Abstract
The National Institute on Aging Interventions Testing Program (ITP) was established to evaluate agents that are hypothesized to increase life span and/or health span in genetically heterogeneous mice. Each compound is tested in parallel at three test sites. It is the goal of the ITP to publish all results, negative or positive. We report here on the results of lifelong treatment of mice, beginning at 4 months of age, with each of five agents, that is, green tea extract (GTE), curcumin, oxaloacetic acid, medium-chain triglyceride oil, and resveratrol, on the life span of genetically heterogeneous mice. Each agent was administered beginning at 4 months of age. None of these five agents had a statistically significant effect on life span of male or female mice, by log-rank test, at the concentrations tested, although a secondary analysis suggested that GTE might diminish the risk of midlife deaths in females only.
Keywords: Longevity, aging, mice, diet, Interventions
By delaying or reducing the onset of multiple late-life diseases, treatments that increase longevity could have a far greater benefit to quality of life than treatments targeted at single late-life diseases such as cancer or heart disease (1–3). There are numerous reports of dietary additives purported to have beneficial effects in rodent models (4–6), but until recently none have been replicated and accepted by the scientific community as a reliable and reproducible result. In 2003, the National Institute on Aging established the NIA Interventions Testing Program (ITP) to evaluate agents that are hypothesized to delay aging in laboratory mice and thus prevent or delay multiple diseases of late life.
The central features of the ITP protocol have been described elsewhere (7–11). Briefly, test agents are nominated by members of the scientific community whose recommendations are reviewed by an expert committee. Interventions are tested in parallel at three sites: The Jackson Laboratory in Bar Harbor, Maine (TJL), the University of Michigan at Ann Arbor (UM), and the University of Texas Health Science Center at San Antonio (UT). A key feature of the program is the use of four-way cross, genetically heterogeneous mice, the progeny of CB6F1 females bred to C3D2F1 males, with breeders supplied to each site at the same time by The Jackson Laboratory. Each animal in the test population is thus genetically unique, avoiding the possibility that an intervention may have an effect limited to a single genotype, yet the genetic heterogeneity of the population is reproducible.
This article presents the full life-span analysis of mice exposed to one of five agents, each initiated at 4 months of age. Resveratrol (RES) has been reported to extend life span in a short-lived vertebrate, the fish Nothobranchius furzeri (12), and to extend median life span in mice given a diet sufficiently high in fat to induce obesity (13). We reported previously that adding RES to the diet starting at 12 months of age did not affect life span (11). However, it was hypothesized that RES, like caloric restriction, might have its greatest effect when started earlier in life (14). Therefore, testing was repeated starting the test diet at 4 months of age. Like RES, curcumin is a polyphenol. Curcumin and its major metabolite tetrahydrocurcumin (TC) have been shown to extend life span in mice and rats (15,16,34). The antioxidant properties of polyphenols contained in green tea have also been evaluated in previous studies (17,18). Green tea polyphenols have been reported to extend life span by almost 6% in the relatively long-lived C57BL/6 strain of mice (15,16). Medium-chain triglyceride oil was reported to reduce fat mass and downregulate expression of adipogenic genes in rats and improve insulin homeostasis (19). Reduction of fat mass is associated with increased life span in mice (20). Therefore, it was hypothesized that medium-chain triglyceride oil would increase life span in mice. Oxaloacetate is an intermediary in oxidative metabolism. It was hypothesized that an excess of oxaloacetate may effectively mimic calorie restriction by raising the NAD+/NADH ratio. Published studies on Caenorhabditis elegans have indicated that excess oxaloacetate can lead to increased life span (21).
Methods
Mouse Production, Maintenance, and Estimation of Life Span
UM-HET3 mice were produced at each of the three test sites as previously described in detail (7,11). The mothers of the test mice were CByB6F1/J, JAX stock #100009, whose female parents are BALB/cByJ and whose male parents are C57BL/6J. The fathers of the test mice were C3D2F1/J, JAX stock #100004, whose mothers are C3H/HeJ and whose fathers are DBA/2J. For breeding cages, each site used Purina 5008 mouse chow. For weanlings prior to 4 months of age, each site used Purina 5LG6.
Mice were housed in plastic cages with metal tops, using ¼-inch corn-cob bedding (Bed O’Cobs, produced by The Andersons, Maumee, OH). Mice were given free access to water, acidified (pH 2.5–2.7) by addition of hydrochloric acid, using water bottles rather than an automated watering system. Mice were housed in ventilated cages and were transferred to fresh cages every 14 days, except that at UT mice were transferred to fresh cages every 7 days. Temperature was maintained within the range of 21°–23°C.
At the age of 42 days, each cage was assigned to a control or test group by use of a random number table. Each mouse was then briefly anesthetized by isoflurane inhalation administered either by nose cone or by an instrument designed for small animal anesthesia. Measures were taken of weight, body length, and tail length; and an electronic ID chip was implanted by sterile syringe beneath the dorsal skin between the shoulder blades, after which the wound was closed by a drop of superglue (Loctite gel, purchased locally, or Nexaband S/C, purchased from Abbott Laboratories, North Chicago, IL). UM and UT used chips purchased from AVID Microchip ID Systems (Folsom, LA, catalog #AVID3002); TJL used chips purchased from Locus Technology (Manchester, MD; catalog #1D-100A). A portion of the distal tail (1 cm) was taken and frozen for later analysis of DNA polymorphisms, after which the mouse was permitted to awaken from the anesthesia. The duration of anesthesia was approximately 1–2 min.
Mice received Purina 5LG6 control diet until the age of 4 months, after which mice in the control group received Purina 5LG6 at all three sites and mice in the treatment groups received Purina 5LG6 containing the additives.
Details of the methods used for health monitoring were provided in Miller et al. (7); in brief, each of the three colonies was evaluated four times each year for infectious agents, including pinworm. All such tests were negative throughout the entire study period.
