Abstract
♦ Background: Studies in hemodialysis patients suggest that the “surprise” question can help to identify a group of patients with a high mortality risk who should receive priority for palliative care interventions. However, the same instrument has not been tested in peritoneal dialysis (PD) patients.
♦ Method: We studied 367 prevalent PD patients from a single dialysis center. Three clinicians independently answered the “surprise” question (Would I be surprised if this patient died within the next 12 months?) according to their clinical impression of the individual patient. Patients are then classified into “yes” (yes, surprised) and “no” (no, not surprised) groups. All patients were followed for 12 months.
♦ Results: In this cohort, 109 patients (29.7%) were allocated to the “no” group, and 258 (70.3%), to the “yes” group. Patients in the “no” group were older and had high prevalences of pre-existing ischemic heart disease, cerebrovascular disease, and peripheral vascular disease. The “no” group had a higher score on the Charlson comorbidity index and a higher malnutrition-inflammation score. At 12 months, 44 patients had died. Mortality was 24.8% in the “no” group and 6.6% in the “yes” group. Multivariate analysis showed that an opinion of “Not surprised if dies in the next 12 months” was an independent predictor of 12-month mortality, with an associated 3.594 excess mortality risk (95% confidence interval: 1.411 to 9.151; p = 0.007). The positive predictive value of this opinion was 24.8%, and its negative predictive value was 93.4%.
♦ Conclusions: The “surprise” question has the potential to help identify a group of PD patients with high short-term mortality. Its use may contribute to a decision to refer PD patients for early palliative care assessment.
Key words: Survival, renal failure, uremia
Long-term dialysis is a life-saving treatment for patients with end-stage renal disease (ESRD). However, in a small number of those patients, clinical conditions and the individual’s level of self-sufficiency raise questions about whether dialysis may actually be futile—worsening the person’s quality of life or simply prolonging the dying process (1). In fact, for elderly patients with advanced chronic kidney disease, the risk of death or functional decline within a relatively short time is substantial (2), favoring a conservative approach to the management of those patients.
Given the evolving epidemiologic scenario in ESRD, there is a growing need to rely on solid data to decide whether to recommend conservative therapy rather than long-term dialysis (3). Unfortunately, little evidence has been published on how to select patients with advanced chronic kidney disease for conservative treatment (1,4,5). Although dialysis is generally associated with longer survival in patients more than 75 years of age, patients with multiple comorbidities— ischemic heart disease in particular—do not survive longer than those treated conservatively (6,7). However, the relevant studies have not touched on the cardinal problem: How to identify ESRD patients who should be treated conservatively?
In nonrenal patients, the “surprise” question (Would I be surprised if this patient died in the next 12 months?) has been recognized as a valid tool for the identification of patients with a poor prognosis who are appropriately offered palliative care (8,9). The “surprise” question has been tested and found to be effective in a primary care population in the Franciscan Health System in Tacoma, Washington, USA (8). Application of the “surprise” question has been well-tested in hemodialysis patients. For example, in a prospective cohort study of 147 patients in 3 hemodialysis dialysis units, Moss et al. (10) found that the “surprise” question was effective in identifying sicker dialysis patients with a high risk for early mortality who should receive priority for palliative care interventions. In another cohort of 512 patients who were receiving long-term hemodialysis at 5 dialysis clinics, Cohen et al. (11) also found that the “surprise” question stood out as an independent predictor of mortality within 6 months, which contributed to an improvement in end-of-life care by providing more accurate prognostic information. However, the applicability of the “surprise” question has not been tested in peritoneal dialysis (PD) patients. The purpose of the present study was to evaluate the clinical characteristics of PD patients who were classified into a “no, I would not be surprised” group in response to the “surprise” question, and to determine the prognostic value of the “surprise” question in identifying PD patients with a high risk for early death.
METHODS
PATIENT SELECTION
The study evaluated all 367 adult patients who, on 1 February 2010, had been receiving PD for more than 1 month at our center. After a baseline assessment, all patients were prospectively followed for 1 year.
“SURPRISE” QUESTION
The “surprise” question was put to 3 independent assessors who were all clinicians. Two were nephrologists, and one was a nephrology trainee. Their clinical experience ranged from 8 to 20 years since graduation. The assessors were directly involved in the long-term care of the PD patients.
