Abstract
Background
Hypoglycaemic effect of kolaviron (KV), (biflavonoid from Garcinia kola) in streptozotocin (STZ)-diabetic rats has been established.
Objectives
To evaluate the possible protective effects of KV on cardiac, renal and hepatic tissues of STZ-diabetic rats.
Methods
This study consists of four groups of 6 rats each. Groups one and two contained non-diabetic and untreated-diabetic rats, respectively. Groups three and four were made up of KV- and glibenclamide (GB) - treated diabetic rats, respectively.
Results
STZ-intoxication caused a significant (p<0.05) increase in the relative weight of liver in diabetic rats. STZ-diabetic rats had significant increase (p<0.05) in the levels of fasting blood glucose (FBG), á-amylase and HbA1c. A marked and significant (p<0.05) increase in the levels of cardiac, renal and liver marker indices such as serum creatine kinase, lactate dehydrogenase, creatinine, urea and alanine aminotransferase were observed in untreated diabetic rats. Also, untreated diabetic rats had significantly (p<0.05) elevated urinary glucose and protein and, lowered creatinine clearance. In KV- and GB- treated groups, the levels of FBG, á-amylase and HbA1c were significantly (p<0.05) reduced, while treatment with KV significantly (p<0.05) attenuated the cardiac, renal and liver marker indices.
Conclusion
KV offered significant antidiabetic and tissues protective effects in the rats.
Keywords: Antidiabetic, Kolaviron, Streptozotocin, Tissues, Protection
Introduction
Diabetes mellitus is one of the most common chronic diseases and is characterized by absolute insulinopaenia or insufficient production of insulin or inadequate peripheral tissue response to physiological levels of insulin. Diabetic patients exhibit symptoms like hyperglycemia, polydipsia, polyuria and glucosuria. The number of adults (aged >20) with diabetes in the world is estimated to increase by 122%, from 135 million in 1995 to 300 million in 20251. Chronic hyperglycemia leads to complications such as cardiovascular diseases, renal failure and retinopathy. Modern drugs, including insulin and other oral hypoglycaemic agents such as sulfonylureas and biguanides are known to control blood glucose levels but with side effects2. Besides, these drugs are expensive and not readily accessible in developing countries3. Hence, herbal remedy may be a potential alternative for the management of diabetes since they contain compounds with antioxidant activity that can play a role in protection against damage induced during diabetes4.
Garcinia kola Heckel (Family; Guttiferae) is a herb grown in Nigeria with a characteristic bitter and resinous taste. The seed is eaten raw by people with the belief that it promotes longevity. Extracts of the plant are used in traditional African medicine for the treatment of laryngitis, cough and liver diseases5. Chemical investigations of the seed revealed the presence of Garcinia biflavanone (GB), xanthones, triterpenes and benzophenones6. Kolaviron (KV), a biflavonoid complex from the Garcinia kola seed is known to elicit hepatoprotective effect in animal model7,8. Several pharmacological effects of KV include anti-hypercholesterolemic activity9, antioxidant activity10, and hypoglycemic effects in diabetic animals11. Accordingly, this study was designed to investigate the possible protective effects of KV on the cardiac, renal and hepatic tissues of STZ-diabetic rats, which are often prone to secondary complications of the disease.
Methods
Chemicals
Streptozotocin was purchased from Sigma Chemical Co., Saint Louis, MO USA. Serum biochemical analysis was done by automated system at Tehran University Teaching Hospital, Tehran, Iran. Other chemicals were of analytical grade and the purest quality available.
Plant material and extraction
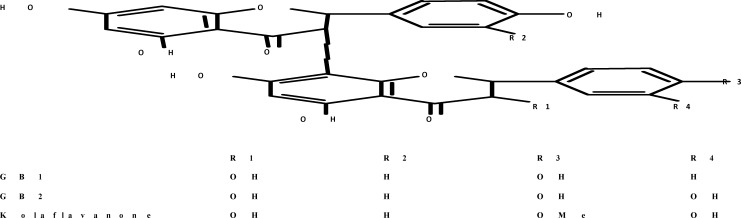
Garcinia kola seeds (Guttiferae heckel) seeds were purchased from a local vendor in Ibadan, Nigeria. Kolaviron was extracted from the fresh seeds of the Kola (3.5 kg) and characterized according to the method of Iwu et al 11, briefly, powdered seeds were extracted with light petroleum ether (b.p. 40–60°C) in a soxhlet extractor for 24 hr. The defatted, dried marc was repacked and then extracted with methanol. The extract was concentrated and diluted to twice its volume with distilled water and extracted with ethyl acetate (6.250 litres). The concentrated ethyl acetate fraction gave a yellow solid known as kolaviron (figure 1). The purity and identity of kolaviron was determined by subjecting it to thin-layer chromatography (TLC) using Silica gel GF 254-coated plates and, solvent mixture of methanol and chloroform in a ratio 1:4 v/v. The separation revealed the presence of three bands which were viewed under UV light at a wavelength of 254 nm with RF values of 0.48, 0.71 and 0.766. The yield of the preparation was 6%.
