Abstract
Background.
Testosterone in Older Men with Mobility Limitations Trial found an increased incidence of cardiovascular events in men randomized to testosterone, resulting in enrollment cessation by trial's Data and Safety Monitoring Board. We evaluated changes in gonadal hormones and markers of inflammation and coagulation to elucidate risk factors associated with cardiovascular events.
Methods.
Men aged 65 years or more, with mobility limitation, total testosterone 100–350 ng/dL, or free testosterone less than 50 pg/mL, were randomized to placebo or 10 g testosterone gel daily for 6 months. Changes in total and free testosterone, estradiol and estrone, C-reactive protein, interleukin 6, fibrinogen, plasminogen activator inhibitor-1, and pro-brain naturetic peptide were compared between groups and within the testosterone group between subjects who experienced cardiovascular events and those who did not.
Results.
Of 209 men randomized (mean age 74 years), gonadal hormones and biomarkers were available in 179 men. Baseline body mass index, gonadal hormones, lipids, Framingham risk scores, and other biomarkers were similar in the two treatment groups. Within the testosterone group, the 6-month increase in free testosterone was significantly greater in men who experienced cardiovascular events than in those who did not [mean (95% confidence interval), 10.6 (4.6–16.7) vs 5.2 (3.0–7.5) ng/dL, p = .05]. In multivariable logistic regression analysis, the change in the serum levels of free testosterone was associated with cardiovascular events.
Conclusion.
Mobility-limited older men who experienced cardiovascular events had greater increases in serum free testosterone levels than those who did not.
Keywords: Testosterone, Older men, Mobility limitation, Cardiovascular disease
The Testosterone in Older Men with Mobility Limitations (TOM) Trial was a placebo-controlled randomized trial, whose aim was to determine whether testosterone therapy in older men with mobility limitation and low total or free testosterone levels improves lower extremity muscle strength and physical function (1). Because of significantly higher incidence of cardiovascular adverse events in men assigned to the testosterone arm (23 vs 5 men in placebo group), in December 2009, the trial's Data and Safety Monitoring Board recommended that further enrollment be stopped and administration of study medications to participants be discontinued (2). In this study, we investigated the risk factors that were associated with cardiovascular events in the TOM Trial.
The divergence in the cardiovascular events between the placebo and testosterone arms of the TOM Trial was apparent within weeks after randomization, making de novo or accelerated atherogenesis a less likely mechanism responsible for these events. Furthermore, the diversity of these cardiovascular events pointed against a single unifying mechanism. We evaluated the relative changes in testosterone and estrogen levels in men who experienced cardiovascular events versus those who did not. Estrogens are prothrombotic, and their administration to men with prostate cancer and for secondary prevention of coronary disease has been associated with an increased risk of myocardial infarction and mortality (3,4). Similarly, novel markers of inflammation such as C-Reactive Protein (CRP), interleukin 6 (IL-6), and fibrinogen have been associated with cardiovascular disease and mortality (5,6). Therefore, we evaluated whether differential changes in serum testosterone and estrogen levels, along with biomarkers of inflammation and coagulation, were associated with cardiovascular events in the two intervention arms. Accordingly, we compared changes in the sex-steroid levels and biomarkers of cardiovascular risk between the testosterone and placebo groups, and within the testosterone group, between those who experienced cardiovascular events and those who did not.
METHODS
The study design, safety results, and the efficacy data of the TOM Trial have been reported previously (1,2,7).
Study Design
Briefly, the TOM Trial was a parallel group, placebo-controlled, double-blind randomized trial, approved by the Institutional Review Board of Boston University Medical Center (BUMC), New England Research Institutes (NERI), Watertown, MA, and the Boston Veterans Administration Health Care System (BVAHC). Subject recruitment took place at BUMC, NERI, and BVAHC, but outcome assessments were performed only at BUMC. All participants provided written informed consent.
Eligibility Criteria
The participants were community-dwelling men, aged 65 years or older, with total testosterone between 100 and 350 ng/dL or free testosterone less than 50 pg/mL, and mobility limitation. The subjects were deemed to have “mobility limitation” if they reported difficulty walking two blocks on a level surface or climbing 10 steps and had a summary score between 4 and 9 on the Short Physical Performance Battery (8), which reflects moderate to mild degree of physical dysfunction.
Men with prostate cancer, lower urinary tract symptom score more than 21, PSA more than 4 ng/mL, unstable angina, uncontrolled congestive heart failure, myocardial infarction within 3 months, uncontrolled hypertension, neuromuscular diseases limiting mobility, transaminase concentrations more than three times the upper limit of normal, creatinine more than 3.5 mg/dL, hemoglobin A1c more than 8.5%, hematocrit more than 48%, untreated severe obstructive sleep apnea, or body mass index more than 40 kg/m2 were excluded. Men using testosterone, growth hormone, or any anabolic therapy, or drugs that affect gonadal function were also excluded.
Randomization and Blinding
Eligible participants were randomized to either placebo or testosterone gel using a concealed computer-generated randomization table and a block size of 6. Subjects were stratified by age (65–75, >75). The participants and outcome assessors were blinded to intervention.
Study Intervention
The participants applied daily 10 g transdermal gel containing either placebo or 100 mg testosterone (Testim 1%; Auxilium Pharmaceuticals, Norristown, PA) for 6 months. This regimen of testosterone gel raises total testosterone concentration into the mid-to-high normal range in hypogonadal men (9). To maintain blinding, all participants applied three tubes of the gel daily that were identical in appearance; those assigned to testosterone group applied two tubes each containing 5 g testosterone gel (containing 50 mg testosterone) plus one tube containing placebo gel; those assigned to placebo group applied three tubes containing placebo. Testosterone was measured 2 weeks after randomization in blood samples drawn 2–4 hours after gel application. If the average of the two testosterone concentrations was less than 500 ng/dL or more than 1000 ng/dL, the unblinded physician either increased the daily dose to 15 g or decreased it to 5 g.
