Abstract
Background
Moringa stenopetala, Baker f. (Moringaceae) is used for food and medicine in Southern Ethiopia.
Objective
To substantiate the hypotensive effect of M. stenopetala in vivo and in vitro.
Methods
An in vivo experiment was carried out on male guinea pigs anaesthetized with pentobarbital. The arterial blood pressure was recorded from a carotid artery filled with heparinized saline via an arterial cannula connected to a pressure transducer. For the in vitro experiment the descending thoracic aorta was removed and kept moistened in Krebs-Henseleit solution and then mounted in a 20ml tissue bath maintained at 37°C and bubbled with a mixture of 95% oxygen and 5% carbon dioxide.
Results
Crude aqueous leaf extract of M. stenopetala caused significant fall in systolic blood pressure (SBP), diastolic blood pressure (DBP) and mean arterial blood pressure (MABP) at doses of 10, 20, 30 and 40 mg/kg in normotensive anaesthetized guinea pigs (n = 12). The effect might have been mediated by non-autonomic nervous system as the effect is not altered by atropine and propranolol. The extract also caused significant dose and time dependent inhibition of K+ induced contraction on guinea pig aorta.
Conclusion
M.stenopetala has blood pressure lowering effect substantiating the use of the plant in traditional medicine.
Keywords: Moringa stenopetala, hypotensive, aqueous extract
Introduction
Moringa stenopetala Baker f. (Moringaceae)1 is commonly grown in Southern parts of Ethiopia at an altitude of 1 100 to 1 600m above sea level. The leaves of M. stenopetala are cooked and eaten as vegetables and the leaves and roots are used to treat malaria, hypertension and gastrointestinal problems2. It has been reported that M. stenopetala leaves and roots showed antitrypanosomal activity3. The antispasmodic effects of the leaves on smooth muscle tissues4 and antibiotic properties of the seeds5,6 were also reported previously. The isolated fractions from the aqueous extract of this plant were also observed to have hypoglycemic and antidiabetic effect in mice7. To further substantiate the claimed use of the plant, the present study reports the hypotensive effect of the aqueous extract of the leaves of M.stenopetala both in in vivo and in vitro experiments.
Methods
Plant material collection, extraction and laboratory animal preparation
Fresh and uncrushed leaves of M. stenopetala were collected from Arbaminch about 505 kms south of Addis Ababa, at an elevation of 1 285 meters above sea level and between 6°30′N to 6°08′N latitude and 37°33′E to 37°37′E longitude Ethiopia during the month of December 2005. The fresh leaves were placed in Erlenmeyer flasks and placed in continuous hot water orbital shaker (GFL, model 3 020, Germany) for 20 minutes. The extract was then filtered with cotton and Whatman filter paper (15.0 cm size) and then freeze dried in a lyophilizer (Vacuubrad, GMBH Germany). Accordingly, from 4kg of fresh leaves of M. stenopetala, 36g of crude extract was obtained and the crude extract was kept in a refrigerator at −20 °C until use for the experiments.
Male guinea pigs were kept in the animal house of the Faculty of Medicine, Addis Ababa University. The animals were housed 5 per cage with water and food ad libitum, ambient temperature 21°C and 12 light/12 dark cycles. Before the experiment, each animal was caged separately and deprived of food for 18 hrs but water ad libitum. The research was conducted in accordance with international accepted principles of laboratory animal use and care.
In vivo experiment on Guinea pigs
The in vivo experiment was carried out according to the method described by Gilani et al.8 and Ghayur et al.9 on twelve male guinea pigs (500 – 600g) anaesthetized with pentobarbital (60 mg/kg, i.p.). The trachea was exposed and cannulated to facilitate spontaneous respiration (Harvard ventilator, model 683 or SN-480). The arterial blood pressure was recorded from the carotid artery filled with heparinized saline via an arterial cannula (Portex cannulae, external diameter 1.02 mm, internal diameter 0.75 mm) connected to a pressure transducer. The aqueous leaf extract of M. stenopetala and the drugs were injected in the form of bolus injection via a cannula inserted into the external jugular vein followed by saline flush (0.2 ml). Pulse pressure was obtained by subtracting diastolic blood pressure (DBP) from systolic blood pressure (SBP) and mean arterial blood pressure (MABP) was also determined from the sum of DBP plus one-third of pulse width. Changes in blood pressure were expressed as the mean ± standard error of the means of control values, obtained before administration of test substances. To study the mechanism of action of the test extract, acetylcholine (1 µg/kg), atropine (1 mg/kg), adrenaline (2 µg/kg), and propranolol (0.1 mg/kg) were used to check whether the extract mediates via cholinergic or adrenergic pathway.