Figure 1.
Structure of Kolaviron
Animals
Male Wistar rats, 220–230 g, were used for the study. The rats were 10–12 weeks of age at the time of this study. They were bred and housed in the Central Animal House, Faculty of Pharmacy, Tehran University of Medical Sciences, University of Tehran, Iran. The animal house was well ventilated with a 12-h light-dark cycle. They were fed on normal laboratory chow and allowed free access to water for two weeks before the commencement and during the period of the experiment. Handling of animals and other protocols conform to the guidelines of the National Institutes of Health (NIH), USA (NIH publication 85-23, 1985)12.
Study design
The effect of kolaviron (KV) administered daily for 3 consecutive weeks was studied in STZ-diabetic rats. The procedure described by Sharma et al.13 was adopted in this study. Rats were fasted overnight and made hyperglycemic by a single intraperitoneal injection of STZ dissolved in 0.05M of citrate buffer (pH 4.3), at a dose of 35mg/kg14. The FBG of these rats were estimated 72 h after STZ administration, and moderately diabetic rats having FBG level above 250 mg/dL were selected and divided into three groups of 6 animals each. Group one contained non-diabetic rats (Normal), group two consisted of untreated STZ-diabetic rats, group three contained STZ-diabetic rats that received KV and group four contained glibenclamide (GB)-treated STZ-diabetic rats. KV and GB were dissolved in corn oil and normal saline, respectively, and given daily to the animals by oral gavage. KV was administered at a dose of 100 mg/kg body weight12 and, GB at a dose of 5 mg/kg15. After the last dose of the drugs, rats were fasted overnight and sacrificed by cervical dislocation. Visceral organs were obtained by dissection and immediately weighed, while blood was collected from the heart of the animals into plain centrifuge tubes. Also, 24-h urine free of food and faeces was collected into ice-cold glass tubes for the determination of protein, glucose and creatinine.
Preparation of Serum
Blood samples were allowed to stand for 1 hour and then centrifuged at 3,000 g for 15 minutes in an MSC bench centrifuge to obtain serum. The clear supernatant (serum) was used for the estimation of urea, creatinine, enzymes and other parameters.
Biochemical assays
Protein determination: Protein contents of serum and urine were determined according to Lowry et al16 using bovine serum albumin (BSA) as a standard.
Alanine and Aspartate aminotransferases (ALT and AST) determination: Serum ALT and AST activities were determined by the combined methods of Mohun and Cook17 and, Reitman and Frankel18.
Creatine kinase, creatinine and urea determination: Creatinine and urea levels were estimated by the methods of Jaffe19 and, Talke and Schubert20, respectively, while creatine kinase was measured in the presence of an antibody to CK-M monomer utilizing a kit purchased from United Diagnostics Industry (Riyadh, SA).
Determination of glucose and total bilirubin levels: Glucose and total bilirubin levels were determined by the methods of Sharma et al13 and, Rutkowski and Debaare21, respectively.
Determination of ã-glutamyl transferase (GGT) activity: The activity of serum GGT was assayed by the method of Fossati et al22.
Alpha amylase and lactate dehydrogenase (LDH) determination: The activities of á-amylase and LDH were determined by the methods of Gella et al23 and, Zimmerman and Weinstein24, respectively.
Determination of alkaline phosphatase (ALP) and HbA1c levels: HbA1c and ALP levels were determined by the methods of Hirokawa et al25 and Williamson26, respectively.
Statistical analysis
All values were expressed as the mean ± S.D. of six animals per group. Data were analyzed using oneway ANOVA followed by the post-hoc Duncan multiple range test for analysis of biochemical data using SPSS (10.0). Values were considered statistically significant at p< 0.05.
