Abstract
♦ Background: The impact of climatic variations on peritoneal dialysis (PD)-related peritonitis has not been studied in detail. The aim of the current study was to determine whether various climatic zones influenced the probability of occurrence or the clinical outcomes of peritonitis.
♦ Methods: Using ANZDATA registry data, the study in cluded all Australian patients receiving PD between 1 October 2003 and 31 December 2008. Climatic regions were defined according to the Köppen classification.
♦ Results: The overall peritonitis rate was 0.59 episodes per patient-year. Most of the patients lived in Temperate regions (65%), with others residing in Subtropical (26%), Tropical (6%), and Other climatic regions (Desert, 0.6%; Grassland, 2.3%). Compared with patients in Temperate regions, those in Tropical regions demonstrated significantly higher overall peritonitis rates and a shorter time to a first peritonitis episode [adjusted hazard ratio: 1.15; 95% confidence interval (CI): 1.01 to 1.31]. Culture-negative peritonitis was significantly less likely in Tropical regions [adjusted odds ratio (OR): 0.42; 95% CI: 0.25 to 0.73]; its occurrence in Subtropical and Other regions was comparable to that in Temperate regions. Fungal peritonitis was independently associated with Tropical regions (OR: 2.18; 95% CI: 1.22 to 3.90) and Other regions (OR: 3.46; 95% CI: 1.73 to 6.91), where rates of antifungal prophylaxis were also lower. Outcomes after first peritonitis episodes were comparable in all groups.
♦ Conclusions: Tropical regions were associated with a higher overall peritonitis rate (including fungal peritonitis) and a shorter time to a first peritonitis episode. Augmented peritonitis prophylactic measures such as antifungal therapy and exit-site care should be considered in PD patients residing in Tropical climates.
Key words: Antibiotics, bacteria, climate, fungus, microbiology, peritonitis, outcomes
Peritonitis is a serious complication of peritoneal dialysis (PD), responsible for significant morbidity and accounting for 30% of technique failures and 21% of infectious deaths in Australian and New Zealand PD patients (1). Studies of organism-specific peritonitis (2-12) have not previously assessed the role of climatic variation in peritonitis and in its microbiologic causes. The temperature and humidity differences in individual climatic regions may influence the development of PD peritonitis by changing patient behavior and hygiene, distribution of normal skin flora, organism virulence, and the chance of contamination.
Previous studies attempting to demonstrate a relationship between changes in temperature and humidity and the occurrence of PD peritonitis have had conflicting outcomes (13-17). Those studies have been limited by small patient numbers, single-center sourcing, retrospective design, and sometimes inappropriate statistical analysis and a relatively narrow range of temperatures and humidity in the studied regions, which were often within one climatic region, thereby possibly underestimating the true effect of climatic variation. To date, no comprehensive, multicenter examination of the impact of climate on PD peritonitis has been reported.
Australia is in a unique position to evaluate the climate question because it encompasses a range of different climatic regions. The aim of the present study was therefore to examine the effect of climatic regional variation on the risk, microbiology, treatment, and clinical outcomes of PD-associated peritonitis in all Australian PD patients, as recorded in the ANZDATA registry.
METHODS
STUDY POPULATION
The study included all Australian adult patients from the ANZDATA registry who were receiving PD between 1 October 2003 (when detailed peritonitis data started to be collected) and 31 December 2008. The registry data include demographics, primary renal diseases, comorbidities at the start of dialysis, smoking status, body mass index (BMI), late referral (defined as commencement of dialysis within 3 months of referral to a nephrologist), microbiology of peritonitis episodes (up to 3 organisms for polymicrobial episodes), and the initial and subsequent antibiotic treatment regimens.
Patients were analyzed according to the climatic region in which they resided, based on postal code. Climatic regions were defined as Desert, Grassland, Temperate, Subtropical, and Tropical according to the Köppen classification scheme used by the Australian Bureau of Meteorology (http://www.bom.gov.au). Patients in the Desert and Grassland regions were grouped together as “Other” in view of the small numbers of patients and peritonitis episodes in the Desert climatic region and the similarity of patient characteristics between the Desert and Grassland groups. Center size was categorized according to quartiles of the numbers of patients receiving care in the individual units over the duration of the study: small (<7 patients), small-medium (7 - 42 patients), medium-large (43 - 140 patients), and large (>140 patients). The data are collected throughout the calendar year by medical and nursing staff in each renal unit and are submitted annually to the ANZDATA registry by the end of March in the following year. The Education and Occupation components of the Socio-Economic Indexes for Areas (SEIFA), based on 2006 Australian Census data, were obtained from the Australian Bureau of Statistics website (http://www.abs.gov.au/) and merged with the ANZDATA dataset according to postal code for the patient’s residence.
Peritonitis was defined as clinical features of peritonitis (abdominal pain or cloudy dialysate) and dialysate leukocytosis (white blood cell count > 100 /μL with >50% neutrophils). Peritonitis rates were calculated according to the standardized recommendations made by the International Society for Peritoneal Dialysis (ISPD) (18). All episodes of peritonitis that occurred while patients were receiving PD were divided by the total number of days that patients were receiving PD during a nominated time period (month, season, year, or study period) to yield a peritonitis rate expressed as episodes per patient-year at risk. In keeping with the ISPD recommendations (18,19), relapsed peritonitis was counted as a single episode, and patients with a PD catheter in situ who were not receiving PD were not included in peritonitis rate calculations. All Australian units follow the ISPD (18) and Caring for Australasians with Renal Impairment PD guidelines (20-22).
The outcomes examined were peritonitis rate, peritonitis-free survival, microbiology, antimicrobial therapy, cure, relapse, peritonitis-associated hospitalization, catheter removal, temporary hemodialysis transfer (in which patients subsequently resumed PD), permanent hemodialysis transfer, and patient death. A peritonitis episode was considered “cured” by antibiotics alone if, at the end of treatment, the patient was symptom-free, the PD effluent was clear, and the episode was not subsequently complicated by relapse, catheter removal, or death. Peritonitis-related death was recorded if the patient’s death was directly attributable to peritonitis in the clinical opinion of the treating nephrologist. In view of the complexities associated with the analysis of multiple events in individuals, in which the assumption of independence of observations is not appropriate, only first episodes of peritonitis in each individual were included when analyzing all outcomes except peritonitis rates.