In vitro experiment preparation on guinea-pig aorta
The in vitro experiment was conducted according to the method described by Ghosh10, Gilani et al.12 and Ghayur et al.10. Guinea-pigs of either sex (400– 600g) were sacrificed by gentle cervical dislocation. The descending thoracic aorta was quickly removed and placed in Krebs-Henseleit solution. Excess fat and connective tissues were trimmed off and the whole length of aorta was then cut spirally resulting in a short strip (2 to 4 cm) that was prepared to be used for the experiment. The strip was mounted in a 20ml tissue bath containing Krebs-Henseleit solution, maintained at 370C and continuously bubbled with a mixture of 95% oxygen and 5% carbon dioxide. A resting tension of 1g was applied to the tissue and an equilibrium period of 1h was allowed to equilibrate before addition of any drug or the test extract. During this period the bath fluid was changed every 15minutes. Effect of extract was first determined on the resting baseline of the tissue to see if it had any vasoconstrictor effect. High K+ (80 mM) was added in the bath to induce sustained contraction of the tissue. The aqueous leaf extract of M. stenopetala was later tested for its ability to inhibit (relax) the contraction induced with high K+ (80 mM). Changes in isometric tension of the strip was measured via a force displacement transducer (FT- 03) using a Grass model 7E polygraph (Grass Instrument Co. Quincy, Mass, USA).
Statistical analysis
The results were analyzed statistically using one-way ANOVA. Post hoc comparison between control and test groups was made with Dunnett's multiple comparison tests using SPSS 10 statistical software package. The values at p < 0.05 were regarded as statistically significant. All data were expressed as mean ± standard error of the mean (M ± SEM).
Results
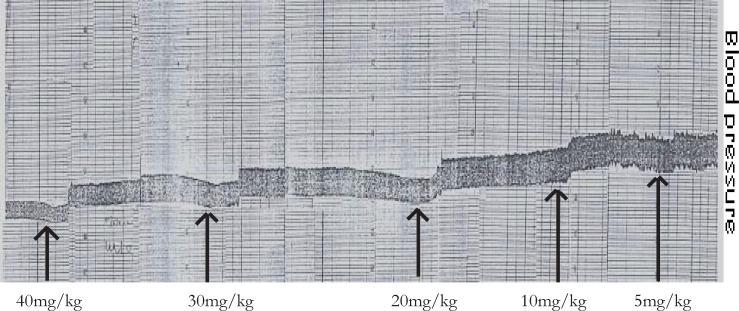
The effect of aqueous leaf extract of M. stenopetala on SBP, DBP, pulse pressure, MABP in anesthetized guinea pigs (n = 12, p < 0.05) is shown in table 1. The iv administration of aqueous leaf extract of M. stenopetala at doses of 10 mg/kg, 20 mg/kg, 30 mg/kg and 40 mg/kg showed statistically significant reduction of SBP, DBP and MABP. On the other hand, table 1 also shows that the decline in pulse pressure was statistically significant only at the dose of 40 mg/kg. A typical tracing of the in vivo experiment is shown in figure 1. The duration of action was longer; that is, about 10 minutes as compared to the one minute action of acetylcholine, and the animals did not show any sign of cardiopulmonary distress after repeated doses of the aqueous extract of M.stenopetala.
Table 1.
The effects of iv infusion of different doses of M. stenopetala aqueous extract in anaesthetized guinea pigs on systolic blood pressure (SBP), diastolic blood pressure (DBP), pulse pressure and mean arterial blood pressure (MABP)
| Doses of M. | SBP | DBP | PP | MABP |
| stenopetala | ||||
| control | 81.91 ± 2.73 | 53.16± 2.70 | 28.74± 2.29 | 62.74± 2.49 |
| 5mg/kg | 76.69 ± 2.59 | 48.22± 2.40 | 28.46± 2.43 | 57.71± 2.18 |
| 10mg/kg | 60.89 ± 3.95** | 38.73±3.19** | 22.15 ± 2.44 | 46.12±3.27** |
| 20mg/kg | 54.65 ± 3.73** | 32.62 ± 2.51** | 22.02 ± 2.59 | 3.97±2.71** |
| 30mg/kg | 47.79 ± 3.02** | 24.89 ± 2.13** | 22.89 ± 2.44 | 32.52±2.18** |
| 40mg/kg | 39.26 ± 3.10** | 20.79 ± 1.56** | 18.47 ± 2.37** | 26.94±1.89** |
Data are expressed as Mean ± SEM (n=12). ** P < 0.05
Figure 1.
