Abstract
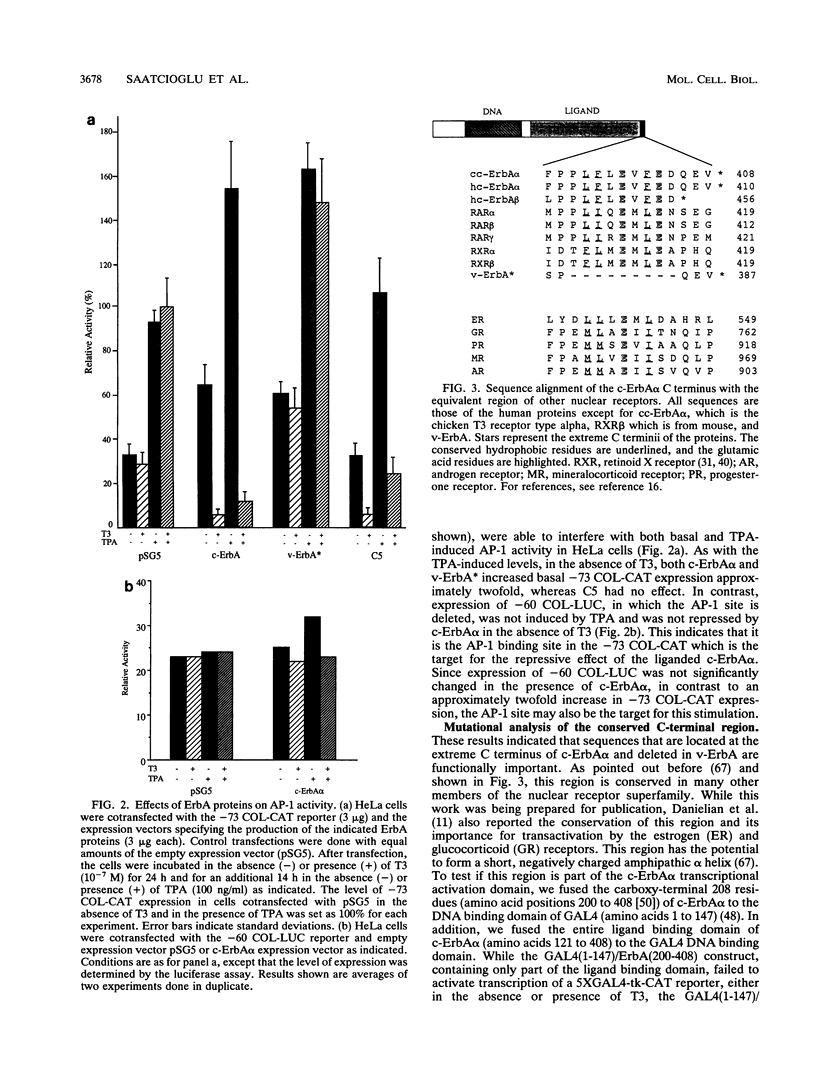
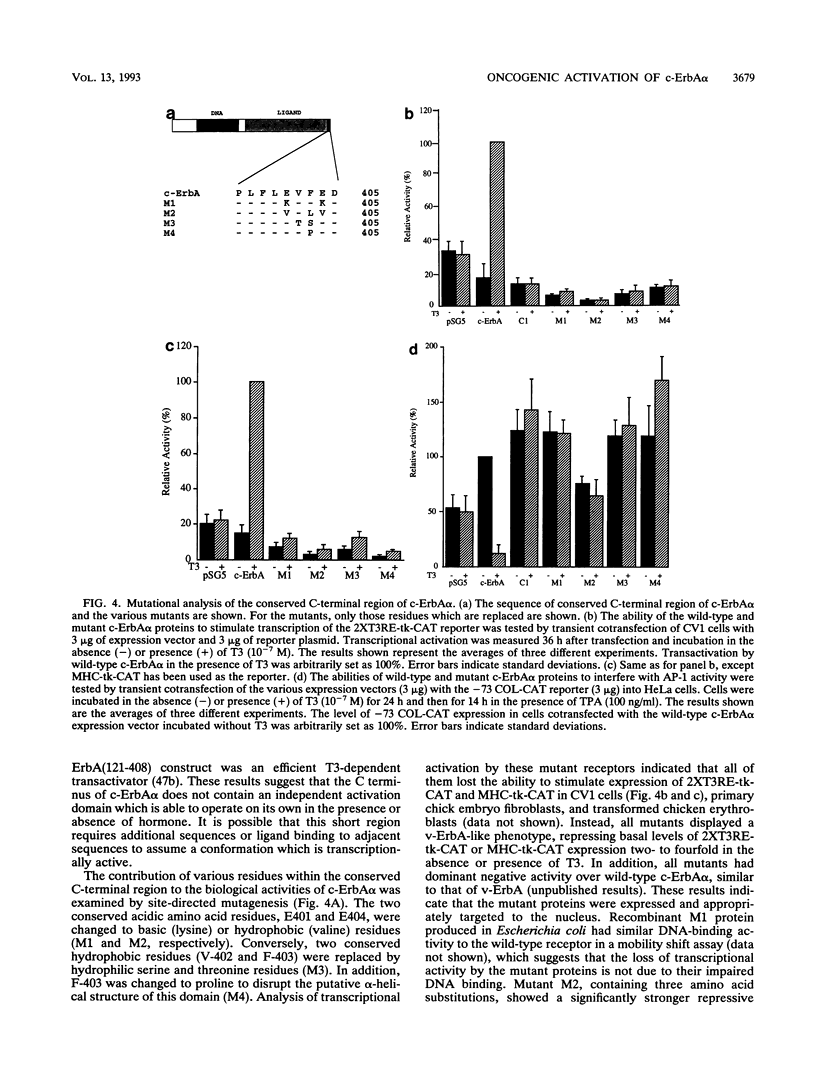
The thyroid hormone (T3) receptor type alpha, the c-ErbA alpha proto-oncoprotein, stimulates transcription of T3-dependent promoters, interferes with AP-1 activity, and induces erythroid differentiation in a ligand-dependent manner. The v-ErbA oncoprotein does not bind hormone and has lost all of these activities. Using c-ErbA/v-ErbA chimeras, we found that a deletion of 9 amino acids, conserved among many members of the nuclear receptor superfamily, which are located at the extreme carboxy terminus of c-ErbA alpha is responsible for loss of both transactivation and transcriptional interference activities. Single, double, and triple amino acid substitutions within this region completely abolished T3-dependent transcriptional activation, interference with AP-1 activity, and decreased T3 binding by c-ErbA alpha. However, the lower T3 binding by these mutants does not fully account for the loss of transactivation and transcriptional interference, since a c-ErbA/v-ErbA chimera which was similarly reduced in T3 binding activity has retained both of these functions. Deletion of homologous residues in the retinoic acid receptor alpha (RAR alpha) resulted in a similar loss of transactivation and transcriptional interference activities. The ability of c-ErbA alpha to induce differentiation of transformed erythroblasts is also impaired by all of the mutations introduced into the conserved carboxy-terminal sequence. We conclude that this 9-amino-acid conserved region is essential for normal biological function of c-ErbA alpha and RAR alpha and possibly other T3 and RA receptors.
