Abstract
Background.
Studies concerning the effect of different types of leisure activities on various cognitive domains are limited. This study tests the hypothesis that mental, physical, and social activities have a domain-specific protection against cognitive decline.
Methods.
A cohort of a geographically defined population in China was examined in 2003–2005 and followed for an average of 2.4 years. Leisure activities were assessed in 1,463 adults aged 65 years and older without cognitive or physical impairment at baseline, and their cognitive performances were tested at baseline and follow-up examinations.
Results.
High level of mental activity was related to less decline in global cognition (β = −.23, p < .01), language (β = −.11, p < .05), and executive function (β = −.13, p < .05) in ANCOVA models adjusting for age, gender, education, history of stroke, body mass index, Apolipoprotein E genotype, and baseline cognition. High level of physical activity was related to less decline in episodic memory (β = −.08, p < .05) and language (β = −.15, p < .01). High level of social activity was associated with less decline in global cognition (β = −.11, p < .05). Further, a dose-response pattern was observed: although participants who did not engage in any of the three activities experienced a significant global cognitive decline, those who engaged in any one of the activities maintained their cognition, and those who engaged in two or three activities improved their cognition. The same pattern was observed in men and in women.
Conclusions.
Leisure activities in old age may protect against cognitive decline for both women and men, and different types of activities seem to benefit different cognitive domains.
Key Words: Cognitive function, Leisure activities, Mental activity, Physical activity, Social activity
A beneficial effect of leisure activities on cognitive function has been observed in both epidemiological and experimental studies (1–4). Participation in leisure activities has also been associated with reduced risk of dementia (4–7), and participation in a broader spectrum of stimulating activities that involve mental, physical, and social components had the strongest protective effect against dementia development (6).
Specifically, a beneficial effect of late life mental activity on cognitive function has been consistently reported in observational studies (4,8,9). A protective effect of physical activity against cognitive decline or impairment has been reported in some studies (2,10–13), but not others (14–18). Fewer studies have examined the association of social activities with cognition (4). Some studies reported a beneficial effect (19–21), but not others (16,18,22).
To date, evidence concerning the effect of different types of activities on various cognitive domains from longitudinal population-based studies is limited. Further, data on gender differences in the activity-cognition relationship are scarce. In this study, we test the hypothesis that leisure activities protect against cognitive decline and that different types of activities may differently affect a specific cognitive domain in a Chinese cohort aged 65 years and older without cognitive or physical impairment. Second, we investigate whether there is a gender-specific beneficial effect of leisure activities on cognitive function.
Methods
Study Population
The study population was derived from a longitudinal population-based study of aging. At baseline, 2000 Chinese age 65 years and older from two counties in Sichuan province and two in Shandong province in China were enrolled during December 2003 to May 2005. For each included village, investigators and interviewers of provincial and county Center for Disease Control conducted a complete census of residents aged 65 years and older in the area. They enrolled eligible residents by going door-to-door and obtained informed consent before conducting the interview. There were no refusals, but a few participants with hearing problems were not enrolled (23). The study was approved by the Indiana University Institutional Review Board in the United States and the Chinese Center for Disease Control and Prevention in China.
Of the 2,000 enrolled participants, we excluded 210 individuals with baseline global cognitive scores in the bottom 10%, a commonly used cutoff point for defining impairment in cognitive research (2,24) with high sensitivity and specificity (25), to minimize the possibility of including people with cognitive impairment or dementia. We excluded 62 participants with physical disabilities to limit the potential influence on participation of leisure activity. There were 265 participants lost for follow-up visit, leaving 1,463 participants in the analyses.
Cognitive Assessment
Cognitive assessment was conducted in face-to-face interviews at the homes of study participants. The following cognitive domains were tested:
Global cognitive function was measured using the Community Screening Instrument for Dementia (CSID) (26), which was developed as a screening tool for dementia in populations with various cultural backgrounds and literacy levels. Details of the instrument have been published elsewhere (26). There were 30 items scored as “correct” or “incorrect” measuring memory, abstract thinking, judgment, and other disturbances of higher cortical function (aphasia, apraxia, agnosia, and constructional difficulty), and the sum scores over all correct answers ranged from 0 to 30. It has demonstrated good two-week test-retest reliability (intraclass correlation = .79) and inter-rater reliability (kappa = 1 for 94% of the items) (26).
Episodic memory was assessed using three tests: Word List Learning, Word List Recall, and IU Story Recall. The Word List Learning test consisted of a 10-item, three-trial word list in which free recall was taken after each learning trial and again after a brief delay (5min). The score was the total number of words recalled across the three learning trials (range 0–30) and at delay (range 0–10) with higher scores indicating better memory. One-month test-retest reliability coefficients of .62 were reported for Word List Learning and of .64 for Word List Recall in participants without dementia (27). The IU Story Recall task was created to be suitable to the culture of the Chinese rural population. The examiner read the story aloud to the participant who attempted to recall it verbatim immediately. The story had 14 units of information that were gist scored (range 0–14) and was found to be acceptable to the villagers (28).