STATISTICAL ANALYSIS
Results are expressed as frequencies and percentages, mean ± standard deviation, or median with inter-quartile range (25th - 75th percentile), as appropriate. Differences between groups were analyzed using the chi-square test for categorical data, analysis of variance for continuous normally distributed data, and the Kruskal-Wallis test for continuous non-normally distributed data. Peritonitis rates were compared using a Poisson regression analysis. Peritonitis-free survival was determined by Kaplan-Meier survival analysis and by multivariate Cox proportional hazards model analysis with backward stepwise elimination based on likelihood ratio until the most parsimonious model was identified. Climatic region, age, sex, racial origin, BMI, estimated glomerular filtration rate (eGFR) at dialysis start, late referral, cause of end-stage renal failure, smoking status, comorbidities, peritoneal transport status, center size, and SEIFA were included in the model as covariates. The independent predictors of the clinical outcomes of peritonitis were determined by multivariate logistic regression. Climatic region, age, sex, racial origin, BMI, eGFR at dialysis start, late referral, cause of end-stage renal failure, smoking status, comorbidities, peritoneal transport status, center size, SEIFA, organism isolated, and initial empiric antibiotic regimen were included in the model as covariates. First-order interaction terms between the significant covariates were examined where appropriate. Data were analyzed using the PASW Statistics for Windows software package (release 18.0: SPSS, North Sydney, Australia). Values of p less than 0.05 were considered statistically significant.
RESULTS
POPULATION CHARACTERISTICS
During the study period, 6610 patients received PD in Australia and were followed for 10 470 patient-years (mean follow-up: 1.58 years per patient). Table 1 depicts the characteristics of those patients. In this group, 6213 episodes of peritonitis occurred in 3128 patients (47%; range: 1 - 15 episodes per patient). The overall rate of peritonitis was 0.59 episodes per patient-year of treatment (equivalent to 1 episode in 20.2 patient-months).
TABLE 1.
Characteristics of Australian Patients Receiving Peritoneal Dialysis During the Study Period, 2003 - 2008, Overall and by Climatic Region
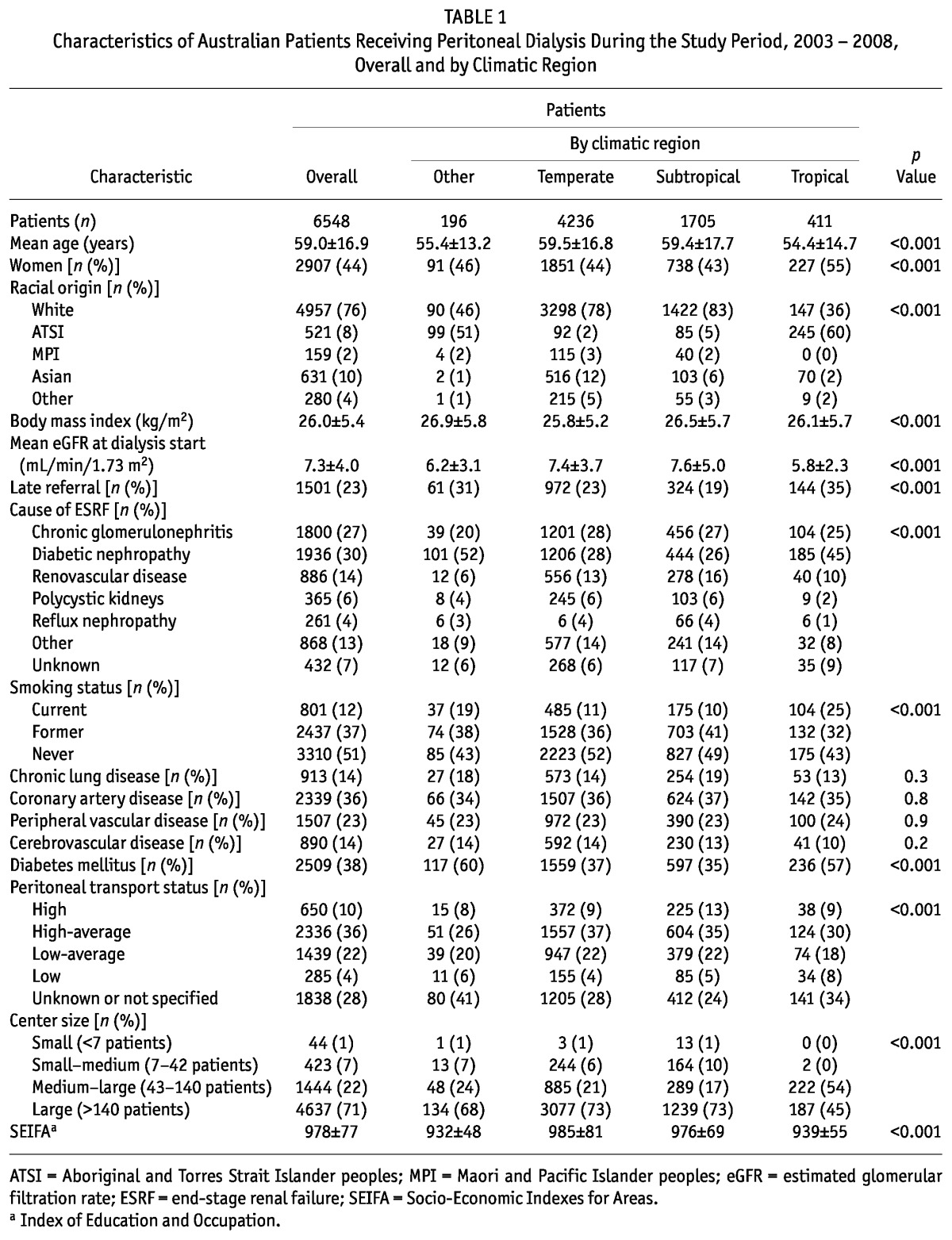
Climatic region was unable to be determined in 62 patients (1%) because of missing postal code data. For the remaining 6548 patients, 4236 (65%) lived in Temperate regions (reference group), 1705 (26%) lived in Subtropical regions, 411 (6%) lived in Tropical regions and 196 (3%) lived in Other climatic regions (Desert, 42; Grassland, 154). Table 1 shows the baseline characteristics of the patients by region.
