Abstract
Background.
Older adults with depression have an increased risk of developing dementia. Low plasma beta-amyloid 42 (Aβ42) and Aβ42/Aβ40 have emerged as promising biomarkers of dementia. The association between depression and plasma Aβ is unclear.
Methods.
In this longitudinal study of 988 community-dwelling elders from the Health Aging and Body Composition study, depression was assessed with the Center for Epidemiologic Studies-Depression Scale 10-item version. We determined the association between Aβ42 and Aβ42/Aβ40 tertile and depression at baseline and over 9 years. We also stratified the models to determine if apolipoprotein E e4 allele status modified the associations.
Results.
Mean baseline age was 74.0 ± 3.0 years, 51 (5.2%) participants had depression, 545 (55.2%) were women, 531 (53.7%) were black, and 286 (30.7%) had one or more apolipoprotein E e4 allele. At baseline, there was no association between Aβ42/Aβ40 or Aβ42 and depression. Over 9 years, 220 (23.5%) participants developed depression. In adjusted Cox proportional hazards models, among those with one or more e4 allele, low Aβ42/Aβ40 was associated with an increased risk of developing depression over time (low 10.8% vs high 3.2%, hazard ratio = 2.38, 95% confidence interval: 1.15–4.92). Among those with no e4 allele, there was no association between Aβ42/Aβ40 and risk of depression over time (13.3% vs 17.5%, hazard ratio = 0.80, 95% confidence interval: 0.52–1.23; p value for interaction = .003).
Conclusions.
The association between low plasma Aβ42/Aβ40 and increased risk of incident depression among those with one or more apolipoprotein E e4 allele implies a synergistic relationship similar to that found with dementia. Future work should investigate the interrelationships among plasma Aβ42/Aβ40, depression, and dementia.
Keywords: Depression, Epidemiology, Plasma beta amyloid
Depression in older adults is associated with an increased risk of cognitive decline, dementia, and mild cognitive impairment (1,2). Although the mechanisms are not fully understood, it has been shown that a history of depression is associated with increased development of amyloid plaques and neurofibrillary tangles—two hallmarks of Alzheimer’s disease (AD; 3–5).
Several studies have indicated that there may be a relationship between plasma Aβ42 and Aβ42/Aβ40 and depression, but these results have been inconclusive (6–8). For example, an association between high plasma Aβ40/Aβ42 (consistent with low Aβ42/Aβ40) and increased risk of depression has been reported (8). Whereas low plasma Aβ42 has also been associated with an increased risk for depression (5,9), others have reported a negative association (6,7). One cross-sectional study also reported an interaction between Aβ, depression, and apolipoprotein E (APOE) e4 allele status, a major genetic risk factor for AD (9). Plasma Aβ42 and Aβ42/Aβ40 have previously been shown to be promising biomarkers for cognitive decline and AD; this is of interest because the plasma levels appear to fluctuate in parallel with cerebrospinal fluid levels but are obtained in a much less invasive manner (10–12).
As prior studies have been inconclusive and limited by small sample size and cross-sectional design, we sought to prospectively investigate the association between plasma Aβ42 and Aβ42/Aβ40 and incident depression in a large cohort of older adults. A second objective was to determine if APOE e4 allele status modified the association between Aβ level and depression. We hypothesized that those with low Aβ42 and Aβ42/Aβ40 would have increased risk for depression and that these associations would be stronger among those with at least one APOE e4 allele.
METHODS
Study Population
Participants were enrolled in the ongoing Health Aging and Body Composition (Health ABC) study. This prospective cohort of 3,075 community-dwelling white and black older adults began in 1997. At baseline, participants were 70–79 years of age and lived in Memphis, Tennessee, or Pittsburgh, Pennsylvania. Participants were recruited from a random sample of Medicare eligible adults living within designated zip codes and were eligible if they reported no difficulties performing activities of daily living, walking a quarter mile, or climbing 10 steps without resting. They also had to be free of life-threatening cancers and plan to remain within the study area for at least 3 years (13).
