Abstract
Objectives
To analyze the concept of “case series” in the medical literature compared with case reports.
Methods
A PubMed search for articles published during 2009 which had “case series” in their title was performed. A total number of 621 articles were retrieved. 586 papers were included in the analysis and 35 were excluded (18 were commentary letters, 5 were not in English, and twelve could not be retrieved by our Library). The number of patients and category of these articles were analyzed.
Results
The median (range) of the number of cases of articles having “case series” in their title was 7 (1–6432) cases. 186/ 586 articles had less than 5 cases (31.7%, 95% CI (28.3–35.1%)). The median (range) of the number of cases of articles having “case report” as their publication type was 4 (1–178) cases. Out of the 219 articles categorized as case reports 114 (52.1%, 95% CI (45.6–58.6%)) had less than five cases.
Conclusions
The concept of “case series” is not well defined in the literature and does not reflect a specific research design. We suggest that a case series should have more than four patients while four paitents or less should be reported individually as case reports.
Keywords: Case report, case series, concept analysis, research design
Introduction
There has been a recent trend in some journals to stop publishing case reports and publish more original articles instead. 1, 2 This is possibly driven by the desire to get a higher impact factor, to properly utilize the limited space in the journals with economical gains, and to effectively use the time of the reviewers. This approach may have a negative impact on understanding the pathophysiology and management of rare diseases. Furthermore, group outcomes may not reflect exactly what happens in individual patients.3, 4 Reporting a study with a small number of patients may turn out to be very useful especially during an epidemic. Historically, reporting case series with small number of patients raised important concerns regarding serious conditions like the relationship between liver adenomas and contraceptive pills, Kaposi's sarcoma and AIDS, and the toxic effects of high concentrations of oxygen on the optic nerve in newborn infants. 5
Some journals have gone around that by accepting case series instead of case reports or alternatively establish a sister journal for case reports so as not to lose this important advantage. 1 An endeavor to launch indexed journals publishing only case reports was also attempted. 6 We have repeatedly noticed, during submitting articles to refereed journals, that the difference between a case series and a case report is not well defined. Both are seperate types of observational studies. 5, 7 A case report is the smallest publishable unit in the medical literature while a case-series is an aggregation of several similar cases. 5 There is no defined limit for the smallest number of a case series. Some authors accepted even three cases to be a case series. 8 Furthermore, the style of reporting each of these types is different.
We were personally lost between the instruction of authors and the personal opinion of editors on defining a case series. Case series were some times rejected on the basis that they were considered by editors as case reports (personal expereince). We could not find a clear distinction between these two types in the medical literature and we aimed in this study to analyze the concept of “case series” as used recently in the medical literature and whether it is different from case reports.
Methods
A PubMed search was made through PubMed website 9 using the term “case series” between brackets. Search was limited to the title filed and the publication date between 1st of January 2009 to 31st of December 2009. The site was accessed on 18th of October 2010. Publications type was accessed on individual abstracts and tabbed. Some articles had more than one type which was vertically organized. The first line was considered as the first type, the second line as the second type, and the third line as the third type.
A total number of 621 articles were displayed. The abstracts of these articles were printed and reviewed manually. The number of cases in each paper was searched manually on the hard copy. 18 papers (letters to the Editor as commentaries on other published articles) and five non-English articles were excluded. 31 abstracts didn't have enough data regarding the number of studied cases. 19 full articles were retrieved through The National Medical Library of the Faculty of Medicine and Health Sciences, UAE University while the other 12 articles could not be retrieved and were excluded from the study. A total number of 586 papers were included in the analysis.
The “publications type” was searched directly from the website as described above. This was available only on 352 out of 586 articles (60.1%).
