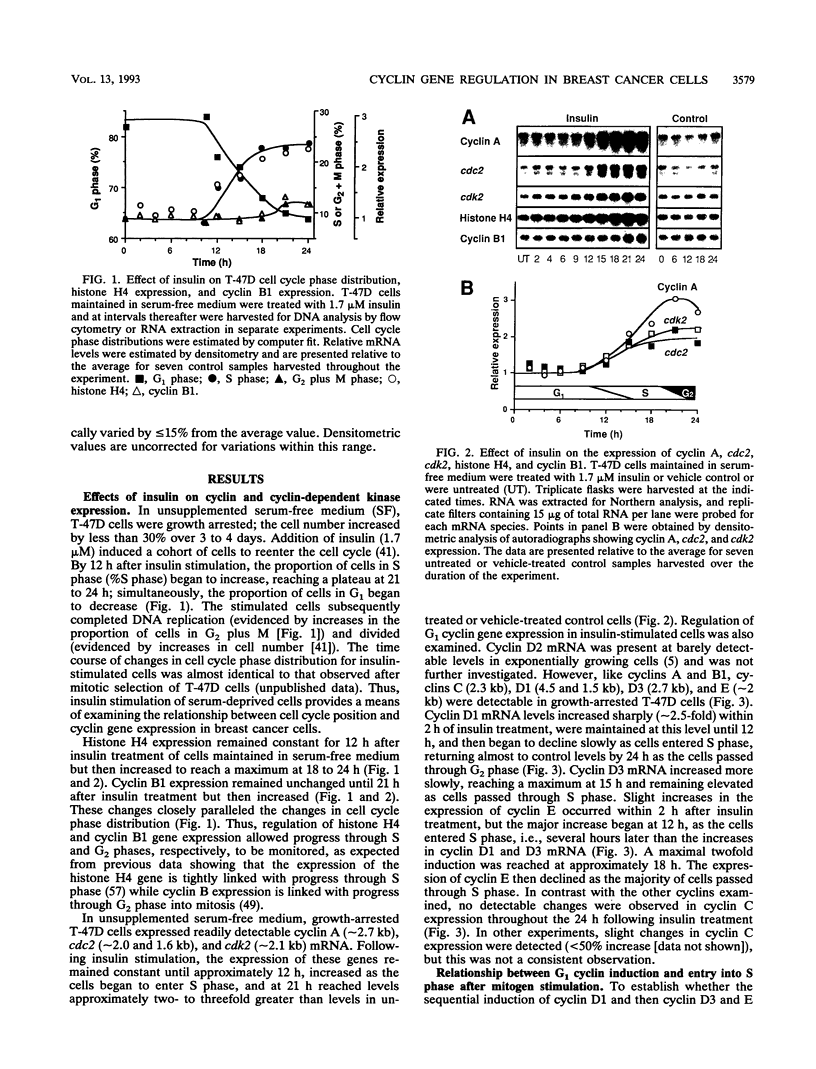
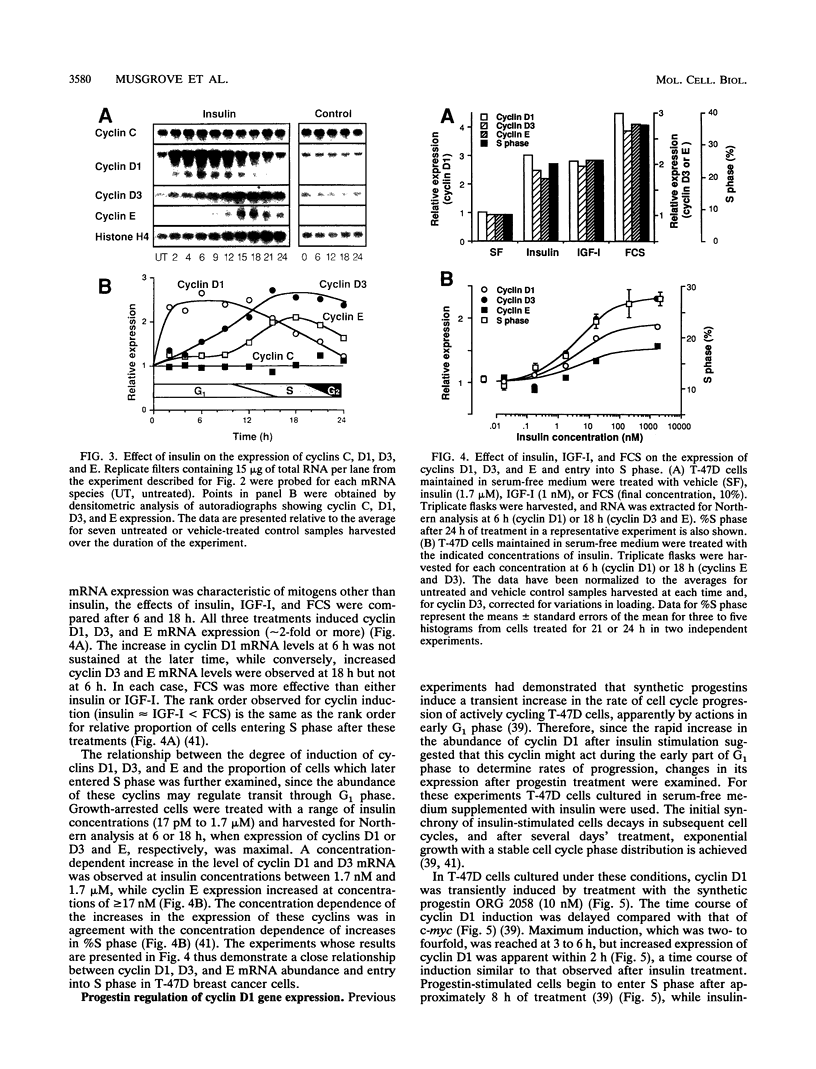
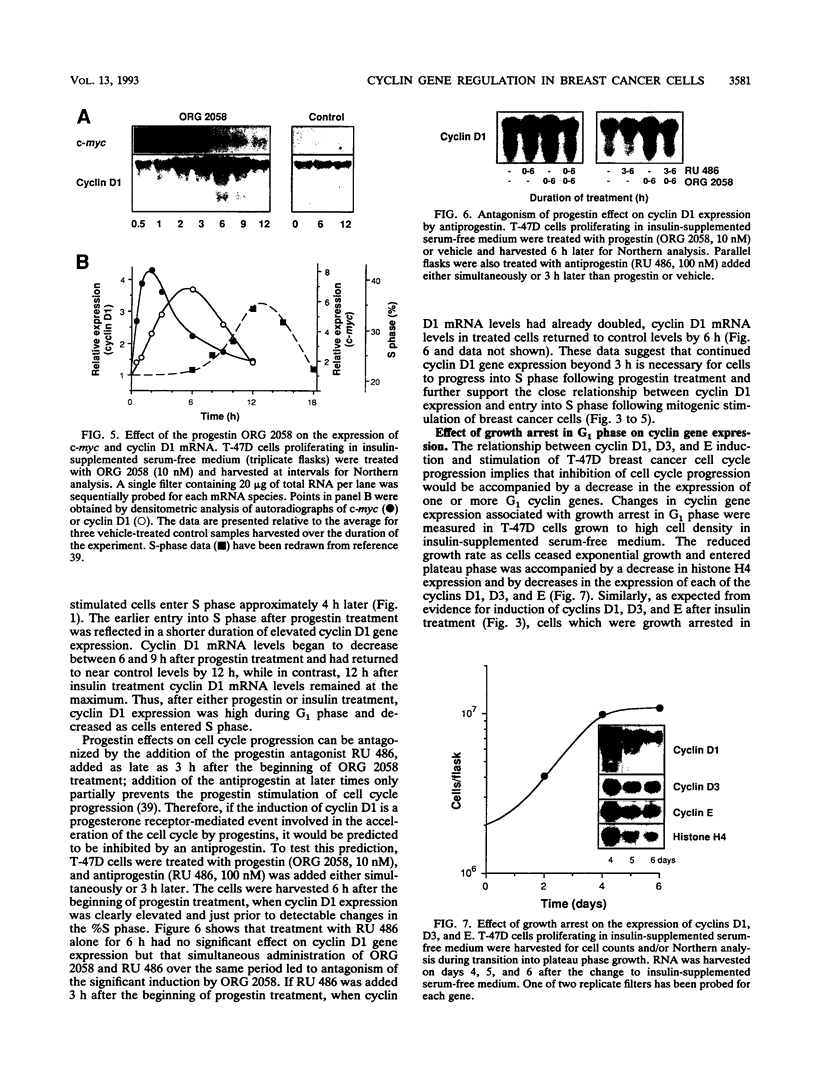
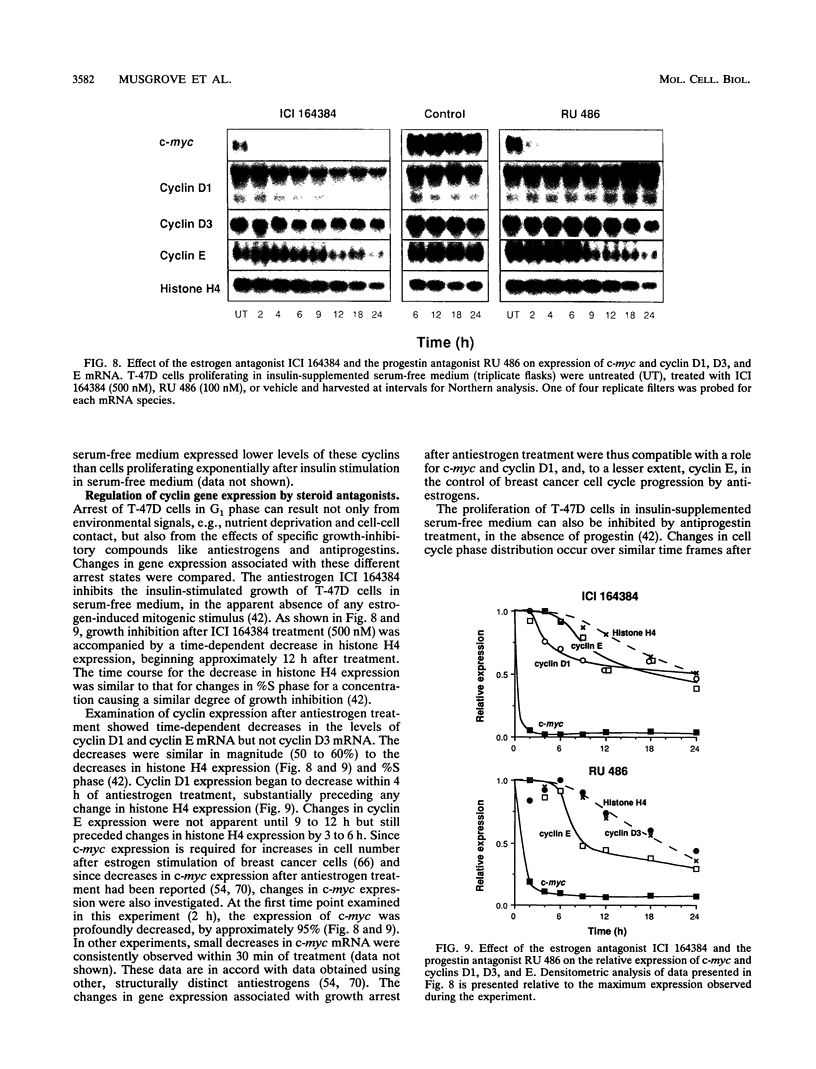
Abstract
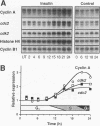
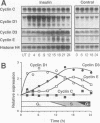
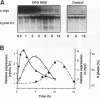
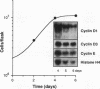
Cyclins and proto-oncogenes including c-myc have been implicated in eukaryotic cell cycle control. The role of cyclins in steroidal regulation of cell proliferation is unknown, but a role for c-myc has been suggested. This study investigated the relationship between regulation of T-47D breast cancer cell cycle progression, particularly by steroids and their antagonists, and changes in the levels of expression of these genes. Sequential induction of cyclins D1 (early G1 phase), D3, E, A (late G1-early S phase), and B1 (G2 phase) was observed following insulin stimulation of cell cycle progression in serum-free medium. Transient acceleration of G1-phase cells by progestin was also accompanied by rapid induction of cyclin D1, apparent within 2 h. This early induction of cyclin D1 and the ability of delayed administration of antiprogestin to antagonize progestin-induced increases in both cyclin D1 mRNA and the proportion of cells in S phase support a central role for cyclin D1 in mediating the mitogenic response in T-47D cells. Compatible with this hypothesis, antiestrogen treatment reduced the expression of cyclin D1 approximately 8 h before changes in cell cycle phase distribution accompanying growth inhibition. In the absence of progestin, antiprogestin treatment inhibited T-47D cell cycle progression but in contrast did not decrease cyclin D1 expression. Thus, changes in cyclin D1 gene expression are often, but not invariably, associated with changes in the rate of T-47D breast cancer cell cycle progression. However, both antiestrogen and antiprogestin depleted c-myc mRNA by > 80% within 2 h. These data suggest the involvement of both cyclin D1 and c-myc in the steroidal control of breast cancer cell cycle progression.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander I. E., Clarke C. L., Shine J., Sutherland R. L. Progestin inhibition of progesterone receptor gene expression in human breast cancer cells. Mol Endocrinol. 1989 Sep;3(9):1377–1386. doi: 10.1210/mend-3-9-1377. [DOI] [PubMed] [Google Scholar]