The hypotensive effect of M. stenopetala crude extract in anaesthetized guinea pig. Arrows indicate the point at which the test extracts were administered
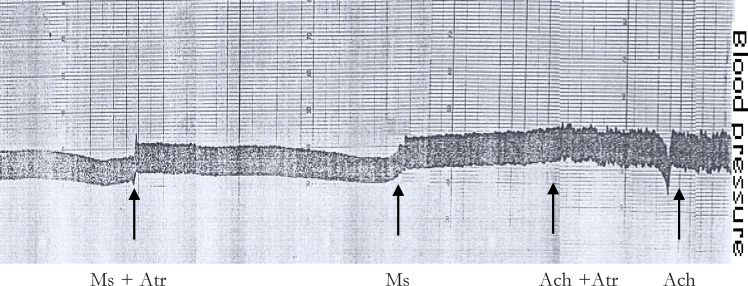
Acetylcholine at a dose of 1 µg/kg produced a considerable drop in blood pressure and pretreatment of animals with atropine (1 mg/kg), the muscarinic blocker of acetylcholine, abolished the effect of acetylcholine on blood pressure. However, atropine pretreatment did not alter the hypotensive effect of the aqueous leaf extract of M. stenopetala in anaesthetized guinea pig as shown in figure 2. Similarly adrenalin at a dose of 2 µg/kg produced a considerable rise in blood pressure and pretreatment of the animals with propranolol (0.1 mg/kg), non selective ?-blocker, abolished the blood pressure raising effect of adrenalin (data not shown). However, pretreatment of the animals with the aqueous leaf extract of M. stenopetala did not abolish the effect of adrenalin, and blocking the adrenergic mechanism with propranolol did not prevent the action of the test extract in anaesthetized guinea pigs.
Figure 2.
The non-muscarinic mechanism of action of M. stenopetala in inducing hypotensive effect in anaestehetized guinea pig. Ms = Moringa stenopetala; Atr = Atropine; Ach = Acetylcholine
Acetylcholine at a dose of 1 µg/kg produced a considerable drop in blood pressure and pretreatment of animals with atropine (1 mg/kg), the muscarinic blocker of acetylcholine, abolished the effect of acetylcholine on blood pressure. However, atropine pretreatment did not alter the hypotensive effect of the aqueous leaf extract of M. stenopetala in anaesthetized guinea pig as shown in figure 2. Similarly adrenalin at a dose of 2 µg/kg produced a considerable rise in blood pressure and pretreatment of the animals with propranolol (0.1 mg/kg), non selective ?-blocker, abolished the blood pressure raising effect of adrenalin (data not shown). However, pretreatment of the animals with the aqueous leaf extract of M. stenopetala did not abolish the effect of adrenalin, and blocking the adrenergic mechanism with propranolol did not prevent the action of the test extract in anaesthetized guinea pigs.
