Abstract
Background
The gap junction plays an important role in spreading of apoptotic and necrotic signals from injured and stressed cells to the neighboring viable cells. The present study was performed to investigate the important role of gap junction communication on rabbits' explosive brain injury.
Methods
Explosion of paper detonators was used to create explosive brain injury model in 60 rabbits, which was randomly divided into control group and experimental group. Octanol, an efficient blocker of gap junction, was injected in the left ventricle to block gap junction communication in the experimental group 2 hours before injury, while the same volume of saline was utilized in the control group.
Results
Penumbra volume around the brain contusion in the experimental group was significantly less than that in the control group at 1d and 3d after brain damage. RT-PCR and Western blotting analysis indicated that the expression of connexin-43 (Cx43) and caspase-3 was significantly lower in the experimental group than that in the control group at all time points.
Conclusion
Rabbits' explosive brain injury can be efficiently attenuated through blocking the gap junction communication, which benefit for deeper understanding the mechanism of brain injury.
Keywords: explosive brain injury, connexin-43 (Cx43), caspase-3
Introduction
In recent years, blast-induced traumatic brain injury has become more and more serious, especially in civilians in war zones or terrorist incidents1. Statistical data have shown that blast-induced traumatic brain injury increases approximately 10-fold over the last 10 years due to terrorism2, which only represents the tip of iceberg. Therefore, elucidation of the mechanism of blast-induced traumatic brain injury and discovery of new therapy target have become an essential task.
The gap junction, an important component of cell-to-cell communication, plays an important role in spreading of apoptotic and necrotic signals from injured and stressed cells to the neighboring viable cells3, 4. The structural unit of gap junction is connexon, which contains six protein subunits, connexins, forming intercellular channels. Based on the difference of molecular weight, connexins can be categorized into Cx43, Cx37 and Cx265. Some blockers of gap junction have been demonstrated to exhibit neuroprotective effects in several models of cerebral injury, including insulin6, octanol7, and carbenoxolone8. The mechanisms might contain reduction of glutamate, and suppression of excitatory amino acid release through volume-regulated anon channels6–8.
In the present study, the attention was given to the effect of gap junction on blast-induced traumatic brain injury. Furthermore, detailed molecular mechanisms were discussed.
Methods
Experimental animals
Animals were supplied by experimental animal centre of Daping Hospital & Research Institute of Surgery, Third Military Medical University. A total of 60 healthy rabbits from New Zealand of either sex (2.0–2.5 kg body weight) were randomly divided in to two groups after paper detonators explosion. In the experiment group, 30 rabbits were injected with octanol through the left ventricle octanol two hours before injury. In the control group, the other 30 rabbits were injected with equal volume of normal saline. Food but not water was forbidden 12 hours before surgery.
Chemicals and equipments
Paper detonators (600 mg TNT) was purchased from Chongqing Basiwu Factory. Octanol was obtained from Sigma (St. Louis, MO). Reverse-transcription PCR Kit was from Promega, USA. PCR Kit was from Takara (Dalian, China). Primer synthesis for â-actin and Caspase-3 were carried out by Sangon Biotech Co. Ltd. (Shanghai, China). Anti-Caspase-3 polyclonal antibody was from Santa Cruz, USA. Anti-rabbit IgG-HRP was from Sigma (St. Louis, MO). 113A31 sensor was from pcb, USA. Wavebook/516A data acquisition system was purchased from Quatronix Inc. (Akron, Ohio). BA61 electronic balance was from Sartorius, Switzerland. Anti-â-actin monoclonal antibody was from Novus Biologicals (Littleton, CO, USA).
Constitution of animal model
The rabbits were head shaved after rabbits' auricular vein intravenous injection with 3% pentobarbital sodium (1 mg/kg). The catheters were inserted via right femoral artery and connected with multiparameter physiological monitor via ductus arteriosus. Precordium was adhibitted with electrode. Rabbits were fixed under explosion shelf with a paper detonator equivalent to 600 mg TNT above head. The position of detonator was set at 1.5 cm deviated from the midline and 2 cm from the front of the binaural line with a vertical distance of 6.5 cm. A Wavebook/516A data acquisition system was used to detect the peak pressure. Local scalp was treated by debridement and stitching after explosion. Brain tissues were collected at 0 °C by killing animals via auricular vein intravenous injection with 20 ml air one or three days after injury. Each group was consisted of 15 rabbits, 10 of which were used for brain contusion and penumbra volume determination and the others were used for protein measurement and RT-PCR.