Cardiovascular Events in the TOM Trial
In the TOM Trial, 23 men in the testosterone arm experienced a cardiovascular event compared to 5 men in the placebo arm (2). On-treatment gonadal hormones and inflammatory markers were assessed in 19 men in the testosterone arm and all 5 men in the placebo arm in whom sufficient sera were available. There were 25 cardiovascular events that occurred in these 24 men. The events were diverse in nature and included myocardial infarction (n = 2), stroke (n = 1), angina (n = 1), congestive heart failure (n = 2), carotid artery occlusion (n = 1), arrhythmia (n = 6), syncope (n = 3), ischemic EKG changes during exercise test (n = 2), lower extremity edema (n = 4), and exacerbation of hypertension (n = 3).
Outcomes
Hormone assays.—
Serum estrogen and cardiovascular biomarkers were measured at baseline and during the 6-month visit in men who completed the intervention phase. Total testosterone level was measured at Quest Diagnostics (San Juan Capistrano, CA) using a Bayer-Advia-Centaur immunoassay with sensitivity 10 ng/dL (10). Intra- and interassay coefficient of variation (CoV) for testosterone were 11.8% and 17%, respectively. Sex hormone binding globulin levels were measured using an immunofluorometric assay with sensitivity 2.5 nmol/L (DELFIA-Wallac, Turku, Finland; intra- and interassay CoV were 8.3% and 7.3%, respectively; 11). Free testosterone was calculated using a published law of mass action equation whose assumptions and binding constants have been reported (12).
Estradiol and estrone were measured using liquid chromatography–tandem mass spectrometry with sensitivity of 2 pg/mL. For estradiol, intraassay CoV were 12.4%, 8.5%, 6.0%, and 4.7% at 7.2, 35, 125, and 394 pg/mL, respectively, whereas interassay CoV were 9.2%, 6.7%, 6.1%, 6.1%, and 7.6% at 7.0, 34, 57, 118, and 381 pg/mL, respectively. For estrone, intraassay CoV were 10.9%, 5.6%, 4.9%, and 6.0% at 6.7, 37, 129, and 400 pg/mL, respectively, whereas interassay CoV were 8.1%, 7.3%, 7.3%, 9.9%, and 8.7% at 6.4, 35, 58, 122, and 391 pg/mL, respectively. High sensitivity C-reactive protein was measured by a latex particle enhanced immunoturbidimetric assay (Roche Diagnostics, Indianapolis, IN). Fibrinogen was measured immunoturbidimetrically using the K-ASSAY Figrinogen Kit (Kamiya Biomedical, Seattle, WA). Quantitative two-site enzyme immunoassay was used to measure interleukin 6 (R & D Systems, Minneapolis, MN) and Plasminogen Activator Inhibitor-1 (Diagnostica Stago, Asnieres, France). N-Terminal Pro-Brain Naturetic Peptide was measured by an automated double incubation sandwich assay (Roche Diagnostics).
Statistical Analyses
The primary analysis reported here is a between-group comparison of the change from baseline in gonadal hormones and cardiovascular biomarkers. In addition, we compared the changes in hormone levels and biomarkers within the testosterone group among men who experienced cardiovascular events and those who did not. Continuous variables were compared on the basis of Student's t tests, and categorical were compared variables using Fisher exact test. Multiple logistic regression models were used to evaluate the association of the change in hormone levels and biomarkers with cardiovascular events (the dependent variable), and the ability of hormone levels and inflammatory markers to account for the difference in proportions of subjects experiencing cardiovascular events between testosterone and placebo arms.
RESULTS
Patient Population
The details of the TOM Trial have been published (1). As reported previously, in December 2009, when the Data and Safety Monitoring Board recommended cessation of further enrollment, 4726 men had been screened, 278 had met the eligibility criteria, and 209 had been randomized, 106 to testosterone and 103 to placebo. Because of early trial cessation and earlier than planned discontinuation of intervention in some participants, serum samples were available for the measurement of estrogen levels and other cardiovascular biomarkers in 179 out of the 209 randomized men (88 and 91 men in the testosterone and placebo arms, respectively).
Baseline Characteristics
The mean age (±SD) of men in the testosterone group was 73.5 (±5.7) and in the placebo was 73.9 (±5.3) years (Table 1). The two groups were similar in their baseline characteristics in terms of age, BMI, body composition, testosterone, estradiol, and estrone levels, and hematocrit. The groups were also similar in the prevalence of hypertension, diabetes, and smoking, although more men in the testosterone group were on antihypertensive therapy. Similarly, slightly higher number of men in the testosterone group had a diagnosis of hyperlipidemia and were receiving lipid-lowering therapy. The two groups were well matched for inflammatory and other cardiovascular markers at baseline. Among men randomized to the testosterone arm (N = 88), those who experienced a cardiovascular event (N = 19) did not differ in their baseline characteristics from those who did not (N = 69), except for serum total testosterone level, which was lower in men who experienced cardiovascular events.
Table 1.