The method for administering the “surprise” question has previously been described (11). Briefly, the assessors were required to answer the “surprise” question for each patient in the entire cohort, taking into account the patient’s recent general clinical progress, overall wellbeing, and impression made. While answering the “surprise” question, the assessors had to respond within 5 minutes and, to minimize assessment bias, were blinded to all clinical and laboratory information, including the patient’s age, sex, and duration on dialysis. By not being allowed to review any clinical information, the assessors had to respond based purely on clinical impression. The “surprise” question would be left unanswered if the assessor could not recall a particular patient.
The patients were then allocated to one of two groups: “Yes, I would be surprised” (the “yes” group) or “No, I would not be surprised” (the “no” group).
COLLECTION OF CLINICAL DATA
A chart review collected baseline demographic, clinical, and biochemical data on the patients assessed using the “surprise” question. Demographics such as sex, age, cause of end-stage renal failure, and duration of dialysis were recorded. Any history of ischemic heart disease, peripheral vascular disease, cerebrovascular disease, chronic lung disease, immunologic disease, and malignancy was also noted, and Charlson comorbidity index (CCI) scores were computed accordingly.
Peritoneal transport parameters, represented as the dialysate-to-plasma (D/P) ratio of creatinine at 4 hours and the mass transfer area coefficient (MTAC) of creatinine, determined using the standard peritoneal equilibration test (PET) at about 1 month after PD initiation, were retrieved for the analysis. Dialysis adequacy was determined during a baseline assessment and then annually by 24-hour dialysate and urine collections. Total weekly Kt/V was determined using standard methods (12). Residual glomerular filtration rate (GFR) was calculated as the average of the 24-hour urinary urea and creatinine clearance (13).
Nutrition status was represented by serum albumin, subjective global assessment (SGA), a comprehensive malnutrition-inflammation score (MIS), normalized protein equivalent of nitrogen appearance (nPNA), and percentage fat-free, edema-free body mass (FEBM). For the SGA, the four-item 7-point scoring system, which has been validated in PD patients (14), was used. Calculation of the MIS has previously been described (15). Briefly, the MIS has 4 main parts and 10 components scored 0 (normal) to 3 (very severe). The total score ranges from 0 to 30. The nPNA was determined using the Bergström formula (16). The FEBM was measured using creatinine kinetics according to the formula of Forbes and Bruining (17).
CLINICAL FOLLOW-UP
After the initial assessment, all patients were followed for 1 year. In general, patients were followed every 8 weeks—or more frequently if clinically indicated. During the follow-up period, patient management was decided by the individual clinician and was not altered by the study. The primary outcome measure was actuarial survival. For the survival analysis, censoring events included transplantation, conversion to hemodialysis, and transfer to another unit.
STATISTICAL ANALYSIS
The statistical analysis was conducted using the SPSS software application for Windows (version 15.0: SPSS, Chicago, IL, USA). Results are presented as mean ± standard deviation unless otherwise stated. Comparisons between groups were performed using the unpaired Student t-test. Correlations were tested using the Pearson or Spearman correlation coefficient as appropriate. A p value less than 0.5 was considered statistically significant. All probabilities were two-tailed.
The interobserver consistency with respect to response to the “surprise” question was calculated using the kappa statistic. Because each assessor would be more familiar with certain patients at a particular time, patients were allocated to the “no” group for the outcome analysis if any single assessor replied “no”; they were allocated to the “yes” group only when no assessor replied “no.”
Survival rates were analyzed using Kaplan-Meier survival curves. Univariate Cox analysis was performed to determine whether the “surprise” question predicted actuarial survival. A multivariate Cox model was then constructed to determine the independent predictors of survival. In addition to the response to the “surprise” question, the variables used in the model included patient age, sex, duration of dialysis, CCI score, systolic blood pressure, peritoneal transport, serum albumin, SGA, MIS, total Kt/V, residual GFR, nPNA, and FEBM. Those variables were selected either because previous studies (18-20) showed them to be important predictors of patient outcome or because they have generally been accepted as important predictors of patient survival.