Full text
PDF






Images in this article
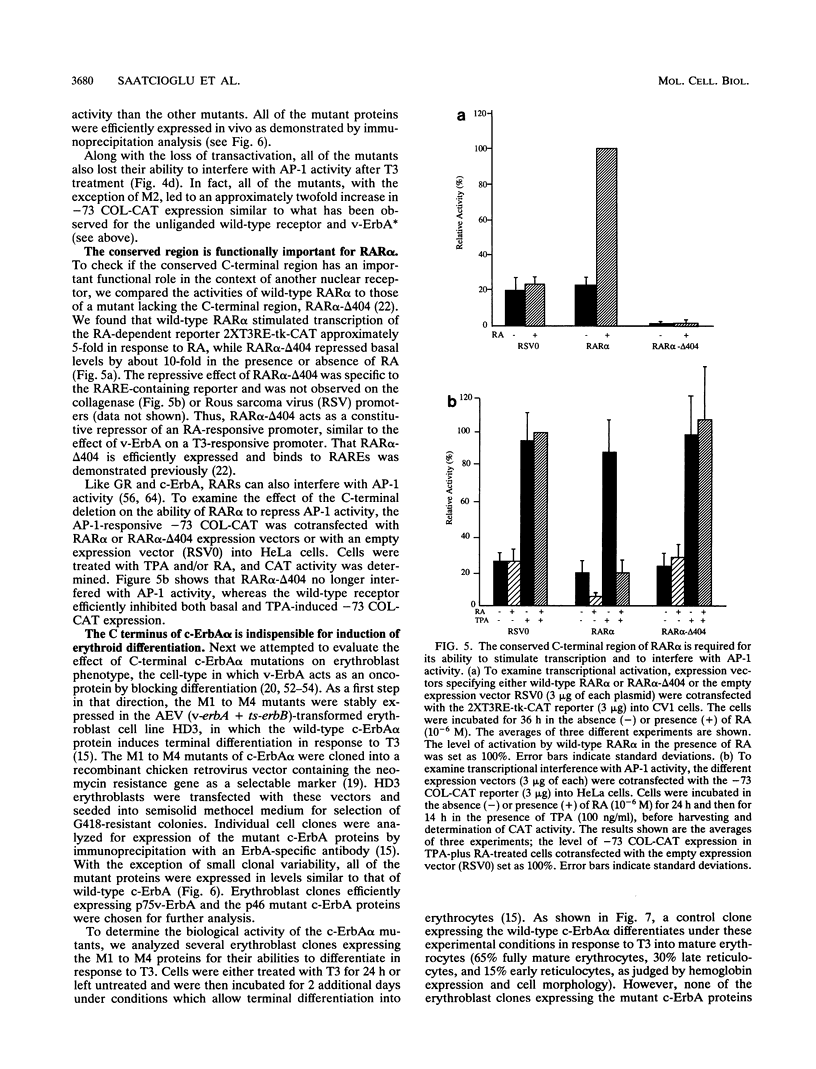
Selected References
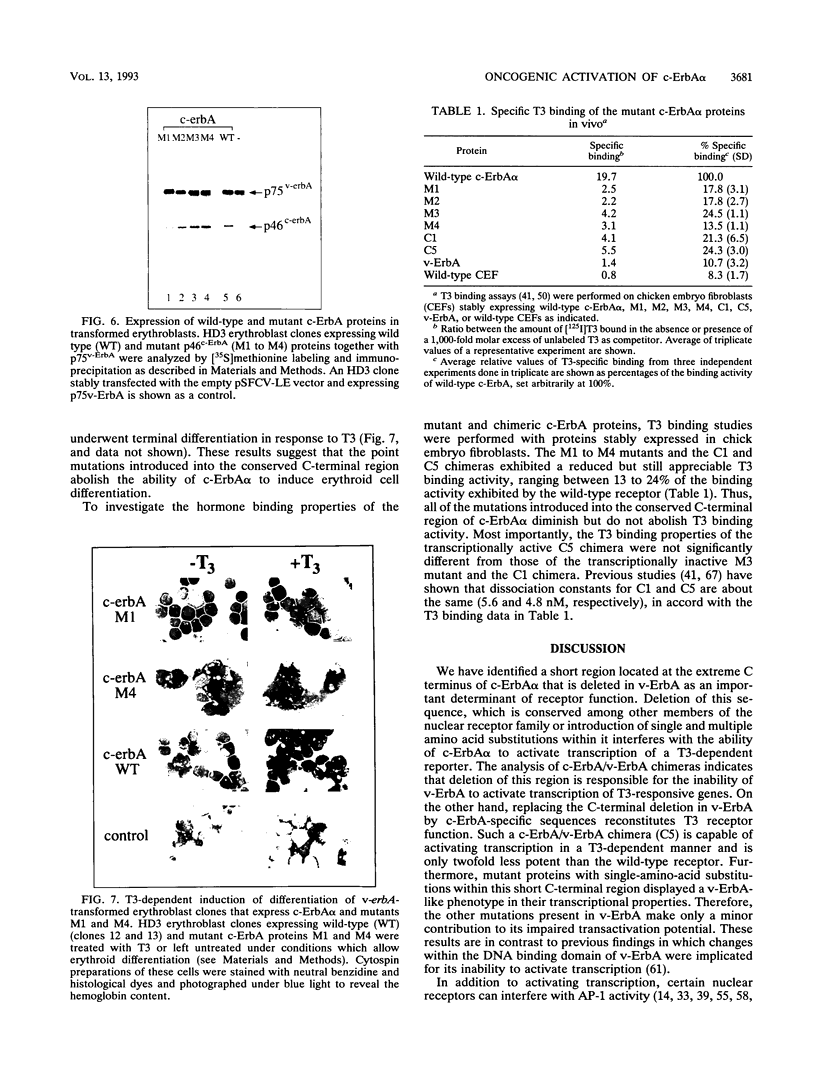
These references are in PubMed. This may not be the complete list of references from this article.