Results
Effect of KV on body weight, á-amylase, HbA1cand FBG of STZ-diabetic rats
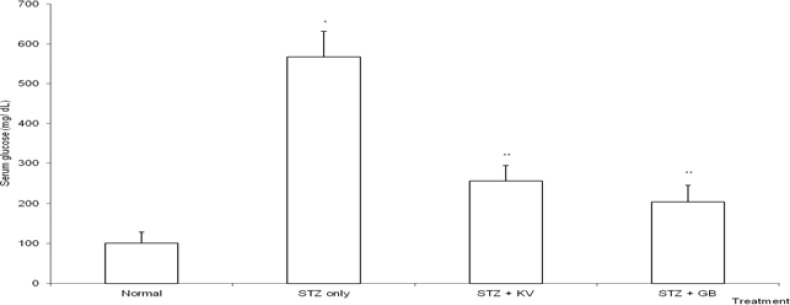
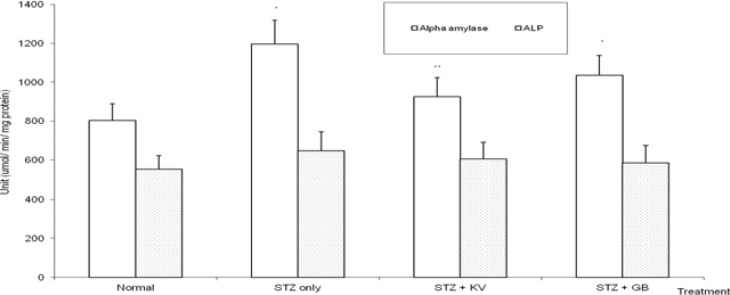
Table 1 shows the effect of KV on body weight and relative weight of organs of STZ-diabetic rats. The final body weight of STZ-diabetic rats decreased significantly (p<0.05) when compared with normal. Treatment with KV and GB significantly (p<0.05) increased the final body weight of the diabetic rats. Also, the decrease in body weight was highest for untreated STZ-diabetic rats when compared with GB- and KV- diabetic rats. In addition, the STZ-induced increase in relative weight of liver was significantly (p<0.05) attenuated in KVand GB- treated diabetic rats. At the end of this study, the FBG levels of STZ-diabetic animals treated with KV and GB decreased significantly (p<0.05) when compared with untreated diabetic group (figure 2). STZ-intoxication caused a significant (p<0.05) increase in the levels of á-amylase and HbA1c of the rats (table 2 and figure 5), however, treatment with both KV and GB significantly (p<0.05) decreased the HbA1c levels of the diabetic rats, while KV alone caused significantly (p<0.05) decrease in the activities of á-amylase when compared to untreated diabetic group. Thus, KV was more effective than GB in lowering the HbA1c levels as well as attenuating the activities of á-amylase in the diabetic rats.
Table 1.
Effect of kolaviron, a biflavonoid fraction from Garcinia kola seeds, on body weight and relative weight of organs in STZ-diabetic rats
| Treatment | Body weight (g) | Weight (g) | Relative weight (as % body weight) |
||||||
| Initial | Final | Change | Liver | Kidney | Heart | Liver | Kidney | Heart | |
| Normal | 225.1±5.3 | 241.3±6.2 | 16.2±5.6 | 6.8±0.5 | 1.3±0.1 | 0.8±0.1 | 2.8±0.3 | 0.5±0.09 | 0.3±0.05 |
| TZ only | 226.3±4.7 | 178.0±9.1* | −48.3±8.3* | 7.1±0.8 | 1.2±0.2 | 0.8±0.1 | 4.0±0.3* | 0.6±0.07 | 0.4±0.05 |
| STZ+KV | 223.4±5.5 | 215.2±4.1** | −8.2±2.4** | 6.9±0.4 | 1.3±0.2 | 0.8±0.2 | 3.2±0.2** | 0.6±0.10 | 0.4±0.03 |
| STZ+GB | 228.4±4.4 | 204.6±8.0** | −23.8±7.3* | 7.2±0.8 | 1.3±0.2 | 0.8±0.1 | 3.5±0.4** | 0.6±0.08 | 0.4±0.08 |
Values are means ± S.D. of 6 rats per group
Significantly different from normal at p< 0.05 STZ= Streptozotocin, KV= Kolaviron at 100 mg/kg
Significantly different from STZ only at p< 0.05 GB= Glibenclamide
Figure 2.
Effect of kolaviron (biflavonoid of Garcinia kola) on the levels of fasting glucose of streptozotocin (STZ)-diabetic rats
*Significantlydifferent from normal (p<0.05), **Significantly different from normal and STZ only (p<0.05), KV= Kolaviron at 100 mg/kg, GB= Glibenclamide
Table 2.