From the original cohort, a random sample of 999 sex- and race-stratified Health ABC participants who had baseline and at least one other cognitive measure over time had plasma Aβ42 and Aβ40 measured from stored plasma at Year 2. Our analytic cohort consisted of 988 participants with plasma Aβ measured and who had baseline plus at least one other depression measure over time. All participants included in this analytic cohort were free of cognitive impairment at baseline; consistent with previous literature, cognitive impairment was defined as a Modified Mini-Mental Status Exam (3MS) score more than 80 (14).
Standard Protocol Approvals, Registrations, and Patient Consents
This study was approved by the institutional review boards of the University of Pittsburgh and the University of Tennessee, Memphis, and that of the coordinating center, the University of California San Francisco. All participants signed a written informed consent.
Beta Amyloid
Stored plasma obtained in Year 2 of Health ABC was used to measure Aβ40 and Aβ42. Plasma was stored at −70°C at Fisher BioServices, Inc. Laboratories and shipped directly to the analytical laboratory. Plasma Aβ was measured at the laboratory of Dr. Steven Younkin at the Mayo Clinic using Innogenetics INNO-BIA assays (12). The detection limit for this assay is 12 pg/mL for Aβ40 and 5 pg/mL for Aβ42. Mean inter-assay coefficient of variation was 9.9% for Aβ40 and 9.3% for Aβ42, and mean intra-assay coefficient of variation was 3.5% for Aβ40 and 2.3% for Aβ42. We categorized Aβ42 and Aβ42/Aβ40 into “low,” “medium,” and “high” tertile groups, consistent with our previous study investigating plasma Aβ and cognitive decline among older adults (12).
Depression
The full 20-item Center for Epidemiologic Studies-Depression Scale (CES-D) was used to assess depressive symptoms at baseline, with scores for the CES-D-10 calculated. The CES-D-10 was used to assess depressive symptoms within the previous week at all follow-up visits (Years 3, 5, 8, and 10) (15). The 10-item version is a shortened version of the original 20-item scale, has been widely used in older populations, and has good validity and reliability (15,16). The shortened scale ranges from 0 to 30 and has good predictive accuracy when compared with the original 20-item scale (17). Previous literature has shown that clinically significant depressive symptoms are indicated by a score more than or equal to 10, which for the purposes of this manuscript will be referred to as depression (17,18). Incident depression over 9 years was defined as the first occurrence of depression from Years 1 to 10 (a score of ≥10).
Covariates
At baseline, demographic information gathered from participants included age, race, sex, and education. Prevalent disease algorithms based on both self-report and physician diagnoses, recorded medications and laboratory data were used to create comorbidity variables indicating presence of diabetes mellitus, hypertension, stroke or transient ischemic attack, and myocardial infarction. Body mass index (kg/m2) was calculated from direct height and weight measurements at baseline. Global cognitive function was measured with the 3MS. Incident dementia was defined as a self-reported previous diagnosis of dementia from a physician or prescription of any medication used to treat the symptoms of dementia, including: Aricept, Galantamine (Razadyne), Rivastigmine (Exelon), Memantine (Namenda), and Tacrine. Cystatin C was measured using baseline plasma stored at −70°C at the Health ABC core laboratory (University of Vermont, Burlington), using a BNII nephelometer (Dade Behring, Deerfield, Illinois), which used a particle-enhanced immunonepholometric assay (N Latex Cystatin C; 19). APOE e4 allele status was determined using standard single-nucleotide polymorphism genotyping techniques and dichotomized into having at least one APOE e4 allele versus having no APOE e4 allele (20).
Statistical Analyses
Pearson chi-square and analysis of variance tests were used, as appropriate, to determine the association between Aβ42 and Aβ42/Aβ40 tertile and baseline participant characteristics and depression at baseline. After excluding the 51 participants who had depression at baseline, unadjusted Cox proportional hazards models were used to determine the association between Aβ42 and Aβ42/Aβ40 and risk of incident depression over time. In order to determine if APOE e4 allele status modified the association between Aβ42 or Aβ42/Aβ40 and depression, we assessed for an interaction, and if the interaction term was significant (p < .05), we stratified the models by APOE e4. Low, medium, and high tertile of Aβ42/Aβ40 (also Aβ42) were compared with the highest tertile as the reference group. Models were also adjusted by all baseline characteristics that were significantly associated with Aβ level at baseline (p ≤ .10), including race, education, diabetes, and baseline 3MS. In order to determine if the association between Aβ42/Aβ40 and depression was independent of an association between Aβ42/Aβ40 and dementia, sensitivity analyses were performed after excluding 153 participants who developed incident dementia over the study period. All statistical analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina).
