Abstract
Cardiorenal syndrome (CRS) type II is a serious condition in which chronic cardiac abnormalities cause worsening kidney function, leading to permanent chronic kidney damage. Management of CRS type II coupled with diuretic-resistant congestive heart failure (CHF) has been an issue of dispute. However, since the early 1990s, reports indicating the clinical usefulness of peritoneal dialysis (PD) as maintenance therapy for intractable CHF in this population have been accumulating. The present manuscript reviews the mechanisms by which kidney dysfunction develops within CHF, and then examines recent experiences of PD as chronic supportive therapy for intractable CRS type II, reviews the contributing mechanisms, and discusses the rationale for using PD as a new therapeutic approach in the nonuremic setting of CHF.
Key words: Congestive heart failure, cardiorenal syndrome type II, nonuremic indications
With an increasing number of patients worldwide developing both congestive heart failure (CHF) and chronic kidney disease (1-3), the co-existence of these two conditions has become a matter of concern. Congestive heart failure often accompanies decreased kidney function, and chronic kidney disease worsens pre-existing CHF. Cardiorenal syndrome (CRS) is a concept that classifies patients with cardiac and kidney dysfunction into four clinical types according to the basic mechanisms of the respective disorders (4):
Type I: abrupt worsening of cardiac function leads to acute kidney injury
Type II: chronic cardiac abnormalities cause worsening kidney function, leading to permanent chronic kidney damage
Type III: abrupt worsening of kidney function leads to acute cardiac injury
Type IV: chronic kidney disease causes chronic cardiac load, leading to permanent chronic cardiac damage
Regardless of type, CRS is a vicious cycle that results in clinical worsening of both kidney and cardiac function.
Ultrafiltration (UF) is a powerful nonpharmacologic, extracorporeal intervention for diuretic-resistant CHF (5). This therapy is best used in patients with acute decompensated heart failure (ADHF) in CRS types I and III. In contrast, the role of UF as a chronic maintenance therapy is less well known in patients with CRS types II and IV, except for uremic patients with end-stage kidney disease who have CRS type IV. Management of CRS types II and IV—and particularly CRS type II with diuretic-resistant CHF—has been an issue of dispute (6). However, since the early 1990s, reports indicating the clinical usefulness of peritoneal dialysis (PD) as maintenance therapy for intractable CHF in this population have been accumulating (7,8).
The present manuscript first reviews the clinical types of CHF and the mechanism by which kidney dysfunction develops within that disorder. It then examines recent experiences of PD as chronic supportive therapy for intractable CRS type II, reviews the contributing mechanisms, and discusses the rationale for using PD as a new therapeutic approach in the nonuremic setting of CHF.
CHF AND KIDNEY DYSFUNCTION
Pathophysiology of the Development of Kidney Dysfunction and CRS type II: Heart failure (HF) is clinically classified into two types: systolic and diastolic (9). Systolic HF is characterized primarily by decreased ejection fraction. It is often complicated by lesions from coronary ischemia. A therapeutic approach using beta-blockers and angiotensin-converting enzyme inhibitors has been established. Diastolic HF is characterized by decreased diastolic capacity, with relatively preserved ejection fraction; it is a common clinical occurrence that is complicated by aging, diabetes, and uremia. Notably, no pharmacologic agents have been proved effective for diastolic HF (9,10), and an increased risk of death in diastolic compared with systolic HF has been reported for patients complicated with CRS type II (11).
In terms of factors leading to the development of kidney dysfunction in CRS type II, several mechanisms have been proposed. The first is altered intrarenal hemodynamics. One of the primary physiologic responses in patients with CHF is maintenance of circulating volume against low cardiac output. During the course of CHF, the following pathologic process is hypothesized (12,13): In the early stage of HF, elevation of renin secretion and angiotensin II increases cardiac afterload because of arterial vasoconstriction. The sympathetic nervous system is activated by elevated angiotensin II, and baroreceptors in the aorta and aortic arch are activated because of hypoperfusion. Those two changes result in vasoconstriction of the afferent arterioles in the kidney, leading to enhancement of sodium reabsorption in the proximal tubules. On the other hand, serum arginine vasopressin markedly increases because of nonosmotic baroreceptor-mediated release from the posterior pituitary, activating water reabsorption via V2 receptors in the collecting duct (14). In addition, increased arginine vasopressin stimulates the V1a receptors of the vascular smooth muscle cells, leading to vasoconstriction of the arterial and venous systems and an increase of preload and afterload (12). These nervous and hormonal changes all accelerate to shift intrarenal hemodynamics from the superficial to the juxtamedullary nephrons (15). The latter change increases oxygen consumption in the thick ascending limb of Henle, rendering this hypoxia-susceptible area at increased risk of hypoxic injury and thus development of tubular damage by prolonged hypoperfusion (16).
