Abstract
Background.
Older heart failure patients with preserved ejection fraction (HFpEF) have severely reduced exercise capacity and quality of life. Both brachial artery flow-mediated dilation (FMD) and peak exercise oxygen uptake (peak VO2) decline with normal aging. However, uncertainty remains regarding whether FMD is reduced beyond the degree associated with normal aging and if this contributes to reduced peak VO2 in elderly HFpEF patients.
Methods.
Sixty-six older (70 ± 7 years) HFpEF patients and 47 healthy participants (16 young, 25 ± 3 years, and 31 older, 70 ± 6 years) were studied. Brachial artery diameter was measured before and after cuff occlusion using high-resolution ultrasound. Peak VO2 was measured using expired gas analysis during upright cycle exercise.
Results.
Peak VO2 was severely reduced in older HFpEF patients compared with age-matched healthy participants (15.2 ± 0.5 vs 19.6 ± 0.6 mL/kg/min, p < .0001), and in both groups, peak VO2 was reduced compared with young healthy controls (28.5 ± 0.8 mL/kg/min; both p < .0001). Compared with healthy young participants, brachial artery FMD (healthy young, 6.13% ± 0.53%) was significantly reduced in healthy older participants (4.0 ± 0.38; p < .0002) and in HFpEF patients (3.64% ± 0.28%; p < .0001). However, FMD was not different in HFpEF patients compared with healthy older participants (p = .86). Although brachial artery FMD was modestly related to peak VO2 in univariate analyses (r = .19; p = .048), it was not related in multivariate analyses that accounted for age, gender, and body size.
Conclusion.
These results suggest that endothelial dysfunction may not be a significant independent contributor to the severely reduced exercise capacity in elderly HFpEF patients.
Keywords: Exercise capacity, Aging, Flow-mediated dilation, Heart failure with preserved ejection fraction, Endothelial function
Approximately 50% of heart failure (HF) patients have preserved left ventricular ejection fraction (HFpEF) (1). HFpEF is increasing in prevalence and is dominant among women and the elderly individuals (1,2). The primary symptom of chronic HFpEF is exercise intolerance. We have shown that peak exercise oxygen uptake (peak VO2) is as severely reduced in older HFpEF patients as in age-matched patients with HF and reduced ejection fraction (HFrEF) and is accompanied by reduced quality of life (3–6). We recently reported that although both cardiac output and arteriovenous oxygen difference (A-VO2Diff) were reduced during exercise in elderly HFpEF patients compared with age-matched healthy controls, the change in A-VO2Diff from rest to peak exercise was the strongest independent predictor of the reduced peak VO2 in HFpEF patients (3). This finding regarding A-VO2Diff suggests that peripheral factors, such as impaired peripheral vascular and/or skeletal muscle function, play an important role in limiting exercise capacity in HFpEF (3).
During the normal response to exercise, there is potent arterial dilation in order to increase muscle blood flow to meet metabolic demands and is mediated primarily by endothelial function (7). With aging, there is a significant decrease in the ability of peripheral arteries to dilate in response to stimuli, including exercise (8–11). Flow-mediated arterial dilation (FMD) assessed by measurement of brachial artery (BA) diameter before and after cuff occlusion with high resolution ultrasound mimics the arterial response to exercise and is an internationally standardized noninvasive index of arterial endothelial function in health and disease (12). We and others have shown that there is progressive reduction of BAFMD with normal healthy aging, and this occurs in tandem with the normal age-related decline in exercise capacity (8,11,13,14).
The aim of this study was to assess BAFMD and peak VO2 in older HFpEF patients in comparison to young and old healthy volunteer control participants. We tested the hypothesis that in older HFpEF patients, BAFMD is reduced beyond the extent that occurs with normal aging and is an important contributor to their severely reduced exercise capacity.
METHODS
Participants
As described in previous studies (3,4,15,16), HFpEF patients had symptoms and signs of HF defined by National Health and Nutrition Examination Survey HF clinical score of 3 or more and criteria by Rich and colleagues (17,18) and had preserved resting systolic function (left ventricle ejection fraction ≥50%, and no segmental wall motion abnormalities) and no significant ischemic or valvular heart disease, pulmonary disease, anemia, or other disorder that could explain the patients’ symptoms.
Normal healthy volunteers were screened and excluded if they had any chronic medical illness, were on any chronic medication, had current complaints or an abnormal physical examination (including blood pressure ≥ 140/90 mmHg), had abnormal results on the screening tests (electrocardiogram, exercise echocardiogram, and spirometry), or were regularly exercising (4,6).