Effect of kolaviron, a biflavonoid fraction from Garcinia kola seeds, on urinary and blood biochemical indices in STZ-diabetic rats
| Treatment | ||||||
| Serum | Urine | Red cell | ||||
| Protein (mg/dL) |
LDH (IU/L) |
Glucose | Protein (mg/ 24h) |
Creatinine (ml/min/ 100g) |
HbA1c (%) |
|
| Normal | 1.82 ± 0.46 | 501.8 ± 31.2 | 2.2 ± 0.4 | 0.7 ±0.06 | 0.62±0.09 | 4.2 ± 0.3 |
| STZ only | 1.63 ± 0.38 | 1007 ± 51.3* | 8.6 ± 1.3* | 3.4 ±0.5* | 0.15±0.04* | 8.3 ± 0.4* |
| STZ+KV | 1.89 ± 0.50 | 613.2 ± 41.0** | 3.2 ± 0.7** | 1.3 ±0.07** | 0.43±0.07** | 5.0 ± 0.4** |
| STZ+GB | 1.86 ± 0.44 | 998.5 ± 39.2* | 2.7 ± 0.5** | 1.1 ± 0.05** | 0.37±0.05** | 6.2 ± 0.2** |
Values are means ± S.D. of 6 rats per group
Significantly different from normal at p< 0.05
Significantly different from STZ only at p< 0.05 STZ= Streptozotocin, KV= Kolaviron at 100 mg/kg, GB= Glibenclamide, HbA1c= Glycosylated hemoglobin, LDH= Lactate dehydrogenase
Figure 5.
Effect of kolaviron (biflavonoid from Garcinia kola) on the activities of serum alkaline phosphatase and á-amylase in streptozotocin (STZ)-diabetic rats
*Significantly different from normal (p<0.05), **Significantly different from STZ only (p<0.05), KV= Kolaviron at 100 mg/kg, GB= Glibenclamide, ALP= Alkaline phosphatase
Effect of KV on urinary and serum markers of kidney functions in STZ diabetic rats
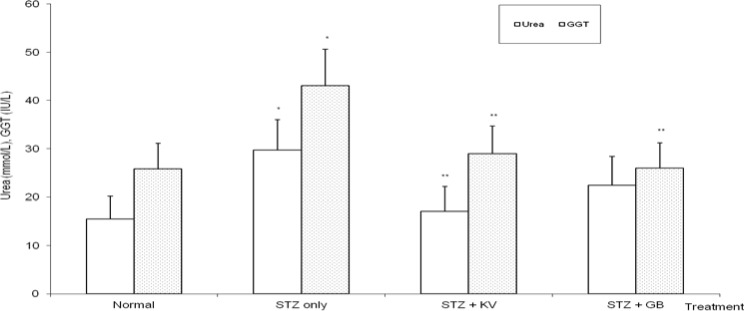
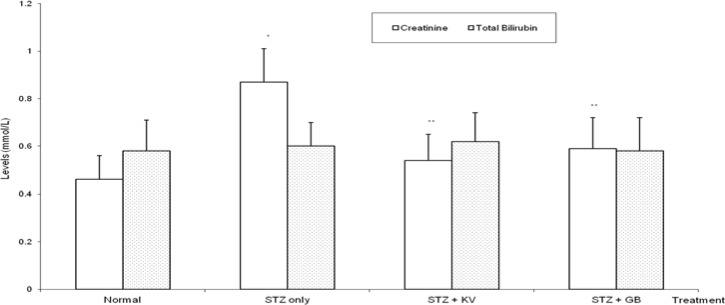
STZ-intoxication caused a significant increase (p< 0.05) in urinary glucose and protein of the rats when compared with normal (table 2). Precisely, the levels of urinary glucose and protein were increased by 291% and 386%, respectively, in untreated diabetic rats. Furthermore, urinary creatinine levels (a measure of creatinine clearance) decreased significantly (p<0.05) in untreated diabetic rats. However, administration of KV and GB significantly (p<0.05) reversed the adverse effects of STZ on urinary biochemical indices of the animals. In untreated STZ-diabetic rats, serum urea and creatinine levels significantly (p<0.05) increased when compared to normal (figures 4 and 6), while both KV- and GB-treated diabetic rats had significantly (p<0.05) lowered serum creatinine levels when compared to untreated diabetic group. KV alone was able to reverse the STZ-induced increase in urea levels to values that were statistically similar (p>0.05) to normal (figure 6).
Figure 4.
Effect of kolaviron (biflavonoid of Garcinia kola) on the levels of serum creatinine and total bilirubin activities in streptozotocin (STZ)-diabetic rats
*Significantly different from normal (p<0.05), **Significantly different from STZ only (p<0.05), KV= Kolaviron at 100 mg/kg, GB= Glibenclamide
Figure 6.