- Ali I. U., Merlo G., Callahan R., Lidereau R. The amplification unit on chromosome 11q13 in aggressive primary human breast tumors entails the bcl-1, int-2 and hst loci. Oncogene. 1989 Jan;4(1):89–92. [PubMed] [Google Scholar]
- Baserga R. The cell cycle: myths and realities. Cancer Res. 1990 Nov 1;50(21):6769–6771. [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Chan Y. L., Gutell R., Noller H. F., Wool I. G. The nucleotide sequence of a rat 18 S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18 S ribosomal ribonucleic acid. J Biol Chem. 1984 Jan 10;259(1):224–230. [PubMed] [Google Scholar]
- Cocks B. G., Vairo G., Bodrug S. E., Hamilton J. A. Suppression of growth factor-induced CYL1 cyclin gene expression by antiproliferative agents. J Biol Chem. 1992 Jun 15;267(17):12307–12310. [PubMed] [Google Scholar]
- Dubik D., Dembinski T. C., Shiu R. P. Stimulation of c-myc oncogene expression associated with estrogen-induced proliferation of human breast cancer cells. Cancer Res. 1987 Dec 15;47(24 Pt 1):6517–6521. [PubMed] [Google Scholar]
- Dulić V., Lees E., Reed S. I. Association of human cyclin E with a periodic G1-S phase protein kinase. Science. 1992 Sep 25;257(5078):1958–1961. doi: 10.1126/science.1329201. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Richman R., Hall F. L., Williams R. T., Lodgson N., Harper J. W. CDK2 encodes a 33-kDa cyclin A-associated protein kinase and is expressed before CDC2 in the cell cycle. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2907–2911. doi: 10.1073/pnas.89.7.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge S. J., Spottswood M. R. A new human p34 protein kinase, CDK2, identified by complementation of a cdc28 mutation in Saccharomyces cerevisiae, is a homolog of Xenopus Eg1. EMBO J. 1991 Sep;10(9):2653–2659. doi: 10.1002/j.1460-2075.1991.tb07808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawell S. E., Lees J. A., White R., Parker M. G. Characterization and colocalization of steroid binding and dimerization activities in the mouse estrogen receptor. Cell. 1990 Mar 23;60(6):953–962. doi: 10.1016/0092-8674(90)90343-d. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Piwnica-Worms H., Ernst T. J., Kanakura Y., Griffin J. D. cdc2 gene expression at the G1 to S transition in human T lymphocytes. Science. 1990 Nov 9;250(4982):805–808. doi: 10.1126/science.2237430. [DOI] [PubMed] [Google Scholar]
- Girard F., Strausfeld U., Fernandez A., Lamb N. J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991 Dec 20;67(6):1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Heikkila R., Schwab G., Wickstrom E., Loke S. L., Pluznik D. H., Watt R., Neckers L. M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. 1987 Jul 30-Aug 5Nature. 328(6129):445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Pines J. Cyclins and cancer. Cell. 1991 Sep 20;66(6):1071–1074. doi: 10.1016/0092-8674(91)90028-w. [DOI] [PubMed] [Google Scholar]
- Inaba T., Matsushime H., Valentine M., Roussel M. F., Sherr C. J., Look A. T. Genomic organization, chromosomal localization, and independent expression of human cyclin D genes. Genomics. 1992 Jul;13(3):565–574. doi: 10.1016/0888-7543(92)90126-d. [DOI] [PubMed] [Google Scholar]
- Keydar I., Chen L., Karby S., Weiss F. R., Delarea J., Radu M., Chaitcik S., Brenner H. J. Establishment and characterization of a cell line of human breast carcinoma origin. Eur J Cancer. 1979 May;15(5):659–670. doi: 10.1016/0014-2964(79)90139-7. [DOI] [PubMed] [Google Scholar]
- Koff A., Cross F., Fisher A., Schumacher J., Leguellec K., Philippe M., Roberts J. M. Human cyclin E, a new cyclin that interacts with two members of the CDC2 gene family. Cell. 1991 Sep 20;66(6):1217–1228. doi: 10.1016/0092-8674(91)90044-y. [DOI] [PubMed] [Google Scholar]
- Koff A., Giordano A., Desai D., Yamashita K., Harper J. W., Elledge S., Nishimoto T., Morgan D. O., Franza B. R., Roberts J. M. Formation and activation of a cyclin E-cdk2 complex during the G1 phase of the human cell cycle. Science. 1992 Sep 18;257(5077):1689–1694. doi: 10.1126/science.1388288. [DOI] [PubMed] [Google Scholar]
- Koga M., Musgrove E. A., Sutherland R. L. Modulation of the growth-inhibitory effects of progestins and the antiestrogen hydroxyclomiphene on human breast cancer cells by epidermal growth factor and insulin. Cancer Res. 1989 Jan 1;49(1):112–116. [PubMed] [Google Scholar]