The second factor is renal congestion. The extent of congestive symptoms in CHF does not necessarily correlate with total fluid volume, and it is known that symptoms develop even in patients who are not overhydrated. The mechanism of congestion includes changes in preload and afterload to the heart caused by increased vascular resistance or decreased reservoir volume of the vasculature, or both. Patients with CHF often have increased arterial resistance and stiffness, and decreased reservoir capacity may also be involved (17). Recent studies have highlighted the clinical significance of kidney congestion as a crucial factor for the exacerbation of kidney function in CHF (18,19). It has been shown that central venous pressure, rather than cardiac output, is closely linked to serum creatinine levels in patients with CRS type II (18), indicating that kidney congestion leads to a decrease in renal plasma flow. Based on these insults triggered by CHF, ischemic lesions might develop in the kidneys. In fact, interstitial inflammatory cellular infiltration and increased fibrosis in the medulla, but only faint abnormalities in the glomerulus, are noted in chronic CHF (20), indicating the significant role of persistent hypoperfusion and hypoxia in the medulla as pathologic factors in the development of CRS type II (21).
Critique of Diuretics and UF Method for CRS type II: Based on the aforementioned pathologies, several clinical factors should be considered in the management of CHF.
The first is the adverse effects of diuretics. The loop diuretic furosemide has been a mainstay in relieving the vicious pathologic cycle of CHF, although evidence supporting its use is lacking. It is supposed that furosemide helps to ameliorate congestive symptoms by decreasing excess fluid accumulation and by directly increasing the venous reservoir (22), a unique ability that helps to improve congestive symptoms before diuresis starts. However, some metabolic adverse effects of diuretics such as hypokalemia and hyperuricemia (23) have to be addressed. Those adverse effects may potentially contribute to excess morbidity and mortality in CHF by increasing the risk for progressive decline in kidney function. Furthermore, in excess, diuretics could activate the renin-angiotensin and the sympathetic nervous systems, which adversely alleviate diuretic effects by decreasing delivery of furosemide to the tubules (24). The unfavorable effects of diuretics on these refractory patients require use of a treatment strategy other than diuretics.