Effect of kolaviron (biflavonoid from Garcinia kola) on the levels of serum gamma glutamyl transferase and urea in streptozotocin (STZ)-diabetic rats
*Significantly different from normal (p<0.05), **Significantly different from STZ only (p<0.05), KV= Kolaviron at 100 mg/kg, GB= Glibenclamide, GGT= Gamma glutamyl transferase
Effect of KV on cardiac and liver function indices in STZ-diabetic rats
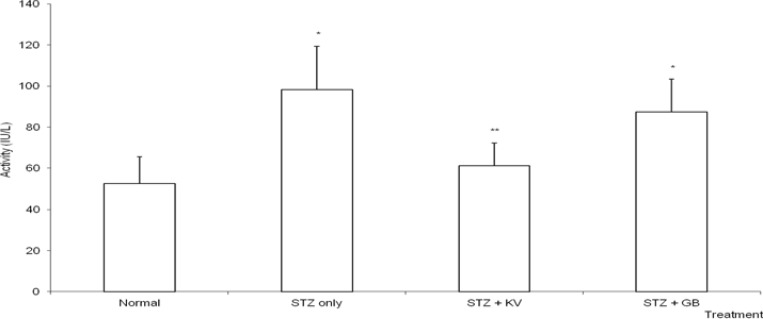
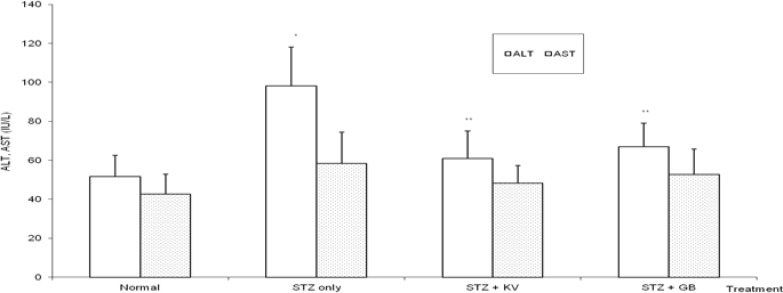
Table 2 and figure 7 show that STZ intoxication caused significant (p<0.05) increase in the activities of serum creatine kinase (CK) and lactate dehydrogenase (LDH) (Cardiac marker enzymes). Treatment with KV alone significantly (p<0.05) ameliorated the adverse effect of STZ on the activities of CK and LDH. Furthermore, the activities of serum alanine aminotransferase (ALT) and gamma glutamyl transferase (GGT) were significantly (p<0.05) elevated in untreated diabetic rats relative to normal (figures 3 and 6). Specifically, the activities of ALT and GGT were increased by 90% and 67%, respectively. However, treatment with either KV or GB significantly (p<0.05) reduced the levels of serum ALT and GGT of the diabetic rats. In contrast, STZ administration produced insignificant (p>0.05) effect on the levels of serum total bilirubin and alkaline phosphatase in the animals.
Figure 7.
Effect of kolaviron (biflavonoid from Garcinia kola) on the activities of serum creatine kinase in streptozotocin (STZ)-diabetic rats
*Significantly different from normal (p<0.05), **Significantly different from STZ only (p<0.05), KV= Kolaviron at 100 mg/kg, GB= Glibenclamide
Figure 3.
Effect of kolaviron (biflavonoid of Garcinia kola) on the activities of alanine and aspartate aminotransferases in streptozotocin (STZ)-diabetic rats
*Significantly different from normal (p<0.05), **Significantly different from STZ only (p<0.05), KV= Kolaviron at 100 mg/kg, GB= Glibenclamide, ALT= Alanine aminotransferase, AST= Aspartate aminotransferase
Discussion
Diabetes mellitus is one of the most common chronic diseases and is associated with hyperglycaemia and comorbidities such as obesity and hypertension. The use of a lower dose of STZ (35 mg/kg) was to produce a partial destruction of â-cells, while the rats also become permanently diabetic27. Since the â-cells are not completely destroyed the rats do not require insulin to survive28. In diabetes, hyperglycaemia is a common feature and can inactivate existing enzymes by glycating their protein, leading to DNA cleavage29. The increased level of blood glucose in STZ -diabetic rats was lowered by both KV and GB. Reports are available regarding the antidiabetic and antihyperlipidemic activities of KV at 100 mg/kg11, which were confirmed in this study. Glycosylated haemoglobin (HbA1c) expresses the percentage of haemoglobin bound to glucose. This sensitive index measures the mean blood glucose level over a period of 6–8 weeks (life span of red blood cells) and it reflects glycemic control in patients30. In this study, the levels of HbA1c significantly increased in untreated diabetic rats. This observation is consistent with the findings of Fuji and Nomoto31 that reported a significant change in the HbA1c level after two weeks of STZ administration in rats. Treatment with KV significantly reduced the HbA1c level relative to untreated diabetic rats, and better than in GB-treated diabetic rats. This result points to the ability of KV to regulate the blood glucose level in the rats better than GB for the 3 weeks duration of this study. It is known that for every 1% drop in HbA1c value may lead to 35% reduction in the risk of microvascular complications, including myocardial infarction in type 2 diabetes32.