Because of the profound influence of atherosclerosis on endothelial function and FMD (19,20), patients and healthy volunteers were screened to exclude those at high risk (hyperlipidemia, cigarette smoking) and those with known or suspected coronary, cerebrovascular, and peripheral arterial disease by record review, history, physical examination, exercise echocardiography, and carotid ultrasound.
Study Protocol
The protocol was approved by the Institutional Review Board, and informed consent was obtained. During a single visit, the outcome measures of peak VO2 and BAFMD were obtained. All tests were performed by and results analyzed by individuals blinded to patient group.
Echocardiography
Echocardiograms were performed as previously described (3,4) using a Hewlett-Packard model Sonos 5500 (Palo Alto, CA) ultrasound imaging system. Standard two-dimensional images and Doppler were obtained and analyzed using a digital workstation as previously described (3,4,15,16).
Cardiopulmonary Exercise Testing
As previously described (3,4,15,16,21,22), exercise testing was performed on an electronically braked upright stationary bicycle. Expired gas analysis was conducted using a metabolic cart (CPX 2000, MedGraphics, Minneapolis, MN), which was calibrated before each test. The initial workload was 12.5 watts for 2 minutes, followed by 25 watts for 3 minutes, and advanced thereafter by 25 watts increments in 3-minute stages. Peak O2 consumption and CO2 values were averaged from the final 30 seconds of the exercise test. A 6-minute-walk test was also performed (23).
Brachial Artery Flow-Mediated Dilation
With participants in the postprandial state and resting quietly in the supine position, BAFMD was measured as previously described in our laboratory according to international standards (8,12,24–26). A cuff was placed on the right forearm below the antecubital fossa. Using a Biosound Phase II ultrasound system, images of the brachial artery at baseline were recorded. The cuff was then inflated to 50mm Hg above systolic pressure for 4 minutes. Images were recorded during the final 30 seconds prior to and 3 minutes following rapid cuff deflation (8). Video frames were automatically digitized and analyzed using previously described techniques (8). To quantify the vasodilator response, the average diameter of the brachial artery was recorded from the final 30 seconds of the baseline scanning period. The maximum diameter from the dilation phase was automatically determined, and the change in diameter and percent change were calculated. We have previously reported the reproducibility of FMD using these techniques in over 4,000 patients in our laboratory (8).
Statistical Analysis
Comparisons were performed using analysis of covariance adjusting for age, gender, and body surface area unless otherwise noted. Intergroup comparisons of baseline characteristics, exercise performance, and brachial artery imaging were made using the global F-test for continuous measures and Fisher’s exact test for categorical measures. Multiple linear regression analysis was performed to determine significant predictors of peak VO2 and to determine the independent effects of these variables on peak VO2. A two-tailed p value of <.05 was required for significance. Values are expressed as means ± SD unless otherwise noted. The Statistical Analysis Software program (SAS) was used for all analyses.
RESULTS
Participant Characteristics
The HFpEF participants and older healthy controls were well matched for age and gender. The HFpEF participants were predominantly older women with a history of hypertension (Table 1). HFpEF patients were stable, New York Heart Association class II and III. Compared with age-matched healthy controls, they had hypertrophic left ventricle remodeling, left atrial dilation, and abnormal diastolic filling, all typical characteristics of HFpEF (Table 2).
Table 1.
Participant Characteristics
| Characteristic | YH (n = 16) | OH (n = 31) | HFpEF (n = 66) | p Value |
| Women | 10 (62) | 21 (68) | 51 (77) | .36 |
| Caucasian | 15 (94) | 29 (94) | 45 (68) | .01 |
| Age (years) | 25 (3) | 70 (6) | 70 (7) | .95 |
| Body weight (kg) | 71.6 (16.2) | 71.1 (13.0) | 87.0 (19.1) | .01 |
| Body mass index (kg/m2) | 23.9 (3.5) | 25.5 (3.5) | 32.5 (7.4) | <.0001 |
| Body surface area (m2) | 1.84 (0.25) | 1.79 (0.18) | 1.93 (0.23) | .01 |
| History of hypertension | — | — | 59 (89%) | — |
| Systolic blood pressure | 120 (16) | 137 (16) | 146 (17) | <.01 |
| Diastolic blood pressure | 73 (8) | 81 (9) | 82 (10) | .80 |
| Diabetes mellitus | — | — | 18(27%) | — |
| Brain natriuretic peptide, pg/mL | — | 30.8 (19.7) | 67.7 (65.5) | .002 |
| Renin, ng/mL/hr | — | 0.7 (0.8) | 3.4 (6.5) | .02 |
| Aldosterone, ng/dL | — | 8.1 (4.7) | 10.8 (6.5) | .03 |
| Angiotensin converting enzyme inhibitors | — | — | 27 (41%) | — |
| Angiotensin receptor blockers | — | — | 4 (6%) | — |
| Digoxin | — | — | 4 (6%) | — |
| Diuretics | — | — | 40 (61%) | — |
| Beta-blockers | — | — | 14 (21%) | — |
| Calcium channel blockers | — | — | 20 (30%) | — |
| Nitrates | — | — | 8 (12%) | — |
| Estrogen | — | 8 (38%) | 19 (37%) | .76 |
Note: YH = young healthy control participants; OH = older healthy control participants; HFpEF = heart failure and preserved ejection fraction patients. Mean (SD) or n (%) where indicated. p value indicates comparison of HFpEF versus OH.