- Koga M., Sutherland R. L. Epidermal growth factor partially reverses the inhibitory effects of antiestrogens on T 47D human breast cancer cell growth. Biochem Biophys Res Commun. 1987 Jul 31;146(2):739–745. doi: 10.1016/0006-291x(87)90591-2. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Norbury C. J., Spurr N. K., Nurse P. Regulated expression and phosphorylation of a possible mammalian cell-cycle control protein. Nature. 1988 Jun 16;333(6174):676–679. doi: 10.1038/333676a0. [DOI] [PubMed] [Google Scholar]
- Leung B. S., Potter A. H. Mode of estrogen action on cell proliferation in CAMA-1 cells: II. Sensitivity of G1 phase population. J Cell Biochem. 1987 Jul;34(3):213–225. doi: 10.1002/jcb.240340307. [DOI] [PubMed] [Google Scholar]
- Lew D. J., Dulić V., Reed S. I. Isolation of three novel human cyclins by rescue of G1 cyclin (Cln) function in yeast. Cell. 1991 Sep 20;66(6):1197–1206. doi: 10.1016/0092-8674(91)90042-w. [DOI] [PubMed] [Google Scholar]
- Lippman M. E., Bolan G. Oestrogen-responsive human breast cancer in long term tissue culture. Nature. 1975 Aug 14;256(5518):592–593. doi: 10.1038/256592a0. [DOI] [PubMed] [Google Scholar]
- Lippman M., Bolan G., Huff K. The effects of estrogens and antiestrogens on hormone-responsive human breast cancer in long-term tissue culture. Cancer Res. 1976 Dec;36(12):4595–4601. [PubMed] [Google Scholar]
- Lu X. P., Koch K. S., Lew D. J., Dulic V., Pines J., Reed S. I., Hunter T., Leffert H. L. Induction of cyclin mRNA and cyclin-associated histone H1 kinase during liver regeneration. J Biol Chem. 1992 Feb 15;267(5):2841–2844. [PubMed] [Google Scholar]
- Matsushime H., Ewen M. E., Strom D. K., Kato J. Y., Hanks S. K., Roussel M. F., Sherr C. J. Identification and properties of an atypical catalytic subunit (p34PSK-J3/cdk4) for mammalian D type G1 cyclins. Cell. 1992 Oct 16;71(2):323–334. doi: 10.1016/0092-8674(92)90360-o. [DOI] [PubMed] [Google Scholar]
- Matsushime H., Roussel M. F., Ashmun R. A., Sherr C. J. Colony-stimulating factor 1 regulates novel cyclins during the G1 phase of the cell cycle. Cell. 1991 May 17;65(4):701–713. doi: 10.1016/0092-8674(91)90101-4. [DOI] [PubMed] [Google Scholar]