The second clinical point to be addressed is the practical aspect of the extracorporeal UF method. As mentioned, the UF method plays a pivotal role in treating diuretic-resistant refractory HF, particularly in patients with ADHF. Silverstein et al. originally used extracorporeal UF in the mid-1970s to treat severe fluid overload (25). Since then, the application of venovenous hemofiltration in patients with ADHF has become common. Recently, a randomized controlled study—the UNLOAD trial—showed the clinical significance of UF compared with diuretic therapy with respect to a reduction in unplanned hospitalizations after treatment of ADHF (26). However, it is not realistic to apply the UF modality outside of hospital as a supportive therapy to prevent worsening of CHF without hampering the patient’s quality of life at home.
PD APPLICATION IN CHF
Clinical Outcomes of PD for CRS Types II and IV with CHF: In contrast to UF, PD is not an established therapeutic option for CHF. However, the therapeutic history of PD for treating CHF is longer than that of venovenous hemofiltration. Schneierson (27) originally applied peritoneal irrigation to treat intractable edema of cardiac origin during the 1940s. Since then, accumulating case series have shown a benefit of PD in managing CHF (28-53), although most studies remain descriptive and do not reveal the precise mechanisms of correcting and preventing CHF.
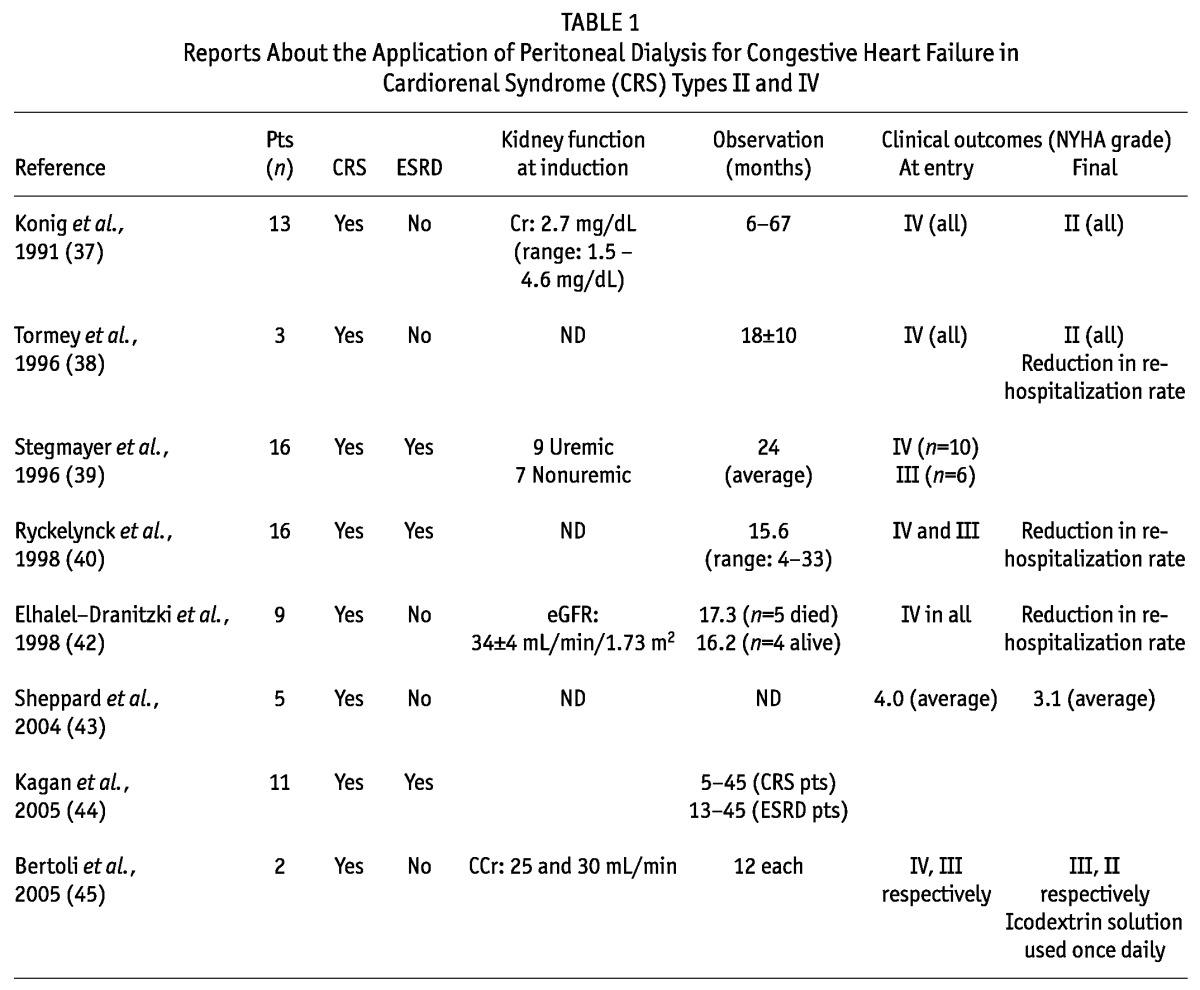
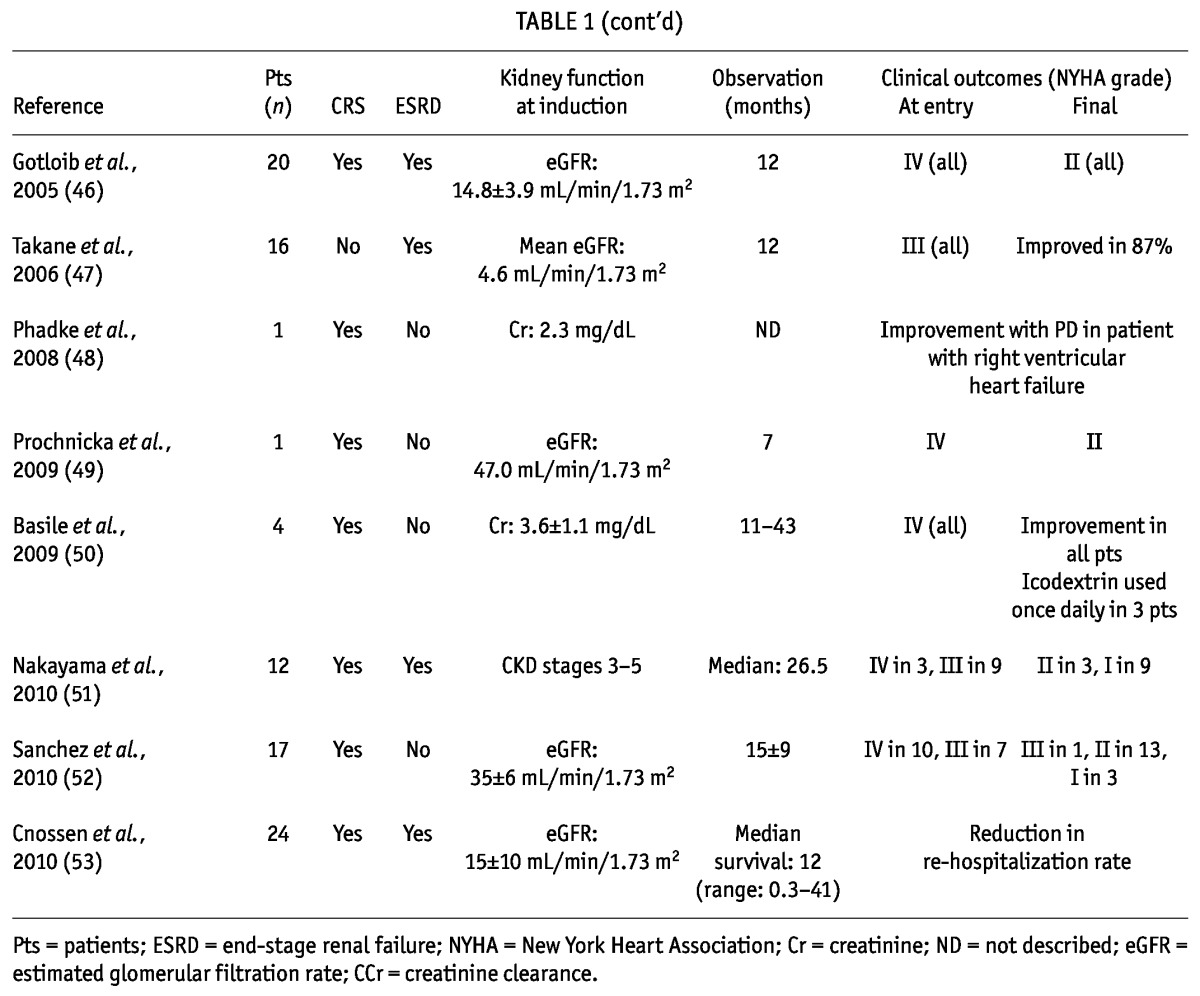
Case series in which PD was applied are classified into two clinical groups by kidney function (Table 1): patients with end-stage renal disease (39,40,43,46,47,51,53) and nonuremic pre-dialysis patients (37-40,42-46,48-53). The former group includes patients with CRS type IV, and case reports for those patients indicate that PD induction can benefit end-stage renal disease patients complicated with severe CHF. With respect to the clinical impact of PD in chronic dialysis patients, a conflicting series was reported from France, showing poorer outcomes in PD patients with CHF than in hemodialysis patients (54). However, that study was a retrospective analysis, and no adjustment was made for clinical CHF score. In addition, the inclusion criteria for severe CHF and the preferential use of PD for CHF patients because hemodialysis was inconvenient in some cases could have affected the findings. Thus, the clinical role of PD for end-stage renal disease patients (CRS type IV), particularly those with severe CHF, should be separately discussed. On the other hand, the nonuremic pre-dialysis patients included some with CRS type II. At an outpatient clinic, PD was applied in those nonuremic patients to manage refractory congestive symptoms. Surprisingly, most patients recovered from a severely ill state (New York Heart Association III/IV) to regain their ability to take part in standard activities of daily living (New York Heart Association I/II). The clinical benefits have been very promising from the viewpoints of reduced hospitalization (38,40,42,53), reduction in the need for diuretics (43), and notably, improved quality of life (39,41,42,52).