The cardiotoxicity of xenobiotics can be evaluated using the serum activity of marker enzymes especially LDH and creatine kinase (CK), which are distributed throughout the body and have isoenzymes that are recognized as markers for liver muscle and heart lesion33. Contradictory reports are available in the literature on the relationship between diabetes and CK activity34,35. However, Hayden and Tyagi28 linked the observed increase in the serum CK and LDH levels of diabetic rats to cardiac muscular damage caused by the disease. Also, Wiernsperger29 stated that the quantity of CK and LDH released is a measure of the state of necrosis in these tissues during diabetes.
In line with the findings of Hayden and Tyagi28, the serum CK and LDH activities of untreated STZ-diabetic rats which were significantly elevated in this study indicates damage to cardiac muscle of the rats. It was observed that treatment of diabetic rats with KV at 100 mg/kg significantly attenuated CK and LDH activities during the 3 weeks of the study. This observation is very remarkable and points to the cardioprotective effect of this biflavonoid in the diabetic animals. In addition, liver enzymes such as ALT, AST, ALP, and GGT reflect different functions of the liver, such as hepatocellular integration, formation, and subsequent free flow of bile and protein synthesis. ALT and AST are important indicators of hepatocellular damage36. In the present study, ALT activities increased by 2-folds in untreated diabetic rats relative to normal. Also, there was a significant increase in the GGT levels in untreated diabetic rats. GGT is found in hepatocytes and biliary epithelial cells and, can leak to blood in pancreatic disease, renal failure, and diabetes36. Oral administration of KV and GB to diabetic rats caused a significant decrease in the activities of both ALT and GGT. This means that KV has some hepatoprotective potentials in diabetic rats by decreasing serum ALT and GGT levels.
In this study, STZ-intoxication caused a significant elevation of urinary glucose and protein, and drastic fall in creatinine clearance. The increase in urinary protein and glucosuria in these animals indicates proximal tubular dysfunction. Furthermore, renal dysfunction in these rats was confirmed by the elevation of serum urea. Elevated levels of blood urea and creatinine have been documented in experimental and human diabetes37. Our data indicate that KV at a dose of 100 mg/kg reduced the levels of serum urea, urinary glucose and protein and, improved creatinine clearance in STZ-diabetic rats. These results show the protective effects of KV against diabetic-induced renal dysfunction in these animals. Previous studies have attributed the biological effects of KV to its strong antioxidant property10,38 which cannot be ruled out in the present study.
Conclusion
This study confirmed that cardiac, renal and hepatic function indices were significantly elevated during STZ-induced diabetes, and that oral administration of KV reduced the levels of some of the indices. Therefore, KV may offer protection for tissues of animals during diabetes. Further studies are required to define a novel pathway by which KV affects diabetes using pancreatic â-cell line.
Acknowledgements
This study was supported by 3 months visiting fellowship (Ref. 3240207975) from TWAS-UNESCO Associateship programme given to OA to work in the laboratory of Professor Ali Moosavi-Movahedi, Institute of Biochemistry and Biophysics, University of Tehran, Iran. The supports from Research Council of the University of Tehran, Tehran University of Medical Science and Iran National Science Foundation (INSF) are gratefully acknowledged.