Removal of Mice from the Longevity Population
As described in detail in Miller et al. (7), mice were removed from the study because of fighting, or accidental death, typically during chip implantation or chip failure, or because they were used for another experimental purpose, such as testing for blood levels of a test agent. For survival analyses, all such mice were treated as alive at the date of their removal from the protocol and lost to follow-up thereafter. These mice were not included in calculations of median longevity. Overall, 3.7% of the mice were removed from the longevity population. There were no significant differences in the proportion of removed mice among the treatment groups. There were also no significant differences in the mice removed at each test site. Mice at UT and UM were somewhat more likely to be removed than mice at TJL (5% vs. 1%, respectively), but this difference did not reach statistical significance.
Activity Testing
Locomotor activity testing was done as previously described in detail (11), using a computer-controlled Micromax apparatus (Accuscan, Columbus, OH). The tested mice were included in the populations evaluated for longevity effects.
Estimation of Age at Death (life Span)
Mice were examined at least daily for signs of ill health. Mice were euthanized for humane reasons if so severely moribund that they were considered, by an experienced technician, unlikely to survive for more than an additional 48 h. A mouse was considered severely moribund if it exhibited more than one of the following clinical signs: (a) inability to eat or to drink, (b) severe lethargy, as indicated by reluctance to move when gently prodded with a forceps, (c) severe balance or gait disturbance, (d) rapid weight loss over a period of one week or more, or (e) an ulcerated or bleeding tumor. The age at which a moribund mouse was euthanized was taken as the best available estimate of its natural life span. Mice found dead were also noted at each daily inspection.
Control and Experimental Diets
TestDiet, Inc., a division of Purina Mills (Richmond, IN), prepared batches of Purina 5LG6 food containing each of the test substances, as well as control diet batches, at intervals of approximately 4 months, and shipped each batch of food at the same time to each of the three test sites. RES was purchased from Lali Lab (Durham, NC) and was mixed with chow at a concentration of 300 mg of RES per kilogram of food (300 ppm). Curcumin was a gift from Sabinsa Corporation (East Windsor, NJ) and used at a dose of 2000 mg of curcumin per kilogram of food. Green tea extract (GTE) was obtained from LKT Laboratories, Inc. (St Paul, MN, catalog #G6817) and used at a dose of 2000 mg of GTE per kilogram of food. Medium-chain triglyceride oil (MCTO) was obtained from Life Enhancement Products, Inc. (Petaluma, CA; Functional Gourmet MCT Oil) and used at 60,000 mg/kg of diet. Oxaloacetic acid (OAA) was obtained from Sigma-Aldrich Co. LLC (St Louis, MO, catalog #O4126-100G) and incorporated into the food at a concentration of 2200 mg/kg of food. On the assumption that each mouse weighs 30 g and consumes 5 g food/day, the estimated daily doses of these agents would be: RES 50; curcumin 333; GTE 333; MCTO 10,000; and OAA 367 mg/kg body weight/day.
Measurement of Curcumin in Lab Chow
Curcumin-containing lab chow was ground to powder using a mortar and pestle, then 100 mg of powdered lab chow was mixed with 10 μL of 10 mg/mL of nordihydroquaiaretic acid (NDGA; internal standard) and 1 mL of mobile phase by vortexing and then shaking for 10 min. Next, the samples were placed in microfilterfuge tubes and centrifuged at 10,000g for 1 min; 100 μL of the sample filtrates were injected into the high-performance liquid chromatography (HPLC) system for quantification of curcumin in lab chow. To quantify curcumin in each sample, the ratio of the peak area of curcumin divided by the peak area of NDGA was compared against a linear regression of ratios of lab chow samples spiked with 0, 10, 50, 500, and 1000 ng curcumin/mg lab chow.
The isocratic HPLC system consisted of a Waters 510 pump, Waters 717 autosampler, Waters 2487 UV detector (214 nm), an Alltima C18 column (4.6 × 150 mm, 5 μ). The mobile phase was (25:29:46) methanol:acetonitrile:KH2PO4 (5.5 mM, pH 2.5), flow rate was 1.5 mL/min, and the column temperature was 23oC. All reagents were HPLC grade and were purchased from Sigma Chemical Co. (St Louis, MO). All solutions were prepared using Milli-Q water. Concentrations of curcumin in lab chow were expressed as ng/mg (parts per million).
Measurement of Oxaloacetic Acid in Lab Chow
Oxaloacetic acid lab chow was ground to powder using a mortar and pestle, and then 20 mg of powdered lab chow was mixed with 10 mL of mobile phase by vortexing and then shaking for 10 min. Next, the samples were placed in microfilterfuge tubes and centrifuged at 10,000g for one min; 100 μL of the sample filtrates were injected into the HPLC system for quantification of oxaloacetic acid in lab chow. To quantify oxaloacetic acid in each sample, the peak area of oxaloacetic acid was compared against a linear regression of peak areas of oxaloacetic acid in lab chow samples spiked with 0, 0.5, 1.5, 2.5, and 3.5 μg/mg lab chow.
The isocratic HPLC system consisted of a Shimadzu low pressure gradient 10AD system coupled to an AB Sciex 3200 tandem mass spectrometer and included an Alltima C18 column (4.6 × 150 mm, 5 μ). The mobile phase was formic acid (0.1% [w/v]) and ammonium formate (10 mM) dissolved in methanol:water (80:20), flow rate was 0.2 mL/min, and the column temperature was 40oC. Oxaloacetic acid was detected at Q1 (132.9 m/z) and quantified at Q3 (87.0 m/z). All reagents were HPLC grade and were purchased from Sigma Chemical Co. All solutions were prepared using Milli-Q water. Concentrations of oxaloacetic acid in lab chow were expressed as μg/mg.
Statistical Methods
Each mouse originally entered into the study was, at the time of analysis, considered to be in one of two categories: either dead (from natural causes) or censored. Mice were considered to be censored at the age at which they were no longer subjected to the mortality risks typical of unmanipulated mice. In some cases, this was because the mouse was removed because of fighting; in other cases, mice died as the result of an accident (eg, death when anesthetized for implantation of a radio-emitting chip). In still other cases, mice were considered censored on the day in which they received an experimental treatment (such as blood sampling or injection with an inflammatory agent) to which the control mice were not exposed. Kaplan–Meier analysis and log-rank comparisons among groups consider censored mice to be lost from follow-up on the day at which they were removed from the longevity protocol. There were no mice remaining alive at the time of the analyses reported here.