Baseline Characteristics of the Study Participants in the TOM Trial
| Testosterone Arm | ||||||
| Testosterone Arm (N = 88) | Placebo Arm (N = 91) | p Value* | CVD-Related Adverse Events (N = 19) | No CVD-Related Adverse Events (N = 69) | p Value* | |
| Age (years) | 73 ± 6 | 74 ± 5 | .62 | 74 ± 6 | 73 ± 6 | .51 |
| BMI (kg/m2) | 30 ± 4 | 30 ± 4 | .56 | 31 ± 4 | 30 ± 4 | .37 |
| Blood pressure (mmHg) | ||||||
| Systolic | 137 ± 14 | 136 ± 15 | .71 | 137 ± 15 | 137 ± 14 | .83 |
| Diastolic | 77 ± 10 | 75 ± 10 | .22 | 76 ± 11 | 77 ± 9 | .70 |
| Mean arterial pressure | 97 ± 9 | 95 ± 10 | .31 | 96 ± 10 | 97 ± 9 | .71 |
| FHS score | 22 ± 6 | 21 ± 6 | .32 | 22 ± 5 | 21 ± 6 | .53 |
| HbA1c (%) | 6.2 ± 0.7 | 6.1 ± 0.9 | .57 | 6.1 ± 0.7 | 6.2 ± 0.7 | .33 |
| Total cholesterol (mg/dL) | 164 ± 35 | 173 ± 40 | .11 | 176 ± 41 | 161 ± 33 | .09 |
| LDL (mg/dL) | 88 ± 29 | 92 ± 33 | .33 | 99 ± 33 | 85 ± 28 | .07 |
| HDL (mg/dL) | 46 ± 13 | 49 ± 18 | .20 | 46 ± 14 | 46 ± 13 | .92 |
| Triglycerides (mg/dL) | 152 ± 92 | 142 ± 71 | .41 | 179 ± 131 | 144 ± 78 | .28 |
| Known CVD | 47 (53) | 41 (45) | .30 | 11 (58) | 36 (52) | .80 |
| Hypertension | 75 (85) | 70 (77) | .18 | 16 (84) | 59 (86) | .89 |
| Hypertension therapy | 75 (85) | 65 (71) | .03 | 16 (84) | 59 (86) | .89 |
| Diabetes | 23 (26) | 26 (29) | .87 | 3 (16) | 20 (29) | .38 |
| Obesity | 40 (45) | 46 (51) | .55 | 10 (53) | 30 (43) | .60 |
| Hyperlipidemia | 57 (65) | 45 (49) | .05 | 12 (63) | 45 (65) | >.99 |
| Statin therapy | 56 (64) | 42 (46) | .02 | 11 (58) | 45 (66) | .59 |
| Smoking status | .58 | .07 | ||||
| Never | 25 (29) | 20 (22) | 9 (47) | 16 (24) | ||
| Former | 56 (64) | 63 (69) | 10 (53) | 46 (68) | ||
| Current | 6 (7) | 8 (8.8) | - | 6 (8.8) | ||
| Hematocrit (%) | 41.0 ± 3.5 | 40.7 ± 3.8 | .60 | 40.8 ± 3.6 | 41.1 ± 3.5 | .77 |
| Hemoglobin (g/dL) | 13.9 ± 1.2 | 13.8 ± 1.3 | .55 | 13.9 ± 1.2 | 13.9 ± 1.2 | .93 |
| Total testosterone (ng/dL) | 248 ± 60 | 233 ± 68 | .11 | 219 ± 59 | 256 ± 57 | .01 |
| Free testosterone (ng/dL) | 4.9 ± 1.2 | 4.2 ± 1.4 | <.01 | 4.7 ± 1.1 | 5.0 ± 1.2 | .29 |
| Estradiol (pg/mL) | 21.5 ± 9.4 | 21.1 ± 8.6 | .76 | 21.2 ± 10.6 | 21.6 ± 9.2 | .87 |
| Free Estradiol (pg/ml) | 0.50 ± 0.23 | 0.46 ± 0.19 | .23 | 0.52 ± 0.26 | 0.50 ± 0.23 | .76 |
| Estrone (pg/mL) | 35 ± 17 | 31 ± 13 | .12 | 36 ± 17 | 35 ± 18 | .95 |
| Free estrone (pg/mL) | 1.36 ± 0.67 | 1.18 ± 0.47 | .06 | 1.39 ± 0.68 | 1.35 ± 0.68 | .84 |
| SHBG (nmol/L) | 33 ± 14 | 40 ± 18 | .01 | 28 ± 13 | 34 ± 13 | .08 |
| Total testosterone to estradiol ratio | 13.1 ± 6.1 | 12.4 ± 4.5 | .44 | 11.1 ± 4.0 | 13.6 ± 6.5 | .07 |
| CRP (ng/mL) | 0.46 ± 0.79 | 0.54 ± 1.03 | .56 | 0.40 ± 0.37 | 0.47 ± 0.87 | .62 |
| IL-6 (pg/mL) | 3.3 ± 2.5 | 4.7 ± 5.4 | .04 | 3.5 ± 2.8 | 3.3 ± 2.5 | .75 |
| Fibrinogen (mg/dL) | 339 ± 134 | 351 ± 104 | .53 | 305 ± 107 | 348 ± 141 | .25 |
| PAI-1 (ng/mL) | 47 ± 30 | 42 ± 31 | .33 | 51 ± 28 | 46 ± 31 | .53 |
| Pro-BNP (ng/mL) | 395 ± 825 | 298 ± 368 | .33 | 561 ± 829 | 352 ± 825 | .36 |
Notes: Mean ± SD or N (%) shown. BMI = body mass index; CRP = C-Reactive Protein; CVD = cardiovascular disease; FHS = Framingham Heart Study Risk Score; HDL = high-density lipoprotein; IL-6 = interleukin 6; LDL = low-density lipoprotein; PAI-1 = plasminogen activator inhibitor-1; Pro-BNP = pro-brain naturetic peptide; SHBG = sex hormone binding globulin.