Table 2.
Supine Resting Left Ventricular Echocardiographic-Doppler Measures
| Variable | HFpEF | OH | p Value |
| Septal wall thickness, cm | 1.4 (0.3) | 1.0 (0.1) | .0001 |
| Posterior wall thickness, cm | 1.2 (0.2) | 1.0 (0.1) | .0001 |
| Mean wall thickness, cm | 1.3 (0.2) | 1.0 (0.1) | .0001 |
| Diastolic cavity dimension, cm | 4.3 (0.7) | 4.3 (0.6) | .89 |
| Relative wall thickness | 0.59 (0.15) | 0.46 (0.1) | .0001 |
| Mass, g | 260 (87) | 169 (48) | .0001 |
| Mass/volume ratio | 3.20 (1.57) | 2.55 (1.10) | .12 |
| End-diastolic volume, mL | 86.6 (29.7) | 71.4 (18.2) | .02 |
| End-systolic volume, mL | 36.1 (13.8) | 25.0 (8.3) | .001 |
| Stroke volume, mL | 50.5 (17.9) | 47.1 (13.3) | .27 |
| Ejection fraction, % | 58.7 (6.6) | 65.4 (7.5) | .001 |
| Left atrial diameter, cm | 3.5 (0.7) | 3.2 (0.5) | .03 |
| Mitral early velocity, cm/s | 73.4 (19.0) | 67.5 (17.5) | .13 |
| Mitral atrial velocity, cm/s | 84.2 (23.3) | 74.5 (13.8) | .007 |
| Early/atrial ratio | 0.99 (0.75) | 0.91 (0.18) | .63 |
| Early deceleration time, ms | 222 (62) | 214 (50) | .28 |
| Isovolumic relaxation time, ms | 82 (22) | 75 (16) | .08 |
| Diastolic function | |||
| Normal | 0 (0) | 24 (77) | <.001 |
| Abnormal relaxation | 30 (49) | 7 (23) | .02 |
| Pseudonormal | 28 (46) | 0 (0) | <.001 |
| Restricted | 3 (5) | 0 (0) | .55 |
Note: HFpEF = heart failure with preserved ejection fraction; OH = older healthy control participants. Data presented as mean ± SD.
Peak Exercise Oxygen Consumption
Peak respiratory exchange ratio was greater than 1.12 in all groups, indicating an exhaustive level of exercise effort. Peak VO2 was significantly reduced in HFpEF patients compared with healthy older and younger controls (15.2 ± 0.5 vs 19.6 ± 0.6 vs 28.5 ± 0.8 mL/kg/min, all comparisons p < .0001). Peak VO2 was also reduced in healthy older versus younger controls (Table 3). Exercise time, peak power output, heart rate, carbon dioxide production, and oxygen pulse were significantly reduced in HFpEF compared with healthy older and younger healthy controls and in older compared with younger healthy control groups (Table 3). The 6-minute-walk distance was significantly reduced in HFpEF patients compared with healthy older controls and between healthy younger and older participants (Table 3).
Table 3.
Cardiopulmonary Performance During Peak Cycle Exercise
| Group | p Value | |||||
| YH | OH | HFpEF | OH vs YH | HFpEF vs YH | HFpEF vs OH | |
| Power output, watts | 151 (6) | 98 (4) | 73 (4) | <.0001 | <.0001 | <.0001 |
| Time, min | 19.3 (0.7) | 13.3 (0.5) | 9.8 (0.4) | <.0001 | <.0001 | <.0001 |
| HR (bpm) | 177 (4) | 147 (3) | 127 (3) | <.0001 | <.0001 | <.0001 |
| SBP, mmHg | 173 (6) | 199 (4) | 190 (3) | .0002 | .009 | .092 |
| DBP, mmHg | 88 (13) | 89 (2) | 89 (2) | .716 | .652 | .938 |
| VO2, mL/min | 2,067 (63) | 1,503 (46) | 1,224 (38) | <.0001 | <.0001 | <.0001 |
| VO2, mL/kg/min | 28.5 (0.8) | 19.6 (0.6) | 15.2 (0.5) | <.0001 | <.0001 | <.0001 |
| O2P, mL/beat | 27.8 (1.2) | 23.1 (0.8) | 18.4 (0.7) | .001 | <.0001 | <.0001 |
| VCO2 , mL/min | 2,369 (77) | 1,770 (56) | 1,366 (47) | <.0001 | <.0001 | <.0001 |
| RER | 1.15 (0.02) | 1.18 (0.01) | 1.12 (0.01) | .307 | .122 | .0002 |
| 6MWD, feet | 2,123 (72) | 1,839 (53) | 1,445 (44) | .002 | <.0001 | <.0001 |
Note: YH = young healthy control participants; OH = older healthy control participants; HFpEF = Heart failure and preserved ejection fraction; HR = heart rate; SBP = systolic blood pressure; DBP = diastolic blood pressure; VO2 = oxygen consumption; O2P = oxygen pulse; VCO2 = carbon dioxide production; RER = respiratory exchange ratio; 6MWD = 6-min walk distance. Data presented as mean ± SE following adjustment for gender and body surface area.