TABLE 1.
Reports About the Application of Peritoneal Dialysis for Congestive Heart Failure in Cardiorenal Syndrome (CRS) Types II and IV
Mechanism of Therapeutic Action of PD: The possible mechanisms of clinical improvement in HF through PD seem to be multifactorial. First, PD continuously draws ultrafiltrate; its physiologic effect therefore has a lesser risk of abrupt hypotension that would exaggerate organ hypoxia and kidney damage. Second, UF in PD is driven by the osmotic power of the PD solution (glucose or glucose polymer) indwelling within the peritoneal cavity which is drained through the extended network of microvessels in the visceral and parietal peritoneum (55,56). This unique process of ultrafiltrate driving fluid from the compartment of microvessels to the peritoneal cavity might decrease the amount of interstitial edema. That mechanism notably highlights the potential of PD to mimic a physiologic state similar to that of expanded peripheral microvessel capacity and increased vascular compliance. This characteristic of PD closely matches the therapeutic principle of CHF treatment: that is, to lessen kidney and central venous congestion. Third, the metabolic effects of PD therapy—such as glucose load from the solution, and correction of acidosis—favor the correction of nutrition and anemia. In fact, the notion of cardiorenal-anemia syndrome was proposed based on clinical experiences in PD patients (57). Fourth, the removal of proinflammatory factors (for example, tumor necrosis factor α and cardiac depressant factor) into the PD fluid might improve cardiac function (58). Finally, PD preserves residual kidney function by slowing fluid removal, leading to less stimulation of the renin-angiotensin system or the sympathetic nervous system, or both (51,52).
In respect to Na removal, excretion of excess Na from the body is supposed to play a crucial role in reducing congestive symptoms (59). In most patients, furosemide renders urinary Na levels hyponatric within the 60 - 70 mmol/L range (60). In addition, PD renders UF fluid hyponatric because of the sodium-sieving effect of the peritoneum through aquaporin (61). However, the ultrafiltrate Na level is about 100 mmol/L (62), which is higher than that in urine produced by furosemide. That difference might contribute to the correction of excess Na retention in patients with CHF.