Unless stated otherwise, all significance tests about survival effects are based upon the two-tailed log-rank test at p < 0.05, stratified by test site, with censored mice included up until their date of removal from the longevity population. Other statistical tests are described in the text; all p values were two tailed.
Results
Effects of Treatments on Body Weights
Figure 1 shows the effects of treatments on body weights during aging, pooled across sites. Mean body weights in curcumin-treated females were significantly less than controls at 6 months (p < .001), 12 months (p < .0001), 18 months, p < .0003) and 24 months (p < .002). There was no significant effect of curcumin treatment on male body weights at any age. Mean body weights were significantly higher in both female (p < .001) and male (p < .02) GTE-treated mice at 24 months of age only. Mean body weights in MCTO-treated females were significantly less than controls at 6 months (p < .02), 12 months (p < .0001), 18 months, p < .0001), and 24 months (p < .004). There was no significant effect of MCTO treatment on male body weights at any age. There was a significant (p = .04) decrease in body weight of RES-treated female mice at 12 months of age only. There were no significant effects of RES on male body weights at any age. There were no significant effects of OAA on male or female body weights at any age.
Figure 1.
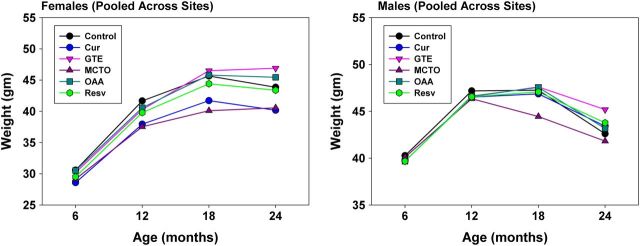
Effect of treatments on body weights at different ages, pooled across sites. Data are expressed as the mean ± SEM of the following number of animals. The number of animals in each age and treatment group was 6 months females—controls = 266, treatment groups = 125–132; 6 months males—controls = 285, treatment groups = 153–157; 12 months females—controls = 268, treatment groups = 126–131; 12 months males—controls = 260, treatment groups = 131–142; 18 months females—controls = 257, treatment groups = 155–129; 18 months males—controls = 217, treatment groups = 115–121; 24 months females—controls = 216, treatment groups =102–111; 18 months males—controls = 158, treatment groups = 86–97.
Survival Analysis
Figure 2 shows survival curves for female mice, pooled across test sites, comparing controls to animals treated with each of the five agents. Figure 2 shows corresponding data from male mice. None of the treatments led to a significant positive or negative effect on life span as evaluated by the log-rank test; the nominal p values, unadjusted for multiple comparisons, ranged from p = .3 to p = .9. Nor did any of the test agents lead to a significant life span extension when data at each site were examined separately (data not shown). Table 1 presents medians and 95% confidence limits for each of the treatment groups, pooled across sites, for each gender.
Figure 2.
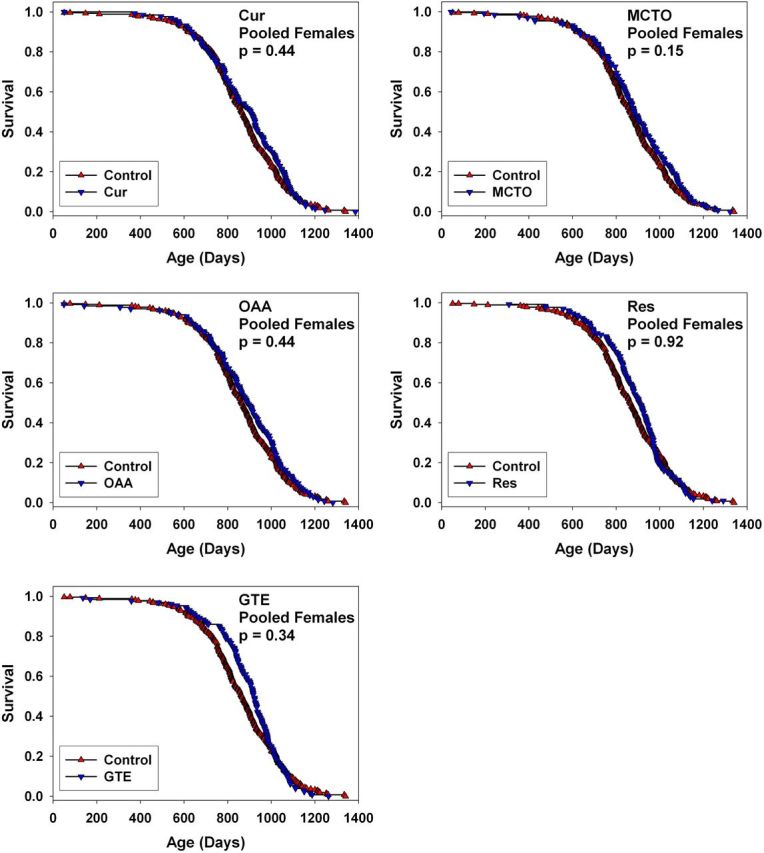
Survival curves for female UM-HET3 mice treated with curcumin, medium-chain length triglyceride oil (MCTO), oxaloacetic acid (OAA), resveratrol (RES), or green tea extract (GTE) from 4 months of age, pooled across the three test sites. Each p > .15 by log-rank test.
Table 1.
Median Survival for Each Intervention, Pooled Across Sites, for Each Gender
| Group | Males | Females |
| Controls | 786 (742–826) | 866 (832–891) |
| Curcumin | 808 (744–838) | 905 (836–933) |
| Green tea extract | 822 (769–880) | 923 (887–939) |
| Medium-chain triglyceride oil | 777 (728–851) | 882 (847–930) |
| Oxaloacetic acid | 819 (756–857) | 889 (854–937) |
| Resveratrol | 813 (763–863) | 907 (871–938) |
Notes: Values given are median age in days, with 95% confidence interval in parentheses. Number of male mice per group ranged from 153 to 158 for experimental groups; N = 294 controls. Number of female mice ranged from 129 to 136 for experimental groups; N = 281 female controls.