Brachial Artery Flow-Mediated Dilation
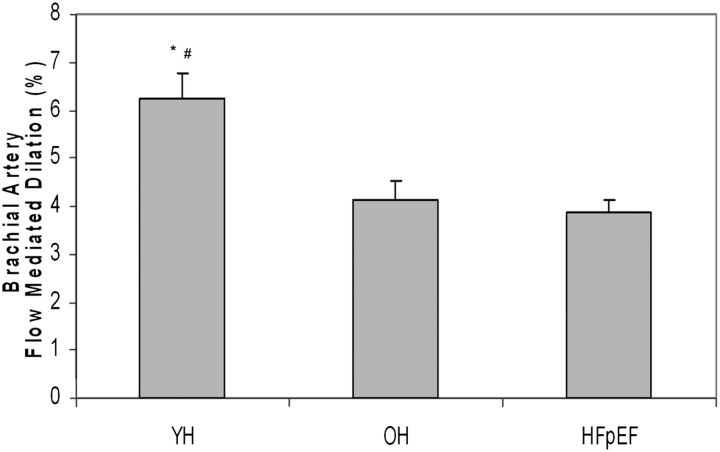
BAFMD was significantly reduced in healthy older compared with healthy young control participants. However, BAFMD was not significantly different in HFpEF patients compared with healthy age-matched older controls (Table 4, Figure 1). The absolute change in brachial artery diameter was reduced in older compared with healthy young control participants, but was also not different in HFpEF patients compared with healthy older controls (Table 4). Both baseline and maximum brachial artery diameter were greater in the HFpEF patients compared with healthy older participants (Table 4).
Table 4.
Brachial Artery Data
| Group | p Value | |||||
| YH (n = 16) | OH (n = 31) | HFpEF (N = 66) | OH vs YH | HFpEF vs YH | HFpEF vs OH | |
| Baseline diameter, mm | 4.13 (0.17) | 3.98 (0.12) | 4.58 (0.09) | .45 | .016 | <.0001 |
| Maximum diameter, mm | 4.37 (0.16) | 4.13 (0.12) | 4.74 (0.09) | .22 | .042 | <.0001 |
| Absolute change in diameter* | 0.25 (0.02) | 0.15 (0.01) | 0.16 (0.01) | .001 | <.0001 | .43 |
| Flow-mediated dilation, % change | 6.13 (0.53) | 4.00 (0.38) | 3.64 (0.28) | .0002 | .0001 | .86 |
Notes: YH = young healthy control participants; OH = older healthy control participants; HFpEF = Heart failure and preserved ejection fraction patients. Data presented as mean ± SE after adjustment for gender and body surface area.
Data also adjusted for baseline diameter.
Figure 1.
Percent brachial artery flow-mediated dilation (FMD) in young healthy volunteer participants (YH), older healthy volunteer participants (OH), and older patients with heart failure and preserved ejection fraction (HFpEF). Data displayed are raw unadjusted means + SE; * denotes p < .001 compared with OH; # denotes p < .0001 YH compared with HFpEF. There was no difference in HFpEF compared with OH (p = .86). p Values represent analyses following adjustment for gender and body size.
Relationship of BAFMD and Peak VO2
In univariate analyses, BAFMD was modestly related to peak VO2 (r = .19; p = .048; Table 5), and age and gender were strongly related to peak VO2 (age r = −.68; p < .001; gender r = .89; p < .001). In a multivariate model, after adjustment for age, gender, and body surface area, there was no significant relationship between BAFMD and peak VO2 (r = .009; p = .93; Table 5). In addition, neither baseline nor maximum brachial artery diameter showed a significant independent relationship to peak VO2 (Table 5). These results were unchanged when adjustments were made to account for medications.