RESULTS
The mean age at baseline of the 988 participants was 74.0 ± 3.0 years, 545 participants (55.2%) were women, 531 (53.7%) were black, 51 (5.2%) had depression, and 286 (28.9%) had one or more e4 allele. The mean (±SD) and median plasma Aβ42 were 33.9 ± 9.7 and 32.83 pg/mL, respectively, and for Aβ42/Aβ40 were 0.19 ± 0.07 and 0.18 pg/mL. At baseline, participants in the low Aβ42/Aβ40 tertile were more likely to be black (χ2 = 9.98, df = 2, p = .007), to have diabetes (χ2 = 6.06, df = 2, p = .05), and to have one or more e4 allele (χ2 = 17.37, df = 2, p < .001; Table 1). Similar patterns were observed for Aβ42 (data not shown).
Table 1.
Baseline Characteristics by Aβ42/Aβ40 Tertile Among the 988 Older Adults
| Baseline Characteristics, M (SD) or n (%) | Low, n = 327 | Medium, n = 334 | High, n = 327 | p Value |
| Age, years | 74.1 (3.0) | 74.1 (2.7) | 73.8 (3.0) | .33 |
| Black (%) | 199 (60.9) | 169 (50.6) | 163 (49.9) | .007 |
| Female (%) | 186 (56.9) | 180 (53.9) | 179 (54.7) | .73 |
| Education ≥ high school (%) | 192 (58.7) | 224 (67.7) | 211 (64.5) | .05 |
| Depression (CES-D-10 score ≥ 10; %) | 12 (3.7) | 20 (6.0) | 19 (5.8) | .78 |
| 3MS score | 90.7 (5.4) | 91.4 (5.4) | 91.1 (5.4) | .25 |
| Stroke/TIA (%) | 33 (10.2) | 34 (10.4) | 28 (8.6) | .72 |
| Diabetes (%) | 93 (29.4) | 74 (22.8) | 68 (21.6) | .05 |
| Myocardial infarction (%) | 38 (11.7) | 32 (9.8) | 35 (10.9) | .73 |
| Hypertension (%) | 226 (69.1) | 219 (65.6) | 206 (63.0) | .25 |
| Body mass index (kg/m2) | 27.6 (4.6) | 27.7 (5.0) | 27.8 (5.2) | .79 |
| Cystatin C (mg/L, natural log) | 0.002 (0.3) | −0.01 (0.2) | −0.01 (0.3) | .67 |
| APOE e4 (%) | 118 (38.2) | 98 (31.1) | 70 (22.7) | <.001 |
Note: APOE = apolipoprotein; ECES-D = Center for Epidemiologic Studies-Depression Scale; TIA = transient ischemic attack.
At baseline, 12 (3.7%) participants in the low Aβ42/Aβ40 tertile, 20 (6.0%) in the medium, and 19 (5.8%) in the high tertile had depression. As the interaction between Aβ42/Aβ40 and APOE e4 (χ2 = 9.08, df = 1, p = .003) was significant, models were stratified by APOE e4 allele status. There was no significant association between Aβ42/Aβ40 and depression at baseline among those with (low 1.8% vs medium 2.8%, vs high 1.4%; χ2 = 1.48, df = 2, p = .48) or without (low 0.77% vs medium 1.7%, vs high 2.2%; χ2 = 2.68, df = 2, p = .26) an e4 allele; results for Aβ42 were similar (data not shown).