- Angel P., Imagawa M., Chiu R., Stein B., Imbra R. J., Rahmsdorf H. J., Jonat C., Herrlich P., Karin M. Phorbol ester-inducible genes contain a common cis element recognized by a TPA-modulated trans-acting factor. Cell. 1987 Jun 19;49(6):729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- Angel P., Karin M. The role of Jun, Fos and the AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991 Dec 10;1072(2-3):129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- Baniahmad A., Köhne A. C., Renkawitz R. A transferable silencing domain is present in the thyroid hormone receptor, in the v-erbA oncogene product and in the retinoic acid receptor. EMBO J. 1992 Mar;11(3):1015–1023. doi: 10.1002/j.1460-2075.1992.tb05140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989 Feb 10;56(3):335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Beug H., Doederlein G., Freudenstein C., Graf T. Erythroblast cell lines transformed by a temperature-sensitive mutant of avian erythroblastosis virus: a model system to study erythroid differentiation in vitro. J Cell Physiol Suppl. 1982;1:195–207. doi: 10.1002/jcp.1041130427. [DOI] [PubMed] [Google Scholar]
- Beug H., Kahn P., Doederlein G., Hayman M. J., Graf T. Characterization of hematopoietic cells transformed in vitro by AEV-H, a v-erbB-containing avian erythroblastosis virus. Haematol Blood Transfus. 1985;29:290–297. doi: 10.1007/978-3-642-70385-0_60. [DOI] [PubMed] [Google Scholar]
- Bohmann D., Bos T. J., Admon A., Nishimura T., Vogt P. K., Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987 Dec 4;238(4832):1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- Bonde B. G., Sharif M., Privalsky M. L. Ontogeny of the v-erbA oncoprotein from the thyroid hormone receptor: an alteration in the DNA binding domain plays a role crucial for v-erbA function. J Virol. 1991 Apr;65(4):2037–2046. doi: 10.1128/jvi.65.4.2037-2046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu R., Boyle W. J., Meek J., Smeal T., Hunter T., Karin M. The c-Fos protein interacts with c-Jun/AP-1 to stimulate transcription of AP-1 responsive genes. Cell. 1988 Aug 12;54(4):541–552. doi: 10.1016/0092-8674(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Damm K., Thompson C. C., Evans R. M. Protein encoded by v-erbA functions as a thyroid-hormone receptor antagonist. Nature. 1989 Jun 22;339(6226):593–597. doi: 10.1038/339593a0. [DOI] [PubMed] [Google Scholar]
- Danielian P. S., White R., Lees J. A., Parker M. G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992 Mar;11(3):1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois C., Aubert D., Legrand C., Pain B., Samarut J. A novel mechanism of action for v-ErbA: abrogation of the inactivation of transcription factor AP-1 by retinoic acid and thyroid hormone receptors. Cell. 1991 Nov 15;67(4):731–740. doi: 10.1016/0092-8674(91)90068-a. [DOI] [PubMed] [Google Scholar]
- Desbois C., Pain B., Guilhot C., Benchaibi M., Ffrench M., Ghysdael J., Madjar J. J., Samarut J. v-erbA oncogene abrogates growth inhibition of chicken embryo fibroblasts induced by retinoic acid. Oncogene. 1991 Nov;6(11):2129–2135. [PubMed] [Google Scholar]
- Diamond M. I., Miner J. N., Yoshinaga S. K., Yamamoto K. R. Transcription factor interactions: selectors of positive or negative regulation from a single DNA element. Science. 1990 Sep 14;249(4974):1266–1272. doi: 10.1126/science.2119054. [DOI] [PubMed] [Google Scholar]