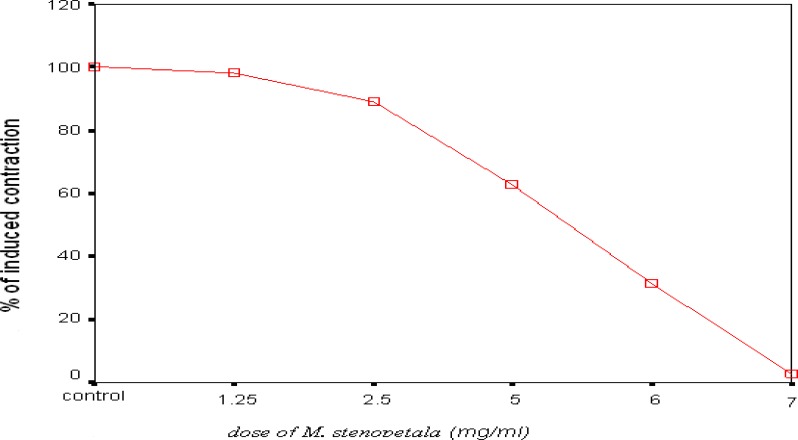
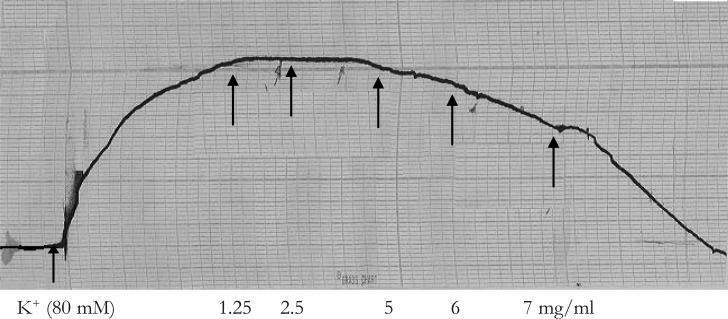
The aqueous leaf extract of M. stenopetala did not exhibit any vasoconstrictor activity on the resting baseline of guinea pig aorta. The extract was then tested on high-K+ (80 mM) induced contraction. The results (figure 3) are expressed as the percentage contraction, taking the control K+-induced contraction before the application of the test extract as 100%. The test extract showed statistically significant dose dependent inhibition of high- K+ induced contraction at concentrations of 5mg/ml (n = 10, p = 0.001), 6 mg/ml (n = 10, p < 0.001) and 7 mg/ml (n = 10, p < 0.001). The inhibition of contraction was within 15 minutes contact time after the application of each dose of the test extract. Thus, the inhibition of contraction was also time dependent (figure 4). The relaxant effect of the test extract was reversible as the tissue regained its spontaneous activity at least within two hrs after repeated washout.
Figure 3.
Dose response curve showing the inhibitory effect of aqueous leaf extract of M. stenopetalaon high K+(80 mM)-induced contraction in isolated aorta of guinea pigs and values are % of induced contraction Mean ± S.E.M
Figure 4.
Concentration and time dependent inhibitory responses of aqueous leaf extract of M. stenopetalain isolated guinea pig aorta. Arrows show the addition of K+ (80 mM) and M. stenopetala
Discussion
An iv administration of aqueous extract of M. stenopetala showed a fall in SBP, DBP and MABP with significant fall in pulse pressure only at higher dose. This result is in agreement with previous in vivo hypotensive studies on leaves of M. oleifera in normotensive wistar rats13. The P-Hydroxybenzaldehyde (PBA) which is aglycone of á-1- rhamnosyloxybenzaldehyde isolated from the leaves of M. oleifera showed 40% and 75% decreases in blood pressure at a dose of 3 and 10mg/kg in anaesthetized normotensive wistar rats, respectively14,15. It was also noted by Gilani et al.12 that the pure compounds isolated from M. oleifera caused dose dependent fall in SBP, DBP and heart rate in anaesthetized normotensive wistar rats. In another similar study, it was described that the aqueous extract of stem bark from M. oliefera produces a dose dependent hypotensive effect in anesthetized mongrel dogs with a maximum effect at 20 mg/kg16.
Acetylcholine at a dose of 1ìg/kg produced a considerable drop in blood pressure. This is in agreement with the study made by Gilani et al12 and a study done in anesthetized dogs16. Thus, the test extract of the present study mediates its hypotensive effect through mechanism(s) independent of muscarinic receptor activation. Similarly, the pure compounds isolated from leaves of M. oleifera did not cause contraction of guinea pig ilea unlike acetylcholine, ruling out the involvement of muscarinic receptor activation12. M. oleifera aqueous extract caused dose dependent negative inotropic effect in isolated frog heart at concentration of 0.1–1 µg and atropine failed to block the negative inotropic effect of the extract16.