Statistics
An excel program was made to enter the “number of cases” and “the publication types” for each article. Data were analyzed with the PASW Statistics 18, SPSS Inc, USA. A standard formula to calculate the 95% confidence interval (95% CI) of proportions was used which is: 95% CI = p ± 1.96 x square root of (pq/n), where p is the studied portion and q is the alternate portion and n is the sample size. 10
Results
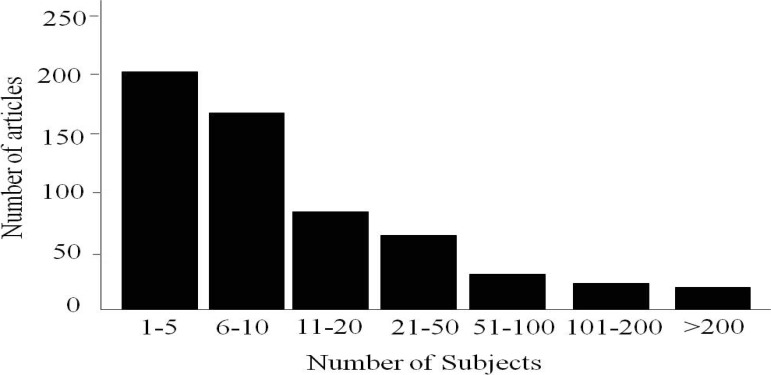
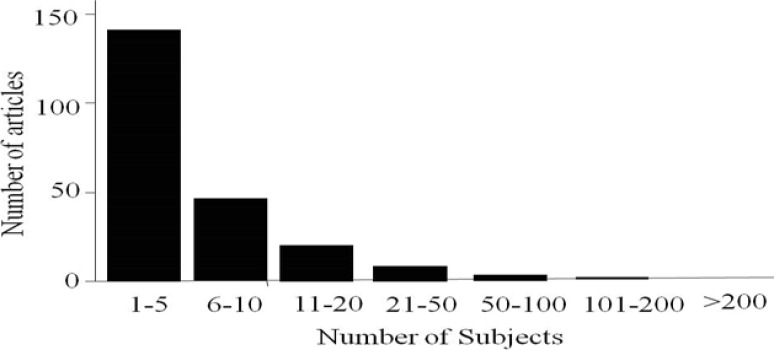
The mean (SD) of the number of cases of articles having “case series” in their title was 57.2 (357.6) cases while the median range of the number of cases was 7 (1–6432) cases. Majority of these articles had 10 cases or less (63%) (figure 1). 186 out of the studied 586 articles had less than 5 cases (31.7%, 95% CI (28.3–35.1%)). One paper had a single case reported as case series in a prestigious journal. 11 The largest number of patients was 6432 patients.12 PubMed categories were available in 352 articles. 219 articles (62.2%, 95% CI (57.4–67%) were categorized as case report (table 1). The mean (SD) of the number of cases of articles having “case report” as their publication type was 8.2 (15.4) cases while the median range of the number of cases was 4 (1–178) cases. 85% of those categorized as case reports had 10 patients or less (figure 2). Out of the 219 articles categorized as case reports 114 (52.1%, 95% CI (45.6–58.6%)). had less than five cases compared with 105 (47.9%, 95% CI (41.4–54.4%)) having 5 or more cases. Only one study (0.5%) categorized as case report had one case. Other categories are shown in table 1. This covered a wide range of research designs including randomized controlled trials in three articles and meta-analysis in one article.
Figure 1.
Number of subjects studied in papers published during 2009 by the U.S. National Library of Medicine, National Institutes of Health “ PubMed” that have “case series” in their title. Total number of publications = 586 papers
Table 1.
Publication type, as defined by the U.S. National Library of Medicine, National Institutes of Health, of articles having “case series” in their title, published during 2009 and indexed by the PubMed website
| Publication type | First type | Second type | Third type |
| Case reports | 219 (62.2%) | ||
| Research support | 41 (11.6%) | 41 (35.6%) | 10 (71.4%) |
| Review | 20 (5.7%) | 48 (41.7%) | 4 (28.6%) |
| Comparative study | 21 (6%) | 6 (5.2%) | |
| Clinical trial | 14 (4%) | 1 (0.9%) | |
| Scientific letter | 11 (3.1%) | 10 (8.7%) | |
| Evaluation study | 7 (1.7%) | ||
| Multicenter study | 2 (0.7%) | 3 (2.6%) | |
| Controlled clinical trial | 5 (1.4%) | ||
| Randomized controlled trial | 2 (0.7%) | 1 (0.9%) | |
| Validation study | 1 (0.3%) | ||
| Historical article | 4 (1.1%) | ||
| English Abstract | 5 (1.4%) | 3 (2.6%) | |
| In vitro | 1 (0.9%) | ||
| Meta analysis | 1 (0.9%) | ||
| Total | 352(100%) | 115 (100%) | 14 (100%) |
Figure 2.