Rationale for PD in a Nonuremic Indication for CHF: Accumulating case series have indicated the clinical merit of PD in patients with CHF, and this unique and important contribution of PD may play a significant palliative role for these severely ill patients, as indicated by the report that hope among hospitalized patients with CHF is stronger than it is among healthy subjects (63). In addition, use of PD may benefit patients by preventing decline into end-stage renal failure, which results in excess hospitalization and related costs.
Regarding the PD prescription, icodextrin solution is a long-acting osmotic agent that allows the patient’s UF volume to gradually increase for up to 12 hours (55,64) and might contribute to a PD-based therapeutic strategy for CHF (45,50). In nonuremic patients, the simple use of this solution once daily could benefit patients or caregivers by reducing their burden at home. With respect to a pragmatic way to achieve isonatric UF removal by PD, hyponatric PD solution permits isonatric fluid removal (65) and is expected to be studied in patients with CHF (66).
After a review of the reported case series, it appears that PD-related complications such as peritonitis, malnutrition because of protein loss, increased intra-abdominal pressure, and socio-economic influences are minimal. Thus, it seems that the benefits of PD therapy outweigh its potential risks.
In consideration of the growing number of patients with CRS type II, studies including prospective analyses to elucidate the clinical significance and possible risk of PD in patients with CHF are warranted. The results of such studies may expand the role of PD in the coming decades as a novel therapeutic modality for severe CHF.
DISCLOSURES
The author has no financial conflicts of interest to declare.
Acknowledgments
The information in this review is based on the experience of the author and literature accumulated over several years. PubMed was searched using various combinations of the terms “congestive heart failure,” “ultrafiltration,” and “peritoneal dialysis.” The author selected mainly English-language articles published since 2000 and searched the references within those articles for further relevant publications.
The author expresses special thanks to Professor Yoshindo Kawaguchi (Shiomidai Hospital, The Jikei University School of Medicine, Tokyo) and Dr. Hirofumi Nakano (Kashima Hospital, Dialysis Center, Iwaki) for reviewing the article and providing valuable comments.
References
- 1. Roger VL, Go AS, Lloyd-Jones DM, Adams RJ, Berry JD, Brown TM, et al. on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2011 update: a report from the American Heart Association. Circulation 2011; 123:e18–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lysaght MJ. Maintenance dialysis population dynamics: current trends and long-term implications. J Am Soc Nephrol 2002; 13(Suppl 1):S37–40 [PubMed] [Google Scholar]
- 3. National Kidney Foundation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis 2002; 39(Suppl 1):S1–266 [PubMed] [Google Scholar]
- 4. Ronco C, Haapio M, House AA, Anavekar N, Bellomo R. Cardiorenal syndrome. J Am Coll Cardiol 2088; 52:1527–39 [DOI] [PubMed] [Google Scholar]
- 5. Kazory A, Ross EA. Ultrafiltration for decompensated heart failure: renal implications. Heart 2009; 95:1047–51 [DOI] [PubMed] [Google Scholar]
- 6. Jois P, Mebazaa A. Cardio-renal syndrome type 2: epidemiology, pathophysiology, and treatment. Semin Nephrol 2012; 32:26–30 [DOI] [PubMed] [Google Scholar]
- 7. Mehrotra R. Peritoneal dialysis in adult patients without end-stage renal disease. Adv Perit Dial 2000; 16:67–72 [PubMed] [Google Scholar]
- 8. Próchnicka A, Olszowska A, Baczyński D, Zelichowski G, Wańkowicz Z. Peritoneal dialysis as a therapeutic approach in congestive heart failure resistant to pharmacological treatment. Pol Arch Med Wewn 2009; 119:815–19 [PubMed] [Google Scholar]
- 9. Jessup M, Brozena S. Heart failure. N Engl J Med 2003; 348:2007–18 [DOI] [PubMed] [Google Scholar]
- 10. Aurigemma GP, Gaasch WH. Clinical practice. Diastolic heart failure. N Engl J Med 2004; 351:1097–105 [DOI] [PubMed] [Google Scholar]