RESULTS
Of the 367 PD patients, 205 (55.9%) were men. Mean age in the entire group was 60.2 ± 12.3 years. The underlying causes of ESRD were diabetes mellitus (34.0%), glomerulonephritis (30.3%), hypertensive nephropathy (10.4%), polycystic kidney disease (4.4%), urologic causes (5.5%), and other specific or unknown causes (12.0%). The mean duration of dialysis was 42 ± 27 months. For 109 of the patients (29.7%), the opinion of at least 1 assessor was “not surprised if died in the next 12 months” (“no” group). For the other 258, the opinion of all assessors was “would be surprised if died in the next 12 months” (“yes” group).
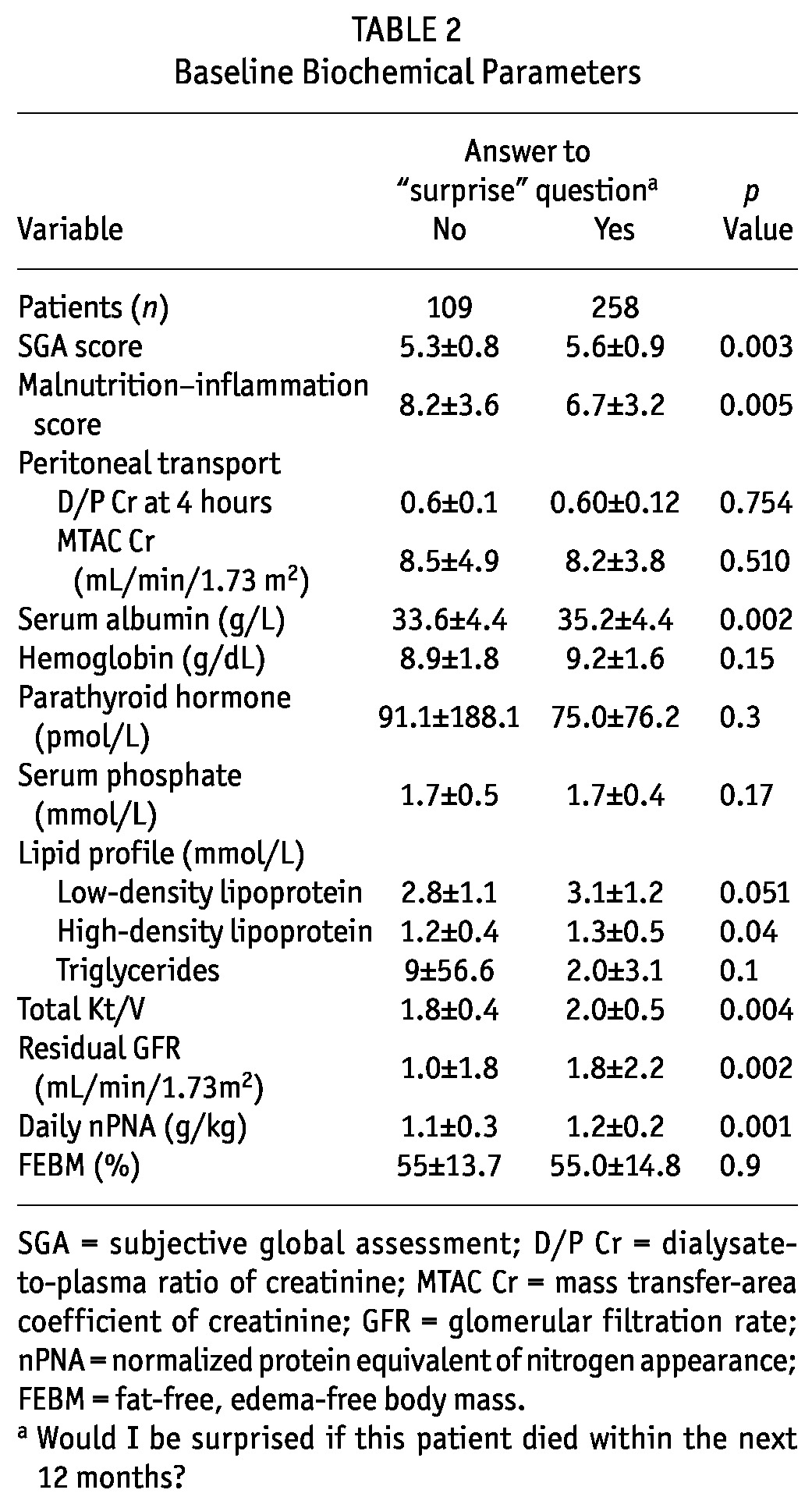
Tables 1 and 2 summarize and compare baseline clinical and biochemical parameters for the patient groups. In short, patients of the “no” group were older and had higher incidences of pre-existing ischemic heart disease, cerebrovascular disease, peripheral vascular disease, and diabetes (although the last comparison did not reach statistical significance). Compared with the “yes” group, the “no” group had higher CCI and malnutrition-inflammation scores and more peritonitis episodes in the past. The “no” group also had a lower SGA score and lower serum albumin, total Kt/V, residual GFR, and nPNA. We observed no significant differences in the use of medications between the groups (data not shown).