Number of subjects of papers published during 2009 by the U.S. National Library of Medicine, National Institutes of Health “PubMed” that have “case series” in their title and categorized as “case report”. Total number of publications = 219 papers
Discussion
An accurate observation may turn out to be the first step towards an important discovery in science. Similarly, many diseases were first observed at the bedside. A good descriptive study has a clear, specific, and measurrable definition of the studied disease. 5 Although it is possible to carry out large-scale randomized trials for rare conditions when resources are available, this may turn out to be extremely difficult and expensive. Furthermore, the design of observational studies and randomized trials answer different questions.
Both case reports and case series lack comparison groups, their data may be biased and incomplete. Despite that, they are useful for generating hypotheses for future studies.13
Definitions
According to the latest version of the Dictionary of Epidemiology, a case series is defined as “a collection of patients with common characteristics used to describe some clinical, pathophysiological or operational aspects of a disease, treatment or diagnostic procedures”. 14 A case report is a “detailed description of a few patients or clinical cases with an unsual disease or complication, uncommon combinations of diseases, and unusual or misleading semiology, cause or outcome”. 14 Interestingly, The fourth version of the same dictionary which was published in 2001 did not have a defnition for a case series neither a case report indicating that epidemiologists have only recently tried to define these terms. 15 Even a standard Evidenced-based Medicine book did not differentiate between a case series and a case report.16 The Centre for Evidence-Based Medicine, University of Oxford, UK has defined case-series as “a report on a series of patients with an outcome of interest”17.
Other definitions indicate that a case series has few patients. The Medical Research Council of South Africa defined it as “an uncontrolled observational study involving an intervention and outcome for more than one person”18. The Centre for training and Research in Public Health, Italy defined case-series as a “report of a number of cases of disease”19. The National Cancer Institute of USA defined case series as “a group or series of case reports involving patients who were given similar treatment”20. Interestingly, the Mesh database site of PubMed does not have a definition for case series neither it was considered as a catgory when classifying the papers having “case series” in their title21. Some investigators do not include “case series” in the list of types of studies because they are generally not planned and do not involve a research hypothesis22.
It is very clear from these definitions that there is no clear distinction between a case report and a case series in the literature. The results of our paper reflect this finding. Some papers, even in prestigious journals, considered one case as a case series11 and another labeled a group of more than 6000 cases as a case series12. We think that this misconception stemmed from clinicians who used the “case series” term linguistically as a series of patients collected over a period of time without considerations to the research design. This misconception was also carried out to the definition of a case report. Interestingly, the Mesh database site of PubMed21 defined case reports as “Clinical presentations that may be followed by evaluative studies that eventually lead to a diagnosis” which is very similar to the definition of a case series. The National Cancer Institute of USA defined a case report as “a detailed report of the diagnosis, treatment, and follow-up of an individual patient containing some demographic information about the patient”20.
Statistical and study design considerations
The variance of the data, the difference between the means, and the power of a study will decide the sample size needed for a study. Finding a difference between two groups depends on the standard error of the mean of each of these groups.