- Disela C., Glineur C., Bugge T., Sap J., Stengl G., Dodgson J., Stunnenberg H., Beug H., Zenke M. v-erbA overexpression is required to extinguish c-erbA function in erythroid cell differentiation and regulation of the erbA target gene CAII. Genes Dev. 1991 Nov;5(11):2033–2047. doi: 10.1101/gad.5.11.2033. [DOI] [PubMed] [Google Scholar]
- Evans R. M. The steroid and thyroid hormone receptor superfamily. Science. 1988 May 13;240(4854):889–895. doi: 10.1126/science.3283939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman B. M., Samuels H. H. Interactions among a subfamily of nuclear hormone receptors: the regulatory zipper model. Mol Endocrinol. 1990 Sep;4(9):1293–1301. doi: 10.1210/mend-4-9-1293. [DOI] [PubMed] [Google Scholar]
- Forman B. M., Yang C. R., Au M., Casanova J., Ghysdael J., Samuels H. H. A domain containing leucine-zipper-like motifs mediate novel in vivo interactions between the thyroid hormone and retinoic acid receptors. Mol Endocrinol. 1989 Oct;3(10):1610–1626. doi: 10.1210/mend-3-10-1610. [DOI] [PubMed] [Google Scholar]
- Fuerstenberg S., Beug H., Introna M., Khazaie K., Muñoz A., Ness S., Nordström K., Sap J., Stanley I., Zenke M. Ectopic expression of the erythrocyte band 3 anion exchange protein, using a new avian retrovirus vector. J Virol. 1990 Dec;64(12):5891–5902. doi: 10.1128/jvi.64.12.5891-5902.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandrillon O., Jurdic P., Pain B., Desbois C., Madjar J. J., Moscovici M. G., Moscovici C., Samarut J. Expression of the v-erbA product, an altered nuclear hormone receptor, is sufficient to transform erythrocytic cells in vitro. Cell. 1989 Jul 14;58(1):115–121. doi: 10.1016/0092-8674(89)90408-x. [DOI] [PubMed] [Google Scholar]
- Giguere V., Ong E. S., Segui P., Evans R. M. Identification of a receptor for the morphogen retinoic acid. Nature. 1987 Dec 17;330(6149):624–629. doi: 10.1038/330624a0. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M., Devary O. V., Rosenfeld M. G. The thyroid hormone receptor binds with opposite transcriptional effects to a common sequence motif in thyroid hormone and estrogen response elements. Cell. 1988 Jul 29;54(3):313–323. doi: 10.1016/0092-8674(88)90194-8. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Lipkin S. M., Devary O. V., Rosenfeld M. G. Positive and negative regulation of gene transcription by a retinoic acid-thyroid hormone receptor heterodimer. Cell. 1989 Nov 17;59(4):697–708. doi: 10.1016/0092-8674(89)90016-0. [DOI] [PubMed] [Google Scholar]
- Glineur C., Zenke M., Beug H., Ghysdael J. Phosphorylation of the v-erbA protein is required for its function as an oncogene. Genes Dev. 1990 Oct;4(10):1663–1676. doi: 10.1101/gad.4.10.1663. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Beug H. Role of the v-erbA and v-erbB oncogenes of avian erythroblastosis virus in erythroid cell transformation. Cell. 1983 Aug;34(1):7–9. doi: 10.1016/0092-8674(83)90130-7. [DOI] [PubMed] [Google Scholar]
- Graupner G., Wills K. N., Tzukerman M., Zhang X. K., Pfahl M. Dual regulatory role for thyroid-hormone receptors allows control of retinoic-acid receptor activity. Nature. 1989 Aug 24;340(6235):653–656. doi: 10.1038/340653a0. [DOI] [PubMed] [Google Scholar]