Language: Animal Fluency Test (29) is a measure of language function, in which a participant was asked to name as many animals as possible in 60 s. One month test-retest correlation coefficient was .67 (27).
Executive function was measured by the IU Token Test (30). A sheet of paper with an array of circles and squares that vary in size and color was given to the participants. The examiner read aloud a series of 12 commands and asked the participant to point to or touch the figures in various combinations and orders. Commands that were correctly executed on the first exposure received 2 points. If an error occurred, the command was repeated and the participant received 1 point for correct response or no points for another failure. The score was the correct number across all 12 commands (range 0–24).
The questionnaires were harmonized, translated into Chinese and back translated into English. To avoid potential bias, this process was accomplished using lay persons who were not familiar with the goals of the interview from Beijing and Indiana. Intensive training sessions for the interviewers were held before the start of the first site, and refresher training was held before interview at each of the other three sites. High inter-rater reliability (95%) was achieved after each interviewer-training course, using volunteers from the community as study participants. All these test scores were standardized. The validity of these cognitive tests has been previously established in the Chinese population and elsewhere (31).
Follow-up evaluation of the cohort was conducted from June 2005 to November 2007 (mean follow-up time 2.4 years with range 2.3–2.6 years), and the same cognitive instruments were used as baseline evaluation.
Leisure Activities Assessment
During the baseline interview, study participants were asked the frequencies of engaging in a predefined list of activities including mental activity (sewing or weaving, reading, playing a music instrument, playing cards, chess, majiang, and attending the Peking opera), physical activity (gardening, walking, attending group exercises), and social activity (visiting family or friends, receiving visitors at home, giving advice). These categorizations were based on previous studies (16) and are mutually exclusive. Individual activity was ascertained on the scale of never, less than once a month, one to three times per month, three to four times per week, five to six times per week, or daily and converted to number of times per week by taking the median activity within each scale. For example, we used two times per month if a participant chose “one to three times per month” and converted this to 0.5 times per week. Activity score for mental, physical, and social was created by summing up the individual activity scores over the type and categorized according to their tertile distributions. After examining their association with cognitive decline, they were dichotomized into low (lower tertile) and a high activity (middle and upper tertiles were collapsed because they had similar effect on cognitive decline) groups.
Apolipoprotein E (APOE) Genotyping
Blood spots on filter paper were collected from all study participants during the baseline evaluation. APOE genotype was determined by eluting DNA from a dried blood spot, followed by HhaI digestion of amplified products (32).
Covariates
Information on age, gender, years of schooling, marital status, household composition, alcohol consumption and smoking, medical history and fracture, as well as height and weight was collected during the baseline examination. Body mass index (BMI) was calculated as body weight in kilograms divided by height in meters squared. BMI was correlated to baseline physical activity score (r = .09, p < .01).
Statistical Analysis
Participants who scored in the bottom 10% of the baseline global cognitive scores were excluded from this analysis. Standardized scores were calculated using means and standard deviations from the remaining 90% for each of the cognitive test. Cognitive decline of each cognitive test was calculated by subtracting the follow-up score from the baseline score. A standardized cognitive decline z-score was created for each of the cognitive domain scores by subtracting the mean and dividing by the standard deviation of the change score. An episodic memory score for each participant was created by using the average of the three z-scores for Word List Learning, Word List Recall, and IU Story. Analysis of covariance (ANCOVA) models were used with standardized cognitive decline scores or baseline leisure activity scores as the dependent variables to identify variables associated with them while adjusting for age, gender, education, and baseline cognition. Significant variables identified in any of the analyses were included as potential covariates in multivariate models.
To examine the combined effect, a four-category leisure activity index was created based on the dichotomized three types of activities: (1) low levels in all three activities, (2) low levels in two, (3) low levels in only one of the three types, and (4) high levels in all three types.
Results
Comparisons of baseline demographic characteristics between participants included in this analysis and those excluded are presented in Table 1. Specifically, those lost to follow-up (n = 265) were significantly older and had lower BMI at baseline and lower cognitive test scores than those included in this analysis. However, there is no difference in the rates of APOE ε4 carriers between the groups.
Table 1.