‘s t test for means and Fisher exact test for proportions.
Changes in Gonadal Hormones
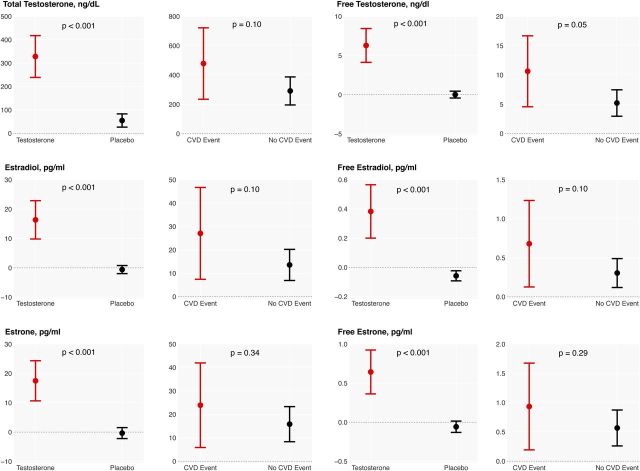
As expected, assignment to the testosterone arm was associated with significantly greater increments in total and free testosterone, estradiol, and estrone levels compared with the placebo arm (Figure 1). Interestingly, within the testosterone arm, men who experienced a cardiovascular event had a greater increase in total (478 [234–722] vs 292 [196–687]; p = .10) and free testosterone (10.6 [4.6–16.7] vs 5.2 [3.0–7.5] ng/dL) levels compared with those who did not (p = .05). Similarly, serum total and free estradiol (27 [7–48] vs 14 [7–20] pg/mL; p = .1; 0.7 [0.13–1.23] vs 0.30 [0.12–0.49] pg/ml; p = .10) and total and free estrone (24 [6–42] vs 16 [8–23]; p = .34; 0.93 [0.20–1.68] vs 0.6 [0.3–0.9]; p = .29) levels increased more in men who experienced a cardiovascular event compared with men who did not (Figure 1).
Figure 1.
Changes in serum levels of sex hormones from baseline to 6 months. The change in each parameter is compared between the treatment groups and within the testosterone group based on cardiovascular disease events. E1 = Estrone; E2 = Estradiol; FT = Free Testosterone; TT = Total Testosterone.
Changes in Hemoglobin and Hematocrit
Hemoglobin and hematocrit levels increased significantly (p < .0001) in men in the testosterone arm (Table 2). No significant changes were seen in the placebo group. Within the testosterone group, the changes in hemoglobin and hematocrit did not differ significantly between men who experienced a cardiovascular event and those who did not.
Table 2.
Change in Biomarkers From Baseline to 6 Months in the TOM Trial
| Testosterone Arm | ||||||
| Testosterone Arm (N = 88) | Placebo Arm (N = 91) | p Value* | CVD-Related Adverse Events (N = 19) | No CVD-Related Adverse Events (N = 69) | p Value* | |
| Hematocrit (%) | 2.9 (2.1 to 3.7) | −0.001 (−0.6 to 0.6) | <.001 | 3.7 (1.8 to 5.6) | 2.7 (1.8 to 3.6) | .29 |
| Hemoglobin (g/dL) | 0.77 (0.5 to 1.0) | −0.04 (−0.2 to 0.2) | < .001 | 0.89 (0.2 to 1.6) | 0.73 (0.5 to 1.0) | .63 |
| Mean arterial pressure | −2.9 (−5.3 to −0.5) | −3.7 (−6.1 to −1.2) | .66 | 0.2 (−4.5 to 4.8) | −3.7 (−6.5 to −1.0) | .18 |
| Total cholesterol (mg/dL) | −11 (−18 to −4) | −4 (−9 to 1) | .11 | −20 (−41 to 2) | −9 (−16 to −1) | .23 |
| LDL (mg/dL) | −3.9 (−9.4 to 1.7) | 2.1 (−1.5 to 5.7) | .07 | −10.0 (−27.6 to 7.6) | −2.4 (−8.1 to 3.3) | .29 |
| HDL (mg/dL) | −1.2 (−2.8 to 0.5) | 2.9 (0.7 to 5.1) | .003 | −2.8 (−6.7 to 1.2) | −0.7 (−2.6 to 1.1) | .33 |
| Triglycerides (mg/dL) | −17 (−32 to −2) | −14 (−26 to −2) | .75 | −38 (−79 to 3) | −12 (−28 to 4) | .17 |
| CRP (ng/mL) | 0.18 (−0.2 to 0.5) | 0.12 (−0.4 to 0.6) | .84 | 0.81 (−0.9 to 2.5) | 0.03 (−0.2 to 0.2) | .98 |
| IL-6 (pg/mL) | 1.1 (−0.1 to 2.2) | −1.0 (−1.9 to −0.1) | .01 | 2.5 (−1.7 to 6.8) | 0.7 (−0.3 to 1.8) | .40 |
| Fibrinogen (mg/dL) | 36 (8 to 65) | 13 (−12 to 37) | .20 | 74 (5 to 143) | 27 (−5 to 59) | .19 |
| PAI-1 (ng/mL) | −10.0 (−19.1 to −0.9) | −3.1 (−9.7 to 3.5) | .21 | −16.6 (−33.3 to −0.003) | −8.6 (−19.2 to 2.0) | .51 |
| Pro-BNP (pg/mL) | 54 (−24 to 132) | 125 (0.2 to 193) | .20 | 85 (−222 to 392) | 46 (−23 to 115) | .78 |
| Total testosterone (ng/dL) | 328 (239 to 418) | 55 (26 to 83) | <.001 | 478 (235 to 722) | 291 (196 to 387) | .10 |
| Free testosterone (ng/dL) | 6.4 (4.1 to 8.5) | 0.01 (−0.4 to 0.5) | <.001 | 10.6 (4.6 to 16.7) | 5.2 (3.0 to 7.5) | .05 |
| Estradiol (pg/mL) | 16.4 (9.8 to 22.9) | −0.6 (−2.0 to 0.8) | <.001 | 27.1 (7.4 to 46.7) | 13.6 (6.9 to 20.2) | .10 |
| Free estradiol (pg/mL) | 0.4 (0.2 to 0.6) | −0.06 (−0.1 to −0.02) | <.001 | 0.7 (0.13 1.2) | 0.3 (0.1 to 0.5) | .10 |
| Estrone (pg/mL) | 17.5 (10.7 to 24.3) | −0.4 (−2.2 to 1.5) | <.001 | 23.9 (5.9 to 42.0) | 15.8 (8.3 to 23.3) | .34 |
| Free estrone (pg/mL) | 0.6 (0.4 to 0.9) | −0.06 (−0.1 to 0.01) | <.001 | 0.9 (0.2 to 1.7) | 0.6 (0.7 to 0.9) | .29 |
| SHBG (nmol/L) | 15.5 (12.3 to 18.7) | 16.1 (11.8 to 20.4) | .83 | 12.1 (5.2 to 19.0) | 16.4 (12.7 to 20.0) | .29 |
Notes: Means and 95% confidence intervals are shown. CRP = C-Reactive Protein; CVD = cardiovascular disease; HDL = high-density lipoprotein; IL-6 = interleukin 6; LDL = low-density lipoprotein; PAI-1 = plasminogen activator inhibitor-1; Pro-BNP = pro-brain naturetic peptide; SHBG = sex hormone binding globulin.