- Green S., Chambon P. Nuclear receptors enhance our understanding of transcription regulation. Trends Genet. 1988 Nov;4(11):309–314. doi: 10.1016/0168-9525(88)90108-4. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada K., Gleason S. L., Levi B. Z., Hirschfeld S., Appella E., Ozato K. H-2RIIBP, a member of the nuclear hormone receptor superfamily that binds to both the regulatory element of major histocompatibility class I genes and the estrogen response element. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8289–8293. doi: 10.1073/pnas.86.21.8289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz Z. D., Yang C. R., Forman B. M., Casanova J., Samuels H. H. Characterization of the domain structure of chick c-erbA by deletion mutation: in vitro translation and cell transfection studies. Mol Endocrinol. 1989 Jan;3(1):148–156. doi: 10.1210/mend-3-1-148. [DOI] [PubMed] [Google Scholar]
- Jonat C., Rahmsdorf H. J., Park K. K., Cato A. C., Gebel S., Ponta H., Herrlich P. Antitumor promotion and antiinflammation: down-modulation of AP-1 (Fos/Jun) activity by glucocorticoid hormone. Cell. 1990 Sep 21;62(6):1189–1204. doi: 10.1016/0092-8674(90)90395-u. [DOI] [PubMed] [Google Scholar]
- Kliewer S. A., Umesono K., Mangelsdorf D. J., Evans R. M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signalling. Nature. 1992 Jan 30;355(6359):446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight J., Zenke M., Disela C., Kowenz E., Vogt P., Engel J. D., Hayman M. J., Beug H. Temperature-sensitive v-sea transformed erythroblasts: a model system to study gene expression during erythroid differentiation. Genes Dev. 1988 Feb;2(2):247–258. doi: 10.1101/gad.2.2.247. [DOI] [PubMed] [Google Scholar]
- König H., Ponta H., Rahmsdorf H. J., Herrlich P. Interference between pathway-specific transcription factors: glucocorticoids antagonize phorbol ester-induced AP-1 activity without altering AP-1 site occupation in vivo. EMBO J. 1992 Jun;11(6):2241–2246. doi: 10.1002/j.1460-2075.1992.tb05283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leid M., Kastner P., Lyons R., Nakshatri H., Saunders M., Zacharewski T., Chen J. Y., Staub A., Garnier J. M., Mader S. Purification, cloning, and RXR identity of the HeLa cell factor with which RAR or TR heterodimerizes to bind target sequences efficiently. Cell. 1992 Jan 24;68(2):377–395. doi: 10.1016/0092-8674(92)90478-u. [DOI] [PubMed] [Google Scholar]
- Lucibello F. C., Slater E. P., Jooss K. U., Beato M., Müller R. Mutual transrepression of Fos and the glucocorticoid receptor: involvement of a functional domain in Fos which is absent in FosB. EMBO J. 1990 Sep;9(9):2827–2834. doi: 10.1002/j.1460-2075.1990.tb07471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf D. J., Ong E. S., Dyck J. A., Evans R. M. Nuclear receptor that identifies a novel retinoic acid response pathway. Nature. 1990 May 17;345(6272):224–229. doi: 10.1038/345224a0. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Zenke M., Gehring U., Sap J., Beug H., Vennström B. Characterization of the hormone-binding domain of the chicken c-erbA/thyroid hormone receptor protein. EMBO J. 1988 Jan;7(1):155–159. doi: 10.1002/j.1460-2075.1988.tb02795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R. C., Mader S., Nagpal S., Leid M., Rochette-Egly C., Chambon P. Negative regulation of the rat stromelysin gene promoter by retinoic acid is mediated by an AP1 binding site. EMBO J. 1990 Dec;9(13):4443–4454. doi: 10.1002/j.1460-2075.1990.tb07895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- När A. M., Boutin J. M., Lipkin S. M., Yu V. C., Holloway J. M., Glass C. K., Rosenfeld M. G. The orientation and spacing of core DNA-binding motifs dictate selective transcriptional responses to three nuclear receptors. Cell. 1991 Jun 28;65(7):1267–1279. doi: 10.1016/0092-8674(91)90021-p. [DOI] [PubMed] [Google Scholar]