Comparisons of Demographic Characteristics for the 1,463 Participants Included in This Study, 265 Participants Lost to Follow-up, 210 Participants Excluded with Baseline Global Cognitive Scores in the Bottom 10% and 62 Participants with Physical Disabilities at Baseline, n (%), or Mean ± SD (standard deviation)
| Included n = 1,463 | Lost to Follow-up n = 265 | Cognitive Impairment n = 210 | Physical Disabily n = 62 | p value* | p value† | |
| Age, y | 71.0±5.0 | 75.0±6.5 | 71.5±4.9 | 74.3±5.9 | <.001 | <.001 |
| Education, n (%) | <.001 | .49 | ||||
| >5 y | 189 (12.9) | 17 (6.4) | 5 (2.4) | 6 (9.7) | ||
| 1–5 y | 443 (30.3) | 63 (23.8) | 14 (6.7) | 22 (35.5) | ||
| No school | 831 (56.8) | 185 (69.8) | 191 (90.9) | 34 (54.9) | ||
| Consume alcohol, n (%) | 675 (46.2) | 108 (40.2) | 58 (27.6) | 31 (50) | <.001 | .92 |
| Smoking, n (%) | 725 (49.6) | 116 (43.8) | 51 (24.4) | 36 (58.1) | <.001 | .87 |
| Body mass index | 22.2±3.5 | 21.5±3.5 | 20.8±3.2 | 22.4±3.9 | <.001 | <.01 |
| APOE ε4 carriers, n (%) | 240 (16.4) | 43 (16.2) | 44 (21.0) | 6 (9.7) | .17 | .70 |
| Cognitive domains (standardized score ranges) | ||||||
| Global cognition (−.9, 1.3) | .3±.7 | −.4 ± 1.2 | −1.8 ± .6 | .1 ± .7 | <.001 | <.001 |
| Episodic memory (−2.2, 3.4) | .2±.8 | −.3±.8 | −.7±.6 | −.04±.8 | <.001 | <.001 |
| Language (−2.0, 5.3) | .2±1.0 | −.4±1.0 | −.7±.8 | −.2±.8 | <.001 | <.001 |
| Executive function (−2.6, 1.5) | .2±.9 | −.3±1.1 | −1.0±1.0 | −.1±.9 | <.001 | <.001 |
Notes: *p values are for testing overall differences among the four groups.
†p values are for testing the difference between included group and lost to follow up group.
The mean age of participants in this analysis was 71.0 years (SD = 5.0). The majority of the participants in this study were illiterate, as only 12.3% had more than 5 years of schooling. A considerable proportion of the participants was smokers (49.6%) and consumed alcohol (46.2%). The prevalence of APOE ε4 carriers was low (16.4%). The proportion of men and women were similar (50.9% and 49.1%, respectively), and there were no gender differences in age, most of the medical histories, and APOE genotype. However, men were more likely to have higher education, consume alcohol, be smokers, and have better cognition in all the cognitive domains than women (Table 2).
Table 2.
Baseline Characteristics and Domains of Cognitive Function of Study Participants by Gender, n (%), or Mean ± SD (standard deviation)
| Total n = 1,463 | Men n = 744 | Women n = 719 | p value | |
| Age, y | 71.0±5.0 | 70.9±5.1 | 71.1±5.1 | .50 |
| Education, n (%) | <.01 | |||
| >5 y | 189 (12.3) | 161 (21.6) | 28 (3.9) | |
| 1–5 y | 443 (30.3) | 339 (45.6) | 104 (14.5) | |
| No school | 831 (56.8) | 244 (32.8) | 587 (81.6) | |
| Consume Alcohol, n (%) | 675 (46.2) | 525 (70.7) | 150 (20.9) | <.01 |
| Smoking, n (%) | 725 (49.6) | 576 (77.4) | 149 (20.7) | <.01 |
| Body mass index | 22.2±3.5 | 21.9±3.1 | 22.4±3.9 | <.05 |
| Medical history, n (%) | ||||
| Cancer | 11 (.8) | 5 (.7) | 6 (.8) | .72 |
| Parkinson’s disease | 12 (.8) | 6 (.8) | 6 (.8) | .95 |
| Diabetes | 38 (2.6) | 10 (1.3) | 28 (3.9) | <.05 |
| Hypertension | 239 (16.3) | 106 (14.2) | 133 (18.5) | <.05 |
| Stroke | 30 (2.1) | 16 (2.2) | 14 (2.0) | .78 |
| Heart attack | 46 (3.1) | 19 (2.6) | 27 (3.8) | .19 |
| Head injury | 73 (5.0) | 43 (5.8) | 30 (4.2) | .16 |
| Fracture | 37 (2.5) | 18 (2.4) | 19 (2.7) | .79 |
| APOE ε4 carriers, n (%) | 240 (16.4) | 110 (14.8) | 130 (18.1) | .09 |
| Cognitive domains (standardized score ranges) | ||||
| Global cognition (−.9, 1.3) | .32 ± .67 | .46 ± .64 | .17 ± .67 | <.01 |
| Episodic memory (−2.2, 3.4) | .15 ± .81 | .25 ± .84 | .05 ± .75 | <.01 |
| Language (−2.0, 5.4) | .17 ± .99 | .42±1.04 | −.08 ± .85 | <.01 |
| Executive function (−2.0, 5.4) | .21 ± .89 | .36 ± .85 | .05 ± .90 | <.01 |
Walking was the most frequent leisure activity, followed by being visited by friends or relatives, gardening, playing cards, reading, and attending group exercise. Men engaged more in mental activities, while men and women had similar participation in social and physical activities (Table 3).