Students t test.
Changes in Cardiovascular Biomarkers
There was considerable variability in the circulating levels of inflammatory and coagulation markers (Table 2). Overall, there was a significantly greater reduction in plasminogen activator inhibitor-1, low-density lipoprotein, and high-density lipoprotein levels and a trend toward greater increase in IL-6 levels in the testosterone group than in the placebo group. Men in the testosterone arm experiencing a cardiovascular event showed a greater numerical increase in IL-6 (2.5 vs 0.7 pg/mL), CRP (0.8 vs 0.03 ng/mL), and fibrinogen (74 vs 27 mg/dL) compared with men who did not experience an event.
However, these changes did not achieve statistical significance.
Association of Biomarkers with Cardiovascular Events
Multivariable logistic regression models were used to determine the association of hormones and other biomarkers with cardiovascular event (Table 3). The changes in serum levels of free testosterone were significantly associated with cardiovascular events.
Table 3.
Logistic Regression Analysis of the Testosterone Group: Association Between the Change in Biomarkers and CVD-Related Adverse Events in the TOM Trial
| Biomarker (SD) | OR (95% CI)* | p Value† |
| Total testosterone (64 ng/dL) | 1.06 (0.98–1.15) | .17 |
| Free testosterone (1.3 ng/dL) | 1.07 (1.00–1.15) | .05 |
| Estradiol (9.0 pg/mL) | 1.15 (0.97–1.36) | .11 |
| Free estradiol (0.2 pg/mL) | 1.12 (0.98–1.29) | .11 |
| Estrone (15.2 pg/mL) | 1.15 (0.87–1.50) | .33 |
| Free estrone (0.6 pg/mL) | 1.15 (0.89–1.48) | .28 |
| SHBG (16.4 nmol/L) | 0.92 (0.56–1.51) | .74 |
| Total trestosterone to estradiol ratio (5.3) | 1.09 (0.93–1.27) | .28 |
| CRP (0.9 ng/mL) | 1.09 (0.94–1.28) | .26 |
| IL-6 (4.3 pg/mL) | 1.29 (0.89–1.88) | .18 |
| Fibrinogen (119 mg/dL) | 1.32 (0.70–2.49) | .40 |
| PAI-1 (30.4 ng/mL) | 1.07 (0.59–1.92) | .83 |
| Pro-BNP (632 pg/ml) | 1.12 (0.53–2.33) | .77 |
Notes: CRP = C-Reactive Protein; CVD = cardiovascular disease; IL-6 = interleukin 6; PAI-1 = plasminogen activator inhibitor-1; Pro-BNP = pro-brain naturetic peptide; SHBG = sex hormone binding globulin.
Multiplicative increase in odds of CVD event per 1 SD difference in change in hormone levels. Magnitude of standard deviation is presented along with each biomarker name.
Wald test. Models control for randomized assignment and baseline marker level. For example, the model for association between circulating total testosterone and CVD events controls for randomization and baseline total testosterone level.
DISCUSSION
TOM Trial's population had a high burden of chronic conditions; nearly a third had diabetes and obesity, more than half had hyperlipidemia, more than three quarters had hypertension, and nearly half the participants had preexisting heart disease. Thus, these participants were at high risk of cardiovascular events at baseline. This is not surprising because functional limitations in older adults are known to be associated with significant subclinical atherosclerotic disease (13). However, the participants who experienced cardiovascular events did not differ in any obvious manner in their baseline characteristics, including the prevalence of diabetes, heart disease, and hypertension from those who did not experience cardiovascular events except for serum total testosterone level, which was lower in men who experienced cardiovascular events. The lower level of baseline total testosterone in these men might indicate greater underlying disease burden, possibility increasing their vulnerability to cardiovascular events. We found that the increase in serum free testosterone was significantly higher in men experiencing cardiovascular events than in those who did not. Importantly, these men also showed a trend toward greater increases in serum free estradiol and free estrone levels. Multivariable logistic regression analyses showed that assignment to testosterone arm was the best predictor of cardiovascular events. Furthermore, change in serum free testosterone was also associated with an increased risk of cardiovascular events. Changes in the circulating concentrations of IL-6, CRP, and fibrinogen levels, although numerically greater in men who experienced cardiovascular events, did not differ significantly from those who did not.