- Petkovich M., Brand N. J., Krust A., Chambon P. A human retinoic acid receptor which belongs to the family of nuclear receptors. Nature. 1987 Dec 3;330(6147):444–450. doi: 10.1038/330444a0. [DOI] [PubMed] [Google Scholar]
- Privalsky M. L., Sharif M., Yamamoto K. R. The viral erbA oncogene protein, a constitutive repressor in animal cells, is a hormone-regulated activator in yeast. Cell. 1990 Dec 21;63(6):1277–1286. doi: 10.1016/0092-8674(90)90423-c. [DOI] [PubMed] [Google Scholar]
- Rauscher F. J., 3rd, Cohen D. R., Curran T., Bos T. J., Vogt P. K., Bohmann D., Tjian R., Franza B. R., Jr Fos-associated protein p39 is the product of the jun proto-oncogene. Science. 1988 May 20;240(4855):1010–1016. doi: 10.1126/science.3130660. [DOI] [PubMed] [Google Scholar]
- Sadowski I., Ptashne M. A vector for expressing GAL4(1-147) fusions in mammalian cells. Nucleic Acids Res. 1989 Sep 25;17(18):7539–7539. doi: 10.1093/nar/17.18.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Damm K., Goldberg Y., Ghysdael J., Leutz A., Beug H., Vennström B. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature. 1986 Dec 18;324(6098):635–640. doi: 10.1038/324635a0. [DOI] [PubMed] [Google Scholar]
- Sap J., Muñoz A., Schmitt J., Stunnenberg H., Vennström B. Repression of transcription mediated at a thyroid hormone response element by the v-erb-A oncogene product. Nature. 1989 Jul 20;340(6230):242–244. doi: 10.1038/340242a0. [DOI] [PubMed] [Google Scholar]
- Sap J., de Magistris L., Stunnenberg H., Vennström B. A major thyroid hormone response element in the third intron of the rat growth hormone gene. EMBO J. 1990 Mar;9(3):887–896. doi: 10.1002/j.1460-2075.1990.tb08186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder C., Gibson L., Beug H. The v-erbA oncogene requires cooperation with tyrosine kinases to arrest erythroid differentiation induced by ligand-activated endogenous c-erbA and retinoic acid receptor. Oncogene. 1992 Feb;7(2):203–216. [PubMed] [Google Scholar]
- Schroeder C., Gibson L., Zenke M., Beug H. Modulation of normal erythroid differentiation by the endogenous thyroid hormone and retinoic acid receptors: a possible target for v-erbA oncogene action. Oncogene. 1992 Feb;7(2):217–227. [PubMed] [Google Scholar]
- Schroeder C., Raynoschek C., Fuhrmann U., Damm K., Vennström B., Beug H. The v-erb A oncogene causes repression of erythrocyte-specific genes and an immature, aberrant differentiation phenotype in normal erythroid progenitors. Oncogene. 1990 Oct;5(10):1445–1453. [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Kliewer S., Ransone L. J., Bolado J., Yang N., Verma I. M., Evans R. M. Functional antagonism between oncoprotein c-Jun and the glucocorticoid receptor. Cell. 1990 Sep 21;62(6):1217–1226. doi: 10.1016/0092-8674(90)90397-w. [DOI] [PubMed] [Google Scholar]
- Schüle R., Rangarajan P., Yang N., Kliewer S., Ransone L. J., Bolado J., Verma I. M., Evans R. M. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif M., Privalsky M. L. V-erbA and c-erbA proteins enhance transcriptional activation by c-jun. Oncogene. 1992 May;7(5):953–960. [PubMed] [Google Scholar]