TABLE 1.
Baseline Patient Demographics and Clinical Parameters
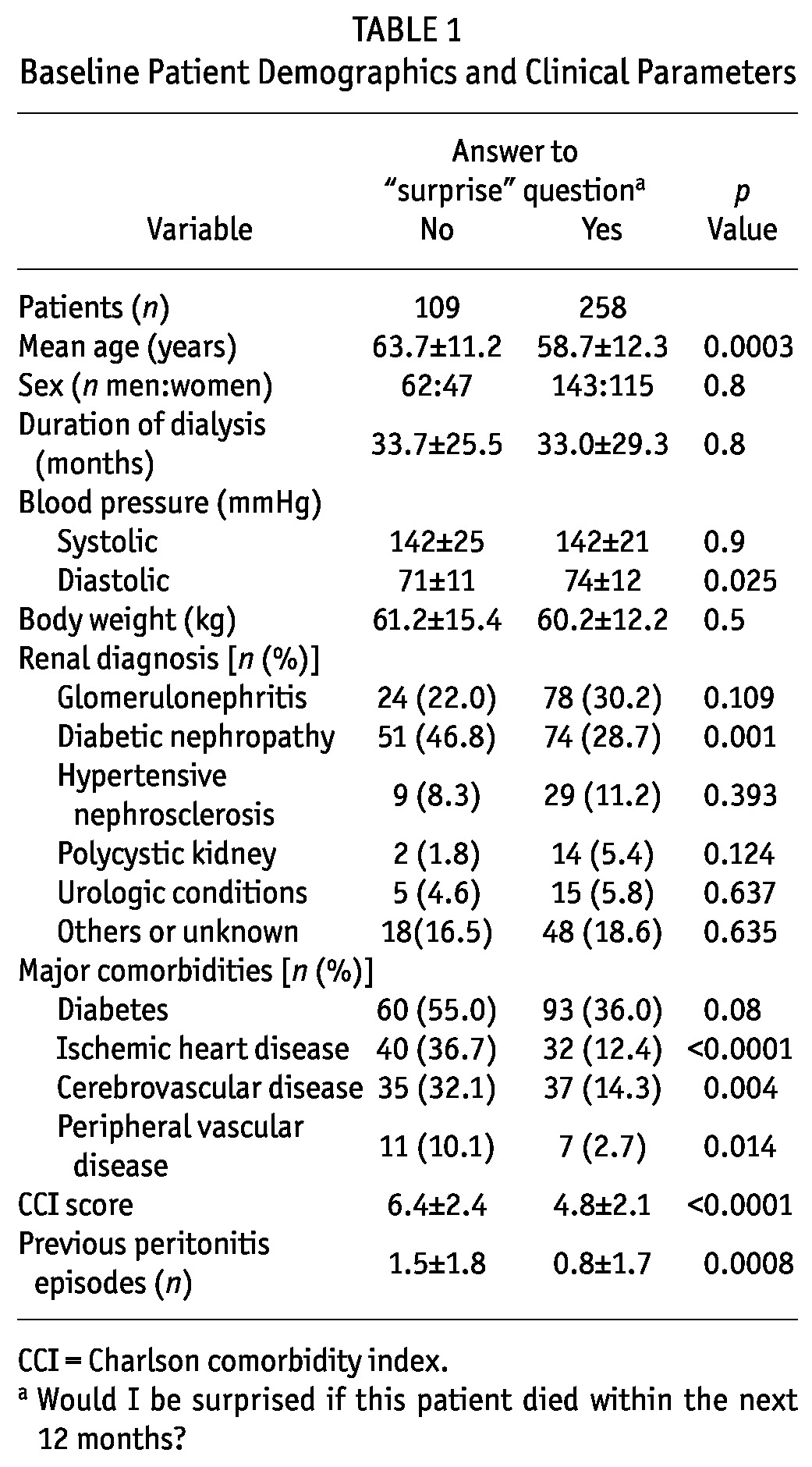
TABLE 2.