It is obvious that if data of a group of subjects are to be summarized statistically, then a minimum number of subjects is needed to be valid. We have found from experience that, five is the minimum reasonable number of independent subjects in a group so as to combine their data 23–25 That is because, the standard error of the mean, which is used for comparisons, will be much larger for a number of subjects less than that. The standard error of the mean equals the standard deviation (a marker of variation in the data) divided by the square root of the sample size. 10 This opinion agrees with Patterson et al who suggested that five cases is the lowest advised number of a case series. 7
Nevertheless, it is important to stress that the number of patients per se will not indicate the required research design. Clincial trials were performed even in a single patient using himself/herself as his/her own control. 26 Using this approcah, randomized controlled trials in a specific patients (N=1 clinical trial) were used to define the best treatment for that patient.26
The style of a case report and a case series
A useful definition of a case report that we have found in the literature was “a description of clincial events of one or several patients in a narrative form”. 27 This, by surface validity, is similar to the patient's medical report. Each patient will have his/her own medical report which is written in a seperate section. Case series will contain individual patients' data like demography, diagnosis, and management. Data of a small case series can be presented as a table and pooled together if needed without the need for individual detailed desription28. This may occasionally alert clinicians to unnoticed serious clincial events.28, 29 This approach also enabled auhtors to develop unique new management algorithms for treating rare serious conditions30, 31. Furhtermore, a case series may have been collected over a specific period of time which should be mentioned in the paper. A case series can be consecutive if all eligible patients were identified by the researchers during the study period. Alternatively, it can be nonconsecutive if it includes some, but not all, of the eligible patients. 32, 33
We think that the distinction between a case report and a case series should be clear in the instruction of authors of different journals. This will save the time of the authors and editors and enhance the review process of medical journals. Some journals will accept only a case series design but not a case report. We suggest that patients less than 5 to be reported individually as case reports and those above 4 to be presented as a case series. Our study has shown that more than 30% of the papers having “case series” in their title have less than 5 cases and more than 50% of those “case series” labeled as a case report have less than 5 cases. The upper limit of a case series could not be defined by us but we suggest ten as an upper limit, similar to what was suggested by the European Urology34. This journal has clear instructions to authors that a “case series” should report on no more than 10 patients. An observational study of more patients based on rates is a different category and should be possibly labeled as a rate-based descriptive study. 7
Conclusion
In summary we have shown that the concept of “case series” is not well defined in the recent literature. We tried to analyze this concept and came with a suggestion that a case series should have more than four patients while four paitents or less should be reported individually as case reports. We hope that our suggestion will be accepted by the scientific community.
References
- 1.International Journal of Surgery. [2nd February 2012]. http://www.elsevier.com/wps/find/journaldescription.cws_home/705107/authorinstructions.
- 2.World Journal of Surgery. [2nd February 2012]. https://mc.manuscriptcentral.com/wjs.
- 3.Mant D. Can randomised trials inform clinical decisions about individual patients? Lancet. 1999;353:743–746. doi: 10.1016/S0140-6736(98)09102-8. [DOI] [PubMed] [Google Scholar]
- 4.Pollock AV. Surgical evaluation at the crossroads. Br J Surg. 1993;80:964–966. doi: 10.1002/bjs.1800800807. [DOI] [PubMed] [Google Scholar]
- 5.Grimes DA, Schulz KF. Descriptive studies: what they can and cannot do. Lancet. 2002;359:145–149. doi: 10.1016/S0140-6736(02)07373-7. [DOI] [PubMed] [Google Scholar]
- 6.Cases Journal. [29th November 2010]. http://casesjournal.com/
- 7.Patterson PD, Weaver M, Clark S, Yealy DM. Case reports and case series in prehospital emergency care research. Emerg Med J. 2010;27:807–809. doi: 10.1136/emj.2009.073668. [DOI] [PubMed] [Google Scholar]
- 8.Hennekens CH, Buring JE, Mayrent SL, editors. Epidemiology in Medicine. First edition. USA: Little, Brown and company Boston/Toronto; 1987. [Google Scholar]
- 9.PubMed website. [18th October 2010]. http://www.ncbi.nlm.nih.gov/pubmed.
- 10.Hassard TH. Estimation. In: Hassard TH, editor. Understanding biostatistics. St. Louis: Mosby year book, Inc.; 1991. pp. 38–51. [Google Scholar]
- 11.Aerts J, Matas A, Sutherland D, Kandaswamy R. Chylous ascites requiring surgical intervention after donor nephrectomy: case series and single center experience. Am J Transplant. 2010;10:124–128. doi: 10.1111/j.1600-6143.2009.02883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheen NJ, Fone D, Phillips CJ, Sparrow JM, Pointer JS, Wild JM. Novel optometrist-led all Wales primary eye-care services: evaluation of a prospective case series. Br J Ophthalmol. 2009;93:435–438. doi: 10.1136/bjo.2008.144329. [DOI] [PubMed] [Google Scholar]
- 13.Kooistra B, Dijkman B, Einhorn TA, Bhandari M. How to design a good case series. J Bone Joint Surg Am. 2009;3:21–26. doi: 10.2106/JBJS.H.01573. [DOI] [PubMed] [Google Scholar]
- 14.Porta M, editor. A dictionary of epidemiology / edited for the International Epidemiological Association. 5th edition. UK: Oxford University Press; 2008. p. 33. [Google Scholar]
- 15.Last JM, editor. A dictionary of epidemiology / edited for the International Epidemiological Association. 4th edition. UK: Oxford University Press; 2001. [Google Scholar]
- 16.Sackett DL, Straus SE, Richardson WS, Rosenberg W, Haynes RB, editors. Evidence-based medicine: How to practice and teach EBM. Second ed. London: Churchill Livingstone; 2000. pp. 105–153. [Google Scholar]
- 17.Centre for Evidence-Based Medicine, University of Oxford, UK. [28 November 2010]. http://www.cebm.net/index.aspx?o=1116.