GTE Effects on Mid-Life Survival in Females
Inspection of the survival curves for mice exposed to GTE (Figure 3) suggested the possibility that GTE might diminish mortality risks in midlife (ie, roughly 600–800 days of age). To evaluate this idea, we conducted a secondary analysis using the Wilcoxon–Breslow test, which gives greater weight to early-life mortality deaths than to those at later ages. Pooling across sites, this test provided evidence for a beneficial effect of GTE in females at p = .03 (females only, stratified by site, two sided). Inspection of the survival curves for female mice at each site (Figure 4) suggested that this effect was similar at each test site, although none of these single-site data sets showed a statistically significant effect, considered individually. The Wilcoxon–Breslow test did not give evidence of any significant effect of GTE in the male mice, either pooled across sites or in any site taken separately.
Figure 3.
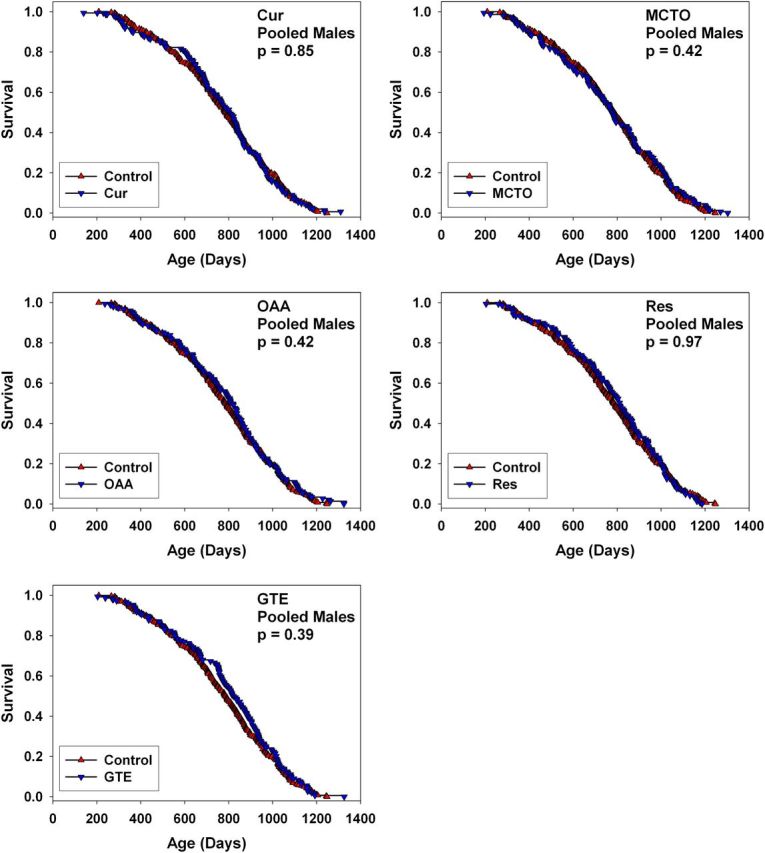
Survival curves for male UM-HET3 mice treated with curcumin, medium-chain length triglyceride oil (MCTO), oxaloacetic acid (OAA), resveratrol (RES), or green tea extract (GTE) from 4 months of age, pooled across the three test sites. Each p > .3 by log-rank test.
Figure 4.
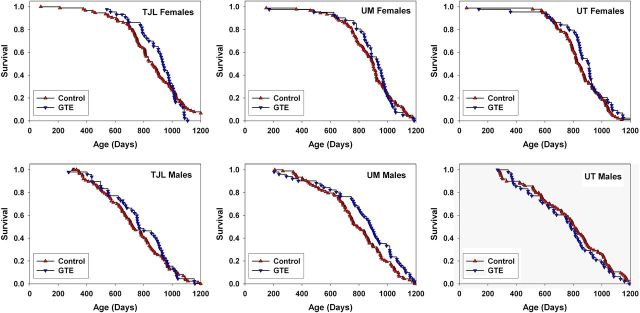
Site-specific survival for male and female UM-HET3 mice treated with green tea extract (GTE) from 4 months of age.
GTE Effects on Locomotor Activity
We also examined the effect of GTE on locomotor activity in 18-month-old mice to determine whether it improved this measure of health span. Pooling data across sites, there was no significant difference in locomotor activity between control and GTE-treated mice (control: 45920 ± 1484; GTE: 46137 ± 1441; mean ± standard error of the mean [SEM]). There were also no significant differences in locomotor activity between control and GTE-treated mice at any one site (TJL: control: 39420 ± 1744; GTE: 43133 ± 1812. UM: control: 55198 ± 3432; GTE: 52344 ± 3003. UT: control: 44193 ± 1785; GTE: 43261 ± 2086).
OAA Stability Study
We also evaluated the idea that the lack of OAA effect on life span might represent degradation of OAA during or after its incorporation into chow. Mouse chow was prepared at the nominal concentration of 2200 mg of OAA per kilogram of food. Samples of food were first sent to a commercial laboratory (CanTest, Burnaby, BC, Canada). This food had been stored at 0–4°C prior to shipping. No detectable OAA (ie, less than 5 ppm) was measured. A second set of OAA–chow samples were tested by one of us (M.J.), according to procedures described in the methods section, after storage at TJL or UM for various periods of time. The two freshest samples, stored at 4°C for 2–5 weeks prior to freezing (−80°C), contained OAA at 555 and 479 ppm, respectively. A sample that had been stored for 22 weeks at 4°C was found to contain OAA at 281 ppm and a sample stored for 28 weeks at 4°C contained OAA at 164 ppm. OAA levels in control chow were undetectable. Since fresh food was produced every 17 weeks, we infer that our measurements indicate that mice in our study consumed food containing OAA at concentrations of no more than 250–560 ppm throughout the study and possibly negligible amounts.