Univariate analysis showed that, compared with patients living in Temperate and Subtropical regions, those living in Tropical and Other climatic regions were significantly more likely to be younger, current smokers, and Aboriginal and Torres Strait Islander peoples. They were also more likely to have been referred late to a renal unit before dialysis, to have commenced dialysis at a lower eGFR, to have diabetes mellitus and diabetic nephropathy, to have a lower SEIFA score for education and occupation, and not to have a recorded baseline peritoneal transport status. Furthermore, patients in Tropical regions were significantly more likely to be female and to be receiving treatment at a larger PD unit. Using multivariate logistic regression, Tropical residence was independently associated with Aboriginal and Torres Strait Islander racial origin [adjusted odds ratio (OR): 25.9; 95% confidence interval (CI): 18.7 to 35.9], lower eGFR at dialysis commencement (OR per mL/min/1.73 m2: 0.89; 95% CI: 0.84 to 0.93), current cigarette smoking (OR: 1.54; 95% CI: 1.07 to 2.21), low transporter status (OR: 2.73; 95% CI: 1.60 to 4.66), and dialysis received at a medium-large (3rd-quartile) center (OR: 4.32; 95% CI: 3.33 to 5.60, using the largest-center quartile as reference). Tropical residence was associated with lower odds of reflux nephropathy (OR: 0.27; 95% CI: 0.10 to 0.77).
OVERALL PERITONITIS RATES
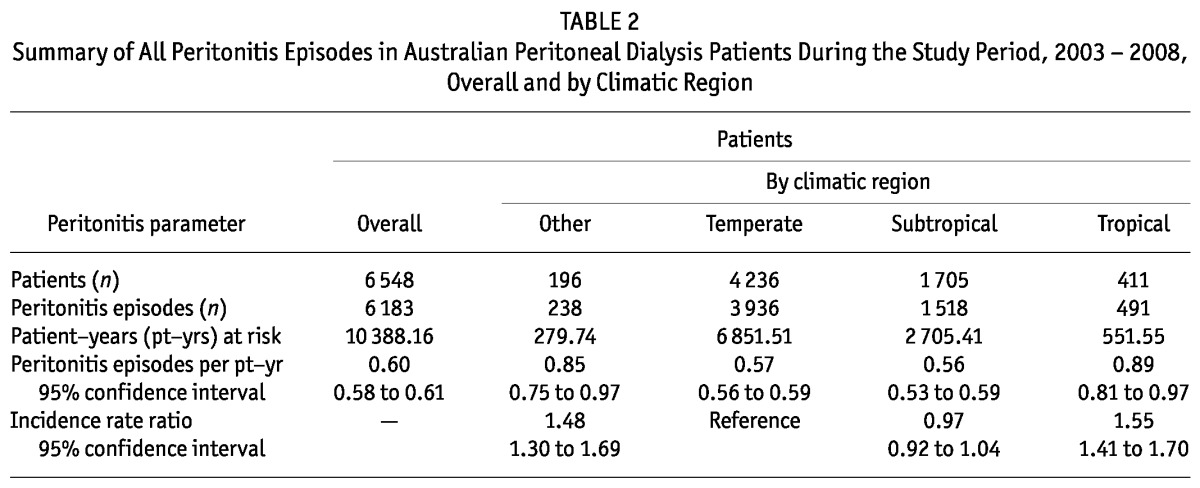
When all peritonitis episodes were considered, the overall peritonitis rate was significantly higher in patients receiving PD in Tropical regions than in Temperate, Subtropical, and Other climatic regions (Table 2).
TABLE 2.
Summary of All Peritonitis Episodes in Australian Peritoneal Dialysis Patients During the Study Period, 2003 - 2008, Overall and by Climatic Region
PERITONITIS-FREE SURVIVAL
Time to a first peritonitis episode was significantly shorter in patients living in Tropical regions than in Temperate, Subtropical, and Other climatic regions (Figure 1). Median (95% CI) peritonitis-free survival rates were 0.95 years (0.78 to 1.12 years), 1.66 years (1.55 to 1.77 years), 1.89 years (1.73 to 2.03 years), and 1.31 years (0.90-1.72 years) respectively (log rank score 46.8, p < 0.001). The 3-year peritonitis-free survival rates were 21%, 30%, 34%, and 26% respectively.
Figure 1.
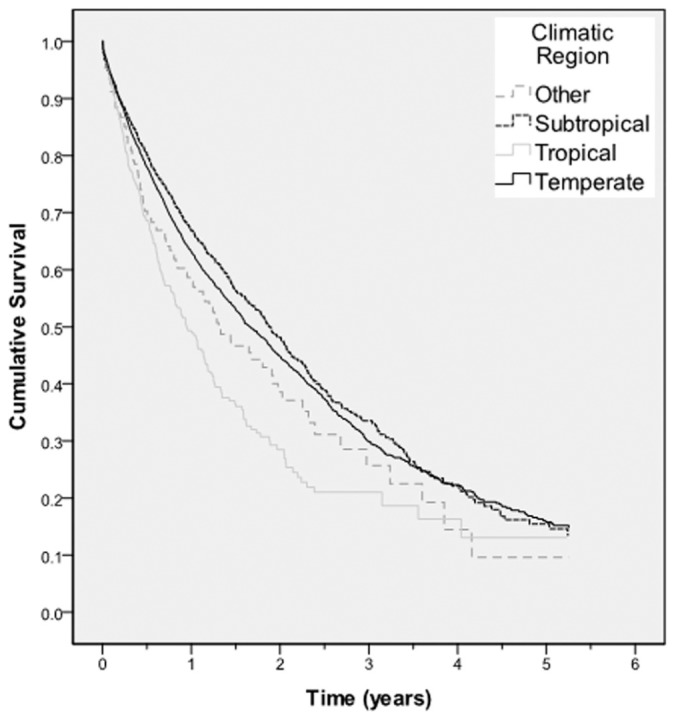
— Kaplan-Meier survival curve for peritonitis-free survival for all Australian patients receiving peritoneal dialysis between 1 October 2003 and 31 December 2008, according to climatic region. The difference between the groups was statistically significant (log rank score: 46.8; p < 0.001).
Using multivariate Cox proportional hazards model analysis, patients receiving PD in Tropical regions experienced a significantly shorter time to a first peritonitis episode (HR: 1.15; 95% CI: 1.01 to 1.31) than did patients receiving PD in Temperate regions (reference group). Peritonitis-free survival rates were comparable in the remaining climatic groups (Figure 2). The other independent predictors of increased peritonitis hazard were Aboriginal and Torres Strait Islander racial origin (HR: 1.49; 95% CI: 1.27 to 1.75, using white racial origin as reference), Maori and Pacific Islander racial origin (HR: 1.28; 95% CI: 1.02 to 1.60, using white racial origin as reference), and a missing peritoneal transport status result (HR: 1.33; 95% CI: 1.19 to 1.49, using low-average transporters as reference). Center size showed an effect on peritonitis-free survival only for small-medium centers (2nd-smallest quartile), in which the hazard was significantly lower (HR: 0.74; 95% CI: 0.61 to 0.91, using the largest-center quartile as reference).