Over 9 years, 220 (23.5%) participants who did not have depression at baseline developed incident depression; this included 93 participants in Year 3 (9.9%), 67 participants in Year 5 (7.2%), 39 participants in Year 8 (4.3%), and 21 participants in Year 10 (2.2%). In unadjusted Cox proportional hazards models, stratified by APOE e4, those with a low ratio and an e4 allele had an increased risk of depression over time (low n = 34 [10.8%] vs high n = 10 [3.2%], hazard ratio [HR] = 2.14, df = 1, 95% confidence interval [CI]: 1.06–4.34) as compared with those with a high ratio (Figure 1). These results remained significant after adjustment for race, education, diabetes, and baseline 3MS (Table 2). Conversely, among those without an e4 allele, there was no association between low Aβ42/Aβ40 and depression, compared to those with high Aβ42/Aβ40 in unadjusted (Figure 1) or adjusted models (low n = 42 [13.3%] vs high n = 54 [17.5%], HR = 0.80, df = 1, 95% CI: 0.52–1.23; Table 2). There was no significant association between Aβ42 and depression over 9 years in either e4 group (data not shown).
Figure 1.
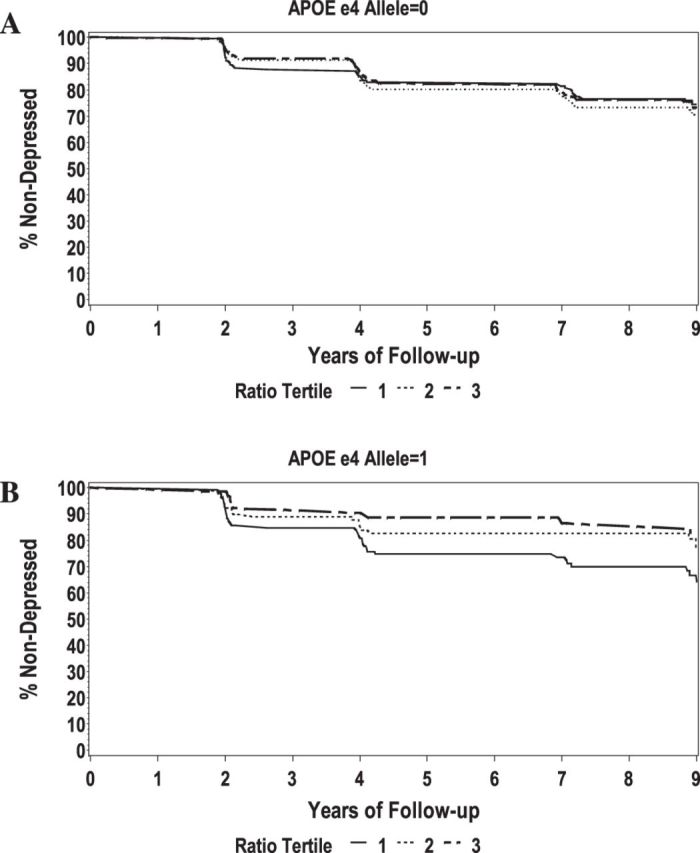
Unadjusted survival curves by Aβ42/Aβ40 tertile, stratified by apolipoprotein E (APOE) e4 allele. (A) No APOE e4 allele and (B) ≥1 APOE e4 allele.
Table 2.
The Adjusted* Association Between Aβ42/Aβ40 and Depression Over Time, Stratified† by APOE e4 Allele Status
| N (%‡) With Depression Over 9 y and Adjusted Hazard Ratio (95% Confidence Interval) | ||||
| No e4 Allele | ≥1 e4 Allele | |||
| Low (n = 315) | 42 (13.3) | 0.80 (0.52–1.23) | 34 (10.8) | 2.38 (1.15–4.92) |
| Medium (n = 314) | 55 (17.5) | 1.08 (0.73–1.61) | 19 (6.1) | 1.57 (0.71–3.47) |
| High (n = 308) | 54 (17.5) | Ref | 10 (3.2) | Ref |
Notes: APOE = apolipoprotein E.
*Adjusted for race, education, diabetes, and baseline 3MS.
p Value for interaction = .003.
Percentages shown are out of the total within in each tertile.