Baseline Biochemical Parameters
INTEROBSERVER VARIATION AND AGREEMENT
The response rates for the 3 assessors were 94.0%, 94.8%, and 100.0%. The kappa statistic for the assessors ranged from 0.34 to 0.41 (p < 0.001 for every comparison between a pair of assessors). In short, the interobserver agreement fell into the fair-to-moderate category. Of the 109 patients allocated to the “no” group, 19 (5.2%) were rated “not surprised” by all 3 assessors; 23 (6.3%), by 2 assessors; and 59 (16.1%), by 1 assessor.
PATIENT SURVIVAL
During the 12 months of post-rating observation, 44 patients (12.0%) died, 10 received a renal graft, and 9 were converted to long-term hemodialysis because of either peritoneal failure or inadequate dialysis. The causes of death among the 44 deceased patients were ischemic heart disease (7 patients), sudden cardiac death (6 patients), cerebrovascular disease (5 patients), peritonitis (7 patients), non-peritonitis infection (10 patients), malignancy (5 patients), liver failure (1 patient), and termination of dialysis (3 patients). Of the 44 patients, 27 (24.8%) were from the “no” group, and 17 (6.6%) were from the “yes” group. At 12 months, actuarial survival was 93.4% for the “yes” group and 75.2% for the “no” group (log rank p < 0.0001, Figure 1). The univariate Cox analysis showed a significant association of the “no” group with 12-month mortality [unadjusted hazard ratio: 4.199; 95% confidence interval (CI): 2.288 to 7.706; p < 0.0001].
Figure 1.
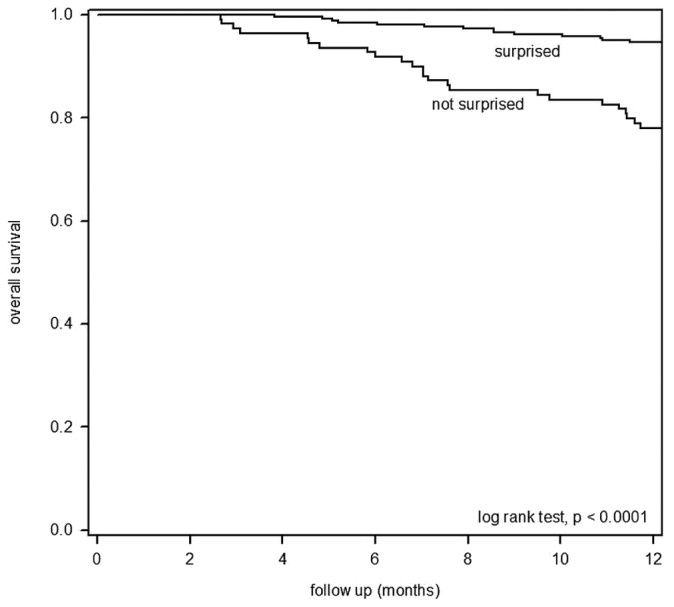
— Kaplan-Meier plot of actuarial survival for peritoneal dialysis patients from the “surprised” and “not surprised” groups.