Figure 2.
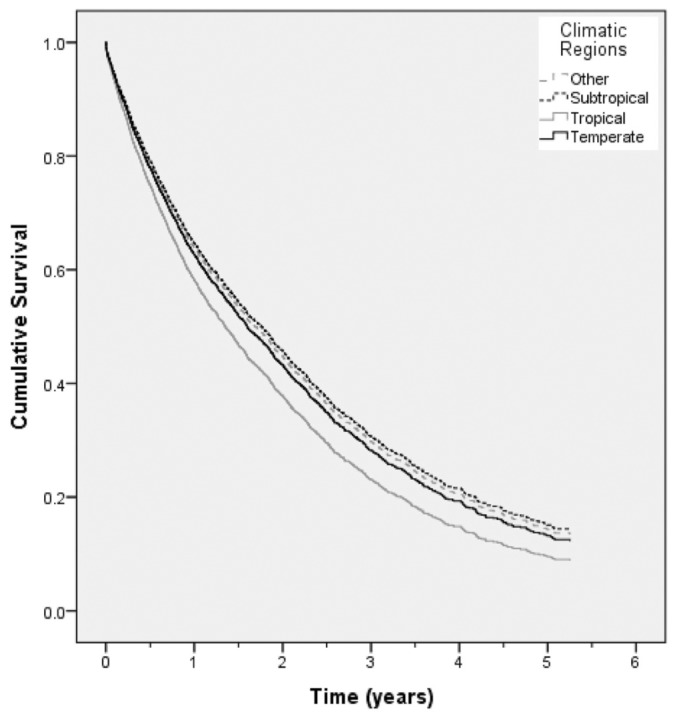
— Cox-adjusted survival curve for peritonitis-free survival for all Australian patients receiving peritoneal dialysis between 1 October 2003 and 31 December 2008, according to climatic region. Compared with temperate-region patients (reference group), tropical-region patients experienced a significantly shorter time to a first peritonitis episode (hazard ratio: 1.18; 95% confidence interval: 1.05 to 1.34).
MICROBIOLOGY OF FIRST PERITONITIS EPISODES
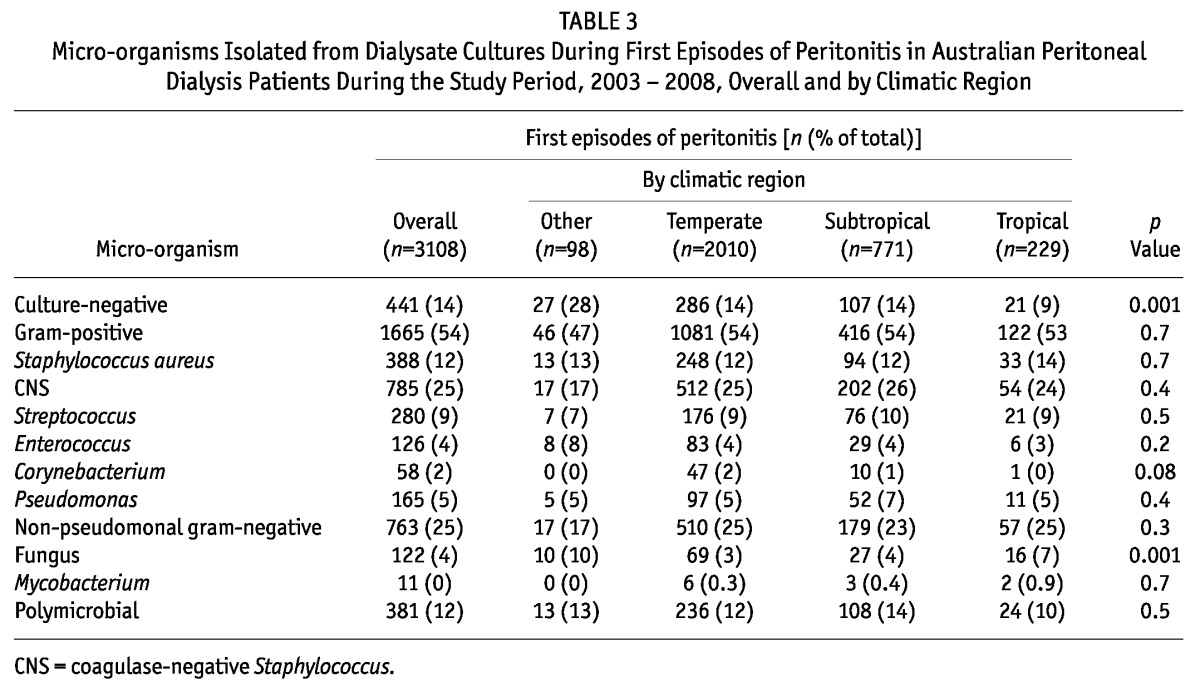
Table 3 summarizes the micro-organisms isolated during the study period from dialysate cultures during first episodes of peritonitis in the various climatic regions. Dialysate cultures were significantly more likely to be negative in the Other climatic regions (Desert and Grassland) than in the remaining regions. Fungi were also more likely to be isolated from first peritonitis episodes in Tropical and Other climatic regions than in the Temperate and Subtropical regions. Using multivariate logistic regression, culture-negative peritonitis was significantly less likely in Tropical regions (adjusted OR: 0.42; 95% CI: 0.25 to 0.73); its occurrence in Subtropical and Other regions was comparable to that in Temperate regions. Fungal peritonitis was independently associated with Tropical regions (OR: 2.18; 95% CI: 1.22 to 3.90, using Temperate as reference) and Other regions (OR: 3.46; 95% CI: 1.73 to 6.91).
TABLE 3.