Table 3.
Baseline Individual and Type of Leisure Activity Scores, Measured by Times per Week, by Gender, Mean ± SD (standard deviation)
| Total n = 1,463 | Men n = 744 | Women n = 719 | p value | |
| Mental activity | ||||
| Sewing or weaving | .12 ± .67 | .10 ± .62 | .14 ± .71 | .22 |
| Reading | .34±1.27 | .62±1.68 | .04 ± .46 | <.01 |
| Playing musical instrument | .09 ± .65 | .09 ± .69 | .08 ± .61 | .59 |
| Playing cards | .43±1.40 | .67±1.71 | .17 ± .91 | <.01 |
| Playing chess | .10 ± .71 | .20 ± .98 | .00 ± .06 | <.01 |
| Playing majiang | .11 ± .69 | .17 ± .86 | .04 ± .45 | <.01 |
| Attending Peking opera | .03 ± .10 | .03 ± .11 | .03 ± .08 | .22 |
| Total mental activity score | 1.21±2.65 | 1.89±3.25 | .51±1.55 | <.01 |
| Physical Activity | ||||
| Gardening | .54±1.64 | .56±1.64 | .52±1.64 | .67 |
| Walking | 1.69±2.74 | 1.83±2.83 | 1.54±2.65 | .04 |
| Attending group exercise | .31±1.28 | .30±1.28 | .33±1.29 | .64 |
| Total physical activity score | 2.54±3.95 | 2.68±3.95 | 2.39±3.94 | .15 |
| Social Activity | ||||
| Visiting family or friends | .25 ± .67 | .20 ± .44 | .29 ± .84 | <.05 |
| Being visited | .56±1.18 | .57±1.25 | .54±1.11 | .63 |
| Giving advice | .09 ± .32 | .11 ± .36 | .06 ± .28 | <.05 |
| Total social activity score | .89±1.61 | .89±1.54 | .90±1.69 | .86 |
As expected, mental, physical, and social activities affected specific cognitive domains differently in ANCOVA models adjusting for covariates (Table 4). Participants in the high mental activity group had significantly less decline in global cognition (p < .01), language (p < .05), and executive function (p < .05) compared with those in the low activity group. For example, participants with mental activity scores greater or equal than seven times per week were associated with .23 standard deviation (SD) and less decline in global function compared with those with low level of the activity.
Table 4.
Adjusted Differences of Cognitive Decline in Standardized Scores (Standard Error Estimates in Parentheses) between Participants with High Level Baseline Leisure Activities and Those with Low Level Activities
| Types of Activities | Global Cognition | Episodic Memory | Language | Executive Function |
| Total population | ||||
| Mental (scores ≥7 vs. <7) | −.23 (.06)** | −.04 (.05) | −.11 (.06)* | −.13 (.06)* |
| Physical (scores >0 vs. 0) | .01 (.05) | −.08 (.04)* | −.15 (.05)** | .04 (.05) |
| Social (scores ≥.25 vs. <.25) | −.13 (.05) * | .01 (.04) | .01 (.05) | .03 (.05) |
| Female | ||||
| Mental (scores ≥7 vs. <7) | −.26 (.09)** | −.08 (.06) | −.11 (.07) | −.14 (.09) |
| Physical (scores >0 vs. 0) | .06 (.08) | −.04 (.05) | −.12 (.06)* | .04 (.08) |
| Social (scores ≥.25 vs. <.25) | −.21 (.08)** | −.03 (.05) | −.02 (.06) | .04 (.08) |
| Male | ||||
| Mental (scores ≥7 vs. <7) | −.19 (.1)* | −.01 (.07) | −.12 (.09) | −.12 (.10) |
| Physical (scores >0 vs. 0) | −.03 (.07) | −.10 (.05) | −.17 (.07)* | .04 (.07) |
| Social (scores ≥.25 vs. <.25) | −.08 (.07) | .03 (.06) | .03 (.07) | .03 (.07) |
Notes:
*p < .05, **p < .01
Results were derived from analysis of covariance (ANCOVA) models adjusting for age, gender, education, history of stroke, body mass index, APOE status, and baseline cognitive scores.
Positive parameter estimates indicate more cognitive decline while negative parameter estimates indicate less cognitive decline.
Participants in the high physical activity group had significantly less decline in episodic memory (p < .05) and language (p < .01). High levels of social activity were related to significantly less decline in global cognition (p < .01). In addition, the observed associations of mental and physical activities with cognitive decline were similar in women and in men. There are no significant gender and activity level interactions (p > .05 for all three activities). However, the estimated effect of social activities on global cognitive decline was attenuated in men (Table 4).