Table 5.
Predictors of Peak Exercise Oxygen Consumption
| Variable | Univariate Predictor | Multivariate Predictor* | ||
| Simple Correlation | p Value | Partial Correlation | p Value | |
| Gender | 0.89 | <.001 | — | — |
| Age | −0.68 | <.001 | — | — |
| Body surface area | −0.15 | .12 | — | — |
| Baseline diameter | 0.07 | .51 | −0.08 | .40 |
| Maximum diameter | 0.09 | .37 | −0.11 | .27 |
| Absolute change in diameter† | 0.24 | .01 | −0.01 | .91 |
| Flow-mediated dilation | 0.19 | .048 | 0.009 | .93 |
Notes: *Data adjusted for age, gender, and body surface area.
Data also adjusted for baseline diameter.
DISCUSSION
Although many studies have examined the mechanisms of exercise intolerance in HFrEF (27–29), in HFpEF the mechanisms of exercise intolerance are not well understood (30), even though the predominantly elderly patients with this disorder represent a large and increasing proportion of the HF population (1,2). As anticipated, the HFpEF patients had severely reduced peak VO2 compared with age-matched healthy participants, in accord with our prior reports in separate groups of patients (3–5,22). BAFMD was greatly reduced in healthy persons 60 years or older compared with those 30 years or younger. This is consistent with prior studies in our laboratory and others that have shown a progressive decline in BAFMD with advancing age in healthy volunteers (8,11,13,14). However, elderly patients with HFpEF did not have reduced BAFMD compared with healthy age-matched peers. Although BAFMD had a modest univariate relationship with peak exercise VO2, there was no significant independent relationship after taking into consideration age, gender, and body size. These data suggest that FMD is not abnormal in older patients with HFpEF and does not significantly contribute to their severely reduced exercise capacity.
These findings are important because understanding mechanisms of exercise intolerance can provide insights into pathophysiology and potential treatment of this common and important disorder (30). Our results suggest that the decreased peak exercise A-VO2Diff in HFpEF patients is not due to reduced large artery vasodilatory reserve function. This further suggests that other peripheral factors, such as impaired skeletal muscle function resulting in reduced oxygen utilization by the active muscle, may play an important role in the reduced exercise capacity in elderly HFpEF patients.
We addressed FMD as a potential mechanism for our recently reported finding of reduced peak A-VO2Diff in HFpEF because studies in HFrEF patients indicated that reduced vasodilator reserve contributes to exercise intolerance in those patients (29,31). Hornig and colleagues (31) reported that BAFMD was severely reduced in HFrEF patients, correlated with the reduced exercise capacity, and improved with exercise training along with peak VO2. Our findings suggest that although HFpEF patients have reductions in peak VO2 that are just as severe as in HFrEF patients, the contributing mechanisms may differ somewhat. The implications of our findings, which point to the potential role of skeletal muscle abnormalities in HFpEF, are supported by a number of studies in healthy elderly individuals and HFrEF patients that indicate that skeletal muscle function is markedly abnormal, contributes to exercise intolerance, and may improve with exercise training (5,32–35).
Using phase-contrast magnetic resonance imaging we previously reported that femoral artery FMD was significantly reduced in elderly HFrEF patients compared with age-matched healthy volunteer participants and HFpEF patients (6). However, femoral artery FMD was relatively preserved in elderly HFpEF patients and was similar to the age-matched healthy control participants (6). The present report significantly extends these prior findings by utilizing high resolution ultrasound of brachial artery FMD in a much larger number of elderly HFpEF patients and age-matched healthy control participants, both of which were well screened to exclude the confounding influence of atherosclerosis, and also by adding a group of young healthy control participants. This allowed us to demonstrate the expected age-related decline in FMD and show that HFpEF patients had no further decline in FMD beyond that due to age alone. Furthermore, the present study examined FMD in a different arterial bed, using techniques that are standardized and well accepted. Taken together, our studies support that abnormal FMD is not a significant contributor to the severely reduced exercise capacity in HFpEF patients (6).
Because of the known profound impact of atherosclerosis on endothelial function and FMD (19,20), our study design included screening for coronary, cerebrovascular, and peripheral vascular disease as described above in both HFpEF patients and healthy older control participants. This is a strength of the present study, as the confounding influence of atherosclerosis has not been uniformly accounted for in prior studies of FMD. Manifest atherosclerotic disease, particularly concomitant ischemic heart disease, is more common in HFrEF than HFpEF (1,36). However, multiple prior studies using animal models and carefully selected patient groups have shown that abnormal endothelial function is present in both brachial and femoral arterial beds in HFrEF, even in the absence of atherosclerosis (37–39). Taken together with our data, this suggests that abnormal FMD is a fundamental component of the pathophysiology of HFrEF but not HFpEF.