Micro-organisms Isolated from Dialysate Cultures During First Episodes of Peritonitis in Australian Peritoneal Dialysis Patients During the Study Period, 2003 - 2008, Overall and by Climatic Region
INITIAL EMPIRIC ANTIBIOTIC TREATMENT OF FIRST PERITONITIS EPISODES
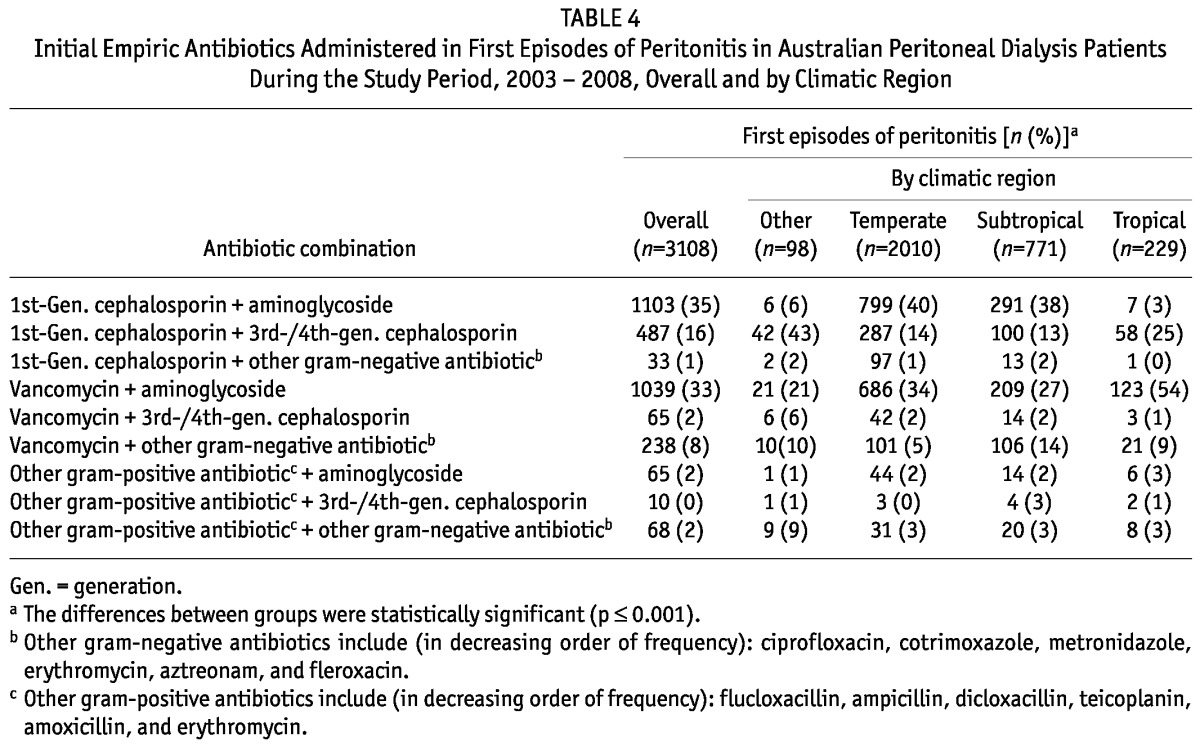
Most patients with peritonitis were treated initially with either intraperitoneal vancomycin or cephazolin in combination with gentamicin (Table 4). Compared with PD patients residing in Temperate and Subtropical regions, those residing in Tropical and Other climatic regions were significantly more likely to be treated empirically with 1st-generation cephalosporins in combination with 3rd- or 4th-generation cephalosporins than with aminoglycosides. Patients in Tropical regions were also significantly more likely to be treated empirically with vancomycin in combination with an aminoglycoside.
TABLE 4.
Initial Empiric Antibiotics Administered in First Episodes of Peritonitis in Australian Peritoneal Dialysis Patients During the Study Period, 2003 - 2008, Overall and by Climatic Region
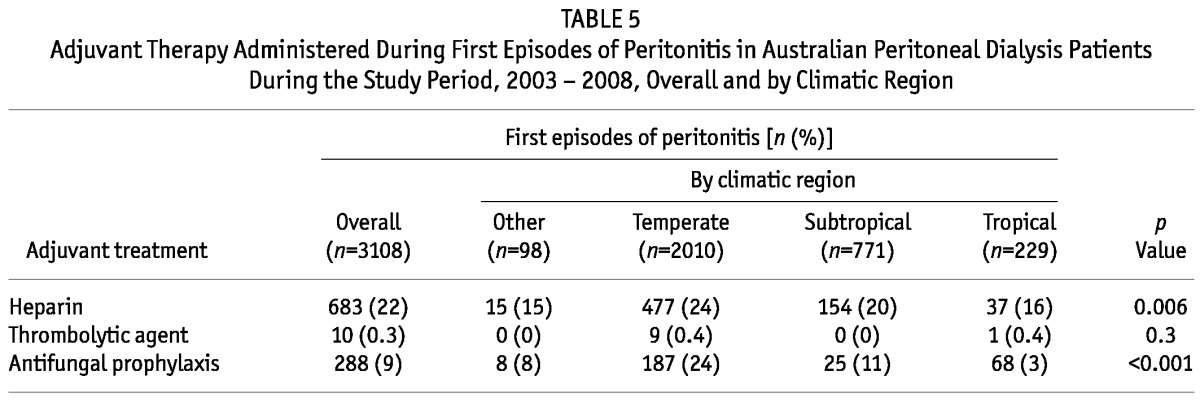
In terms of adjunctive therapy for peritonitis, heparin was significantly less likely to be added to PD exchanges for patients living in Tropical and Other climatic regions than for those living in Temperate and Subtropical regions (Table 5). Moreover, patients in Subtropical regions were significantly less likely than those in all other groups to be co-prescribed antifungal prophylaxis, primarily nystatin (Table 5). No significant differences in the administration of thrombolytic agents was observed between the groups (Table 5).
TABLE 5.