On the other hand, adrenaline produced a considerable rise in blood pressure, and pretreatment of the animals with propranolol abolished this effect. However, pretreatment with aqueous leaf extract of M. stenopetala did not abolish the effect of adrenalin. Thus, the extract does not act through the same mechanism as that of propranolol. It is also evident from this study that the crude extract of leaves of M. stenopetala does not utilize the adrenergic mechanism because blocking the adrenergic mechanism with propranolol did not prevent the action of the present test extract suggesting that the extract is acting at a different site (i.e. non-adrenergic). This is consistent with the in vivo study of the aqueous extract of M. oleifera on anesthetized dogs that produced dose dependent hypotensive effect and the response was not altered by propranolol and pheniramine16. Therefore, the hypotensive effect of M. stenopetala observed in the present in vivo study may be attributed to a direct action of the extract on the vascular system.
The present in vivo hypotensive property of the extract of M. stenopetala is substantiated by in vitro investigation on the isolated aorta of guinea pig where the test extract inhibited high K+-induced contraction. As noted by Gilani et al.8, K+ at high doses (>30mM) is known to cause smooth muscle contractions through opening of voltagedependent slow Ca2+ channels, thus allowing influx of extracellular Ca2+ causing a contractile effect. The percent dose-dependent inhibition of K+-induced contraction in isolated aortic preparation (table 2 and figures 3 & 4) is consistent with dose-dependent inhibition of high-K+ (80 mM)-induced contraction by isolated compounds from crude extracts of M. oleifera on isolated aorta of rabbit12.
Table 2.
The percent inhibition of high-K+ (80 mM)-induced contraction by aqueous leaf extract of M. stenopetala on isolated aorta of guinea pig (n = 10)
| Treatment | % of induced | % inhibition of high | Level of |
| contraction | K+ (80 mM) | Significance | |
| induced | |||
| contraction | |||
| Control(K+ of 80 mM) | 100.0 ± 0.0 | 0.00 ± 0.00 | |
| 1.25 mg/ml | 98.3 ± 0.6 | 1.47 ± 0.49 | 0.772 |
| 2.5 mg/ml | 88.9 ± 2.7 | 11.12 ± 2.86 | 0.255 |
| 5 mg/ml | 62.8 ± 8.5** | 36.55 ± 8.64** | 0.001 |
| 6 mg/ml | 31.4 ± 9.7** | 67.76 ± 9.55** | 0.001 |
| 7 mg/ml | 2.9 ± 2.9** | 95.56 ± 3.14** | 0.001 |
Data are Mean ± SEM, ** p value < 0.05
In the present study, the mechanism of the dose-dependent relaxation of high K+-induced contraction by M. stenopetala in isolated aorta preparation may be mediated through the calcium channel blockade. As shown in other studies calcium channel blockers inhibit concentration dependent contraction induced by high K+10,17. Thus, in this study the dose-dependent inhibition of high K+-induced contraction by M.stenopetala in isolated guinea pig aortic tissue preparation may be as a result of restricted Ca2+ entry through voltage-dependent slow calcium channels.
The in vitro study of M. stenopetala is also supported by previous in vitro experiments. The crude extract of the leaves of M. stenopetala resulted in time and concentration dependent decline in acetylcholine induced contractions of both guinea pig ileum and mouse duodenum tissues4. The extract also abolished rhythmic spontaneous contractions of both tissue preparations4. Thus, the direct depressant effect of the leaf extract of M. stenopetala on isolated frog heart, smooth muscle tissue preparation of guinea pig aorta, guinea pig ileum and mouse duodenum is probably responsible for its hypotensive effects observed in the in vivo study on anaesthetized animal model.
Conclusion
The results of this study demonstrated that the intravenous administration of fresh leaf extract of M. stenopetala exhibited blood pressure lowering effect in normotensive anaesthetized guinea pigs. The mode of action is neither cholinergic nor adrenergic pathway because its effect is not altered by atropine which is a muscarinic receptor blocker of acetylcholine and propranolol which is a non-selective blocker of ?-adrenergic receptors. In tissue preparation, the crude leaf extract of M. stenopetala exhibited vasodilatation effect on high K+-induced contraction of isolated aorta that may be acting through blockade of the voltage sensitive Ca2+ channels.
Acknowledgements
The authors acknowledge Addis Ababa University, in Addis Ababa Ethiopia for funding the study.