Using an automated finger tonometry assessment technique, Borlaug and colleagues (40) recently reported that finger blood flow was reduced in elderly HFpEF patients compared with age-matched healthy control participants but was not different in HFpEF patients compared with hypertensive control participants without HF. This supports that abnormal FMD is not a unique aspect of HFpEF pathophysiology apart from concomitant disease, such as hypertension which is present in about 90% of HFpEF patients (1). The disparity between study findings may be attributable to differences in patient characteristics and methodology. In our study, we excluded participants with known atherosclerosis. In contrast, one third of the elderly HFpEF patients in the Borlaug study had known, clinically significant underlying coronary artery disease, and information regarding hyperlipidemia, carotid, cerebrovascular, and peripheral arterial disease was not reported. We assessed endothelial function using high resolution ultrasound in response to cuff ischemia which reflects large conduit artery vasodilation. It is an internationally standardized well-accepted technique shown by our group and others to be related to clinical outcomes (25,26) and is sensitive to changes after therapy (8,31). Borlaug and colleagues on the other hand assessed the change in finger pulse amplitude in response to cuff ischemia, which is a newer technique that is thought to assess microvascular function in the terminal vascular bed (41). Responses measured by this technique are influenced by a number of factors other than endothelial function, including autonomic function as well as potentially intrinsic arterial stiffness (41).
Studies in HFrEF patients have shown that regular physical exercise increases limb blood flow and improves endothelial function and that this improvement is associated with enhanced exercise capacity (30,31,42,43). All groups of participants in the present study were sedentary and not involved in regular exercise, suggesting that this potential confounding factor was not primarily responsible for our results.
Limitations
For ethical reasons, cardiac medications were withheld for only 12 hours before exercise testing and brachial artery imaging in patients. However, the overall results were unchanged after adjustments to take cardiovascular-active medications into account.
This study assessed FMD in the brachial artery; however, the primary working muscle beds during upright cycle exercise are in the lower limb and are supplied primarily by the femoral artery. However, as discussed above, we have reported that femoral artery FMD is similar between elderly HFpEF patients and age-matched healthy participants despite marked differences in peak VO2 (6).
Our HFpEF patients were predominantly women, reflecting the sex distribution found in population studies (1). Celermajer reported that FMD is reduced in men compared with women (13). However, there was no difference in sex distribution between our HFpEF patients and age-matched controls, and results were unchanged after adjustment for sex.
CONCLUSION
Elderly HFpEF patients had severely decreased exercise capacity compared with age-matched healthy participants (3–5,22), and BAFMD was greatly reduced in healthy older versus younger individuals; however, elderly HFpEF patients did not have reduced brachial artery FMD compared with healthy age-matched controls. Further, BAFMD did not have a significant independent relationship to peak VO2 in these patients. This suggests that peripheral arterial endothelial dysfunction may not be a primary significant contributor to reduced exercise capacity in HFpEF. This suggests that future studies should examine other factors including skeletal muscle function in elderly HFpEF patients and their potential contribution to exercise intolerance, the primary chronic symptom in the large and growing number of patients with this syndrome.
FUNDING
This work was supported by the following research grants from the National Institutes of Health (NIH): R37AG18915 and RO1AG12257; The Claude D. Pepper Older Americans Independence Center of Wake Forest University N I H P30AG21332.