Adjuvant Therapy Administered During First Episodes of Peritonitis in Australian Peritoneal Dialysis Patients During the Study Period, 2003 - 2008, Overall and by Climatic Region
OUTCOMES OF FIRST PERITONITIS EPISODES
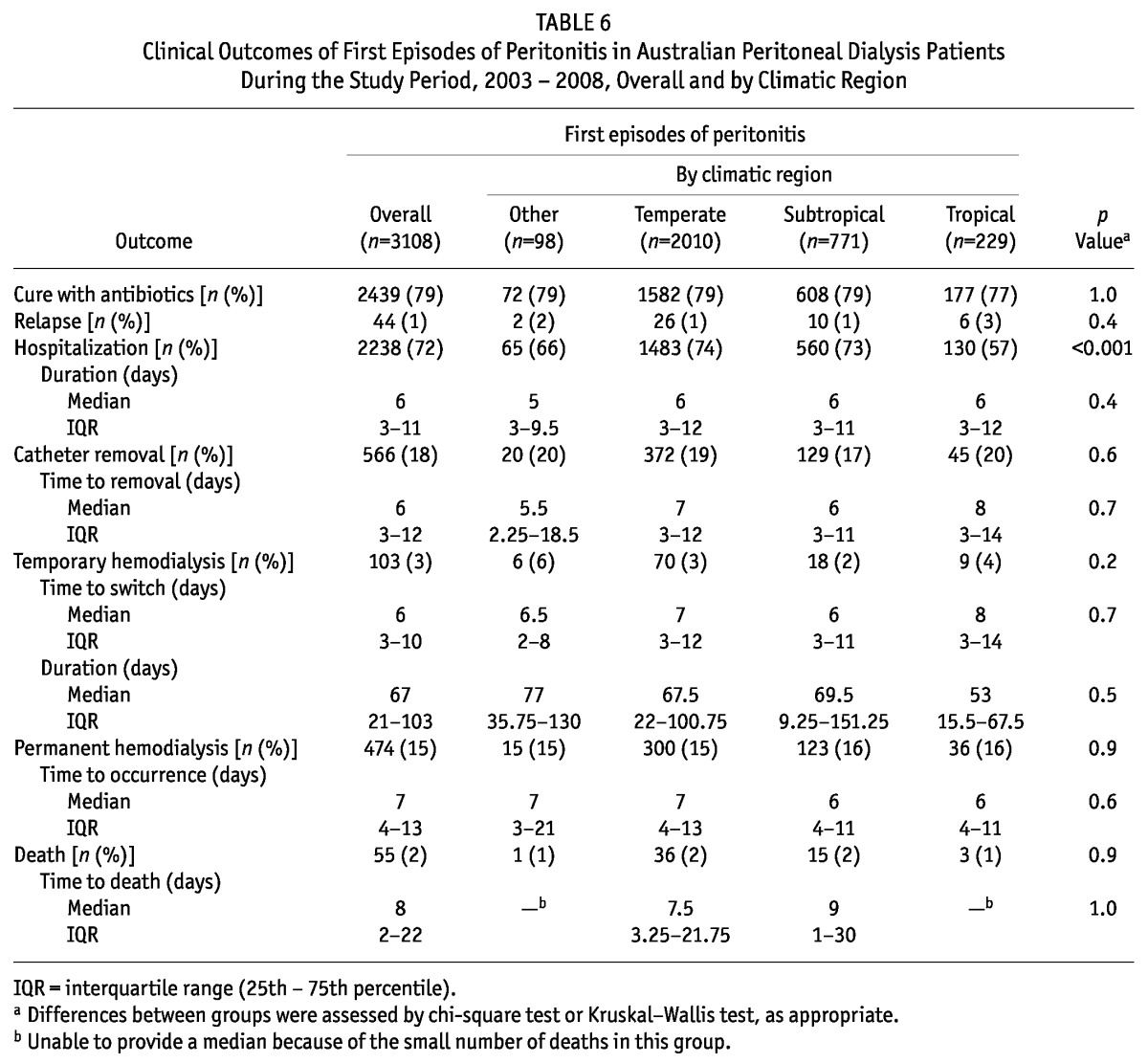
Table 6 shows the outcomes of first peritonitis episodes according to climatic region. In general, outcomes were comparable in all the groups. One exception was a significantly lower hospitalization rate for peritonitis episodes in Tropical regions. Using multivariate logistic regression, living in a Tropical region, compared with living in a Temperate region (reference group), was independently predictive of a lower probability of hospitalization (OR: 0.42; 95% CI: 0.28 to 0.61).
TABLE 6.
Clinical Outcomes of First Episodes of Peritonitis in Australian Peritoneal Dialysis Patients During the Study Period, 2003 - 2008, Overall and by Climatic Region
No other significant independent associations were observed between climatic region and the outcomes of either catheter removal or interim or permanent hemodialysis transfer (data not shown). The events for death and relapse were too small in number to permit adequate statistical analysis.
DISCUSSION
The present study represents the first detailed examination to date of the effects of climatic region on the frequency and clinical outcomes of PD-related peritonitis. Its key findings are that PD patients residing in Tropical regions experience higher overall peritonitis rates and shorter times to a first peritonitis episode, even after adjustment for differences in demographic and clinical factors. Furthermore, Tropical and Other (Desert and Grassland) climatic regions are associated with higher rates of fungal peritonitis, and culture-negative peritonitis is significantly less likely to occur in Tropical regions. The initial empiric antibiotics prescribed for peritonitis also differed by climatic region, with patients from Tropical regions being more likely to receive treatment with vancomycin in combination with an aminoglycoside or a 1st-generation cephalosporin in combination with a 3rd- or 4th-generation cephalosporin. Moreover, in spite of higher rates of fungal peritonitis, antifungal chemoprophylaxis was less commonly used in Tropical and Other climatic regions than in Subtropical regions. Nevertheless, outcomes after a first peritonitis episodes were comparable across all climatic regions.
Increased episodes of peritonitis in warmer climatic conditions have been reported by a number of earlier single-center studies (13,14,16). In a study of 80 PD patients in a Brazilian centre, Alves et al. (16) observed significantly higher rates of PD catheter-related infections during months with a mean maximal temperature of 32°C or more than during months with a mean temperature below 28°C. Similarly, Kim et al. (13) and Szeto et al. (14) found that the occurrence of peritonitis paralleled temperature and relative humidity in a Korean center and a Hong Kong center respectively. In contrast, Chan et al. (17) could not find evidence of an association between relative humidity and peritonitis in 239 PD patients over 4.5 years at another Hong Kong center. Unfortunately, the findings of the foregoing studies are potentially limited by their single-center nature, often within a single climatic zone. In some cases, inappropriate statistical analyses were also used to compare peritonitis rates, leading to uncertainty regarding the findings.
Recently, an observational cohort study involving 2032 patients from 114 dialysis centers in Brazil (BRAZPD) observed significantly higher peritonitis rates in the warmest (northeast) part of the country, even after adjustment for other demographic, clinical, and educational factors (23). Those findings, together with the results of the present study, suggest that Tropical climate may substantially increase the risk of PD peritonitis.
Like the BRAZPD study (23), the present investigation also observed an increased hazard of peritonitis in non-white races. However, unlike BRAZPD, the present study did not find an association between educational level and peritonitis risk. This apparent disparity may relate to our use of an education and occupation index based on postal code rather than of individual data (as in the BRAZPD study). Alternatively, the universal free education system in Australia may have mitigated the impact of education status on peritonitis risk. Moreover, although the BRAZPD study found a significantly higher risk of peritonitis in the smallest centers, a parallel risk was not observed in Australia. Indeed, the second-smallest quartile of center sizes was associated with a significantly lower peritonitis risk. That finding may reflect operational inefficiencies associated with very large centers, leading to poorer performance.