The role of gonadal steroids in the pathogenesis or exacerbation of cardiovascular disease remains unclear. Among men who were randomized to the testosterone arm, those who incurred cardiovascular events had higher circulating free testosterone levels. Although the mean on-treatment testosterone levels were not substantially different from those achieved in other randomized testosterone trials (2), there was substantial variation in testosterone levels in men randomized to testosterone arm of the trial. In spite of the adjustment of testosterone dose based on testosterone levels at 2 weeks, some subjects had testosterone levels above the target range of 500–1,000 ng/dL. It is conceivable that high testosterone levels in men with high burden of chronic conditions, who were at high baseline risk of cardiovascular disease, might render them susceptible to additional cardiovascular events.
Testosterone has been shown to stimulate human platelet aggregation by increasing thromboxane A2 receptor density (14) while castration reverses this phenomenon (15). Hence, it is conceivable that high serum testosterone concentrations could have promoted thrombosis on plaque surface. Men with heart failure treated with transdermal testosterone and those using anabolic steroids experience myocardial remodeling and left ventricular dysfunction (16–18). Although not evaluated in the TOM Trial, it is possible that myocardial remodeling might have contributed to some of these events. Testosterone also promotes salt and water retention, particularly in older men (11,19). Although pro-brain naturetic peptide showed a greater increase in men in the testosterone arm, no difference was seen within the testosterone arm based on the event status of the participants.
An interesting finding of this analysis is that the increases in serum total and free estradiol and estrone levels were approximately twice as high in men who experienced cardiovascular events compared with those who did not (although not statistically significant), reflecting, in part, the higher on-treatment testosterone levels. Because inflammation is known to promote aromatase activity, the greater increase in estradiol and estrone levels could be due to the underlying inflammatory milieu in frail men (20,21). The use of estrogen therapy in men has been associated with increased risk of cardiovascular events. For instance, in the Coronary Drug Project, the men with known coronary disease who were administered conjugated estrogens for secondary prevention experienced significantly higher rates of nonfatal myocardial infarction than men in the placebo group (3). Similarly, men with prostate cancer receiving estrogens for androgen deprivation therapy experience higher rates of myocardial infarction, stroke, pulmonary embolism, and congestive heart failure compared with men undergoing conventional androgen deprivation (4,22). Furthermore, in men who experience myocardial infarction, elevated serum estrogen levels are associated with coronary thrombosis (23), whereas venous thromboembolism remains a major complication in male-to-female transsexuals treated with oral estrogens (24). Estrogen promotes thrombosis by stimulating platelet aggregation, increasing serum concentration of coagulation factors and imparting resistance to activated protein C (25–27). Population studies have also shown that serum estradiol levels are independently associated with the risk of lower extremity peripheral arterial disease and stroke (28,29). In the Honolulu-Asia Aging Study, men in the highest quintile of estradiol levels had twice the risk of stroke compared with men in the lower quintiles (29) with the mean estradiol level in the top quintile being 34 pg/mL, substantially lower than on-treatment mean estradiol level of 51 pg/mL in men experiencing cardiovascular events in the TOM Trial. Frail men also have increased levels of factor VIII and D-dimer compared with non-frail men (30). Hence, it is possible that high testosterone and estrogen levels in older men with functional limitations might render them more susceptible to thrombosis and cardiovascular events.
In addition to the conventional cardiovascular risk factors, novel biomarkers of inflammation and coagulation have been associated with cardiovascular disease and mortality (31). In the TOM Trial, the men experiencing cardiovascular events had greater numerical increase (though not statistically significant) in IL-6, CRP, and fibrinogen levels compared with men who did not. The increase in CRP levels in men encountering cardiovascular events was 1 ng/mL. In longitudinal population studies, absolute CRP values greater than 1.57 ng/mL have been associated with 1.5-fold increase in all-cause mortality (32). Hence, an increase of 1 ng/mL in a 6-month period could be important. In older men, estradiol levels are associated with inflammation (33); hence, higher estradiol levels in men with cardiovascular events could have promoted inflammation. IL-6, a pleotropic cytokine, promotes CRP production (34), coagulation, and neutrophil aggregation (35), and is associated with cardiovascular disease and mortality in the elderly participants. In a case–control study, men experiencing myocardial infarction at 6-year follow-up had baseline IL-6 levels of 1.81 pg/mL compared with 1.46 pg/mL among participants who did not (31). In the TOM Trial, the increase in serum IL-6 levels in men encountering cardiovascular events during the 6-month intervention was 2.2 pg/mL. Fibrinogen is another risk factor for cardiovascular disease (36). Men in the TOM Trial experiencing cardiovascular events showed an increase in fibrinogen concentration of approximately 80 mg/dL after the 6-month intervention. Hence, the greater numerical increases in these biomarkers in men encountering cardiovascular events suggest that these markers could potentially be also contributing to these events. The relatively small number of men who experienced cardiovascular events limited the statistical power to detect these numerical differences in inflammatory markers.