References
- 1.Kitzman DW, Gardin JM, Gottdiener JS, et al. Importance of heart failure with preserved systolic function in patients > or = 65 years of age. CHS Research Group. Cardiovascular Health Study. Am J Cardiol. 2001;87:413–419. doi: 10.1016/s0002-9149(00)01393-x. [DOI] [PubMed] [Google Scholar]
- 2.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 3.Haykowsky MJ, Brubaker PH, John JM, Stewart KP, Morgan TM, Kitzman DW. Determinants of exercise intolerance in elderly heart failure patients with preserved ejection fraction. J Am Coll Cardiol. 2011;58:265–274. doi: 10.1016/j.jacc.2011.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kitzman DW, Little WC, Brubaker PH, et al. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA. 2002;288:2144–2150. doi: 10.1001/jama.288.17.2144. [DOI] [PubMed] [Google Scholar]
- 5.Kitzman DW, Higginbotham MB, Cobb FR, Sheikh KH, Sullivan MJ. Exercise intolerance in patients with heart failure and preserved left ventricular systolic function: failure of the Frank-Starling mechanism. J Am Coll Cardiol. 1991;17:1065–1072. doi: 10.1016/0735-1097(91)90832-t. [DOI] [PubMed] [Google Scholar]
- 6.Hundley WG, Bayram E, Hamilton CA, et al. Leg flow-mediated arterial dilation in elderly patients with heart failure and normal left ventricular ejection fraction. Am J Physiol Heart Circ Physiol. 2007;292:H1427–H1434. doi: 10.1152/ajpheart.00567.2006. [DOI] [PubMed] [Google Scholar]
- 7.Richardson RS, Saltin B. Human muscle blood flow and metabolism studied in the isolated quadriceps muscles. Med Sci Sports Exerc. 1998;30:28–33. doi: 10.1097/00005768-199801000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Herrington DM, Fan L, Drum M, et al. Brachial flow-mediated vasodilator responses in population-based research: methods, reproducibility and effects of age, gender and baseline diameter. J Cardiovasc Risk. 2001;8:319–328. doi: 10.1177/174182670100800512. [DOI] [PubMed] [Google Scholar]
- 9.Donato AJ, Uberoi A, Wray DW, Nishiyama S, Lawrenson L, Richardson RS. Differential effects of aging on limb blood flow in humans. Am J Physiol Heart Circ Physiol. 2006;290:H272–H278. doi: 10.1152/ajpheart.00405.2005. [DOI] [PubMed] [Google Scholar]
- 10.Beere PA, Russell SD, Morey MC, Kitzman DW, Higginbotham MB. Aerobic exercise training can reverse age-related peripheral circulatory changes in healthy older men. Circulation. 1999;100:1085–1094. doi: 10.1161/01.cir.100.10.1085. [DOI] [PubMed] [Google Scholar]
- 11.Parker BA, Ridout SJ, Proctor DN. Age and flow-mediated dilation: a comparison of dilatory responsiveness in the brachial and popliteal arteries. Am J Physiol Heart Circ Physiol. 2006;291:H3043–H3049. doi: 10.1152/ajpheart.00190.2006. [DOI] [PubMed] [Google Scholar]
- 12.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39:257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 13.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Deanfield JE. Aging is associated with endothelial dysfunction in healthy men years before the age-related decline in women. J Am Coll Cardiol. 1994;24:471–476. doi: 10.1016/0735-1097(94)90305-0. [DOI] [PubMed] [Google Scholar]
- 14.Black MA, Cable NT, Thijssen DH, Green DJ. Impact of age, sex, and exercise on brachial artery flow-mediated dilatation. Am J Physiol Heart Circ Physiol. 2009;297:H1109–H1116. doi: 10.1152/ajpheart.00226.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitzman DW, Brubaker PH, Morgan TM, Stewart KP, Little WC. Exercise training in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. Circ Heart Fail. 2010;3:659–667. doi: 10.1161/CIRCHEARTFAILURE.110.958785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kitzman DW, Hundley WG, Brubaker PH, et al. A randomized double-blind trial of enalapril in older patients with heart failure and preserved ejection fraction: effects on exercise tolerance and arterial distensibility. Circ Heart Fail. 2010;3:477–485. doi: 10.1161/CIRCHEARTFAILURE.109.898916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schocken DD, Arrieta MI, Leaverton PE, Ross EA. Prevalence and mortality rate of congestive heart failure in the United States. J Am Coll Cardiol. 1992;20:301–306. doi: 10.1016/0735-1097(92)90094-4. [DOI] [PubMed] [Google Scholar]
- 18.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 19.Celermajer DS, Sorensen KE, Bull C, Robinson J, Deanfield JE. Endothelium-dependent dilation in the systemic arteries of asymptomatic subjects relates to coronary risk factors and their interaction. J Am Coll Cardiol. 1994;24:1468–1474. doi: 10.1016/0735-1097(94)90141-4. [DOI] [PubMed] [Google Scholar]
- 20.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. J Am Coll Cardiol. 1995;26:1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 21.Brubaker PH, Marburger CT, Morgan TM, Fray B, Kitzman DW. Exercise responses of elderly patients with diastolic versus systolic heart failure. Med Sci Sports Exerc. 2003;35:1477–1485. doi: 10.1249/01.MSS.0000084416.71232.EA. [DOI] [PubMed] [Google Scholar]