- McGowan C. H., Russell P., Reed S. I. Periodic biosynthesis of the human M-phase promoting factor catalytic subunit p34 during the cell cycle. Mol Cell Biol. 1990 Jul;10(7):3847–3851. doi: 10.1128/mcb.10.7.3847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson M., Enders G. H., Wu C. L., Su L. K., Gorka C., Nelson C., Harlow E., Tsai L. H. A family of human cdc2-related protein kinases. EMBO J. 1992 Aug;11(8):2909–2917. doi: 10.1002/j.1460-2075.1992.tb05360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Motokura T., Keyomarsi K., Kronenberg H. M., Arnold A. Cloning and characterization of human cyclin D3, a cDNA closely related in sequence to the PRAD1/cyclin D1 proto-oncogene. J Biol Chem. 1992 Oct 5;267(28):20412–20415. [PubMed] [Google Scholar]
- Murray A. W., Kirschner M. W. Dominoes and clocks: the union of two views of the cell cycle. Science. 1989 Nov 3;246(4930):614–621. doi: 10.1126/science.2683077. [DOI] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Musgrove E. A., Lee C. S., Sutherland R. L. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor alpha, epidermal growth factor receptor, c-fos, and c-myc genes. Mol Cell Biol. 1991 Oct;11(10):5032–5043. doi: 10.1128/mcb.11.10.5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove E. A., Sutherland R. L. Steroids, growth factors, and cell cycle controls in breast cancer. Cancer Treat Res. 1991;53:305–331. doi: 10.1007/978-1-4615-3940-7_15. [DOI] [PubMed] [Google Scholar]
- Musgrove E. A., Wakeling A. E., Sutherland R. L. Points of action of estrogen antagonists and a calmodulin antagonist within the MCF-7 human breast cancer cell cycle. Cancer Res. 1989 May 1;49(9):2398–2404. [PubMed] [Google Scholar]
- Ninomiya-Tsuji J., Nomoto S., Yasuda H., Reed S. I., Matsumoto K. Cloning of a human cDNA encoding a CDC2-related kinase by complementation of a budding yeast cdc28 mutation. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9006–9010. doi: 10.1073/pnas.88.20.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauli U., Chrysogelos S., Stein G., Stein J., Nick H. Protein-DNA interactions in vivo upstream of a cell cycle-regulated human H4 histone gene. Science. 1987 Jun 5;236(4806):1308–1311. doi: 10.1126/science.3035717. [DOI] [PubMed] [Google Scholar]