The TOM Trial had several strengths and some limitations. The trial had many attributes of good trial design: concealed randomization, placebo control, parallel group design, blinding, and an intent-to-treat analytical strategy. The testosterone dose was adjusted to maintain testosterone levels within the target range. However, the strength of the inferences is limited by the post hoc nature of these analyses, as they were not a part of the prespecified hypotheses. Furthermore, the relatively small number of men who experienced cardiovascular events constrained statistical power. Therefore, these findings should be viewed as hypothesis-generating and need confirmation in prospective randomized trials. Furthermore, in light of the unique nature of TOM Trial's population, the observed associations of these markers with cardiovascular events might be specific to this population and should not be extended to young men with classical hypogonadism.
As recently reported, the men randomized to testosterone in the TOM Trial did experience clinically meaningful improvements in measures of muscle strength and some aspects of physical function (7). Thus, there is a trade-off between the beneficial anabolic effects and the potential adverse effects. Novel strategies are needed to dissociate the beneficial anabolic effects and the potential adverse effects. In this regard, several selective androgen receptor modulators that are non-aromatizable, but possess anabolic properties of testosterone, are in early development. The combined application of low-dose testosterone in conjunction with functional training, such as the physical activity intervention used in the LIFE Trial (37), is also an option. Combined administration of testosterone plus an aromatase inhibitor has also been considered, although this approach is limited by the potential concerns about the long-term effects of aromatase inhibitors on bone health. Further trials are needed to test the efficacy and safety of these approaches.
In conclusion, mobility-limited older men who experienced cardiovascular events in the TOM Trial had greater increase in serum free testosterone levels than those who did not. Research on alternative strategies that may benefit mobility-limited men is needed. In the interim, the benefits of testosterone supplementation in this population should be weighed against the risk of adverse events.
FUNDING
This study was supported primarily by a grant from the National Institutes on Aging administered under a cooperative agreement (1UO1AG14369). Additional support was provided by Boston Claude D. Pepper Older Americans Independence Center grant (5P30AG031679) and a BU Clinical and Translational Science Institute grant (1UL1RR025771). Testosterone and placebo gel for the study were provided by Auxilium Pharmaceuticals, Inc., Norristown, PA.
References
- 1.LeBrasseur NK, Lajevardi N, Miciek R, et al. Effects of testosterone therapy on muscle performance and physical function in older men with mobility limitations (The TOM Trial): design and methods. Contemp Clin Trials. 2009;30:133–140. doi: 10.1016/j.cct.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Basaria S, Coviello AD, Travison TG, et al. Adverse events associated with testosterone administration. N Engl J Med. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The Coronary Drug Project. Initial findings leading to modifications of its research protocol. JAMA. 1970;214:1303–1313. [PubMed] [Google Scholar]
- 4.Hedlund PO, Johansson R, Damber JE, et al. Significance of pretreatment cardiovascular morbidity as a risk factor during treatment with parenteral oestrogen or combined androgen deprivation of 915 patients with metastasized prostate cancer: evaluation of cardiovascular events in a randomized trial. Scand J Urol Nephrol. 2011;45:346–353. doi: 10.3109/00365599.2011.585820. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Cannon CP, Morrow D, et al. Pravastatin or Atorvastatin Evaluation and Infection Therapy-Thrombolysis in Myocardial Infarction 22 (PROVE IT-TIMI 22) Investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 6.Maggio M, Basaria S, Ble A, et al. Correlation between testosterone and the inflammatory marker soluble interleukin-6 receptor in older men. J Clin Endocrinol Metab. 2006;91:345–347. doi: 10.1210/jc.2005-1097. [DOI] [PubMed] [Google Scholar]
- 7.Travison TG, Basaria S, Storer TW, et al. Clinical meaningfulness of the changes in muscle performance and physical function associated with testosterone administration in older men with mobility limitation. J Gerontol A Biol Sci Med Sci. 2011;66:1090–1099. doi: 10.1093/gerona/glr100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guralnik JM, Ferrucci L, Simonsick EM, et al. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332:556–561. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Steidle C, Schwartz S, Jacoby K, et al. AA2500 testosterone gel normalizes androgen levels in aging males with improvements in body composition and sexual function. J Clin Endocrinol Metab. 2003;88:2673–2681. doi: 10.1210/jc.2002-021058. [DOI] [PubMed] [Google Scholar]
- 10.Salameh WA, Redon-Goldman MM, Clarke NJ, et al. Validation of a total testosterone assay using high turbulence liquid chromatography tandem mass spectrometry: total and free testosterone reference ranges. Steroids. 2010;75:165–175. doi: 10.1016/j.steroids.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Bhasin S, Woodhouse L, Casaburi R, et al. Older men are as responsive as young men to the anabolic effects of graded doses of testosterone on the skeletal muscle. J Clin Endocrinol Metab. 2005;90:678–688. doi: 10.1210/jc.2004-1184. [DOI] [PubMed] [Google Scholar]