- 11. Ahmed A, Rich MW, Sanders PW, Perry GJ, Bakris GL, Zile MR, et al. Chronic kidney disease associated mortality in diastolic versus systolic heart failure: a propensity matched study. Am J Cardiol 2007; 99:393–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sarraf M, Schrier RW. Cardiorenal syndrome in acute heart failure syndromes. Int J Nephrol 2011; 2011:293938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sarraf M, Masoumi A, Schrier RW. Cardiorenal syndrome in acute decompensated heart failure. Clin J Am Soc Nephrol 2009; 4:2013–26 [DOI] [PubMed] [Google Scholar]
- 14. Schrier RW, Berl T, Anderson RJ. Osmotic and nonosmotic control of vasopressin release. Am J Physiol 1979; 236:F321–32 [DOI] [PubMed] [Google Scholar]
- 15. Whitehouse T, Stotz M, Taylor V, Stidwill R, Singer M. Tissue oxygen and hemodynamics in renal medulla, cortex, and corticomedullary junction during hemorrhage-reperfusion. Am J Physiol Renal Physiol 2006; 291:F647–53 [DOI] [PubMed] [Google Scholar]
- 16. Eckardt KU, Rosenberger C, Jürgensen JS, Wiesener MS. Role of hypoxia in the pathogenesis of renal disease. Blood Purif 2003; 21:253–7 [DOI] [PubMed] [Google Scholar]
- 17. Cotter G, Metra M, Milo-Cotter O, Dittrich HC, Gheorghiade M. Fluid overload in acute heart failure—re-distribution and other mechanisms beyond fluid accumulation. Eur J Heart Fail 2008; 10:165–9 [DOI] [PubMed] [Google Scholar]
- 18. Mullens W, Abrahams Z, Francis GS, Sokos G, Taylor DO, Starling RC, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol 2009; 53:589–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Testani JM, Khera AV, St John Sutton MG, Keane MG, Wiegers SE, Shannon RP, et al. Effect of right ventricular function and venous congestion on cardio-renal interactions during the treatment of decompensated heart failure. Am J Cardiol 2010; 105:511–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Tanaka M, Yoshida H, Furuhashi M, Togashi N, Koyama M, Yamamoto S, et al. Deterioration of renal function by chronic heart failure is associated with congestion and oxidative stress in the tubulointerstitium. Intern Med 2011; 50:2877–87 [DOI] [PubMed] [Google Scholar]
- 21. Colombo PC, Ganda A, Lin J, Onat D, Harxhi A, Iyasere JE, et al. Inflammatory activation: cardiac, renal, and cardio-renal interactions in patients with the cardiorenal syndrome. Heart Fail Rev 2012; 17:177–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dikshit K, Vyden JK, Forrester JS, Chatterjee K, Prakash R, Swan HJ. Renal and extrarenal hemodynamic effects of furosemide in congestive heart failure after acute myocardial infarction. N Engl J Med 1973; 288:1087–90 [DOI] [PubMed] [Google Scholar]
- 23. Reungjui S, Pratipanawatr T, Johnson RJ, Nakagawa T. Do thiazides worsen metabolic syndrome and renal disease? The pivotal roles for hyperuricemia and hypokalemia. Curr Opin Nephrol Hypertens 2008; 17:470–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ellison DH. Diuretic therapy and resistance in congestive heart failure. Cardiology 2001; 96:132–43 [DOI] [PubMed] [Google Scholar]
- 25. Silverstein ME, Ford CA, Lysaght MJ, Henderson LW. Treatment of severe fluid overload by ultrafiltration. N Engl J Med 1974; 291:747–51 [DOI] [PubMed] [Google Scholar]
- 26. Costanzo MR, Guglin ME, Saltzberg MT, Jessup ML, Bart BA, Teerlink JR, et al. on behalf of the UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49:675–83 [DOI] [PubMed] [Google Scholar]
- 27. Schneierson SJ. Continuous peritoneal irrigation in the treatment of intractable edema of cardiac origin. Am J Med Sci 1949; 218:76–9 [DOI] [PubMed] [Google Scholar]
- 28. Nora JJ, Trygstad CW, Mangos JA, Gibbons JE, Jegier W. Peritoneal dialysis in the treatment of intractable congestive heart failure of infancy and childhood. J Pediatr 1966; 68:693–8 [DOI] [PubMed] [Google Scholar]
- 29. Mailloux LU, Swartz CD, Onesti G, Heider C, Ramirez O, Brest AN. Peritoneal dialysis for refractory congestive heart failure. JAMA 1967; 199:873–8 [PubMed] [Google Scholar]
- 30. Cairns KB, Porter GA, Kloster FE, Bristow JD, Griswold HE. Clinical and hemodynamic results of peritoneal dialysis for severe cardiac failure. Am Heart J 1968; 76:227–34 [DOI] [PubMed] [Google Scholar]