Over the study period, 153 (16.3%) participants developed incident dementia and were excluded in sensitivity analyses. In unadjusted Cox proportional hazards models, stratified by APOE e4, those with a low ratio and an e4 allele had an increased risk of depression over time (low n = 23 [9.0%] vs high n = 5 [1.8%], HR = 3.28, df = 1, 95% CI: 1.25–8.63) as compared with those with a high ratio. These results remained significant after adjustment for race, education, diabetes, and baseline 3MS (HR = 3.88, df = 1, 95% CI: 1.39–10.26). Conversely, among those without an e4 allele, there was no association between low Aβ42/Aβ40 and depression, compared with those with high Aβ42/Aβ40 in unadjusted (low n = 34 [13.3%] vs high n = 46 [16.6%], HR = 0.98, df = 1, 95% CI: 0.63–1.52) or adjusted models (HR = 0.81, df = 1, 95% CI: 0.51–1.30). Again, there was no significant association between Aβ42 and depression over 9 years in either e4 group after excluding those with incident dementia (data not shown).
DISCUSSION
In this prospective cohort study of white and black community-dwelling older adults, those with low Aβ42/Aβ40 and an APOE e4 allele had an increased risk for depression over 9 years; this relationship was not observed for those with low Aβ42/Aβ40 and no e4 allele. This association remained even after excluding participants who developed incident dementia over the study period, indicating that circulating Aβ levels in the periphery may contribute to the pathogenesis of both depression and AD but that they are independent of each other. In another recent Health ABC study, older adults with low plasma Aβ42/Aβ40 were also at an increased risk for cognitive decline over time (12). Together, these results suggest that older adults with low plasma Aβ42/Aβ40 and an APOE e4 allele are likely at an increased risk for both depression and AD.
These results are consistent with previous studies showing those with high Aβ40/Aβ42 (consistent with low Aβ42/Aβ40) have an increased risk of depression (8,9). They further add to existing literature in demonstrating that APOE e4 allele status modifies the association between plasma Aβ42/Aβ40 and depression. It has been suggested that such an interaction may reflect the presence of a subtype of depression that is a prodromal form of AD (9). For example, one cross-sectional study of homebound older adults with no APOE e4 allele found those with depression had lower plasma Aβ42, higher Aβ40/Aβ42, and poorer performance on cognitive tests than those without depression (9). Conversely, older adults with an APOE e4 allele had lower plasma Aβ42 and higher Aβ40/Aβ42 than older adults without an APOE e4 allele, regardless of depression status (9). Our results differed from previous studies in that we found no association between Aβ42 or and depression in older adults at baseline or over time, even after stratifying by APOE e4 allele and excluding those with incident dementia (9). The difference in findings could be due to the cross-sectional nature of the previous studies; differences may also exist due to different study populations (community dwelling with no major functional impairment in Health ABC, compared with homebound older adults in the previous studies; 8,9).
The hypothesis that depression associated with low plasma Aβ42/Aβ40 may represent prodromal dementia postulates that depression in older adults has various pathologies and etiologies, and depression occurring in the earliest preclinical phases of dementia is a particular subtype (8,9). It has been suggested that plasma Aβ42/Aβ40 may be a marker of this subtype of depression because of the previously described associations between plasma Aβ42/Aβ40 and dementia and between plasma Aβ42/Aβ40 and depression (8,10,12,21) Because having an APOE e4 allele is a risk factor for AD, this theory is strengthened if those with APOE e4 allele status have a stronger association between Aβ and depression. Our results support the hypothesis that among those with at least one APOE e4 allele, low Aβ42/Aβ40 is associated with an increased risk of depression and that this depression could be prodromal dementia. This finding is noteworthy because distinguishing the prodromal dementia subtype of depression from other depression in older adults may allow for earlier intervention or cognitive training in those at an increased risk for dementia. However, it also important to note that our results remained significant even after excluding those with incident dementia, indicating that although depression and dementia may have a common underlying pathway, Aβ42/Aβ40 likely exerts some independent effects on depression.