When the three types of activities were integrated into a composite activity index, a dose-response pattern was observed: the more types of activity the stronger the associations (p for trend = .001 when treating the four groups as an ordinal variable). Although participants who engaged in low levels of activity experienced a significant global cognitive decline, those who engaged in high levels of any activity maintained their cognition, and those who engaged in two or three activities improved their cognition (Figure 1). Similar patterns were seen in men and in women, despite the gender differences in scores at baseline and at follow-up.
Figure 1.
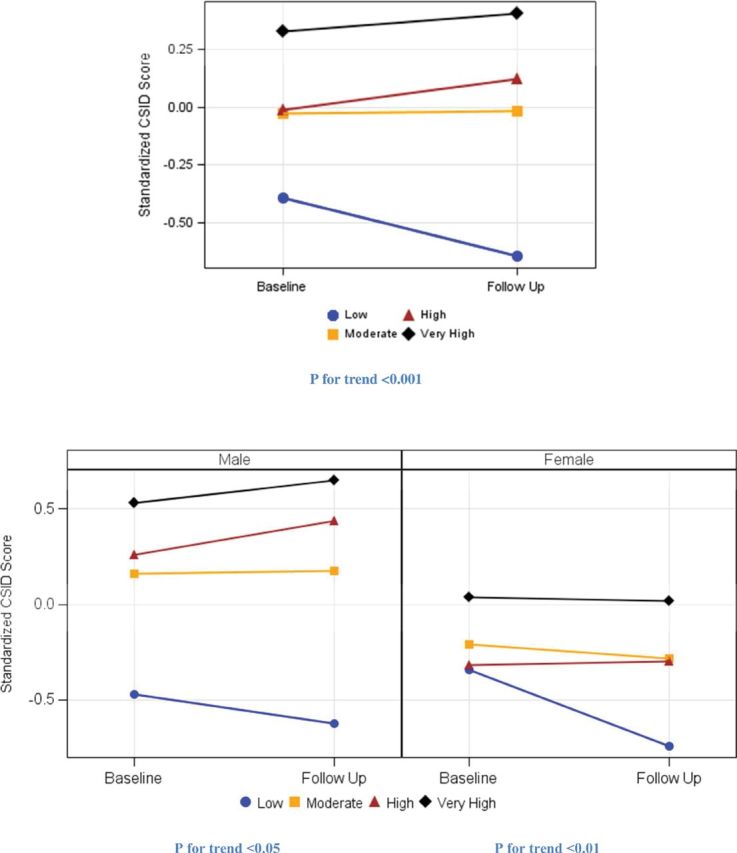
Baseline and predicted follow-up global cognitive function by leisure activity index for the total population (upper graph) and by male and female (lower graph) adjusting for age, education, stroke, BMI, APOE ε4, and baseline CSID score.
Discussion
In this population-based study of Chinese people aged 65 and older, participation in leisure activities was related to a decreased risk of subsequent cognitive decline over an average of 2.4 years of follow-up. Different types of activities protected against cognitive decline on different cognitive domains. Mental activity was associated with global cognition, language, and executive function; physical activity was associated with memory and language; and social activity was associated with global cognition. Further, although all types of activities protected against cognitive decline in women, only mental and physical activities were associated with less cognitive decline in men. The observed effects could not be explained by age, gender, education, body mass index, history of stroke, and APOE. In addition, there was a dose-response pattern of the number of activities with cognitive function: with more types of activities showing stronger protection. Furthermore, participation in at least one type of activity was related to maintained or improved cognitive function in both men and women.
There are a number of proposed hypotheses about the mechanism through which leisure activities impact cognition. Cognitive reserve is the most relevant hypothesis that proposes that life experience may influence neural processing and synaptic organization by permitting neurological processes to become more efficient, adaptive, and plastic, thus allowing some people to cope with progressing dementia pathology better than others (33). Although environments that involve diverse cognitive stimuli may be the most conducive in increasing cognitive reserve, physiological benefits of physical activity have been related to changes in hormone levels, improved cerebral blood flow, and an increased number of neuronal synapses. Social activities may offer a stimulating social environment that involves not only navigating social cues, dealing with complex and challenging social issues, but also physical movement and information processing that in turn enhance cognitive reserve.
Leisure activities may also have beneficial effects through psychological and behavioral pathways by lowering stress, having a better diet and healthier lifestyle, promoting psychological well-being, and lowering inflammation, consequently reducing the risk of developing various diseases that are associated with worse cognitive function. Social activities predominantly affect the immune system (34) and influence inflammatory processes in the brain. Active individuals are more likely to engage with others, leading to positive emotional states and lower stress (35), protecting against loss of hippocampal neurons (36). Leisure activities could also protect people against cognitive decline via their beneficial effect on cardiovascular and cerebrovascular diseases. The additive or synergistic interactions between vascular factors and Alzheimer’s disease pathology may promote the development of cognitive impairment.