- 18.Medical Research Council of South Africa, author. Evidence-Based Medicine. [28 November 2010]. http://www.sahealthinfo.org/evidence/c.htm.
- 19.The Centre for training and Research in Public Health, Caltanissetta, Italy. [28 November 2010]. http://www.cefpas.it/ebm/tools/glossary.htm.
- 20.National Cancer Institute, USA. [28 November 2010]. http://ww w.cancer.g ov/Templa tes/db_alpha.aspx?CdrID=44006.
- 21.Mesh database. [28 November 2010]. http://www.ncbi.nlm.nih.gov/mesh.
- 22.Dawson B, Trapp RG. “Chapter 2. Study Designs in Medical Research” (Chapter) In: Dawson B, Trapp RG, editors. Basic & Clinical Biostatistics. 4e. [30 November 2010]. http://www.accessmedicine.com/content.aspx?aID=2046062. [Google Scholar]
- 23.Karlsson H, Abu-Zidan FM, Walther S, Lennquist S. Heparin ameliorates pulmonary hypertension induced by platelet-activating factor in pigs. Thromb Res. 1998;90:223–228. doi: 10.1016/s0049-3848(98)00064-4. [DOI] [PubMed] [Google Scholar]
- 24.Abu-Zidan FM, Walther S, Lennquist S. BB-882 is a potent antagonist of the haemodynamic changes induced by platelet-activating factor in pigs. Pharmacol Toxicol. 1996;78:23–27. doi: 10.1111/j.1600-0773.1996.tb00175.x. [DOI] [PubMed] [Google Scholar]
- 25.Abu-Zidan FM, Siösteen AK, Wang J, al-Ayoubi F, Lennquist S. Establishment of a teaching animal model for sonographic diagnosis of trauma. J Trauma. 2004;56:99–104. doi: 10.1097/01.TA.0000038546.82954.3D. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt G, Sackett D, Adachi J, Roberts R, Chong J, Rosenbloom D, Keller J. A clinician's guide for conducting randomized trials in individual patients. CMAJ. 1988;139:497–503. [PMC free article] [PubMed] [Google Scholar]
- 27.Hu X, Wright JG, McLeod RS, Lossing A, Walters BC. Observational studies as alternatives to randomized clinical trials in surgical clinical research. Surgery. 1996;119:473–475. doi: 10.1016/s0039-6060(96)80150-4. [DOI] [PubMed] [Google Scholar]
- 28.Hefny AF, Eid HO, Al-Bashir M, Abu-Zidan FM. Blast injuries of large tyres: case series. Int J Surg. 2010;8:151–154. doi: 10.1016/j.ijsu.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Webster C, Mercer S, Schrager J, Carrell TW, Bowley D. Indirect colonic injury after military wounding: a case series. J Trauma. 2011;71:147–157. doi: 10.1097/TA.0b013e31822af672. [DOI] [PubMed] [Google Scholar]
- 30.Jawas A, Abu-Zidan FM. Management algorithm for complete blunt renal artery occlusion in multiple trauma patients: case series. Int J Surg. 2008;6:317–322. doi: 10.1016/j.ijsu.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 31.Hill SM, Elwood ET. Pediatric lower extremity mower injuries. Ann Plast Surg. 2011;67:279–287. doi: 10.1097/SAP.0b013e3181fab9ba. [DOI] [PubMed] [Google Scholar]
- 32.National Cancer Institute, USA, author. Consecutive case series. [28 November 2010]. http://www.cancer.gov/Templates/db_alpha.aspx?CdrID=285747.
- 33.National Cancer Institute, USA, author. Non consecutive case series. [28 November 2010]. http://www.cancer.gov/dictionary/?CdrID=44575.
- 34.European Urolgy, Instruction for authors. [28 November 2010]. http://www.europeanurolog y.com/about-thejournal/for-authors.