- 12.Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74:512–519. doi: 10.1016/j.steroids.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Newman AB, Gottdiener JS, Mcburnie MA, et al. Associations of subclinical cardiovascular disease with frailty. J Gerontol A Biol Sci Med Sci. 2001;56:M158–M166. doi: 10.1093/gerona/56.3.m158. [DOI] [PubMed] [Google Scholar]
- 14.Ajayi AA, Mathur R, Halushka PV. Testosterone increases human platelet thromboxane A2 receptor density and aggregation responses. Circulation. 1995;91:2742–2747. doi: 10.1161/01.cir.91.11.2742. [DOI] [PubMed] [Google Scholar]
- 15.Ajayi AA, Halushka PV. Castration reduces platelet thromboxane A2 receptor density and aggregability. QJM. 2005;98:349–356. doi: 10.1093/qjmed/hci054. [DOI] [PubMed] [Google Scholar]
- 16.Malkin CJ, Pugh PJ, West JN, et al. Testosterone therapy in men with moderate severity heart failure: a double-blind randomized placebo controlled trial. Eur Heart J. 2006;27:57–64. doi: 10.1093/eurheartj/ehi443. [DOI] [PubMed] [Google Scholar]
- 17.D’Andrea A, Caso P, Salerno G, et al. Left ventricular early myocardial dysfunction after chronic misuse of anabolic androgenic steroids: a Doppler myocardial and strain imaging analysis. Br J Sports Med. 2007;41:149–155. doi: 10.1136/bjsm.2006.030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karila TA, Karjalainen JE, Mäntysaari MJ, et al. Anabolic androgenic steroids produce dose-dependent increase in left ventricular mass in power athletes, and this effect is potentiated by concomitant use of growth hormone. Int J Sports Med. 2003;24:337–343. doi: 10.1055/s-2003-40702. [DOI] [PubMed] [Google Scholar]
- 19.Johannsson G, Gibney J, Wolthers T, et al. Independent and combined effects of testosterone and growth hormone on extracellular water in hypopituitary men. J Clin Endocrinol Metab. 2005;90:3989–3994. doi: 10.1210/jc.2005-0553. [DOI] [PubMed] [Google Scholar]
- 20.Simpson ER, Misso M, Hewitt KN, et al. Estrogen—the good, the bad, and the unexpected. Endocr Rev. 2005;26:322–330. doi: 10.1210/er.2004-0020. [DOI] [PubMed] [Google Scholar]
- 21.Spratt DI, Morton JR, Kramer RS, et al. Increases in serum estrogen levels during major illness are caused by increased peripheral aromatization. Am J Physiol Endocrinol Metab. 2006;291:E631–E638. doi: 10.1152/ajpendo.00467.2005. [DOI] [PubMed] [Google Scholar]
- 22.Mikkola AK, Ruutu ML, Aro JL, et al. Parenteral polyoestradiol phosphate vs orchidectomy in the treatment of advanced prostatic cancer. Efficacy and cardiovascular complications: a 2-year follow-up report of a national, prospective prostatic cancer study. Br J Urol. 1998;82:63–68. doi: 10.1046/j.1464-410x.1998.00688.x. [DOI] [PubMed] [Google Scholar]
- 23.Phillips GB, Pinkernell BH, Jing TY. The association of hyperestrogenemia with coronary thrombosis in men. Arterioscler Thromb Vasc Biol. 1996;16:1383–1387. doi: 10.1161/01.atv.16.11.1383. [DOI] [PubMed] [Google Scholar]
- 24.van Kesteren PJ, Asscheman H, Megens JA, et al. Mortality and morbidity in transsexual subjects treated with cross-sex hormones. Clin Endocrinol (Oxf) 1997;47:337–342. doi: 10.1046/j.1365-2265.1997.2601068.x. [DOI] [PubMed] [Google Scholar]
- 25.Boudoulas KD, Montague CR, Goldschmidt-Clermont PJ, et al. Estradiol increases platelet aggregation in Pl(A1/A1) individuals. Am Heart J. 2006;152:136–139. doi: 10.1016/j.ahj.2005.07.036. [DOI] [PubMed] [Google Scholar]
- 26.Tchaikovski SN, Rosing J. Mechanisms of estrogen-induced venous thromboembolism. Thromb Res. 2010;126:5–11. doi: 10.1016/j.thromres.2010.01.045. [DOI] [PubMed] [Google Scholar]
- 27.Sudhir K, Komesaroff PA. Cardiovascular actions of estrogens in men. J Clin Endocrinol Metab. 1999;84:3411–3415. doi: 10.1210/jcem.84.10.5954. [DOI] [PubMed] [Google Scholar]
- 28.Tivesten A, Mellström D, Jutberger H, et al. Low serum testosterone and high serum estradiol associate with lower extremity peripheral arterial disease in elderly men. The MrOS Study in Sweden. J Am Coll Cardiol. 2007;50:1070–1076. doi: 10.1016/j.jacc.2007.04.088. [DOI] [PubMed] [Google Scholar]
- 29.Abbott RD, Launer LJ, Rodriguez BL, et al. Serum estradiol and risk of stroke in elderly men. Neurology. 2007;68:563–568. doi: 10.1212/01.wnl.0000254473.88647.ca. [DOI] [PubMed] [Google Scholar]
- 30.Walston J, McBurnie MA, Newman A, et al. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- 31.Ridker PM, Rifai N, Stampfer MJ, et al. Plasma concentration of interleukin-6 and the risk of future myocardial infarction among apparently healthy men. Circulation. 2000;101:1767–1772. doi: 10.1161/01.cir.101.15.1767. [DOI] [PubMed] [Google Scholar]
- 32.Harris TB, Ferrucci L, Tracy RP, et al. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 33.Maggio M, Ceda GP, Lauretani F, et al. Estradiol and inflammatory markers in older men. J Clin Endocrinol Metab. 2009;94:518–522. doi: 10.1210/jc.2008-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumann H, Gauldie J. Regulation of hepatic acute phase plasma protein genes by hepatocyte stimulating factors and other mediators of inflammation. Mol Biol Med. 1990;7:147–159. [PubMed] [Google Scholar]
- 35.Cermak J, Key NS, Bach RR, et al. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–520. [PubMed] [Google Scholar]
- 36.Danesh J, Lewington S, Thompson SG, et al. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 37.Fielding RA, Rejeski WJ, Blair S, et al. The Lifestyle Interventions and Independence for Elders Study: design and methods. J Gerontol A Biol Sci Med Sci. 2011;66:1226–1237. doi: 10.1093/gerona/glr123. [DOI] [PMC free article] [PubMed] [Google Scholar]