Our finding that mental activity had a protective effect against global cognitive decline is consistent with previous studies on the topic. A protective effect of social activity on cognitive decline found in our study is in line with some studies on the topic (19–21), but not the others (16,18,22).
Most previous studies reported a protective role of physical activity on cognitive decline, but our study together with others, including a Chinese study, failed to detect such an effect (14–18). This could be due to the fact that walking was the most frequently engaged activity that dominated the physical activity score in the Chinese cohort, which may lead to differences in results from other cohorts with physical activity dominated by more intensive physical activities than walking. However, in agreement with other studies (2,37,38) we found a protective effect on other cognitive domains, such as episodic memory and language, although physical activity has been associated with executive function and processing speed in other studies (39,40).
Our observation that different types of activities protected against cognitive decline on different domains is in agreement with most previous studies. Mental activity has been related to enhanced memory, executive function, language, and cognitive skill (41), and less decline in perceptual speed (22). Physical activity has been related to less decline on memory (42), cognition, and attention (2). Studies on social activity in relation to different cognitive domains are still scarce. A previous study reported that no activity types including social activity affected any of the cognitive domains (18). However, another study reported that engaging in a broad range of everyday activities, including physical, mental, and social activities, accounted for a notable amount of the variance in change scores for various domains of cognition (21).
Previously, a dose-response association between levels of physical activity and cognitive impairment was reported (10), but no study has examined the dose-response influence of the combination of different types of leisure activities on cognition. Indeed, the finding of the current study is in line with previous findings that the broader spectrum of leisure activities was associated with stronger protection on the risk of dementia (6).
There are limited studies of gender differences on the effect of late life leisure activities on cognitive function. A recent study in random samples of the Swedish population aged 46–75 years reported that engaging in physical activities in middle age had a protective effect on global cognitive function in later life in women but not in men (43). Another study reported that gender may modify the effect of exercise on cognitive impairment in a healthy cohort aged >84 years (44). To our knowledge, this is the first study reporting that participation in any type of leisure activity may help maintain cognition for both women and men, and more types of activities were associated with stronger protection.
The first limitation of the current study is the relatively short follow-up time. Although we excluded persons with a baseline global cognitive score in bottom 10% and those with impaired physical function, and controlled for baseline cognition in all the analyses, it is possible that persons with subclinical cognitive impairment may still remain in the study population, we could not rule out the possible reverse causation. However, our results were supported by previous studies with longer follow-up (24,45). Second, leisure activities were collected at enrollment and no information was available on previous activities, and therefore cumulative effects of lifetime activities could not be examined. Third, while a number of confounders were controlled for, latent and unmeasured differences might have contributed to the associations of mental, physical, and social activities with cognitive decline. Fourth, it is uncertain whether there is a biological variance based on the observed change scores in cognitive performance. Moreover, although the categorization of activities was mutually exclusive, the components in each of the activity categories were not necessarily exclusive. For example, social activities may simultaneously include also mental and physical components. This uncertainty may also influence our results of the specific type of activities in relation to different cognitive domains. Finally, this rural elderly Chinese cohort had lower levels of literacy, higher rates of smoking, but lower rates of medical conditions than studies conducted in European or American populations, which may limit the generalizability of the results to other populations.
The current study provided evidence on the limited knowledge concerning the effect of different types of activities on specific cognitive domains. It is one of the few longitudinal population-based studies on the topic carried out in Asia, and our results are comparable with studies carried out in Western countries.
In summary, leisure activities had a protective effect against cognitive decline. Although different types of activities affected different cognitive domains, there was a dose-response association of the number of activity types with global cognition for both women and men. While these findings need to be confirmed by more longitudinal studies, this study underscores the importance of encouraging older adults participating in leisure activities to maintain cognition or prevent cognitive decline. This would lead to significant public health benefits because currently no efficient treatment for cognitive impairment is available.
Funding
This research was supported by grants from the National Institutes of Health (R01 AG019181), Swedish Council for Working Life and Social Research, Swedish Brain Power, Gamla Tjänarinnor Foundation, Söderström-Königska Sjukhemmet Foundation, and Gun and Bertil Stohnes foundation.
Conflict of Interest Statement
The authors have no conflict of interest to declare.