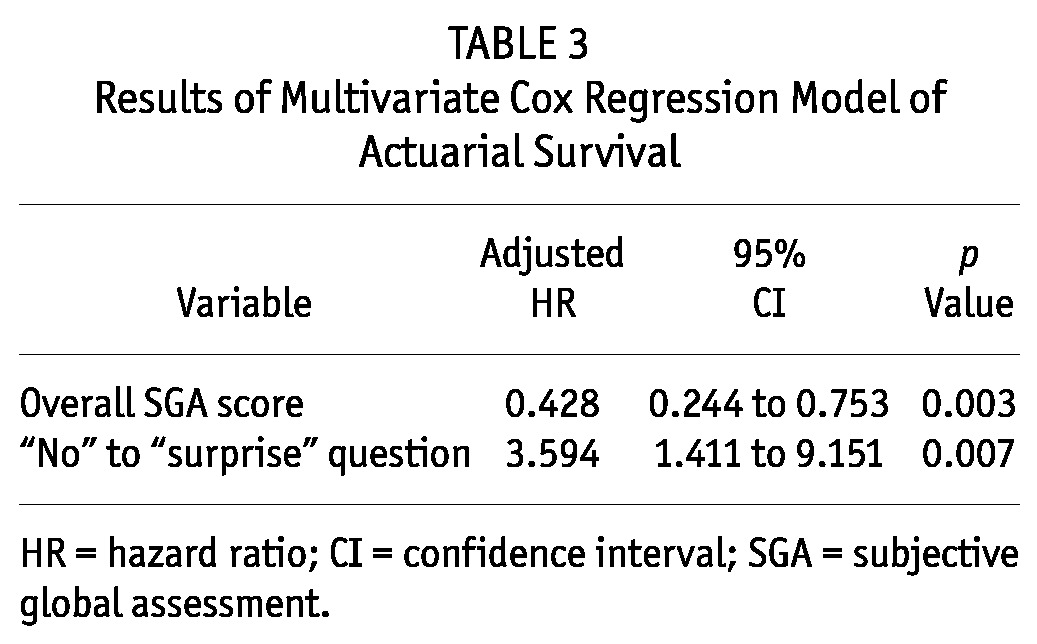
A multivariate Cox regression analysis explored the independent predictors of 12-month mortality. Table 3 summarizes the final model. In that model, overall SGA score and membership in the “no” group were independent predictors of 12-month mortality. The assessor opinion “Not surprised if dies in the next 12 months” was associated with a 3.594 excess risk of death (95% CI: 1.411 to 9.151; p = 0.007).
TABLE 3.
Results of Multivariate Cox Regression Model of Actuarial Survival
“SURPRISE” QUESTION AS A TRIAGE TOOL
We further explored the role of the “surprise” question as a triage tool for advising palliative care. As already mentioned, 27 patients from the “no” group (24.8%) and 17 patients from the “yes” group (6.6%) died within the 12-month observation. The sensitivity of the “surprise” question was 61.4%, its specificity was 70.0%, its positive predictive value was 24.8%, and its negative predictive value was 93.4%.
If the cut-off for “not surprised” had been adjusted to 2 or 3 assessors (and not just 1), then the sensitivity would have been 38.6%; the specificity, 92.3%; the positive predictive value, 40.5%; and the negative predictive value, 91.7%. If the cut-off had been adjusted to require the agreement of all 3 clinicians, then the sensitivity would have been 21.5%; the specificity, 96.7%; the positive predictive value, 42.1%; and the negative predictive value, 91.4%.
DISCUSSION
In the present study, the response to the “surprise” question was found to be an independent predictor of 12-month mortality (Table 3). We believe that the result of the present study could be extrapolated to other PD centers. Our study population was unbiased in that it included all patients managed at our center. The distribution of underlying renal diagnoses was similar to that reported by the Hong Kong Renal Registry and by the US Renal Data System. In our cohort, the 1-year mortality was 12%, which accords with previous reports from our center (20) and from other centers in Hong Kong (21). However, it is important to note that the mortality of dialysis patients in Hong Kong is much lower than the approximately 22% reported from the United States (22).
The results of the present study greatly resemble those from two previous studies in hemodialysis patients (10,11). As in those previous reports, the patients in the “no” group in the present study were older and had more comorbid conditions, a lower functional score, and a worse nutrition status than did patients in the “yes” group. In addition, most of the deaths were caused by cardiovascular disease (including sudden cardiac death, ischemic heart disease, and cerebrovascular disease) and infection, each accounting for about 40% of the total mortality. This distribution of causes of death is similar to that in an earlier report from our center (20) and for the overall Hong Kong PD population (21). Because of the limited number of events, we did not perform further subgroup analyses to explore whether the “surprise” question tends to predict infection-related or cardiovascular-related mortality.
There is an important difference between our study and the two earlier ones from Hong Kong. In the present study, nearly 30% of the PD patients were allocated to the “no” group, but the proportion in the other studies was 16% - 23% (10,11). In our analysis, we allocated patients to the “no” group if any of the 3 assessors gave the “not surprised” response. Our rationale was that each assessor might be more familiar with particular patients, and a “not surprised” response for a given patient by any one assessor therefore probably had good reasoning behind it. Unlike the situation in the two earlier studies, the local medical system makes it difficult to pinpoint for each patient the most suitable clinician to answer the “surprise” question. In general, all patients in the study had been directly evaluated within the preceding 3 months by at least 1 of the 3 assessors who took part in the study. We believe that if a patient had such an uneventful course of dialysis that the assessor could not recall the patient, then that patient should be allocated to the “yes” group.