References
- 1.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 2.Nissen SE, Wolski K. Effects of rosiglitazone on the risk of myocardial infarction and death from cardiovascular cause. N Engl J Med. 2007;356:2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 3.Adeneye AA, Agbaje EO. Pharmacological evaluation of oral hypoglycaemic and antidiabetic effects of fresh leaves ethanol extract of Morinda lucida Benth. in normal and alloxan-induced diabetic rats. Afr J Biomed Res. 2008;11:65–71. [Google Scholar]
- 4.Vaya J, Belinky PA, Aviram M. Antioxidant constituents from licorice roots: isolation, structure elucidation and antioxidative capacity toward LDL oxidation. Free Radic Biol Med. 1997;23:302–313. doi: 10.1016/s0891-5849(97)00089-0. [DOI] [PubMed] [Google Scholar]
- 5.Iwu MM, Igboko OA. Flavonoids of Garcinia kola seeds. J Nat Prod. 1982;45:650–651. [Google Scholar]
- 6.Cotterhill PJ, Scheinmann F, Stenhouse TA. Extractives from Guttiferae: kolaflavanone, a new biflavanone from the nuts of Garcinia kola Heckel. J Chem Soc Perkin Trans. 1978;1:246. [Google Scholar]
- 7.Iwu MM, Igboko OA, Onwuchekwa U, Okunji CO. Evaluation of the antihepatotoxicity of the biflavonoids of Garcinia kola seeds. J Ethnopharmacol. 1987;21:127–138. doi: 10.1016/0378-8741(87)90123-1. [DOI] [PubMed] [Google Scholar]
- 8.Adaramoye OA, Adeyemi EO. Hepatoprotection of D-galactosamineinduced toxicity in mice by purified fractions from Garcinia kola seed. Basic Clin Pharmacol Toxicol. 2006a;98:135–141. doi: 10.1111/j.1742-7843.2006.pto_256.x. [DOI] [PubMed] [Google Scholar]
- 9.Adaramoye OA, Nwaneri VO, Anyanwu KC, Farombi EO, Emerole GO. Possible antiatherogenic effect of kolaviron (A Garcinia kola seed extract) in hypercholesterolemic rats. Clin Exp Pharmacol Physiol. 2005a;32:40–46. doi: 10.1111/j.1440-1681.2005.04146.x. [DOI] [PubMed] [Google Scholar]
- 10.Adaramoye OA, Farombi EO, Adeyemi EO, Emerole GO. Inhibition of human low-density lipoprotein oxidation by flavonoids of Garcinia kola seeds. Pak J Med Sci. 2005b;21:331–339. [Google Scholar]
- 11.Iwu MM, Igboko OA, Okunji CO, Tempesta MS. Anti-diabetic and aldose reductase activities of biflavanones of Garcinia kola. J Pharm Pharmacol. 1990;42:290–292. doi: 10.1111/j.2042-7158.1990.tb05412.x. [DOI] [PubMed] [Google Scholar]
- 12.National Institutes of Health (US), author Guide for the Care and Use of Laboratory Animals. NIH Publication No. 85-23. Revised 1985. [Google Scholar]
- 13.Sharma SR, Dwivedi SK, Swarup D. Hypoglycemic, antihyperglycemic and hypolipidemic activities of Caesalpinia bonducella seeds in rats. J Ethnopharmacol. 1997;58:39–44. doi: 10.1016/s0378-8741(97)00079-2. [DOI] [PubMed] [Google Scholar]
- 14.Nakhoda A, Wong HA. The induction of diabetes in rats by intramuscular administration of streptozotocin. Experientia. 1979;35:1679–1680. doi: 10.1007/BF01953269. [DOI] [PubMed] [Google Scholar]
- 15.Farswan M, Mazumder PM, Percha V. Protective effect of Cassia glauca Linn. on the serum glucose and hepatic enzymes level in streptozotocin induced NIDDM in rats. Indian J Pharmacol. 2009;41:19–22. doi: 10.4103/0253-7613.48887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Mohun AF, Cook LJ. Simple method for measuring serum level of glutamate-oxaloacetate and glutamate-pyruvate transaminases in laboratories. J Clin Pathol. 1957;10:394–399. doi: 10.1136/jcp.10.4.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reitman S, Frankel S. A colorimetric method for the determination of serum level of glutamate-oxaloacetate and pyruvate transaminases. Am J Clin Pathol. 1957;28:56–63. doi: 10.1093/ajcp/28.1.56. [DOI] [PubMed] [Google Scholar]
- 19.Jaffe M. Ueber den Neiderschlag, welchen Pikrinsäure im normalen harn Erzeught und über eine neue Reaction des Kreatinins. Z Physiol Chem. 1886;10:391–400. (Ger). [Google Scholar]