Measurement of brain contusion and penumbra volume
Brain tissues were stored at −20 °C. Brain slice with the biggest contusion area was photographed in order to determine the contusion proportion of the whole brain after series slice to 5 mm-thick. Brain tissues were initially fixed with 4% paraformaldehyde for 4–6 h (4 °C), dehydrated by graded ethanol, vitrified by dimethylbenzene, and then embedded in paraffin. 5 µm-thick slices were cut and stained with hematoxylin and eosin. Histopathological indices of centre brain contusion and penumbra were recorded using optical microscope. Image Pro-Plus 5.1 was used to calculate relative area of penumbra.
Gap junction protein Cx43 measurement using Western-blot
The tissues were sonicated in lysis buffer to extract and determine total protein. Each sample was electrophoresed on 10% SDS-PAGE and then transferred to nitrocellulose membranes. After overnight incubation of the membranes with blocking solution (1×PBS containing 5% dry nonfat milk), blotting was performed by using a polyclonal rabbit anti-Cx43 antibody and secondary polyclonal anti-rabbit antibodies conjugated to horseradish peroxidase. Immunopositive bands on membranes exposed to ECL reagents were detected with X-ray films. Membranes were reblotted with anti-mouse â-actin antibodies for quantification of Cx43 expression levels.
Caspase-3 measurement using reverse transcription-PCR (RT-PCR)
RT-PCR was carried out as previously described 9. Total RNA was extracted using TRIzol reagent (Invitrogen, USA) according to the manufacturer's instructions. The RNA yield and purity were assessed by spectrophotometric analysis. Total RNA (500 ng) from each sample was subjected to reverse transcription with random nonamer, dNTP and AMV reverse transcriptase in a 10 µl reaction mixture. The PCR of cDNA was carried out using Takara Ex Taq Hotstart polymerase, dNTPs and the following related primers: 5′-GGAGCTGGACTGTGGCATTGA-3′ (sense) and 5′-CAGTTCTTTCGTGAGCATGGA-3′ (antisense) for caspase-3; 5′-CTCTGGAAAGCTGTGGCGTGAT-3′ (sense) and 5′-CTGGGATGGAATT GTGAGGGGG-3′ (antisense) for GAPDH. After denaturation for 3 min at 94 °C, the total amount of reaction products was amplified for 30 cycles (94 °C, 30 s; 58 °C, 30 s; 72 °C, 120 s) on the TAKARA PCR Thermal Cycler.
Statistical analysis
The results were expressed as mean ± standard deviation (SD). Statistical differences were evaluated using the two-tailed Student's t-test and considered significant at the *p < 0.05, **p <0.01 level.
Results
Pathological change and comparison of brain contusion volume
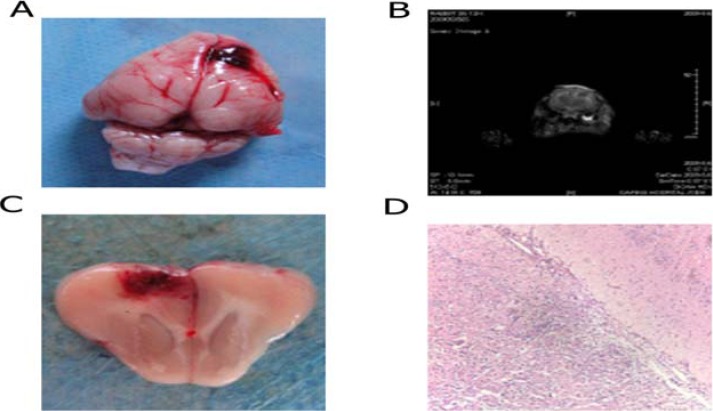
As shown in figure 1, anatomical physiology showed that focal cerebral lacerated wound was mainly located in posterior parietal cortex. Observation under microscope showed that continuous fracture, bleeding and contusion alteration appears in cerebral cortex. Neurons exhibited disorganized feature, degeneration and necrosis. The structure of cell nuclear was not clear, and some exhibits pyknosis. One ecotone was observed between central region of brain contusion and normal brain tissue. This region underwent slight dyeing, and interstitial space exhibited enlargement and edema. The structure and shape of most neurons were normal, and microglia cells exhibied normal shape. Based on these properties, this region can be recognized as ischemic penumbra. Compared with control group, the ratio of ischemic penumbra region to central brain contusion was lower in experimental group (p<0.05) at 1d and 3d after brain damage. However, the central brain contusion didn't show significant alteration (table 1).