Finally, our study observed an increased risk of peritonitis in patients with missing peritoneal equilibration test results. That finding may reflect unmeasured associated factors, such as patient noncompliance, distance of patient residence from the PD unit, or periods of ill health.
An association between climatic conditions (such as temperature and humidity) and PD peritonitis rates is biologically plausible because hot and humid climates promote skin perspiration and may influence human behavior, such that people living in Tropical regions may be more likely to participate in outdoor activities such as swimming in oceans or waterholes. Furthermore, humid environments can also enhance the growth and persistence of bacteria on dialysis tubing and other environmental reservoirs (24).
In the present study, residence in a Tropical, Desert, or Grassland region was found to significantly and specifically heighten the risk of fungal peritonitis by a factor of approximately 2 - 3. Fungi are widely found in human environments, being part of the normal flora of the skin and mucosa, but these organisms can become pathogenic under certain conditions. The strongest risk factors for fungal peritonitis in PD patients—prolonged use of antibiotics and previous bacterial peritonitis— have already been recognized (10,25). Our study suggests that climates with high ambient temperatures may also represent an important risk factor for fungal peritonitis. Higher temperatures may directly affect the virulence of Candida species, responsible for most fungal peritonitis in Australia (10), by promoting the development of tubular, branching-type hyphal cells that facilitate deep penetration into human tissues rather than the unicellular budding yeast state (26). The ensuing heightened risk of peritonitis may be further compounded by lower rates of co-prescription of antifungal prophylaxis in Tropical (11%) and Other climatic regions (8%) compared with Subtropical regions (24%). A previous randomized controlled trial by Lo et al. (27) showed that antifungal prophylaxis during all courses of antibiotics prolonged the time to Candida peritonitis. More recently, another randomized controlled trial (28) of fluconazole 200 mg every 48 hours during all courses of antibiotics (compared with no fluconazole in a control group) demonstrated significant reductions in fungal peritonitis (p = 0.0051). Consequently, the ISPD guidelines recommend that fungal prophylaxis during antibiotic therapy may prevent some cases of Candida peritonitis in programs that have high rates of fungal peritonitis. Based on the findings of the present investigation, we suggest that the practice of prophylaxis should extend to hot climates, such as in Tropical regions. However, we cannot exclude the possibility that differences in choice of empirical antibiotic therapy of peritonitis between the various climatic regions may have influenced the subsequent risk of fungal peritonitis.
Interestingly, we also observed a lower incidence of culture-negative peritonitis in Tropical climatic regions. That finding does not appear to be attributable to infection caused by unusual or highly virulent organisms in non-Tropical regions, because we observed no significant differences in the clinical outcomes of first peritonitis episodes between the various climatic zones. Although hospitalization rates were lower in Tropical regions, raising the possibility of clinically milder peritonitis episodes in those areas, the difference persisted after adjustment for demographic, socio-economic, facility, and clinical factors (including causative organism and empiric antibiotic regimen). Whether the findings for culture-negative peritonitis according to climatic region reflect regional differences in recent antibiotic exposure, sample collection, or culture methods cannot be addressed because the ANZDATA registry does not collect that information.
The strengths of the present study include its very large sample size and inclusiveness. We included all patients receiving PD in Australia during the study period, such that a variety of centers with varying approaches to the microbiologic diagnosis and treatment of peritonitis were included. That inclusiveness greatly enhanced the external validity of our findings. Those strengths should be balanced against the study’s limitations, which include limited depth of data collection. The ANZDATA registry does not collect certain important information such as the presence of concomitant exit-site and tunnel infections, antimicrobial susceptibilities of isolated micro-organisms, patient compliance, patient living conditions, individual unit management protocols and practices (including fungal prophylaxis), duration of cloudy dialysate, laboratory values (such as C-reactive protein and dialysate white cell counts), severity of comorbidities, PD modality (ambulatory or continuous ambulatory PD) at time of peritonitis, disconnect systems used, prescribed PD dialysate (especially icodextrin), antibiotic dosages or routes of antibiotic administration, peritoneal dialysate sampling and culture methodology, or previous antibiotic exposure for any indication. Even though we adjusted for a large number of patient characteristics, the possibility of residual confounding cannot be excluded. In common with other registries, ANZDATA is voluntary, and no external audit of data accuracy is undertaken, including for the diagnosis of peritonitis. Consequently, the possibility of coding or classification bias cannot be excluded.
CONCLUSIONS
The present study represents the largest investigation to date and the first comprehensive multicenter examination of the effects of climatic region on the risk, microbiology, treatment, and clinical outcomes of PD-associated peritonitis. The findings clearly demonstrate that PD patients living in Tropical areas experienced higher overall peritonitis rates (particularly fungal peritonitis) and shorter times to a first peritonitis episode. Augmented peritonitis prophylactic measures—such as antifungal therapy and more intensive exit-site care—should be considered in PD patients residing in Tropical climates.
DISCLOSURES
DWJ is a consultant for Baxter Healthcare and has previously received research funds from that company. He has also received speakers’ honoraria and research grants from Fresenius Medical Care, and he is a current recipient of a Queensland Government Health Research Fellowship. KMB is a consultant for Baxter Healthcare. FGB is a consultant for Baxter Healthcare and Fresenius and has received travel grants from Amgen and Roche. SPM has received speaking honoraria from Amgen Australia, Fresenius Australia, and Solvay Pharmaceuticals, and travel grants from Amgen Australia, Genzyme Australia, and Janssen-Cilag. NB has previously received research funds from Roche, travel grants from Roche, Amgen, and Janssen-Cilag, and speaking honoraria from Roche. The remaining authors have no competing financial interests to declare.
Acknowledgments
The authors gratefully acknowledge the substantial contributions of the entire Australia and New Zealand nephrology community (physicians, surgeons, database managers, nurses, renal operators, and patients) in providing information for and maintaining the ANZDATA database.