There are several possible mechanisms underlying the association of Aβ42/Aβ40 and depression. For example, high plasma Aβ40/Aβ42 (consistent with low Aβ42/Aβ40) has been associated with amygdala atrophy and decreased total brain volume; both the amygdala and brain volume are implicated in depression and dementia (5). Another possible mechanism underlying the relationship between depression and Aβ42/Aβ40 is the development of amyloid plaques. Although amyloid plaques are a hallmark of AD (22,23), a lifetime history of depression has also been linked with the formation of amyloid plaques in the medial temporal lobe (4). This evidence would further support the idea that an association between plasma Aβ42/Aβ40 and depression is indicative of a prodromal dementia subtype of depression. There are other possible mechanisms underlying the association between depression and plasma Aβ42/Aβ40 that are not directly dementia related, such as hippocampal atrophy (5,24). To explain, it has been reported that high Aβ40/Aβ42 (consistent with low Aβ42/Aβ40) may be related to increased hippocampal atrophy, which in turn is related to increased risk of depression (5,25). Another possible underlying mechanism is stress; chronic stress has been shown to be associated with both depression and increased glucocorticoid levels (24,26). In animal models, chronic glucocorticoid administration was also associated with an increased plasma Aβ42/Aβ40 level, but future studies are needed to investigate if there is also a relationship in humans (27). Finally, another potential mechanism is a shared genetic risk for depression and AD, possibly indicated by APOE e4 status; at least one longitudinal study of older men found that depression is associated with an increased risk of dementia but only among those with APOE e4 (24,28).
This study has several strengths including a prospective study design and a relatively large sample size. Furthermore, depression was measured at multiple time points, and we were able to adjust for a large number of potential confounders. We also repeated analyses including those who were taking an antidepressant medication but who did not have a score consistent with depression on the CES-D-10, and this did not significantly change our results. Furthermore, as Health ABC also had the Geriatric Depression Scale measured at one study visit, we performed sensitivity analyses to see if the association changed for those with depression on this scale, but again, results did not significantly change. Another strength is that Health ABC consists of community-dwelling older adults who were fairly high functioning and likely dementia free at baseline. Because of detailed follow-up data, we were able to exclude participants who developed incident dementia over the 9-year study period, and this is another strength. Finally, Innogenetics INNO-BIA assays were used to measure Aβ42 and Aβ40, which may provide more accurate measurements of these plasma markers than enzyme-linked immunosorbent assays because of the high sensitivity, low variability, and high reproducibility (29).
There are also several weaknesses that should be taken into consideration when interpreting these results. For example, the CES-D-10 measures self-reported depressive symptoms and is not a diagnostic assessment of depression (15). Another weakness is that we only had depressive symptoms measured at discrete study visit time points and did not have information on whether or not depressive symptoms were experienced at times between study visits. Thus, if participants had transient depression between study visits, they may have been missed. However, if the depression associated with low Aβ42/Aβ40 is prodromal dementia, we would expect it to be continuously expressed. This was supported by a sensitivity analysis where we examined the association between Aβ42/Aβ40 and “chronic depression” (those with depression at three or more visits) and found those with a low ratio and APOE e4 allele had a borderline significant increased risk of chronic depression (odds ratio = 3.00, df = 1, 95% CI: 0.97–9.26); this association did not exist among those with no APOE e4 (odds ratio = 0.84, df = 1, 95% CI: 0.41–1.75). Aβ40 and Aβ42 were measured at only one time, so we were unable to assess change in Aβ over time.
Our results suggest that those with low Aβ42/Aβ40 and at least one APOE e4 allele had an increased risk for depression over 9 years, and these results remained significant even after excluding participants who developed incident dementia. These results suggest that plasma Aβ42/Aβ40 may be a useful biomarker for depression independent of dementia. These results also support previous hypotheses that plasma Aβ may be a useful biomarker for a depression subtype representing prodromal dementia. This is of public health significance because of the fast-growing population of older adults who are at an increased risk for both depression and dementia, and the large number of older adults that depression and dementia already affect (30,31). Future studies are needed to determine how the level of plasma Aβ40 and Aβ42 fluctuate over time and how this fluctuation is associated with depression and dementia, in those with and without APOE e4 alleles. This will help elucidate the complex relationships between Aβ, depression, and dementia.
