 10.1016/j.neuroscience.2006.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BA, Daneman M, Murphy DR. Speech comprehension difficulties in older adults: cognitive slowing or age-related changes in hearing? Psychol Aging. 2005;20:261–271. doi: 10.1037/0882-7974.20.2.261. [DOI] [PubMed] [Google Scholar]
- Shaddock Palombi P, Backoff PM, Caspary DM. Responses of young and aged rat inferior colliculus neurons to sinusoidally amplitude modulated stimuli. Hear Res. 2001;153:174–180. doi: 10.1016/s0378-5955(00)00264-1. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Shaywitz BA. Dyslexia (specific reading disability) Biol Psychiatry. 2005;57:1301–1319. doi: 10.1016/j.biopsych.2005.01.043. [DOI] [PubMed] [Google Scholar]
- Tadros SF, D’Souza M, Zettel ML, Zhu X, Waxmonsky NC, Frisina RD. Glutamate-related gene expression changes with age in the mouse auditory midbrain. Brain Res. 2007;1127:1–9. doi: 10.1016/j.brainres.2006.09.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma-Nelson P, Bentley A, Weaver GC, Wink D. Peer-led team learning for undergraduate research: a Handbook for implementation, unpublished manuscript [Google Scholar]
- Walton JP, Simon H, Frisina RD. Age-related alterations in the neural coding of envelope periodicities. J Neurophysiol. 2002;88:565–578. doi: 10.1152/jn.2002.88.2.565. [DOI] [PubMed] [Google Scholar]
- Weaver GC, Wink DJ, Varma-Nelson P, Lytle F, Morris R, Fornes W, Russell CB, Boone WJ. Developing a new model to provide first and second-year undergraduates with chemistry research experience: Early findings of the Center for Authentic Science Practice in Education (CASPiE) Chem Educ. 2006;11:129–125. [Google Scholar]
- Weaver GC, Russell CB, Wink DJ. Inquiry-based and research-based laboratory pedagogies in undergraduate science. Nat Chem Biol. 2008;4:577–580. doi: 10.1038/nchembio1008-577. [DOI] [PubMed] [Google Scholar]
- Wilson AN, Howitt SM, Wilson KF, Roberts P. Academics’ perceptions of the purpose of undergraduate research experiences in a research-intensive degree. Studies in Higher Education. 2011 (in press) [Google Scholar]
- Winer JA. The functional architecture of the medial geniculate body and the primary auditory cortex. In: Fay RR, Popper AN, Webster DB, editors. The mammalian auditory pathway: Neuroanatomy. New York: Springer-Verlag; 1992. pp. 222–409. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Plan for Independent Experiment- Worksheet (30pts): This worksheet is to be completed by your group. It is designed to help you through the experimental design process by forcing you to be very deliberate in the way you approach designing the experiment to test your hypothesis. I will read this and we will discuss it in lab.
We acknowledge equal effort toward this assignment and approve of its contents:
______________ ______________ ______________
Chalk talk on preliminary findings :: (20 pts)
This presentation is meant to give you an opportunity to organize and interpret your preliminary findings and communicate them to the class. You should put together a brief Powerpoint presentation (5–6 slides) to do this. I want you to pay particular attention to describing what your stain labels and what that shows and also are there any promising trends in the data (see instructions in the text below). Please refer to the Guide to Figures and Graphs handout to help you decide on the appropriate way to best display your data and consult with me for any inferential/test statistics you could perform.
You will be assessed according to the following rubric where:
| Metric | Outcomes | Score | |||
| Presentation (3 pts each) |
√ □ - □ x □ o □ Rationale and hypothesis |
√ □ - □ x □ o □ Basic experimental approach |
√ □ - □ x □ o □ Description of the figure(s) |
√ □ - □ x □ o □ Conclusion and future direction |
/12 |
| Figure(s) (1.5 pt each) |
√ □ - □ x □ o □ Appropriate display |
√ □ - □ x □ o □ Labeled axes and title |
√ □ - □ x □ o □ Descriptive and inferential/test statistics |
√ □ - □ x □ o □ Visually accessible |
/6 |
| Oral Communication (1 pts each) |
√ □ - □ x □ o □ Appropriate detail |
√ □ - □ x □ o □ Clarity |
/2 | ||
| Total | /20 |
√ = 100% v = 75% - = 50% o = 0%
Relevant background and rationale- state what information you know, have learned, or gathered regarding the model you wish to use (aging or dyslexia (physical model)), the brain region (inferior colliculus, medial geniculate body, and/or auditory cortex), and parameters that you can measure (cell size, density, axons, synapses, etc) that leads you to your question. You should cite papers you/we have read.
Question - The question should logically flow from the background information you present.
Hypothesis - your take-a-stand statement about how things will be based on your observations and reading.
Experimental protocol – you need to talk about what histological method(s) that you are going to use and why. You have Nissl stain and immunohistochemistry as options (refer to the table in lab 5 for antibodies to choose from). There are several types of controls to consider. There are whole animal level controls, how you will actually do the staining, and immunohistochemical controls (negative and positive), for example. Be specific about what you are actually going to measure (cell number, size, axon terminal sizes, etc.)
(8pts) Relevant background information and rationale
(2pts) Question
(2pts) Rationale and hypothesis
-
(18pts) Experimental protocol - PLEASE BE VERY SPECIFIC ABOUT YOUR EXPERIMENTAL CONDITIONS AND WHAT YOU ARE GOING TO MEASURE!!!
Experiment/procedure to test your hypothesis (please describe this very carefully with the manipulation, how many animals, how sections/cells, etc.)
control condition/comparison condition
what is to be quantified and descriptive statistics (inferential statistics, if appropriate) (be very specific in what you are going to measure and what are you going to specifically compare)
-
Presentation
-
Rationale and hypothesis (1 slide for bullet point rationale and 1 slide for hypothesis)
-
The rationale for performing the experiment
State any previous observations you have made in lab that led you to ask the question you are asking
-
provide background information that leads to your hypothesis
review papers we discussed
other ideas?
-
-
Basic experimental approach (1 slide of bullet points)
A very brief overview of what was manipulated and measured in the experiment
Describe what your stain labels and what that might tell you
-
Description of figure (s) (1 slide or more, depending on data presentation)
A walk-through of the figure saying what is plotted as a function of what and a statement of the conclusion
-
Future direction (1 slide of bullet points)
-
What would you do next?
repeat the same experiment?
change?
-
-
-
Figure(s)
-
Appropriate display – REMEMBER: A well-designed graph is better than a table with numbers!
Use of a bar graph, scatter plot, or line graph appropriate to the data
-
Labeled axes and title
Descriptive title for the graph that summarizes the main finding and axes that are clearly labeled with units
-
Descriptive and inferential/test statistics
Plots of averages with standard deviations (descriptive statistics) are preferred to single observation data points. If appropriate, control data should be plotted on the same graph and any results of test statistics (t test) should be noted on the figure (ex. An asterisk over data points that are statistically different from control values).
-
Visually accessible
Is the main finding clear by eye to the observer
-
-
Oral Communication
Appropriate detail
-
Clarity
Information communicated clearly
Pixel intensity distribution for VGluT2 staining. Example of a pixel intensity distribution from VGluT2 immunostaining in the MGB (200x200 micron area). This staining is the darkest staining we observed in the class.