- Shemshedini L., Knauthe R., Sassone-Corsi P., Pornon A., Gronemeyer H. Cell-specific inhibitory and stimulatory effects of Fos and Jun on transcription activation by nuclear receptors. EMBO J. 1991 Dec;10(12):3839–3849. doi: 10.1002/j.1460-2075.1991.tb04953.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeal T., Binetruy B., Mercola D., Grover-Bardwick A., Heidecker G., Rapp U. R., Karin M. Oncoprotein-mediated signalling cascade stimulates c-Jun activity by phosphorylation of serines 63 and 73. Mol Cell Biol. 1992 Aug;12(8):3507–3513. doi: 10.1128/mcb.12.8.3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesono K., Murakami K. K., Thompson C. C., Evans R. M. Direct repeats as selective response elements for the thyroid hormone, retinoic acid, and vitamin D3 receptors. Cell. 1991 Jun 28;65(7):1255–1266. doi: 10.1016/0092-8674(91)90020-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberger C., Thompson C. C., Ong E. S., Lebo R., Gruol D. J., Evans R. M. The c-erb-A gene encodes a thyroid hormone receptor. Nature. 1986 Dec 18;324(6098):641–646. doi: 10.1038/324641a0. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Chambard J. C., Sun Y. L., Smeal T., Schmidt T. J., Drouin J., Karin M. Transcriptional interference between c-Jun and the glucocorticoid receptor: mutual inhibition of DNA binding due to direct protein-protein interaction. Cell. 1990 Sep 21;62(6):1205–1215. doi: 10.1016/0092-8674(90)90396-v. [DOI] [PubMed] [Google Scholar]
- Yang-Yen H. F., Zhang X. K., Graupner G., Tzukerman M., Sakamoto B., Karin M., Pfahl M. Antagonism between retinoic acid receptors and AP-1: implications for tumor promotion and inflammation. New Biol. 1991 Dec;3(12):1206–1219. [PubMed] [Google Scholar]
- Yu V. C., Delsert C., Andersen B., Holloway J. M., Devary O. V., När A. M., Kim S. Y., Boutin J. M., Glass C. K., Rosenfeld M. G. RXR beta: a coregulator that enhances binding of retinoic acid, thyroid hormone, and vitamin D receptors to their cognate response elements. Cell. 1991 Dec 20;67(6):1251–1266. doi: 10.1016/0092-8674(91)90301-e. [DOI] [PubMed] [Google Scholar]
- Zenke M., Kahn P., Disela C., Vennström B., Leutz A., Keegan K., Hayman M. J., Choi H. R., Yew N., Engel J. D. v-erbA specifically suppresses transcription of the avian erythrocyte anion transporter (band 3) gene. Cell. 1988 Jan 15;52(1):107–119. doi: 10.1016/0092-8674(88)90535-1. [DOI] [PubMed] [Google Scholar]
- Zenke M., Muñoz A., Sap J., Vennström B., Beug H. v-erbA oncogene activation entails the loss of hormone-dependent regulator activity of c-erbA. Cell. 1990 Jun 15;61(6):1035–1049. doi: 10.1016/0092-8674(90)90068-p. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Hoffmann B., Tran P. B., Graupner G., Pfahl M. Retinoid X receptor is an auxiliary protein for thyroid hormone and retinoic acid receptors. Nature. 1992 Jan 30;355(6359):441–446. doi: 10.1038/355441a0. [DOI] [PubMed] [Google Scholar]
- Zhang X. K., Wills K. N., Graupner G., Tzukerman M., Hermann T., Pfahl M. Ligand-binding domain of thyroid hormone receptors modulates DNA binding and determines their bifunctional roles. New Biol. 1991 Feb;3(2):169–181. [PubMed] [Google Scholar]
- Zhang X. K., Wills K. N., Husmann M., Hermann T., Pfahl M. Novel pathway for thyroid hormone receptor action through interaction with jun and fos oncogene activities. Mol Cell Biol. 1991 Dec;11(12):6016–6025. doi: 10.1128/mcb.11.12.6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Verneuil H., Metzger D. The lack of transcriptional activation of the v-erbA oncogene is in part due to a mutation present in the DNA binding domain of the protein. Nucleic Acids Res. 1990 Aug 11;18(15):4489–4497. doi: 10.1093/nar/18.15.4489. [DOI] [PMC free article] [PubMed] [Google Scholar]