References
- 1.Edwards S, Tadesse M, Demissew S, Hedberg J. Flora of Ethiopia and Eritrea: Mangnoliaceae to Flacourtiacea. Vol. 1. Ethiopia: The National Herbarium; 2002. pp. 155–163. [Google Scholar]
- 2.Mekonnen Y, Gessesse A. Documentation on the uses of Moringa stenopetala and its possible antileishmanial effect. SINET: Ethipi J Sci. 1998;21:287–295. [Google Scholar]
- 3.Mekonnen Y, Yardley V, Rock P, Croft S. In vitro antitrypanosomal activity of M. stenopetala leaves and roots. Phytotherapy Research. 1999;13(6):538–539. doi: 10.1002/(sici)1099-1573(199909)13:6<538::aid-ptr486>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 4.Mekonnen Y. Effect of ethanol extract of Moringa stenopetala leaves on guineapigs and mouse smooth muscle. Phytother Res. 1999;13:442–444. doi: 10.1002/(sici)1099-1573(199908/09)13:5<442::aid-ptr476>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Eilert U, Walters B, Nahrstedt A. The antibiotic principle of seeds of Moringa oleifera and Moringa stenopatala. Planta Med. 1981;42(1):55–61. doi: 10.1055/s-2007-971546. [DOI] [PubMed] [Google Scholar]
- 6.Mekonnen Y, Draeger B. Glucosinolates from Moringa stenopetala. Planta Medica. 69;2003:380–382. doi: 10.1055/s-2003-38881. [DOI] [PubMed] [Google Scholar]
- 7.Mussa A, Makonnen E, Urga K. Effects of the Crude Aqueous Extract and Isolated Fractions of Moringa stenopetala leaves in Normal and Diabetic Mice. Pharmacology online. 2008;3:1049–1055. [Google Scholar]
- 8.Debella A. Manual for phytochemical screening of medicinal plants. Addis Ababa, Ethiopia: Department of Drug Research, Ethiopian Nutrition and Research Institute; 2002. [Google Scholar]
- 9.Gilani AH, Jabeen Q, Ghayur MN, Janbaz KH, Akhtar MS. Studies on antispasmodic, bronchodilator and hepatoprotective activities of the Carum copticum seed extract. J Ethnopharmacol. 2005;98:127–135. doi: 10.1016/j.jep.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Ghayur MN, Gilan AN. Ginger lowers blood pressure through blockade of voltage-dependant calcium channels. J Cardiovasc Pharmacol. 2005;45:74–80. doi: 10.1097/00005344-200501000-00013. [DOI] [PubMed] [Google Scholar]
- 11.Gosh MN. Fundamentals of experimental pharmacology. 2nd Ed. Calcutta: Scientific book agency; 1984. pp. 60–100. [Google Scholar]
- 12.Gilani HA, Aftab K, Suria A, Siddiqui S, Salam R, Siddiqui BS, Faizi S. Pharmacological studies on hypotensive and spasmolytic activities of pure compounds from Moringa oleifera. Phytother Res. 1994;8:87–91. [Google Scholar]
- 13.Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani A. Novel hypotensive agents, Niazimin A, Niazimin B, Niazicin A and Niazicin B From Moringa stenopetala: Isolation of first occurring carbamates. J Chem Soc Perkin Trans. 1994;1:3035–3040. [Google Scholar]
- 14.Faizi S, Siddiqui BS, Saleem R, Siddiqui S, Aftab K, Gilani A. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochem. 1995;38:957–963. doi: 10.1016/0031-9422(94)00729-d. [DOI] [PubMed] [Google Scholar]
- 15.Faizi S, Siddiqui BS, Saleem R, Aftab K, Shaheen F, Gilani A. Hypotensive Constituents from the pods of Moringa oleifera. Planta Med. 1998;64:225–228. doi: 10.1055/s-2006-957414. [DOI] [PubMed] [Google Scholar]
- 16.Limaye AD, Nimbkar YA, Jain R, Ahmad M. Cardiovascular effects of the aqueous extrats of Moringa ptergosperma. Phytother Res. 1995;9:37–40. [Google Scholar]
- 17.Keiichi O, Kenji I, Mitsuaki N. Effects of Aranidipine a novel calcium channel blocker, on mechanical responses of the isolated Rat portal vein: Comparison with typical calcium channel blockers and potassium channel openers. J Cardiovasc Pharmacol. 1997;29:209–215. doi: 10.1097/00005344-199702000-00009. [DOI] [PubMed] [Google Scholar]