Acknowledgments
All coauthors have no financial interests in the manuscript. Professor Sujuan Gao has full access to all the data used in this study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1. Fratiglioni L, Wang HX. . Brain reserve hypothesis in dementia. J Alzheimers Dis. 2007;12(1):11–22 [DOI] [PubMed] [Google Scholar]
- 2. Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–1461 [DOI] [PubMed] [Google Scholar]
- 3. Lautenschlager NT, Cox KL, Flicker L, et al. Effect of physical activity on cognitive function in older adults at risk for Alzheimer disease: a randomized trial. JAMA. 2008;300(9):1027–1037 [DOI] [PubMed] [Google Scholar]
- 4. Wang HX, Xu W, Pei JJ. Leisure activities, cognitive function and dementia. Biochim Biophys Acta. 2012;1822(3):482–91. [DOI] [PubMed] [Google Scholar]
- 5. Wang HX, Karp A, Winblad B, Fratiglioni L. Late-life engagement in social and leisure activities is associated with a decreased risk of dementia: a longitudinal study from the Kungsholmen project. Am J Epidemiol. 2002;155(12):1081–1087 [DOI] [PubMed] [Google Scholar]
- 6. Karp A, Paillard-Borg S, Wang HX, Silverstein M, Winblad B, Fratiglioni L. Mental, physical and social components in leisure activities equally contribute to decrease dementia risk. Dement Geriatr Cogn Disord. 2006;21(2):65–73 [DOI] [PubMed] [Google Scholar]
- 7. Podewils LJ, Guallar E, Kuller LH, et al. Physical activity, APOE genotype, and dementia risk: findings from the Cardiovascular Health Cognition Study. Am J Epidemiol. 2005;161(7):639–651 [DOI] [PubMed] [Google Scholar]
- 8. Saczynski JS, Jonsdottir MK, Sigurdsson S, et al. White matter lesions and cognitive performance: The role of cognitively complex leisure activity. J Geront A Biol Sci Med Sci. 2008;63(8):848–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dodge HH, Kita Y, Takechi H, Hayakawa T, Ganguli M, Ueshima H. Healthy cognitive aging and leisure activities among the oldest old in Japan: Takashima Study. J Geront A Biol Sci Med Sci. 2008;63(11):1193–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504 [DOI] [PubMed] [Google Scholar]
- 11. Yaffe K, Barnes D, Nevitt M, Lui LY, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14):1703–1708 [DOI] [PubMed] [Google Scholar]
- 12. van Gelder BM, Tijhuis MA, Kalmijn S, Giampaoli S, Nissinen A, Kromhout D. Physical activity in relation to cognitive decline in elderly men: the FINE Study. Neurology. 2004;63(12):2316–2321 [DOI] [PubMed] [Google Scholar]
- 13. Etgen T, Sander D, Huntgeburth U, Poppert H, Forstl H, Bickel H. Physical activity and incident cognitive impairment in elderly persons: the INVADE study. Arch Intern Med. 2010;170(2):186–193 [DOI] [PubMed] [Google Scholar]
- 14. Wilson RS, Mendes De, Leon CF, Barnes LL, et al. Participation in cognitively stimulating activities and risk of incident Alzheimer disease. JAMA. 2002;287:742–748 [DOI] [PubMed] [Google Scholar]
- 15. Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology. 2006;66(6):821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang JY, Zhou DH, Li J, et al. Leisure activity and risk of cognitive impairment: the Chongqing Aging Study. Neurology. 2006;66(6):911–913 [DOI] [PubMed] [Google Scholar]
- 17. Scarmeas N, Luchsinger JA, Brickman AM, et al. Physical activity and Alzheimer disease course. Am J Geriatr Psychiatry. 2011;19(5):471–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aartsen MJ, Smits CH, van Tilburg T, Knipscheer KC, Deeg DJ. Activity in older adults: cause or consequence of cognitive functioning? A longitudinal study on everyday activities and cognitive performance in older adults. J Gerontol B Psychol Sci Soc Sci. 2002;57(2):P153–P–162 [DOI] [PubMed] [Google Scholar]
- 19. Bassuk SS, Glass TA, Berkman LF. Social disengagement and incident cognitive decline in community-dwelling elderly persons. Ann Intern Med. 1999;131(3):165–173 [DOI] [PubMed] [Google Scholar]
- 20. Barnes LL, Mendes de Leon CF, Wilson RS, Bienias JL, Evans DA. Social resources and cognitive decline in a population of older African Americans and whites. Neurology. 2004;63(12): 2322–2326 [DOI] [PubMed] [Google Scholar]
- 21. Newson RS, Kemps EB. General lifestyle activities as a predictor of current cognition and cognitive change in older adults: a cross-sectional and longitudinal examination. J Gerontol B Psychol Sci Soc Sci. 2005;60(3):P113–P–120 [DOI] [PubMed] [Google Scholar]
- 22. Ghisletta P, Bickel JF, Lovden M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. J Gerontol B Psychol Sci Soc Sci. 2006;61(5):P253–P–261 [DOI] [PubMed] [Google Scholar]