FUNDING
This work was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and National Institute of Nursing Research grant R01-NR012459. This research was supported in part by the Intramural Research Program on the National Institutes of Health (NIH) , N IA.
CONFLICT OF INTEREST
The authors have no conflicts of interest and no financial disclosures to make.
Acknowledgments
Andrea Metti would like to acknowledge Dr. Robert Boudreau for his statistical advice and guidance. An abstract detailing the results from this study is currently under review for presentation at the Alzheimer’s Association International Conference in Vancouver, July 2012.
References
- 1.Barnes DE, Alexopoulos GS, Lopez OL, Williamson JD, Yaffe K. Depressive symptoms, vascular disease, and mild cognitive impairment. Findings from the Cardiovascular Health Study. Arch Gen Psychiatry. 2006;63:273–280. doi: 10.1001/archpsyc.63.3.273. [DOI] [PubMed] [Google Scholar]
- 2.Jorm AF. History of depression as a risk factor for dementia: an updated review. Aust N Z J Psychiatry. 2001;35:776–781. doi: 10.1046/j.1440-1614.2001.00967.x. [DOI] [PubMed] [Google Scholar]
- 3.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 4.Rapp MA, Schnaider-Beeri M, Grossman HT, et al. Increased hippocampal plaques and tangles in patients with Alzheimer disease with a lifetime history of major depression. Arch Gen Psychiatry. 2006;63(2):161–167. doi: 10.1001/archpsyc.63.2.161. [DOI] [PubMed] [Google Scholar]
- 5.Sun X, Bhadelia R, Liebson E, et al. The relationship between plasma amyloid-β peptides and the medial temporal lobe in the homebound elderly. Int J Geriatr Psychiatry. 2010;26:593–601. doi: 10.1002/gps.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blasko I, Kemmler G, Jungwirth S, et al. Plasma amyloid beta-42 independently predicts both late-onset depression and Alzheimer disease. Am J Geriatr Psychiatry. 2010;18(11):973–982. doi: 10.1097/JGP.0b013e3181df48be. [DOI] [PubMed] [Google Scholar]
- 7.Moon Y, Kang S, No H, et al. The correlation of plasma Aβ42 levels, depressive symptoms, and cognitive function in the Korean elderly. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:1603–1606. doi: 10.1016/j.pnpbp.2011.05.013. [DOI] [PubMed] [Google Scholar]
- 8.Sun X, Steffens DC, Au R, et al. Amyloid-associated depression: a prodromal depression of Alzheimer disease? Arch Gen Psychiatry. 2008;65(5):542–550. doi: 10.1001/archpsyc.65.5.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun X, Chiu CC, Liebson E, et al. Depression and plasma amyloid β peptides in the elderly with and without the apolipoprotein E4 allele. Alzheimer Dis Assoc Disord. 2009;23:238–244. doi: 10.1097/WAD.0b013e31819cb3ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graff-Radford NR, Crook JE, Lucas J, et al. Association of low plasma Aβ42/Aβ40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64:354–362. doi: 10.1001/archneur.64.3.354. [DOI] [PubMed] [Google Scholar]
- 11.Pesaresi M, Lovati C, Bertora P, et al. Plasma levels of beta-amyloid (1-42) in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27:904–905. doi: 10.1016/j.neurobiolaging.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Yaffe K, Weston A, Graff-Radford NR, et al. Association of plasma β-amyloid level and cognitive reserve with subsequent cognitive decline. JAMA. 2011;305(3):261–266. doi: 10.1001/jama.2010.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaffe K, Barnes D, Lindquist K, et al. Endogenous sex hormone levels and risk of cognitive decline in an older biracial cohort. Neurobiol Aging. 2007;28:171–178. doi: 10.1016/j.neurobiolaging.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 14.Slinin Y, Paudel ML, Taylor BC, et al. 25-Hydroxyvitamin D levels and cognitive performance and decline in elderly men. Neurology. 2010;74(1):33–41. doi: 10.1212/WNL.0b013e3181c7197b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1(3):385–401. [Google Scholar]