References
- 1. Johnson DW, Chang S, Excell L, Livingston B, Bannister K, McDonald SP. Peritoneal dialysis. In: McDonald S, Chang S, Excell L, eds. The Twenty-Ninth Report Australia and New Zealand Dialysis and Transplant Registry 2006. Adelaide, Australia: ANZDATA Registry; 2007: 87–103 [Google Scholar]
- 2. Barraclough K, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Polymicrobial peritonitis in peritoneal dialysis patients in Australia: predictors, treatment, and outcomes. Am J Kidney Dis 2010; 55:121–31 [DOI] [PubMed] [Google Scholar]
- 3. Barraclough K, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Corynebacterium peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 82 cases. Nephrol Dial Transplant 2009; 24:3834–9 [DOI] [PubMed] [Google Scholar]
- 4. Edey M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Enterococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 116 cases. Nephrol Dial Transplant 2009; 25:1272–8 [DOI] [PubMed] [Google Scholar]
- 5. Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Culture-negative peritonitis in peritoneal dialysis patients in Australia: predictors, treatment, and outcomes in 435 cases. Am J Kidney Dis 2009; 55:690–7 [DOI] [PubMed] [Google Scholar]
- 6. Fahim M, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Coagulase-negative staphylococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 936 cases. Nephrol Dial Transplant 2010; 25:3386–92 [DOI] [PubMed] [Google Scholar]
- 7. Govindarajulu S, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Staphylococcus aureus peritonitis in Australian peritoneal dialysis patients: predictors, treatment, and outcomes in 503 cases. Perit Dial Int 2009; 30:311–19 [DOI] [PubMed] [Google Scholar]
- 8. Jarvis EM, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Predictors, treatment, and outcomes of non-Pseudomonas gram-negative peritonitis. Kidney Int 2010; 78:408–14 [DOI] [PubMed] [Google Scholar]
- 9. Jose MD, Johnson DW, Mudge DW, Tranæus A, Voss D, Walker R, et al. Peritoneal dialysis practice in Australia and New Zealand: a call to action. Nephrology (Carlton) 2011; 16:19–29 [DOI] [PubMed] [Google Scholar]
- 10. Miles R, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Predictors and outcomes of fungal peritonitis in peritoneal dialysis patients. Kidney Int 2009; 76:622–8 [DOI] [PubMed] [Google Scholar]
- 11. O’Shea S, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Streptococcal peritonitis in Australian peritoneal dialysis patients: predictors, treatment and outcomes in 287 cases. BMC Nephrol 2009; 10:19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Siva B, Hawley CM, McDonald SP, Brown FG, Rosman JB, Wiggins KJ, et al. Pseudomonas peritonitis in Australia: predictors, treatment, and outcomes in 191 cases. Clin J Am Soc Nephrol 2009; 4:957–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim MJ, Song JH, Park YJ, Kim GA, Lee SW. The influence of seasonal factors on the incidence of peritonitis in continuous ambulatory peritoneal dialysis in the temperate zone. Adv Perit Dial 2000; 16:243–7 [PubMed] [Google Scholar]
- 14. Szeto CC, Chow KM, Wong TY, Leung CB, Li PK. Influence of climate on the incidence of peritoneal dialysis-related peritonitis. Perit Dial Int 2003; 23:580–6 [PubMed] [Google Scholar]
- 15. Quinn MJ, Hasbargen JA, Hasbargen BJ. When does peritonitis occur? Perit Dial Int 1994; 14:172–4 [PubMed] [Google Scholar]
- 16. Alves FR, Dantas RC, Lugon JR. Higher incidence of catheter-related infections in a tropical climate. Adv Perit Dial 1993; 9:244–7 [PubMed] [Google Scholar]
- 17. Chan MK, Chan CY, Cheng IK, Ng WS. Climatic factors and peritonitis in CAPD patients. Int J Artif Organs 1989; 12:366–8 [PubMed] [Google Scholar]
- 18. Piraino B, Bailie GR, Bernardini J, Boeschoten E, Gupta A, Holmes C, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int 2005; 25:107–31 [PubMed] [Google Scholar]
- 19. Piraino B, Bernardini J, Brown E, Figueiredo A, Johnson DW, Lye WC, et al. ISPD position statement on reducing the risks of peritoneal dialysis-related infections. Perit Dial Int 2011; 31:614–30 [DOI] [PubMed] [Google Scholar]
- 20. Johnson D, Brown F, Lammi H, Walker R. on behalf of Caring for Australians with Renal Impairment (CARI). The CARI guidelines. Dialysis adequacy (PD) guidelines. Nephrology (Carlton) 2005; 10(Suppl 4):S81–107 [DOI] [PubMed] [Google Scholar]
- 21. The CARI guidelines. Evidence for peritonitis treatment and prophylaxis: type of peritoneal dialysis catheter. Nephrology 2004; 9(Suppl 3):S59–64 [DOI] [PubMed] [Google Scholar]
- 22. The CARI guidelines. Evidence for peritonitis treatment and prophylaxis: treatment of peritoneal dialysis-associated peritonitis in adults. Nephrology (Carlton) 2004; 9(Suppl 3):S91–106 [DOI] [PubMed] [Google Scholar]
- 23. Martin LC, Caramori JC, Fernandes N, Divino-Filho JC, Pecoits-Filho R, Barretti P. on behalf of the Brazilian Peritoneal Dialysis Multicenter Study BRAZPD Group. Geographic and educational factors and risk of the first peritonitis episode in Brazilian Peritoneal Dialysis Study (BRAZPD) patients. Clin J Am Soc Nephrol 2011; 6:1944–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stinghen AE, Barretti P, Pecoits-Filho R. Factors contributing to the differences in peritonitis rates between centers and regions. Perit Dial Int 2007; 27(Suppl 2):S281–5 [PubMed] [Google Scholar]
- 25. Matuszkiewicz-Rowinska J. Update on fungal peritonitis and its treatment. Perit Dial Int 2009; 29(Suppl 2):S161–5 [PubMed] [Google Scholar]
- 26. Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol 2002; 5:366–71 [DOI] [PubMed] [Google Scholar]
- 27. Lo WK, Chan CY, Cheng SW, Poon JF, Chan DT, Cheng IK. A prospective randomized control study of oral nystatin prophylaxis for Candida peritonitis complicating continuous ambulatory peritoneal dialysis. Am J Kidney Dis 1996; 28:549–52 [DOI] [PubMed] [Google Scholar]
- 28. Restrepo C, Chacon J, Manjarres G. Fungal peritonitis in peritoneal dialysis patients: successful prophylaxis with fluconazole, as demonstrated by prospective randomized control trial. Perit Dial Int 2010; 30:619–25 [DOI] [PubMed] [Google Scholar]