- 23. Gao S, Jin Y, Hall KS, et al. Selenium level and cognitive function in rural elderly Chinese. Am J Epidemiol. 2007;165(8):955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yaffe K, Barnes D, Nevitt M, Lui L-Y, Covinsky K. A prospective study of physical activity and cognitive decline in elderly women: women who walk. Arch Intern Med. 2001;161(14): 1703–1708 [DOI] [PubMed] [Google Scholar]
- 25. Ganguli M, Belle S, Ratcliff G, et al. Sensitivity and specificity for dementia of population-based criteria for cognitive impairment: the MoVIES project. J Gerontol. 1993;48(4):M152–M–161 [DOI] [PubMed] [Google Scholar]
- 26. Hall KS, Ogunniyi AO, Hendrie HC, et al. A cross-cultural community based study of dementias: methods and performance of the survey instrument: Indianapolis, U.S.A. and Ibadan, Nigeria. Int J Methods Psychiatr Res. 1996;6:129–142 [Google Scholar]
- 27. Morris JC, Heyman A, Mohs RC, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39(9):1159–1165 [DOI] [PubMed] [Google Scholar]
- 28. Emsley CL, Gao S, Li Y, et al. Trace element levels in drinking water and cognitive function among elderly Chinese. Am J Epidemiol. 2000;151(9):913–920 [DOI] [PubMed] [Google Scholar]
- 29. Isaacs B, Akhtar AJ. The set test: a rapid test of mental function in old people. Age Ageing. 1972;1(4):222–226 [DOI] [PubMed] [Google Scholar]
- 30. Yamamoto K, Evans JD, Johnson KE, Unverzagt FW. Clinical utility of IU Token Test in the diagnosis of dementia. J Int Neuropsychol Soc. 2003;9:316 [Google Scholar]
- 31. Prince M, Acosta D, Chiu H, Scazufca M, Varghese M. Dementia diagnosis in developing countries: a cross-cultural validation study. Lancet. 2003;361(9361):909–917 [DOI] [PubMed] [Google Scholar]
- 32. Yang M, Hendrie HC, Hall KS, Oluwole OS, Hodes ME, Sahota A. Improved procedure for eluting DNA from dried blood spots. Clin Chem. 1996;42(7):1115–1116 [PubMed] [Google Scholar]
- 33. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8(3):448–460 [PubMed] [Google Scholar]
- 34. Seeman T. Social ties and health: the benefits of social integration. Ann Epidemiol. 1996;6(5):442–451 [DOI] [PubMed] [Google Scholar]
- 35. Crowe M, Andel R, Pedersen NL, Johansson B, Gatz M. Does participation in leisure activities lead to reduced risk of Alzheimer's disease? A prospective study of Swedish twins. J Gerontol B Psychol Sci Soc Sci. 2003;58(5):P249–P–255 [DOI] [PubMed] [Google Scholar]
- 36. Sapolsky RM, Krey LC, McEwen BS. The neuroendocrinology of stress and aging: the glucocorticoid cascade hypothesis. Endocr Rev. 1986;7(3):284–301 [DOI] [PubMed] [Google Scholar]
- 37. Sturman MT, Morris MC, Mendes de Leon CF, Bienias JL, Wilson RS, Evans DA. Physical activity, cognitive activity, and cognitive decline in a biracial community population. Arch Neurol. 2005;62(11):1750–1754 [DOI] [PubMed] [Google Scholar]
- 38. Albert MS, Jones K, Savage CR, et al. Predictors of cognitive change in older persons: MacArthur studies of successful aging. Psychol Aging. 1995;10(4):578–589 [DOI] [PubMed] [Google Scholar]
- 39. Chang M, Jonsson PV, Snaedal J, et al. The effect of midlife physical activity on cognitive function among older adults: AGES Reykjavik Study. J Gerontol A Biol Sci Med SciDec2010;65(12):1369–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Angevaren M, Vanhees L, Nooyens AC, Wendel-Vos CG, Verschuren WM. Physical activity and 5-year cognitive decline in the Doetinchem Cohort Study. Ann Epidemiol. 2010;20(6):473–479 [DOI] [PubMed] [Google Scholar]
- 41. Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61(6):812–816 [DOI] [PubMed] [Google Scholar]
- 42. Richards M, Hardy R, Wadsworth ME. Does active leisure protect cognition? Evidence from a national birth cohort. Soc Sci Med. 2003;56(4):785–792 [DOI] [PubMed] [Google Scholar]
- 43. Kareholt I, Lennartsson C, Gatz M, Parker MG. Baseline leisure time activity and cognition more than two decades later. Int J Geriatr Psychiatry. 2011;26(1):65–74 [DOI] [PubMed] [Google Scholar]
- 44. Sumic A, Michael YL, Carlson NE, Howieson DB, Kaye JA. Physical activity and the risk of dementia in oldest old. J Aging Health. 2007;19(2):242–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vercambre M-N, Grodstein F, Manson JE, Stampfer MJ, Kang JH. Physical activity and cognition in women with vascular conditions. Arch Intern Med. 2011;171(14):1244–1250 [DOI] [PMC free article] [PubMed] [Google Scholar]