- 16.Beekman AT, Deeg DJ, Van Limbeek J, Braam AW, De Vries MZ, Van Tilburg W. Criterion validity of the Center for Epidemiologic Studies Depression Scale (CES-D): results from a community-based sample of older subjects in the Netherlands. Psychol Med. 1997;27(1):231–235. doi: 10.1017/s0033291796003510. [DOI] [PubMed] [Google Scholar]
- 17.Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults: evaluation of a short form of the CES-D (Center for Epidemiologic Studies Depression Scale) Am J Prev Med. 1994;10(2):77–84. [PubMed] [Google Scholar]
- 18.Vogelzangs N, Kritchevsky SB, Beekman AT, et al. Obesity and onset of significant depressive symptoms: results from a prospective community-based cohort study of older men and women. J Clin Psychiatry. 2010;71(4):391–399. doi: 10.4088/JCP.08m04743blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erlandsen E, Randers E, Kristensen J. Evaluation of the Dade Behring N Latex Cystatin C assay on the Dade Behring Nephelometer II System. Scand J Clin Lab Invest. 1999;59:1–8. doi: 10.1080/00365519950185940. [DOI] [PubMed] [Google Scholar]
- 20.Hixson J, Vernier D. Restriction isotyping of human apolipoprotein E by gen amplification and cleavage with Hhal. J Lipid Res. 1990;31(3):545–548. [PubMed] [Google Scholar]
- 21.Qiu WQ, Sun X, Selkoe DJ, et al. Depression is associated with low plasma Aβ42 independently of cardiovascular disease in the homebound elderly. Int J Geriatr Psychiatry. 2007;22:536–542. doi: 10.1002/gps.1710. [DOI] [PubMed] [Google Scholar]
- 22.Buckner R, Snyder A, Shannon B, et al. Molecular, structural and functional characterization of Alzheimer's disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rowe C, Ng S, Ackermann U, et al. Imaging β-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 24.Byers A, Yaffe K. Depression and risk of developing dementia. Nat Rev Neurol. 2011;7:323–331. doi: 10.1038/nrneurol.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cole J, Costafreda S, McGuffin P, Fu C. Hippocampal atrophy in first episode depression: A meta-analysis of magnetic resonance imaging studies. J Affect Disord. 2011;134:483–487. doi: 10.1016/j.jad.2011.05.057. [DOI] [PubMed] [Google Scholar]
- 26.Martocchia A, Curto M, Toussan L, Stefanelli M, Falaschi P. Pharmacological strategies agains glucocorticoid-mediated brain damage during chronic disorders. Recent Pat CNS Drug Discov. 2011;6:196–204. doi: 10.2174/157488911796958020. [DOI] [PubMed] [Google Scholar]
- 27.Kulstad J, McMillan P, Leverenz J, et al. Effects of chronic glucocorticoid administration on insulin-degrading enzyme and amyloid-beta peptide in the aged macaque. J Neuropathol Exp Neurol. 2005;64(2):139–146. doi: 10.1093/jnen/64.2.139. [DOI] [PubMed] [Google Scholar]
- 28.Irie F, Masaki K, Petrovitch H, et al. Apolipoprotein E e4 allele genotype and the effect of depressive symptoms on the risk of dementia in men: the Honolulu-Asia Aging Study. Arch Gen Psychiatry. 2008;65:906–912. doi: 10.1001/archpsyc.65.8.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Innogenetics. New Prospects for Research into Alzheimer's Disease with INNO-BIA Plasma Aβ Forms. A Standardized Research Test for Measuring the Concentrations of Beta-Amyloid Isoforms in Blood. A Literature Review. 2007. http://www.innogenetics.be/documenten/BIA_plasma_litreview.pdf. Updated 2007. Accessed March 3, 2011. [Google Scholar]
- 30.Alexopoulos GS, Chester JG. Outcomes of geriatric depression. Clin Geriatr Med. 1992;8:363–376. [PubMed] [Google Scholar]
- 31.Prince M, Jackson J. World Alzheimer Report. London, UK: Alzheimer's Disease International; 2009. [Google Scholar]