- 31. Raja RM, Krasnoff SO, Moros JG, Kramer MS, Rosenbaum JL. Repeated peritoneal dialysis in treatment of heart failure. JAMA 1970; 213:2268–9 [PubMed] [Google Scholar]
- 32. Sharma BK, Chander M, Singh R, Chugh KS. Peritoneal dialysis in resistant congestive heart failure and pulmonary oedema. J Indian Med Assoc 1972; 58:159–62 [PubMed] [Google Scholar]
- 33. McKinnie JJ, Bourgeois RJ, Husserl FE. Long-term therapy for heart failure with continuous ambulatory peritoneal dialysis. Arch Intern Med 1985; 145:1128–9 [PubMed] [Google Scholar]
- 34. Rubin J, Ball R. Continuous ambulatory peritoneal dialysis as treatment of severe congestive heart failure in the face of chronic renal failure. Report of eight cases. Arch Intern Med 1986; 146:1533–5 [PubMed] [Google Scholar]
- 35. Shilo S, Slotki IN, Iaina A. Improved renal function following acute peritoneal dialysis in patients with intractable congestive heart failure. Isr J Med Sci 1987; 23:821–4 [PubMed] [Google Scholar]
- 36. König P, Geissler D, Lechleitner P, Spielberger M, Dittrich P. Improved management of congestive heart failure. Use of continuous ambulatory peritoneal dialysis. Arch Intern Med 1987; 147:1031–4 [DOI] [PubMed] [Google Scholar]
- 37. König PS, Lhotta K, Kronenberg F, Joannidis M, Herold M. CAPD: a successful treatment in patients suffering from therapy-resistant congestive heart failure. Adv Perit Dial 1991; 7:97–101 [PubMed] [Google Scholar]
- 38. Tormey V, Conlon PJ, Farrell J, Horgan J, Walshe JJ. Long-term successful management of refractory congestive cardiac failure by intermittent ambulatory peritoneal ultrafiltration. QJM 1996; 89:681–3 [DOI] [PubMed] [Google Scholar]
- 39. Stegmayr BG, Banga R, Lundberg L, Wikdahl AM, Plum-Wirell M. PD treatment for severe congestive heart failure. Perit Dial Int 1996; 16(Suppl 1):S231–5 [PubMed] [Google Scholar]
- 40. Ryckelynck JP, Lobbedez T, Valette B, Le Goff C, Mazouz O, Levaltier B, et al. Peritoneal ultrafiltration and refractory congestive heart failure. Adv Perit Dial 1997; 13:93–7 [PubMed] [Google Scholar]
- 41. Ryckelynck JP, Lobbedez T, Valette B, Le Goff C, Mazouz O, Levaltier B, et al. Peritoneal ultrafiltration and treatment-resistant heart failure. Nephrol Dial Transplant 1998; 13(Suppl 4):56–9 [DOI] [PubMed] [Google Scholar]
- 42. Elhalel-Dranitzki M, Rubinger D, Moscovici A, Haviv YS, Friedlaender MM, Silver J, et al. CAPD to improve quality of life in patients with refractory heart failure. Nephrol Dial Transplant 1998; 13:3041–2 [DOI] [PubMed] [Google Scholar]
- 43. Sheppard R, Panyon J, Pohwani AL, Kapoor A, Macgowan G, McNamara D, et al. Intermittent outpatient ultrafiltration for the treatment of severe refractory congestive heart failure. J Card Fail 2004; 10:380–3 [DOI] [PubMed] [Google Scholar]
- 44. Kagan A, Rapoport J. The role of peritoneal dialysis in the treatment of refractory heart failure. Nephrol Dial Transplant 2005; 20(Suppl 7):vii28–31 [DOI] [PubMed] [Google Scholar]
- 45. Bertoli SV, Ciurlino D, Maccario M, Martino S, Bigatti G, Traversi L, et al. Home peritoneal ultrafiltration in patients with severe congestive heart failure without end-stage renal disease. Adv Perit Dial 2005; 21:123–7 [PubMed] [Google Scholar]
- 46. Gotloib L, Fudin R, Yakubovich M, Vienken J. Peritoneal dialysis in refractory end-stage congestive heart failure: a challenge facing a no-win situation. Nephrol Dial Transplant 2005; 20(Suppl 7):vii32–6 [DOI] [PubMed] [Google Scholar]
- 47. Takane H, Nakamoto H, Arima H, Shoda J, Moriwaki K, Ikeda N, et al. Continuous ambulatory peritoneal dialysis is effective for patients with severe congestive heart failure. Adv Perit Dial 2006; 22:141–6 [PubMed] [Google Scholar]
- 48. Phadke G, Mahale A, Ahlin T, Chemiti G, Levitski T. Continuous ambulatory peritoneal dialysis in a patient with isolated right heart failure and ascites: a case report. Adv Perit Dial 2008; 24:111–12 [PubMed] [Google Scholar]
- 49. Próchnicka A, Olszowska A, Baczyński D, Zelichowski G, Lubas A, Wiśniewska M, et al. Peritoneal dialysis as a therapeutic approach in congestive heart failure resistant to pharmacological treatment: case report. Pol Arch Med Wewn 2009; 119:834–7 [PubMed] [Google Scholar]