- Pines J. Cyclins: wheels within wheels. Cell Growth Differ. 1991 Jun;2(6):305–310. [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Reddel R. R., Murphy L. C., Sutherland R. L. Factors affecting the sensitivity of T-47D human breast cancer cells to tamoxifen. Cancer Res. 1984 Jun;44(6):2398–2405. [PubMed] [Google Scholar]
- Reed S. I. G1-specific cyclins: in search of an S-phase-promoting factor. Trends Genet. 1991 Mar;7(3):95–99. doi: 10.1016/0168-9525(91)90279-Y. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Santos G. F., Scott G. K., Lee W. M., Liu E., Benz C. Estrogen-induced post-transcriptional modulation of c-myc proto-oncogene expression in human breast cancer cells. J Biol Chem. 1988 Jul 15;263(20):9565–9568. [PubMed] [Google Scholar]
- Sarup J. C., Rao K. V., Fox C. F. Decreased progesterone binding and attenuated progesterone action in cultured human breast carcinoma cells treated with epidermal growth factor. Cancer Res. 1988 Sep 15;48(18):5071–5078. [PubMed] [Google Scholar]
- Sutherland R. L., Hall R. E., Pang G. Y., Musgrove E. A., Clarke C. L. Effect of medroxyprogesterone acetate on proliferation and cell cycle kinetics of human mammary carcinoma cells. Cancer Res. 1988 Sep 15;48(18):5084–5091. [PubMed] [Google Scholar]
- Sutherland R. L., Hall R. E., Taylor I. W. Cell proliferation kinetics of MCF-7 human mammary carcinoma cells in culture and effects of tamoxifen on exponentially growing and plateau-phase cells. Cancer Res. 1983 Sep;43(9):3998–4006. [PubMed] [Google Scholar]
- Taylor I. W., Hodson P. J., Green M. D., Sutherland R. L. Effects of tamoxifen on cell cycle progression of synchronous MCF-7 human mammary carcinoma cells. Cancer Res. 1983 Sep;43(9):4007–4010. [PubMed] [Google Scholar]
- Theillet C., Adnane J., Szepetowski P., Simon M. P., Jeanteur P., Birnbaum D., Gaudray P. BCL-1 participates in the 11q13 amplification found in breast cancer. Oncogene. 1990 Jan;5(1):147–149. [PubMed] [Google Scholar]
- Tsai L. H., Harlow E., Meyerson M. Isolation of the human cdk2 gene that encodes the cyclin A- and adenovirus E1A-associated p33 kinase. Nature. 1991 Sep 12;353(6340):174–177. doi: 10.1038/353174a0. [DOI] [PubMed] [Google Scholar]