Several limitations to the study need to be considered.
First, almost all of the patients in this study were Chinese in ethnicity, and Hong Kong has a “PD First” policy. In addition, the study was conducted in a single tertiary referral center, and all 3 assessors were working in the same hospital. Moreover, the medical and nursing practice of our hospital may be different from that in other centers. In view of those considerations, the possibility of extrapolating our results to other PD populations requires validation in other studies.
Second, 3 assessors were involved in this study. That number is rather limited, and the interobserver agreement for response to the “surprise” question was at best modest. Also, the agreement reliability between the observers was rated fair-to-moderate only. In our analysis, we allocated patients to the “no” group if any assessor gave the “not surprised” response. However, in a large PD center in which each patient is managed by several clinicians, it remains uncertain whether the opinion of a single clinician should be considered significant, or whether a threshold number of clinicians must give the same response to reach a consensus opinion. Based on our data, it seems reasonable to argue that, if a patient were to be allocated to the “no” group by 2 or more assessors, then the mortality rate in the resulting group would be much higher and the associated cut-off might be used to make a decision to refer the patient for palliative care. However, even though our study found that the “surprise” question was an effective prognostic tool for 12-month survival in our PD patients, the positive predictive value was low, and more than 70% of the “no” group remained alive at 12 months. That result implies that we should not apply this tool in the decision to refer patients to palliative care. By contrast, allocation to the “yes” group implies that further aggressive treatment is justified.
It is also important to note that the present study examined existing PD patients. It remains unknown whether the “surprise” question could also be applied to ESRD patients newly referred to the PD program. To the best of our knowledge, no simple clinical tool has been tested for prognostic stratification in this important group of patients. Further study in this area is certainly necessary.
Finally, although the response to the “surprise” question was highly predictive for 12-month mortality, the sensitivity and specificity of the question as a single tool for identifying high-risk patients are not entirely satisfactory. Notably, the positive predictive value for death after an opinion of “Not surprised if dies in the next 12 months” was only 24.8%—that is, more than three quarters of the patients did not die. In other words, it may not be appropriate to decide to refer a PD patient for palliative care according to the response to the “surprise” question alone. Nonetheless, based on the multivariate analysis, it seems possible to derive a simple score, based on a combination of the response to the “surprise” question and a few clinical parameters, that will accurately identify a small group of patients likely to die in the near future. Such an approach would need further study for validation.
CONCLUSIONS
We found that the “surprise” question has the potential to help identify a group of PD patients who have a higher prospect of short-term mortality and who may be suitable for early palliative care assessment. Further studies are needed to validate the “surprise” question in other PD populations.
DISCLOSURES
All authors declare that they have no financial conflicts of interest.
Acknowledgments
This study was supported in part by the Richard Yu Chinese University of Hong Kong Peritoneal Dialysis Research Fund.