Comparisons of Survival and Body Weight Data of Untreated Control Groups to Previous ITP Cohorts
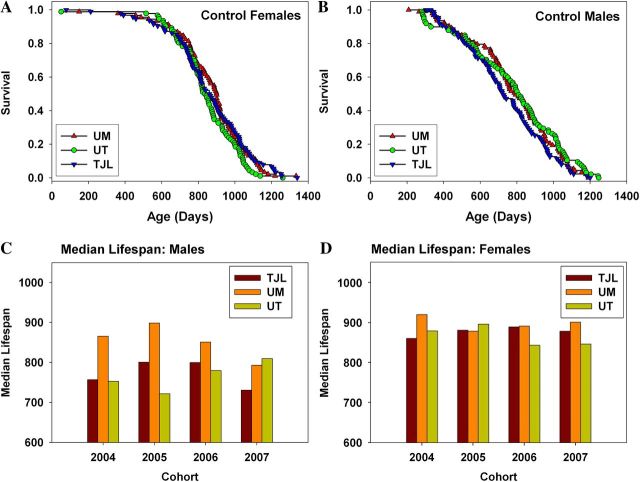
As reported previously, for ITP cohorts born in 2004, 2005, and 2006, there was significant variation among the three test sites in the diets provided to pregnant and nursing breeders and to weanlings up until the age at which drug-containing food was supplied to the test mice (10). Each site used a commercial diet based on the NIH-31 standard, but there was some variation in the components used by the different vendors. We noted a significant difference (p = .0001) in survival of untreated male mice among the three test sites in the 2004–2006 cohorts, with UM males living longer than males at either of the other two sites (8). We found no difference in survival of female mice among sites. These sex-specific disparities in survival of control mice were seen in each of three consecutive annual cohorts and thus clearly reflected replicable disparities in one or more aspects of husbandry, water quality, commensal infections, or other environmental variables among the three test sites, with dietary differences a leading hypothesis. Indeed, we noted site-specific differences in the weight trajectory of control mice (7), with both male and female mice at UM lighter in weight than mice at either of the other two sites and speculated that these site effects might also reflect differences in the composition of food provided during development and early adult life.
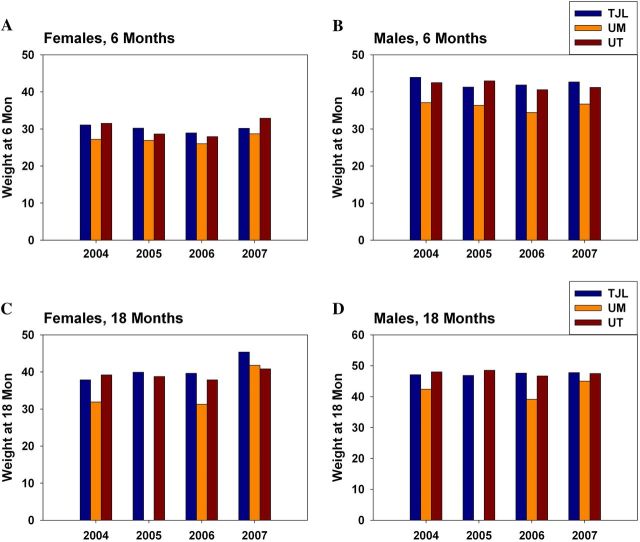
In the current cohort, we modified the protocol to eliminate differences in dietary composition. All three sites used the same diet formulation for breeders, nursing mothers, and weanlings, following the diet regimen used in previous years at TJL, to see if this would provide greater uniformity among sites in male survival curves and body weight trajectories. The disparity among sites in survival of control males (Figure 5b) has been eliminated by diet standardization. As in previous years, there are no significant differences in survival of control females (Figure 5a and d). We note, however (Figure 5c), that the newly standardized regime reduced average survival of UM males compared with earlier cohorts, instead of improving survival of males at UT and TJL. Figure 6 shows a historical comparison of mean body weights measured at 6 and 18 months of age, pooled across sites, for the first 4 years of this collaborative program. In the first three cohorts, UM males and females were lighter than controls at the other two sites. In Cohort 2007, the site difference for females is now all but eliminated. Additionally, the site effect on males, though still apparent, is smaller than in previous years. The comparison shown in Figures 5c and 6 must be interpreted cautiously because it involves historical data sets developed in separate annual cohorts, rather than simultaneous controls.
Figure 5.
Comparison of site-specific survival. (A) Female controls in cohort 2007. (B) Male controls in cohort 2007. (C) Historical comparison of median life spans of males from all cohorts. (D) Historical comparison of median life spans of females from all cohorts.
Figure 6.
Historical comparison of site-specific body weights. (A) Female controls at 6 months. (B) Male controls at 6 months. (C) Female controls at 18 months. (D) Male controls at 18 months of age.
Discussion
Interest in the possibility that RES may retard aspects of the aging process in mice has stimulated considerable research. Studies in yeast, flies, and nematodes suggested that longevity could be increased by modulating levels of members of the sirtuin family of proteins (13,22,23). Further interest in RES was spurred by the report that it prolonged life span in the fish N. furzeri (12), thus extending the results in invertebrate models to a vertebrate species. On the other hand, others have disputed the sirtuin dependence of life span extension in C. elegans and failed to detect an effect in Drosophila melanogaster, demonstrating that life-span extension in response to RES is very protocol dependent (24). RES was reported to extend median life span of mice made obese by feeding a high fat diet (13). In a subsequent study, C57BL/6JNia mice were treated with high fat and control diets containing multiple doses of RES, up to 200 mg/kg body weight/day, beginning at 12 months of age (14). RES increased longevity of mice made obese by feeding a high fat diet, but not of mice-fed control diets and the effect on the high-fat–fed mice was attributed primarily to alleviation of congestion and edema in the thoracic cavity associated with extreme hepatosteatosis (14). We recently confirmed the absence of a life-span effect in genetically heterogeneous UM-HET3 mice, reporting that feeding RES at doses of 50 and 200 mg/kg/day, beginning at 12 months did not extend life span, although these doses led to detectable levels of RES in blood at each dose (11). In the report by Pearson and colleagues (14), it was speculated that because the mice were a year old before treatment started, the increased age of the animals at the start of the experiment might have diminished the potential effects of RES on life span in mice on a standard chow diet. However, in the present study, there was no effect of RES treatment when begun earlier, at 4 months of age. Thus, to date, effects of RES on mouse life span have been seen only in mice subjected to diets so high in fat that the principal cause of death was hepatosteatosis. It is possible that RES may be able to provide protection against the metabolic stresses induced by high-fat diets in mice and may help to delay age-related changes in function that do not modulate longevity in mice on a regular diet.