- 20.Talke H, Schubert GE. Enzymatische Harnstoff bestimmung in Blut and serum in Optischen Test nach Warburg. Klin Wochschr. 1965;43:174. doi: 10.1007/BF01484513. [DOI] [PubMed] [Google Scholar]
- 21.Rutkowski RB, Debaare L. An ultra-micro colorimetric method for determination of total and direct serum bilirubin. Clin Chem. 1966;12:432–438. [PubMed] [Google Scholar]
- 22.Fossati R, Melzid'Eril GV, Turenghi G, Precipe L, Berti G. A kinetic colorimetric assay of gamma-glutamyltransferase. Clin Chem. 1986;32:1581–1584. [PubMed] [Google Scholar]
- 23.Gella FJ, Gubern G, Vidal R, Canalias F. Determination of total and pancreatic alphaamylase in human serum with 2-chloro-4-nitrophenyl-alpha-D-maltotrioside as substrate. Clin Chim Acta. 1997;259:147–160. doi: 10.1016/s0009-8981(96)06481-9. [DOI] [PubMed] [Google Scholar]
- 24.Zimmerman HJ, Weinstein BS. Lactic dehydrogenase activity in human serum. J Lab & Clin Med. 1956;48:607–609. [PubMed] [Google Scholar]
- 25.Hirokawa K, Shimoji K, Kajiyama N. An enzymatic method for the determination of hemoglobin A1c. Biotechnol Lett. 2005;27:963–968. doi: 10.1007/s10529-005-7832-x. [DOI] [PubMed] [Google Scholar]
- 26.Williamson T. A comparison between the phosphastrate and phenyl phosphate methods of alkaline phosphatase assay. Med Lab Technol. 1972;29:182–187. [PubMed] [Google Scholar]
- 27.Aybar MJ, Sánchez Riera AN, Grau A, Sánchez SS. Hypoglycemic effects of water extract of Smallanthus soncifolius (yacan) leaves in normal and diabetic rats. J Ethanopharmacol. 2001;74:125–132. doi: 10.1016/s0378-8741(00)00351-2. [DOI] [PubMed] [Google Scholar]
- 28.Hayden MR, Tyagi SC. Intimal redox stress: accelerated atherosclerosis in metabolic syndrome and type 2 diabetes mellitus. Atheroscleropathy Cardiovasc Diabetol. 2002;1:3–8. doi: 10.1186/1475-2840-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wiernsperger NF. Oxidative stress as a therapeutic target in diabetes: revisiting the controversy. Diabetes Metab. 2003;29:579–585. doi: 10.1016/s1262-3636(07)70072-1. [DOI] [PubMed] [Google Scholar]
- 30.Ghacha R, Sinha AK, Karkar AM. HbA1c and serum fructosamine as marker of chronic glycemic state in type 2 diabetic hemodialysis patients. Dialysis Transplant. 2001;30:214–217. [Google Scholar]
- 31.Fuji E, Nomoto T. Changes in glycosylated hemoglobin in short and semi long term streptozotocin-diabetic mice and rats. Japan J Pharmacol. 1984;34:113–115. doi: 10.1254/jjp.34.113. [DOI] [PubMed] [Google Scholar]
- 32.UKPDS group, author. Intensive blood glucose control with sulphonylurea or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes. Lancet. 1998;352:837–853. [PubMed] [Google Scholar]
- 33.Aldrich JE. Clinical enzymology. In: Anderson SC, Cockayne S, editors. Clinical Chemistry: Concept and Applications. New York: McGraw Hill; 2003. pp. 261–284. [Google Scholar]
- 34.Zhao X, Bassirat M, Zeinab K, Helme RD. Effects of diabetes on creatine kinase activity in streptozotocin-diabetic rats. Chin Med J (Engl) 1999;112:1028–1031. [PubMed] [Google Scholar]
- 35.Al-Shabanah AO, El-Kashef HA, Badary OA, Al-Bekairi AM, Elmazar MM. Effect of streptozotocin-induced hyperglycemia on intravenous pharmacokinetics and acute cardiotoxicity of doxorubicin in rats. Pharmacol Res. 2000;41:31–37. doi: 10.1006/phrs.1999.0568. [DOI] [PubMed] [Google Scholar]
- 36.Limidi JK, Hyde GM. Evaluation of abnormal liver function tests. Postgrad Med J. 2003;79:307–312. doi: 10.1136/pmj.79.932.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yousef WM, Omar AH, Ghanayem NM. Effect of some calcium channel blockers in experimentally induced diabetic nephropathy in rats. Int J Diabetes Metab. 2006;14:39–49. [Google Scholar]
- 38.Farombi EO, Shrotriya S, Surh YJ. Kolaviron inhibits dimethyl nitrosamine-induced liver injury by suppressing COX-2 and iNOS expression via NF-kappaB and AP-1. Life Sci. 2009;84:149–155. doi: 10.1016/j.lfs.2008.11.012. [DOI] [PubMed] [Google Scholar]