- 50. Basile C, Chimienti D, Bruno A, Cocola S, Libutti P, Teutonico A, et al. Efficacy of peritoneal dialysis with icodextrin in the long-term treatment of refractory congestive heart failure. Perit Dial Int 2009; 29:116–18 [PubMed] [Google Scholar]
- 51. Nakayama M, Nakano H, Nakayama M. Novel therapeutic option for refractory heart failure in elderly patients with chronic kidney disease by incremental peritoneal dialysis. J Cardiol 2010; 55:49–54 [DOI] [PubMed] [Google Scholar]
- 52. Sánchez JE, Ortega T, Rodríguez C, Díaz-Molina B, Martín M, Garcia-Cueto C, et al. Efficacy of peritoneal ultrafiltration in the treatment of refractory congestive heart failure. Nephrol Dial Transplant 2010; 25:605–10 [DOI] [PubMed] [Google Scholar]
- 53. Cnossen TT, Kooman JP, Konings CJ, Uszko-Lencer NH, Leunissen KM, van der Sande FM. Peritoneal dialysis in patients with primary cardiac failure complicated by renal failure. Blood Purif 2010; 30:146–52 [DOI] [PubMed] [Google Scholar]
- 54. Sens F, Schott-Pethelaz AM, Labeeuw M, Colin C, Villar E. on behalf of the REIN Registry. Survival advantage of hemodialysis relative to peritoneal dialysis in patients with end-stage renal disease and congestive heart failure. Kidney Int 2011; 80:970–7 [DOI] [PubMed] [Google Scholar]
- 55. Ho-dac-Pannekeet MM, Schouten N, Langendijk MJ, Hiralall JK, de Waart DR, Struijk DG, et al. Peritoneal transport characteristics with glucose polymer based dialysate. Kidney Int 1996; 50:979–86 [DOI] [PubMed] [Google Scholar]
- 56. Flessner MF. Peritoneal ultrafiltration: mechanisms and measures. Contrib Nephrol 2006; 150:28–36 [DOI] [PubMed] [Google Scholar]
- 57. Silverberg DS, Wexler D, Blum M, Sheps D, Schwartz D, Yachnin T, et al. Aggressive therapy of congestive heart failure and associated chronic renal failure with medications and correction of anemia stops or slows the progression of both diseases. Perit Dial Int 2001; 21(Suppl 3):S236–40 [PubMed] [Google Scholar]
- 58. Zemel D, Imholz AL, de Waart DR, Dinkla C, Struijk DG, Krediet RT. Appearance of tumor necrosis factor-alpha and soluble TNF-receptors I and II in peritoneal effluent of CAPD. Kidney Int 1994; 46:1422–30 [DOI] [PubMed] [Google Scholar]
- 59. Costanzo MR, Saltzberg MT, Jessup M, Teerlink JR, Sobotka PA. on behalf of the Ultrafiltration Versus Intravenous Diuretics for Patients Hospitalized for Acute Decompensated Heart Failure (UNLOAD) Investigators. Ultrafiltration is associated with fewer rehospitalizations than continuous diuretic infusion in patients with decompensated heart failure: results from UNLOAD. J Card Fail 2010; 16:277–84 [DOI] [PubMed] [Google Scholar]
- 60. Ali SS, Olinger CC, Sobotka PA, Dahle TG, Bunte MC, Blake D, et al. Loop diuretics can cause clinical natriuretic failure: a prescription for volume expansion. Congest Heart Fail 2009; 15:1–4 [DOI] [PubMed] [Google Scholar]
- 61. Ni J, Verbavatz JM, Rippe A, Boisdé I, Moulin P, Rippe B, et al. Aquaporin-1 plays an essential role in water permeability and ultrafiltration during peritoneal dialysis. Kidney Int 2006; 69:1518–25 [DOI] [PubMed] [Google Scholar]
- 62. Nakayama M, Yokoyama K, Kubo H, Matsumoto H, Hasegawa T, Shigematsu T, et al. The effect of ultra-low sodium dialysate in CAPD. A kinetic and clinical analysis. Clin Nephrol 1996; 45:188–93 [PubMed] [Google Scholar]
- 63. Rustøen T, Howie J, Eidsmo I, Moum T. Hope in patients hospitalized with heart failure. Am J Crit Care 2005; 14:417–25 [PubMed] [Google Scholar]
- 64. Ota K, Akiba T, Nakao T, Nakayama M, Maeba T, Park MS, et al. on behalf of the Icodextrin Study Group. Peritoneal ultrafiltration and serum icodextrin concentration during dialysis with 7.5% icodextrin solution in Japanese patients. Perit Dial Int 2003; 23:356–61 [PubMed] [Google Scholar]
- 65. Nakayama M, Kasai K, Imai H. on behalf of TRM-280 Study Group. Novel low Na peritoneal dialysis solutions designed to optimize Na gap of effluent: kinetics of Na and water removal. Perit Dial Int 2009; 29:528–35 [PubMed] [Google Scholar]
- 66. Nakayama M. Fluid status and its management in Japanese peritoneal dialysis patients. Perit Dial Int 2006; 26:144–9 [PubMed] [Google Scholar]