Curcumin is a polyphenol that is purified from the root of the plant rhizome Curcuma longa, a source of the spice turmeric. It and its major blood metabolite TC have been reported to have a number of anti-aging properties. Curcumin and TC have a broad range of effects that could influence several mechanisms related to aging (25). Curcumin has widely reported anticancer properties reviewed in Frank and colleagues (26) and it has been in testing in human clinical trials for colon cancer (27). It has also been reported to be protective in mouse and rat models of Alzheimer’s disease (28,29). In rat models of diabetes, curcumin has been reported to reduce advanced glycation end product accumulation and serum lipid peroxidation and to inhibit formation of cataracts (30,31). It has also been reported to lower blood lipid peroxides, total cholesterol, and LDL cholesterol and to raise HDL cholesterol in mice and humans (32,33). It has been reported that male F344/N rats fed curcumin (2000 ppm in diet) from weaning had a 10% greater probability of survival between 75 and 100 weeks (34). Similarly, it was reported that male C57BL/6NNia mice, fed TC the primary blood metabolite of curcumin, at a concentration of 2000 ppm in diet starting at 13 months, had an 11.7% greater mean life span as compared with mice fed the control diet, with a 6.5% extension of the age at which 90% of the mice had died (16). There was no significant effect of TC in mice in which treatment began at 19 months of age (16). In aggregate, these findings were highly suggestive that curcumin would extend life span in our mouse model. The intended concentration of curcumin used in the present study (2000 ppm in the diet) and the method of delivery (in the diet) were the same as those used in previous life-span studies. The actual concentration of curcumin measured in randomly chosen pellets in the present study was reasonably close to 2000 ppm, that is, 1682 ± 126 ppm (data not shown). Moreover, in the present study, curcumin reduced body weights of female mice at all ages. This indicates that at this dose, curcumin has a biological effect, at least in females. Given its reported effects on extending life span in both rats and mice, and the broadly anti-aging effects of curcumin, it is not clear why it had no effect on life span in UM-HET3 mice at any of the three ITP sites.
Like curcumin, green tea polyphenols have been reported to have a number of health benefits (35). GTE has been reported to provide protection in animal models of Alzheimer’s disease and Parkinson disease and is reportedly protective against diseases that appear to share oxidative stress as an etiology, such as atherosclerosis and coronary heart disease (35). Green tea also has been reported to be a cancer chemopreventive and to have anti-angiogenic effects (36). These health benefits of green tea are mainly attributed to antioxidant properties and the ability of some constituents to scavenge reactive oxygen species (18,37). Indeed, green tea catechins, particularly epigallocatechin-3-gallate, have been reported to induce antioxidant enzymes including glutathione peroxidase, catalase, and glutathione S-transferase (38). Green tea intake in humans has also been reported to increase plasma antioxidant capacity (39). Luczaj and coworkers (40) reported that GTE, administered in drinking water, protected against age-dependent ethanol-induced oxidative stress in rats. Moreover, green tea polyphenols administered in drinking water (80 mg/L) to mice beginning at 13 months of age, but not at 19 months, were reported to increase life span in C57BL/6NNia mice (16). The average life span was significantly longer than in the control mice, although the age at which the groups reached 10% survival was not significantly affected (16). These authors speculated that larger increases in life span might have been seen if the treatment had begun earlier than 13 months of age. The lack of effect in the current study cannot be attributed to starting the treatment at a late age because treatment of the mice was begun at 4 months of age. Moreover, GTE increased body weights of both male and female mice at 24 months of age, indicating an effect at the dose used, if only at that one age. It is possible that the disparity in the effect on life span in this study and the previous study by Kitani and coworkers could be because of differences in the many constituents found in green tea used previously and in this study or because of the peculiarities of the C57BL/6 strain of mice used in the earlier work. Quality control and batch consistency are always a concern when testing extracts, and it is possible that not all components with biological activity, both positive and negative, have been identified in GTE or are equally stable in diet preparations. Alternatively, the use of genetically heterogeneous mice versus an inbred strain may underlie the different responses seen in this study compared with earlier ones.
The secondary analysis of GTE-treated mice, using the Wilcoxon–Breslow test, must be interpreted very cautiously. Pooling across sites, this test indicated a significant effect of GTE in females at a two-sided criterion of p = .03. The primary analytical plan for the ITP is to evaluate survival effects using the log-rank test, which assumes that intergroup differences in mortality risk are the same at each age. The Wilcoxon–Breslow test gives greater emphasis to early deaths and is thus particularly suited to situations in which early events have greater importance that later ones. Since it is unclear, and controversial, to what extent early deaths give information about the biology of aging and age-dependent diseases, we consider the results of the Wilcoxon–Breslow test to be of less general value than those of the log-rank test. In addition, we note the well-established inflation of Type I error rate when analytical methods are selected after inspection of the data, as in this case, and the further risk of Type I errors in a situation involving multiple comparisons (in this case five agents in each of two sexes). Nonetheless, the pattern of survival in the GTE-treated females, and in the males at TJL and UM, are consistent with the idea that this agent might reduce mortality rate at early ages while at the same time increasing it at later ages. On the other hand, measures of locomotor activity as an indicator of health span did not show any differences between GTE-treated groups and controls. Further studies, in which GTE is provided to young and middle-aged adults but then removed at later ages, might well be justified by these initial observations.