- Vignon F., Bouton M. M., Rochefort H. Antiestrogens inhibit the mitogenic effect of growth factors on breast cancer cells in the total absence of estrogens. Biochem Biophys Res Commun. 1987 Aug 14;146(3):1502–1508. doi: 10.1016/0006-291x(87)90819-9. [DOI] [PubMed] [Google Scholar]
- Wakeling A. E. Regulatory mechanisms in breast cancer. Steroidal pure antiestrogens. Cancer Treat Res. 1991;53:239–257. [PubMed] [Google Scholar]
- Watson P. H., Pon R. T., Shiu R. P. Inhibition of c-myc expression by phosphorothioate antisense oligonucleotide identifies a critical role for c-myc in the growth of human breast cancer. Cancer Res. 1991 Aug 1;51(15):3996–4000. [PubMed] [Google Scholar]
- Webster N. J., Green S., Jin J. R., Chambon P. The hormone-binding domains of the estrogen and glucocorticoid receptors contain an inducible transcription activation function. Cell. 1988 Jul 15;54(2):199–207. doi: 10.1016/0092-8674(88)90552-1. [DOI] [PubMed] [Google Scholar]
- Withers D. A., Harvey R. C., Faust J. B., Melnyk O., Carey K., Meeker T. C. Characterization of a candidate bcl-1 gene. Mol Cell Biol. 1991 Oct;11(10):4846–4853. doi: 10.1128/mcb.11.10.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Won K. A., Xiong Y., Beach D., Gilman M. Z. Growth-regulated expression of D-type cyclin genes in human diploid fibroblasts. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9910–9914. doi: 10.1073/pnas.89.20.9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong M. S., Murphy L. C. Differential regulation of c-myc by progestins and antiestrogens in T-47D human breast cancer cells. J Steroid Biochem Mol Biol. 1991 Jul;39(1):39–44. doi: 10.1016/0960-0760(91)90010-3. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Connolly T., Futcher B., Beach D. Human D-type cyclin. Cell. 1991 May 17;65(4):691–699. doi: 10.1016/0092-8674(91)90100-d. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Menninger J., Beach D., Ward D. C. Molecular cloning and chromosomal mapping of CCND genes encoding human D-type cyclins. Genomics. 1992 Jul;13(3):575–584. doi: 10.1016/0888-7543(92)90127-e. [DOI] [PubMed] [Google Scholar]