Figure 1.
Description of animal model. A. Anatomical physiology of rabbits' brain; B. NMR scan of rabbits' brain with injury; C. Cross section of rabbits' brain contusion; D
Table 1.
Brain contusion in control group and experimental group
| n | Central brain contusion | ischemic penumbra | |||
| 1d | 3d | 1d | 3d | ||
| Experimental group | 5 | 12.86±0.55 | 16.00±0.60 | 27.86±0.48* | 34.96±1.25* |
| Control group | 5 | 13.30±0.52 | 16.58±0.31 | 38.34±4.02 | 50.72±1.57 |
p<0.05, compared with control group
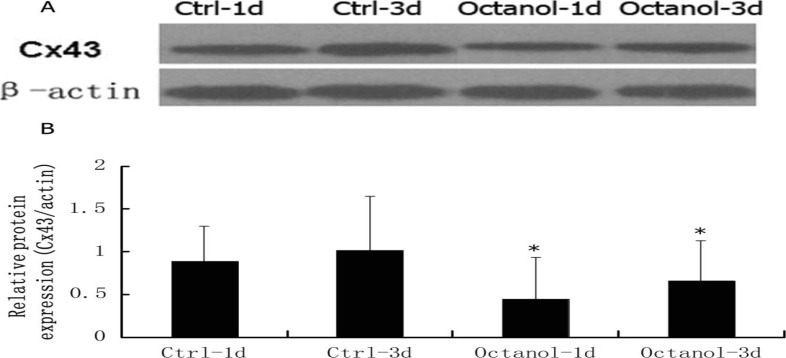
Blockage of gap junction significantly decreased the expression of C × 43
As shown in figure 2, blockage of gap junction using octanol significantly induced the reduction of Cx43 expression.
Figure 2.
Western analysis of Cx43 expression. A. Representative figure of Cx43 expression. B. Statistical analysis of alteration of Cx43 expression after treated with Octanol
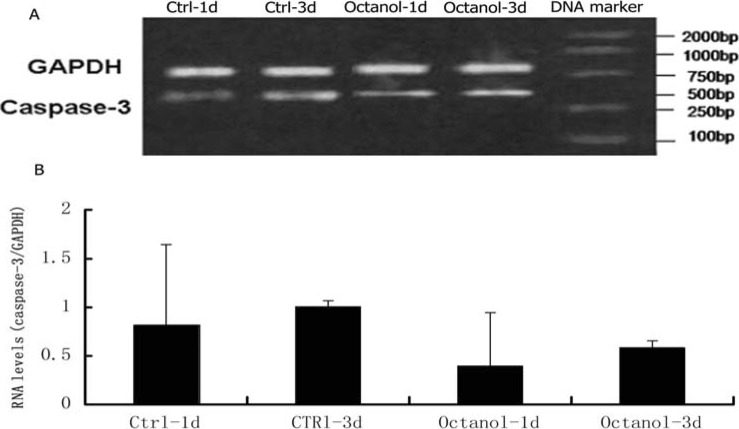
The alteration of RNA levels of caspase-3
The RNA level of caspase-3 in octanol-treated group was lower than that in control group (p<0.05) (figure 3).
Figure 3.
RNA levels of caspase-3. A. Representative figure of RT-PCR of caspase-3. B. Statistical analysis of alteration of RNA of caspase-3 after treated with Octanol
Discussion
The neuroprotective effect of gap function blockers has been demonstrated using in vitro and in vivo experiments. De Pina-Benabou et al. have employed an intrauterine hypoxia-ischemia model to demonstrate that immediate carbenoxolone post-treatment ameliorates long-term developmental deficits and markedly decreases rate of mortality of new born pups8. Carbenoxolone and octanol, two typical blockers of gap function, have been demonstrated to decrease cell death 48 hours after induction of ischemia in organotypic hippocampal slices in glucosefree medium4. Our recent results are consistent with these reports that octanol is an efficient blocker of gap function, which can lower the ratio of ischemic penumbra region to central brain contusion.
In previous studies, the effect of Cx43 in a variety of central neurous system (CNS) pathologies has been reported 10. Cx43 was demonstrated to be upregulated in in Alzheimer's disease11. When brain suffers from mechanical lesions and ischemia, Cx43 down-regulation was detected 12, 13. Protein expression was determined in our present study, and the results showed the decreased expression of Cx43 after blockage of gap function by octanol.