Medium-chain length triglyceride oil (MCTO) is a mixture of triglycerides composed of fatty acids with a chain length of 6–10 carbons. Use of MCTO for weight control has been investigated in both animals and humans. In general, feeding MCTO results in less weight gain reduces serum leptin concentration and enhances insulin sensitivity (19,41,42). It has been reported that MCTO diets improve performance of patients with Alzheimer’s disease and that it inhibits liver and intestinal inflammation (43,44). Since no previous studies reported on the effects of MCTO on life span, MCTO was tested in the present study to determine its effects on life span. Despite its many reported health benefits, there was no statistically significant effect of MCT on median or maximum life span in the current study.
Oxaloacetate (OAA) is an intermediary in oxidative metabolism. It was hypothesized that an excess of oxaloacetate may effectively mimic calorie restriction by raising the NAD+/NADH ratio (21). It has been reported that oxaloacetate increases both median and maximal life span in C. elegans in a dose-dependent manner, an effect that requires the DAF-16 transcription factor and the nutrient sensing AMPK gene (21). In the present study, we evaluated whether OAA could increase life span in mice under the ITP protocol. No indication of a life-span benefit in either sex was found. However, we also determined that OAA is quite labile under the storage conditions used, such that the amount of OAA received by the mice was no more than 25% of the intended amount.
Funding
This work was supported by the National Institute on Aging at the National Institutes of Health (U01-AG022303 to R.A.M., U01-AG025707 and U01-AG022308 to D.E.H., AG13925 to J.L.K., and U01-AG022307 and P30-AG13319 to R.S.) and the Department of Veterans Affairs (R.S.). R.d.C. is supported by the Intramural Research Program of the National Institute on Aging.
Acknowledgments
We wish to thank Elizabeth Adler, Lisa Burmeister, Vivian Diaz, Sabrina Friedline, Melissa Han, Patricia J. Harrison, Bill Kohler, Pamela J. Krason, and Bee Stork for reliable technical assistance.
References
- 1.Miller RA. Extending life: scientific prospects and political obstacles. Milbank Q. 2002;80(1):155–174. doi: 10.1111/1468-0009.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Olshansky SJ, Carnes BA, Cassel C. In search of Methuselah: estimating the upper limits to human longevity. Science. 1990;250(4981):634–640. doi: 10.1126/science.2237414. [DOI] [PubMed] [Google Scholar]
- 3.Olshansky SJ. Commentary: prescient visions of public health from Cornaro to Breslow. Int J Epidemiol. 2006;35(1):22–23. doi: 10.1093/ije/dyi283. [DOI] [PubMed] [Google Scholar]
- 4.Archer JR, Harrison DE. L-Deprenyl treatment in aged mice slightly increases life spans, and greatly reduces fecundity by aged males. J Gerontol A Biol Sci Med Sci. 1996;51(6):B448–B453. doi: 10.1093/gerona/51a.6.b448. [DOI] [PubMed] [Google Scholar]
- 5.Schneider EL, Reed JD., Jr Life extension. N Engl J Med. 1985;312(18):1159–1168. doi: 10.1056/NEJM198505023121805. [DOI] [PubMed] [Google Scholar]
- 6.Cotzias GC, Miller ST, Tang LC, Papavasiliou PS. Levodopa, fertility, and longevity. Science. 1977;196(4289):549–551. doi: 10.1126/science.850799. [DOI] [PubMed] [Google Scholar]
- 7.Miller RA, Harrison DE, Astle CM, et al. An Aging Interventions Testing Program: study design and interim report. Aging Cell. 2007;6(4):565–575. doi: 10.1111/j.1474-9726.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- 8.Strong R, Miller RA, Astle CM, et al. Nordihydroguaiaretic acid and aspirin increase lifespan of genetically heterogeneous male mice. Aging Cell. 2008;7(5):641–650. doi: 10.1111/j.1474-9726.2008.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nadon NL, Strong R, Miller RA, et al. Design of aging intervention studies: the NIA Interventions Testing Program. Age (Dordr) 2008;30(4):187–199. doi: 10.1007/s11357-008-9048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison DE, Strong R, Sharp ZD, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460(7253):392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miller RA, Harrison DE, Astle CM, et al. Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J Gerontol A Biol Sci Med Sci. 2011;66(2):191–201. doi: 10.1093/gerona/glq178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Valenzano DR, Terzibasi E, Genade T, Cattaneo A, Domenici L, Cellerino A. Resveratrol prolongs lifespan and retards the onset of age-related markers in a short-lived vertebrate. Curr Biol. 2006;16(3):296–300. doi: 10.1016/j.cub.2005.12.038. [DOI] [PubMed] [Google Scholar]
- 13.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pearson KJ, Baur JA, Lewis KN, et al. Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 2008;8(2):157–168. doi: 10.1016/j.cmet.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kitani K, Yokozawa T, Osawa T. Interventions in aging and age-associated pathologies by means of nutritional approaches. Ann N Y Acad Sci. 2004;1019:424–426. doi: 10.1196/annals.1297.075. [DOI] [PubMed] [Google Scholar]
- 16.Kitani K, Osawa T, Yokozawa T. The effects of tetrahydrocurcumin and green tea polyphenol on the survival of male C57BL/6 mice. Biogerontology. 2007;8(5):567–573. doi: 10.1007/s10522-007-9100-z. [DOI] [PubMed] [Google Scholar]
- 17.Salah N, Miller NJ, Paganga G, Tijburg L, Bolwell GP, Rice-Evans C. Polyphenolic flavanols as scavengers of aqueous phase radicals and as chain-breaking antioxidants. Arch Biochem Biophys. 1995;322(2):339–346. doi: 10.1006/abbi.1995.1473. [DOI] [PubMed] [Google Scholar]