- 22.Hundley WG, Kitzman DW, Morgan TM, et al. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 23.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 1985;132:919–923. [PMC free article] [PubMed] [Google Scholar]
- 24.Yeboah J, Burke GL, Crouse JR, Herrington DM. Relationship between brachial flow-mediated dilation and carotid intima-media thickness in an elderly cohort: the Cardiovascular Health Study. Atherosclerosis. 2008;197:840–845. doi: 10.1016/j.atherosclerosis.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yeboah J, Crouse JR, Hsu FC, Burke GL, Herrington DM. Brachial flow-mediated dilation predicts incident cardiovascular events in older adults: the Cardiovascular Health Study. Circulation. 2007;115:2390–2397. doi: 10.1161/CIRCULATIONAHA.106.678276. [DOI] [PubMed] [Google Scholar]
- 26.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–509. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson JR, Mancini DM, Dunkman WB. Exertional fatigue due to skeletal muscle dysfunction in patients with heart failure. Circulation. 1993;87:470–475. doi: 10.1161/01.cir.87.2.470. [DOI] [PubMed] [Google Scholar]
- 28.Sullivan MJ, Knight JD, Higginbotham MB, Cobb FR. Relation between central and peripheral hemodynamics during exercise in patients with chronic heart failure. Muscle blood flow is reduced with maintenance of arterial perfusion pressure. Circulation. 1989;80:769–781. doi: 10.1161/01.cir.80.4.769. [DOI] [PubMed] [Google Scholar]
- 29.Esposito F, Mathieu-Costello O, Shabetai R, Wagner PD, Richardson RS. Limited maximal exercise capacity in patients with chronic heart failure: partitioning the contributors. J Am Coll Cardiol. 2010;55:1945–1954. doi: 10.1016/j.jacc.2009.11.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kitzman DW. Understanding results of trials in heart failure with preserved ejection fraction: remembering forgotten lessons and enduring principles. J Am Coll Cardiol. 2011;57:1687–1689. doi: 10.1016/j.jacc.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hornig B, Maier V, Drexler H. Physical training improves endothelial function in patients with chronic heart failure. Circulation. 1996;93:210–214. doi: 10.1161/01.cir.93.2.210. [DOI] [PubMed] [Google Scholar]
- 32.Konopka AR, Trappe TA, Jemiolo B, Trappe SW, Harber MP. Myosin heavy chain plasticity in aging skeletal muscle with aerobic exercise training. J Gerontol A Biol Sci Med Sci. 2011;66:835–841. doi: 10.1093/gerona/glr088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murias JM, Kowalchuk JM, Ritchie D, Hepple RT, Doherty TJ, Paterson DH. Adaptations in capillarization and citrate synthase activity in response to endurance training in older and young men. J Gerontol A Biol Sci Med Sci. 2011;66:957–964. doi: 10.1093/gerona/glr096. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan MJ, Higginbotham MB, Cobb FR. Exercise training in patients with severe left ventricular dysfunction. Hemodynamic and metabolic effects. Circulation. 1988;78:506–515. doi: 10.1161/01.cir.78.3.506. [DOI] [PubMed] [Google Scholar]
- 35.Warburton DE, Taylor A, Bredin SS, Esch BT, Scott JM, Haykowsky MJ. Central haemodynamics and peripheral muscle function during exercise in patients with chronic heart failure. Appl Physiol Nutr Metab. 2007;32:318–331. doi: 10.1139/h06-085. [DOI] [PubMed] [Google Scholar]
- 36.Gottdiener JS, Bednarz J, Devereux R, et al. American Society of Echocardiography recommendations for use of echocardiography in clinical trials. J Am Soc Echocardiogr. 2004;17:1086–1119. doi: 10.1016/j.echo.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser L, Spickard RC, Olivier NB. Heart failure depresses endothelium-dependent responses in canine femoral artery. Am J Physiol. 1989;256:H962–H967. doi: 10.1152/ajpheart.1989.256.4.H962. [DOI] [PubMed] [Google Scholar]
- 38.Drexler H, Hayoz D, Munzel T, et al. Endothelial function in chronic congestive heart failure. Am J Cardiol. 1992;69:1596–1601. doi: 10.1016/0002-9149(92)90710-g. [DOI] [PubMed] [Google Scholar]
- 39.Drexler H, Lu W. Endothelial dysfunction of hindquarter resistance vessels in experimental heart failure. Am J Physiol. 1992;262:H1640–H1645. doi: 10.1152/ajpheart.1992.262.6.H1640. [DOI] [PubMed] [Google Scholar]
- 40.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56:845–854. doi: 10.1016/j.jacc.2010.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patvardhan EA, Heffernan KS, Ruan JM, Soffler MI, Karas RH, Kuvin JT. Assessment of vascular endothelial function with peripheral arterial tonometry: information at your fingertips? Cardiol Rev. 2010;18:20–28. doi: 10.1097/CRD.0b013e3181c46a15. [DOI] [PubMed] [Google Scholar]
- 42.Hambrecht R, Fiehn E, Weigl C, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- 43.Katz SD, Yuen J, Bijou R, LeJemtel TH. Training improves endothelium-dependent vasodilation in resistance vessels of patients with heart failure. J Appl Physiol. 1997;82:1488–1492. doi: 10.1152/jappl.1997.82.5.1488. [DOI] [PubMed] [Google Scholar]