References
- 1. Fenwick S, Saxena R, Harper JM. Dialysis: when to start or when to stop? Nephrol Dial Transplant 2004; 19:1022 [DOI] [PubMed] [Google Scholar]
- 2. Wenger NS, Shekelle PG. Assessing care of vulnerable elders: ACOVE project overview. Ann Intern Med 2001; 135:642–6 [DOI] [PubMed] [Google Scholar]
- 3. Galla JH. Clinical practice guideline on shared decision-making in the appropriate initiation of and withdrawal from dialysis. The Renal Physicians Association and the American Society of Nephrology. J Am Soc Nephrol 2000; 11:1788–91 [DOI] [PubMed] [Google Scholar]
- 4. Chandna SM, Schulz J, Lawrence C, Greenwood RN, Farrington K. Is there a rationale for rationing chronic dialysis? A hospital based cohort study of factors affecting survival and morbidity. BMJ 1999; 318:217–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Smith C, Da Silva-Gane M, Chandna S, Warwicker P, Greenwood R, Farrington K. Choosing not to dialyse: evaluation of planned non-dialytic management in a cohort of patients with end-stage renal failure. Nephron Clin Pract 2003; 95:c40–6 [DOI] [PubMed] [Google Scholar]
- 6. Brunori G, Viola BF, Maiorca P, Cancarini G. How to manage elderly patients with chronic renal failure: conservative management versus dialysis. Blood Purif 2008; 26:36–40 [DOI] [PubMed] [Google Scholar]
- 7. Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? A comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant 2007; 22:1955–62 [DOI] [PubMed] [Google Scholar]
- 8. Pattison M, Romer AL. Improving care through the end of life: launching a primary care clinic-based program. J Palliat Med 2001; 4:249–54 [DOI] [PubMed] [Google Scholar]
- 9. Della Penna R. Asking the right question. J Palliat Med 2001; 4:245–8 [DOI] [PubMed] [Google Scholar]
- 10. Moss AH, Ganjoo J, Sharma S, Gansor J, Senft S, Weaner B, et al. Utility of the “surprise” question to identify dialysis patients with high mortality. Clin J Am Soc Nephrol 2008; 3:1379–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cohen LM, Ruthazer R, Moss AH, Germain MJ. Predicting six-month mortality for patients who are on maintenance hemodialysis. Clin J Am Soc Nephrol 2010; 5:72–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nolph KD, Moore HL, Twardowski ZJ, Khanna R, Prowant B, Meyer M, et al. Cross-sectional assessment of weekly urea and creatinine clearances in patients on continuous ambulatory peritoneal dialysis. ASAIO J 1992; 38:M139–42 [DOI] [PubMed] [Google Scholar]
- 13. van Olden RW, Krediet RT, Struijk DG, Arisz L. Measurement of residual renal function in patients treated with continuous peritoneal dialysis. J Am Soc Nephrol 1996; 7:745–50 [DOI] [PubMed] [Google Scholar]
- 14. Enia G, Sicuso C, Alati G, Zoccali C. Subjective global assessment of nutrition in dialysis patients. Nephrol Dial Transplant 1993; 8:1094–8 [PubMed] [Google Scholar]
- 15. Kalantar-Zadeh K, Kopple JD, Block G, Humphreys MH. A malnutrition-inflammation score is correlated with morbidity and mortality in maintenance hemodialysis patients. Am J Kidney Dis 2001; 38:1251–63 [DOI] [PubMed] [Google Scholar]
- 16. Bergström J, Heimbürger O, Lindholm B. Calculation of the protein equivalent of total nitrogen appearance from urea appearance. Which formulas should be used? Perit Dial Int 1998; 18:467–73 [PubMed] [Google Scholar]
- 17. Forbes GB, Bruining GJ. Urinary creatinine excretion and lean body mass. Am J Clin Nutr 1976; 29:1359–66 [DOI] [PubMed] [Google Scholar]
- 18. Shiao CC, Kao TW, Hung KY, Chen YC, Wu MS, Chu TS, et al. Seven-year follow-up of peritoneal dialysis patients in Taiwan. Perit Dial Int 2009; 29:450–7 [PubMed] [Google Scholar]
- 19. Gao N, Kwan BC, Chow KM, Chung KY, Pang WF, Leung CB, et al. Arterial pulse wave velocity and peritoneal transport characteristics independently predict hospitalization in Chinese peritoneal dialysis patients. Perit Dial Int 2010; 30:80–5 [DOI] [PubMed] [Google Scholar]
- 20. Szeto CC, Wong TY, Leung CB, Wang AY, Law MC, Lui SF, et al. Importance of dialysis adequacy in mortality and morbidity of Chinese CAPD patients. Kidney Int 2000; 58:400–7 [DOI] [PubMed] [Google Scholar]
- 21. Wong PN, Mak SK, Lo KY, Tong GM, Wong Y, Wong AK. Adverse prognostic indicators in continuous ambulatory peritoneal dialysis patients without obvious vascular or nutritional comorbidities. Perit Dial Int 2003; 23(Suppl 2):S109–15 [PubMed] [Google Scholar]
- 22. Mehrotra R, Story K, Guest S, Fedunyszyn M. Neighborhood location, rurality, geography, and outcomes of peritoneal dialysis patients in the United States. Perit Dial Int 2011; 32:322–31 [DOI] [PMC free article] [PubMed] [Google Scholar]