- 18.Pietta PG. Flavonoids as antioxidants. J Nat Prod. 2000;63(7):1035–1042. doi: 10.1021/np9904509. [DOI] [PubMed] [Google Scholar]
- 19.Han J, Hamilton JA, Kirkland JL, Corkey BE, Guo W. Medium-chain oil reduces fat mass and down-regulates expression of adipogenic genes in rats. Obes Res. 2003;11(6):734–744. doi: 10.1038/oby.2003.103. [DOI] [PubMed] [Google Scholar]
- 20.Bluher M, Kahn BB, Kahn CR. Extended longevity in mice lacking the insulin receptor in adipose tissue. Science. 2003;299(5606):572–574. doi: 10.1126/science.1078223. [DOI] [PubMed] [Google Scholar]
- 21.Williams DS, Cash A, Hamadani L, Diemer T. Oxaloacetate supplementation increases lifespan in Caenorhabditis elegans through an AMPK/FOXO-dependent pathway. Aging Cell. 2009;8(6):765–768. doi: 10.1111/j.1474-9726.2009.00527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5(6):493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 23.Wood JG, Rogina B, Lavu S, et al. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- 24.Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128(10):546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Maheshwari RK, Singh AK, Gaddipati J, Srimal RC. Multiple biological activities of curcumin: a short review. Life Sci. 2006;78(18):2081–2087. doi: 10.1016/j.lfs.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 26.Frank N, Knauft J, Amelung F, Nair J, Wesch H, Bartsch H. No prevention of liver and kidney tumors in Long-Evans Cinnamon rats by dietary curcumin, but inhibition at other sites and of metastases. Mutat Res. 2003;523–524:127–135. doi: 10.1016/s0027-5107(02)00328-7. [DOI] [PubMed] [Google Scholar]
- 27.Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res. 2004;10(20):6847–6854. doi: 10.1158/1078-0432.CCR-04-0744. [DOI] [PubMed] [Google Scholar]
- 28.Frautschy SA, Hu W, Kim P, et al. Phenolic anti-inflammatory antioxidant reversal of Abeta-induced cognitive deficits and neuropathology. Neurobiol Aging. 2001;22(6):993–1005. doi: 10.1016/s0197-4580(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 29.Lim GP, Chu T, Yang F, Beech W, Frautschy SA, Cole GM. The curry spice curcumin reduces oxidative damage and amyloid pathology in an Alzheimer transgenic mouse. J Neurosci. 2001;21(21):8370–8377. doi: 10.1523/JNEUROSCI.21-21-08370.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kumar PA, Suryanarayana P, Reddy PY, Reddy GB. Modulation of alpha-crystallin chaperone activity in diabetic rat lens by curcumin. Mol Vis. 2005;11:561–568. [PubMed] [Google Scholar]
- 31.Suryanarayana P, Saraswat M, Mrudula T, Krishna TP, Krishnaswamy K, Reddy GB. Curcumin and turmeric delay streptozotocin-induced diabetic cataract in rats. Invest Ophthalmol Vis Sci. 2005;46(6):2092–2099. doi: 10.1167/iovs.04-1304. [DOI] [PubMed] [Google Scholar]
- 32.Soni KB, Kuttan R. Effect of oral curcumin administration on serum peroxides and cholesterol levels in human volunteers. Indian J Physiol Pharmacol. 1992;36(4):273–275. [PubMed] [Google Scholar]
- 33.Soudamini KK, Unnikrishnan MC, Soni KB, Kuttan R. Inhibition of lipid peroxidation and cholesterol levels in mice by curcumin. Indian J Physiol Pharmacol. 1992;36(4):239–243. [PubMed] [Google Scholar]
- 34.Alden CJ. Toxicology and Carcinogenesis Studies of Turmeric Oleoresin (Major component 79%–85% curcumin) in F344/N Rats and B6C3F1 Mice. Washington, DC: U.S. Dept. of Health and Human Services, Public Health Service, National Institutes of Health; 1993. National Toxicology Program Technical Report Series No. 427. [Google Scholar]
- 35.Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78(18):2073–2080. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer—part 1. Curr Oncol. 2006;13(1):14–26. doi: 10.3747/co.v13i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang JA, Choi JH, Rhee SJ. Effects of green tea catechin on phospholipase A2 activity and antithrombus in streptozotocin diabetic rats. J Nutr Sci Vitaminol (Tokyo) 1999;45(3):337–346. doi: 10.3177/jnsv.45.337. [DOI] [PubMed] [Google Scholar]
- 38.Khan SG, Katiyar SK, Agarwal R, Mukhtar H. Enhancement of antioxidant and phase II enzymes by oral feeding of green tea polyphenols in drinking water to SKH-1 hairless mice: possible role in cancer chemoprevention. Cancer Res. 1992;52(14):4050–4052. [PubMed] [Google Scholar]
- 39.Kimura M, Umegaki K, Kasuya Y, Sugisawa A, Higuchi M. The relation between single/double or repeated tea catechin ingestions and plasma antioxidant activity in humans. Eur J Clin Nutr. 2002;56(12):1186–1193. doi: 10.1038/sj.ejcn.1601471. [DOI] [PubMed] [Google Scholar]
- 40.Luczaj W, Waszkiewicz E, Skrzydlewska E, Roszkowska-Jakimiec W. Green tea protection against age-dependent ethanol-induced oxidative stress. J Toxicol Environ Health A. 2004;67(7):595–606. doi: 10.1080/15287390490425579. [DOI] [PubMed] [Google Scholar]
- 41.Crozier G, Bois-Joyeux B, Chanez M, Girard J, Peret J. Metabolic effects induced by long-term feeding of medium-chain triglycerides in the rat. Metabolism. 1987;36(8):807–814. doi: 10.1016/0026-0495(87)90122-3. [DOI] [PubMed] [Google Scholar]
- 42.St Onge MP, Ross R, Parsons WD, Jones PJ. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res. 2003;11(3):395–402. doi: 10.1038/oby.2003.53. [DOI] [PubMed] [Google Scholar]
- 43.Kono H, Fujii H, Asakawa M, et al. Protective effects of medium-chain triglycerides on the liver and gut in rats administered endotoxin. Ann Surg. 2003;237(2):246–255. doi: 10.1097/01.SLA.0000048450.44868.B1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reger MA, Henderson ST, Hale C, et al. Effects of beta-hydroxybutyrate on cognition in memory-impaired adults. Neurobiol Aging. 2004;25(3):311–314. doi: 10.1016/S0197-4580(03)00087-3. [DOI] [PubMed] [Google Scholar]