Apoptosis, characterized morphologically by condensed nuclei, cell shrinkage, chromatin marginalization and membrane blebbing, has been suggested to an important process in blast-induced traumatic brain injury. The pro-apoptosis factors including Bcl-2 and Bax have been indicated to alter in explosive brain damage 14. Our recent data demonstrate the change of downstream pro-apoptotic factor caspase-3, which furtherly supports the role of cell apoptosis on explosive brain damage.
Conclusion
Our present study demonstrated the neuroprotective role of gap function inhibitors on explosive brain damage, and change of the expression of Cx43 and caspase-3 were involved in this process.
References
- 1.Risdall JE, Menon DK. Traumatic brain injury. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366(1562):241–250. doi: 10.1098/rstb.2010.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wolf SJ, Bebarta VS, Bonnett CJ, Pons PT, Cantrill SV. Blast injuries. The Lancet. 2009;374(9687):405–415. doi: 10.1016/S0140-6736(09)60257-9. [DOI] [PubMed] [Google Scholar]
- 3.Lin JHC, Weigel H, Cotrina ML, et al. Gap-junction-mediated propagation and amplification of cell injury. Nature neuroscience. 1998;1(6):494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 4.Frantseva MV, Kokarovtseva L, Velazquez JLP. Ischemia-induced brain damage depends on specific gap-junctional coupling. Journal of Cerebral Blood Flow & Metabolism. 2002;22(4):453–462. doi: 10.1097/00004647-200204000-00009. [DOI] [PubMed] [Google Scholar]
- 5.De Maio A, Vega VL, Contreras JE. Gap junctions, homeostasis, and injury. Journal of cellular physiology. 2002;191(3):269–282. doi: 10.1002/jcp.10108. [DOI] [PubMed] [Google Scholar]
- 6.Homma N, Alvarado JL, Coombs W, et al. A particle-receptor model for the insulin-induced closure of connexin43 channels. Circulation research. 1998;83(1):27–32. doi: 10.1161/01.res.83.1.27. [DOI] [PubMed] [Google Scholar]
- 7.Rawanduzy A, Hansen A, Hansen TW, Nedergaard M. Effective reduction of infarct volume by gap junction blockade in a rodent model of stroke. Journal of Neurosurgery. 1997;87(6):916–920. doi: 10.3171/jns.1997.87.6.0916. [DOI] [PubMed] [Google Scholar]
- 8.de Pina-Benabou MH, Szostak V, Kyrozis A, et al. Blockade of gap junctions in vivo provides neuroprotection after perinatal global ischemia. Stroke. 2005;36(10):2232–2237. doi: 10.1161/01.STR.0000182239.75969.d8. [DOI] [PubMed] [Google Scholar]
- 9.Fang ZZ, Nian Y, Li W, et al. Cycloartane triterpenoids from Cimicifuga yunnanensis induce apoptosis of breast cancer cells (MCF7) via p53 dependent mitochondrial signaling pathway. Phytotherapy Research. 2011;25(1):17–24. doi: 10.1002/ptr.3222. [DOI] [PubMed] [Google Scholar]
- 10.Oyamada M, Oyamada Y, Takamatsu T. Gap junctions in health and disease. Medical Electron Microscopy. 1998;31(3):115–120. [Google Scholar]
- 11.Nagy J, Li W, Hertzberg E, Marotta C. Elevated connexin43 immunoreactivity at sites of amyloid plaques in Alzheimer's disease. Brain research. 1996;717(1–2):173–178. doi: 10.1016/0006-8993(95)01526-4. [DOI] [PubMed] [Google Scholar]
- 12.Rouach N, Avignone E, Meme W, et al. Gap junctions and connexin expression in the normal and pathological central nervous system. Biology of the Cell. 2002;94(7–8):457–475. doi: 10.1016/s0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 13.Nagy J, Li W. A brain slice model for in vitro analyses of astrocytic gap junction and connexin43 regulation: actions of ischemia, glutamate and elevated potassium. European Journal of Neuroscience. 2000;12(12):4567–4572. [PubMed] [Google Scholar]
- 14.Wennersten A, Holmin S, Mathiesen T. Characterization of Bax and Bcl-2 in apoptosis after experimental traumatic brain injury in the rat. Acta neuropathologica. 2003;105(3):281–288. doi: 10.1007/s00401-002-0649-y. [DOI] [PubMed] [Google Scholar]