Abstract
Background.
The study assessed the impact of prostate-specific antigen (PSA) testing in the United States by comparing the rates of PSA testing in U.S. counties to the rates of prostate biopsies and newly treated prostate cancer and to deaths from prostate cancer.
Methods.
We examined the association between the percentage of men aged 66–74 from a nationally representative 5% Medicare sample who received PSA testing in each U.S. county in 1997 and the percent of men who received prostate biopsies or treatment for newly diagnosed prostate cancer in 1997 as well as mortality from prostate cancer and from all other causes from 1998 to 2007.
Results.
Analyses of 1,067 U.S. counties showed a significant relationship between the rate of PSA testing and both the rate of men undergoing treatment for prostate cancer and prostate cancer mortality (both p < .001) but no relationship with mortality from other causes. For every 100,000 men receiving a PSA test in 1997, an additional 4,894 men underwent prostate biopsy and 1,597 additional men underwent prostate cancer treatment in 1997, and 61 fewer men died from prostate cancer during 1998–2006. Analyses stratified by age and race produced similar results.
Conclusions.
PSA testing was associated with modest reductions in prostate cancer mortality and large increases in the number of men overdiagnosed with and overtreated for prostate cancer. The results are similar to those obtained by the large European randomized prospective trial of PSA testing.
Keywords: PSA screening, Cancer deaths, Medicare, Health disparities
There is a growing consensus that routine screening for prostate cancer with prostate-specific antigen (PSA) results in the diagnosis and treatment of prostate cancer in men who otherwise would not have been diagnosed during their lives (1–12). What is less clear is whether routine PSA screening has any benefit—whether it results in fewer deaths from prostate cancer. Prostate cancer differs from most other cancers in that, with the exception of metastatic disease, stage at diagnosis has a small impact on overall survival (13). The two large randomized prospective trials of PSA screening have produced conflicting results (14–17). A study of 76,693 men aged 55–74 in the United States found no decrease in prostate cancer mortality in men who received annual testing compared with usual care in 10 and 13 years of follow-up (14, 15). However, the usual care group had high rates of PSA testing. In contrast, a European Study of 162,243 men aged 50–74 found a 19% decrease in prostate cancer mortality in 9 years of follow-up and a 29% reduction after 11 years follow-up (16, 17). This translated to one prostate cancer death prevented for each 1,410 men screened at 9 years and one less death per 1,055 men at 11 years. This study also found a high risk of overdiagnosis, with 48 additional men diagnosed and treated for prostate cancer for each prostate cancer death prevented.
In addition to prospective trials, a number of investigators have explored the impact of the PSA testing through ecological analyses, comparing the rates of PSA testing in different geographic areas to rates of prostate cancer diagnoses and mortality (5, 18–20). Most find greater incidence and treatment of prostate cancer in the high PSA testing area but no differences in prostate cancer mortality.
In this study, we take advantage of the fact that PSA screening rates vary considerably from one area of the country to another, and ask whether differences in rates of PSA testing across U.S. counties are associated with differences in the rate of men treated for prostate cancer and also deaths from prostate cancer. We hypothesized that the findings from this analysis would be similar to those of the prospective trial of PSA testing in Europe (16, 17) because the ecological analysis of U.S. data avoids the issue of the high underlying rate of PSA testing that complicated the U.S. trial of PSA testing (14, 15).
METHODS
We selected cohorts of male Medicare beneficiaries 66–74 years of age from the nationally representative 5% Medicare sample who had complete enrollment in parts A and B and no health maintenance organization (HMO) enrollment in the year prior to and the year of selection for inclusion in the study. Men with claims consistent with a prior diagnosis of or treatment for prostate cancer in the preceding 12 months were excluded.
Our main analysis compared the association of the PSA testing rate in men aged 66–74 in 1997 in each county with the rate of prostate biopsies and new prostate cancer treatments in 1997 in those counties as well as lagged age-group–specific mortality rates for prostate cancer and nonprostate cancer from 1998 to 2007.
Mortality data were drawn from the National Vital Statistics System’s bridged-race files. Population denominators for each county were derived from the 5% Medicare sample denominator file, including all male beneficiaries regardless of HMO status or enrollment in parts A and B. Lagged age-group–specific mortality rates were calculated for prostate cancer mortality (The International Statistical Classification of Diseases and Related Health Problems [ICD]-9 Code 185 and ICD-10 Code C61) and nonprostate cancer mortality (all other deaths). In lagging the rates, we generated numerators and denominators that were shifted 1 year for each year out from PSA testing.
Rates were calculated at the county level for PSA testing (Current Procedural Terminology codes 84152–84154), prostate biopsy (Current Procedural Terminology codes 55700–55705), and prostate cancer treatment, which included parenteral gonadotropin-releasing hormone (GnRH) agonists (Healthcare Common Procedure Coding System codes J9202, J1950, J9217, J9218, J9219, and J3315), orchiectomy (Current Procedural Terminology codes 54520–54522 and 54530–54535), prostatectomy (Current Procedural Terminology codes 55810–55845 and ICD-9 procedure 60.5), or radiation therapy (Healthcare Common Procedure Coding System codes 77401–77499, 77520, 77523, 77750–77799, G0256, G0261 and ICD-9 procedures 92.21–92.29).
The Area Resource File includes percent of county living in poverty, percent of county that was black, percent uninsured, and housing density. County HMO market penetration was calculated as the number of male beneficiaries aged 66 and older who had one or more months of HMO coverage divided by the total number of male beneficiaries aged 66 and older. Latitude was obtained from the U.S. Census Bureau and is dichotomized to above and below the 40th parallel in the analyses (21).
We performed linear regression models to examine the association of PSA testing in 1997 with biopsies, treatments, and mortality. These models were weighted by the number of men in each county in the relevant group. In each model, we controlled for age group (66–69 and 70–74), percent poverty, percent black, percent uninsured, housing density, and HMO market penetration. For these analyses, we only included the 1,067 counties with 50 or more men aged 66–74 with parts A and B and no HMO in the 5% Medicare sample. This equated to more than 1,000 men in that age group in each county.
We also performed analyses stratified by age (66–69 and 70–74) and race (black and white). In supplemental analyses, we repeated the main analyses without restricting the county size to 50+ beneficiaries in the 5% sample and instead included all 3,120 linkable U.S. counties. In another analysis, we examined the cross-sectional association between PSA testing in 2007 with mortality in 2007. We also examined the association of PSA testing in men aged 65–74 in 1997 with age-standardized mortality from prostate cancer for men aged 65–74 or 70–79 years from 1998 to 2007. We generated age-standardized mortality rates using the direct method and the U.S. 2000 standard million for 1998–2007 (22). We next looked at the rate of men who received PSA testing in either 1997 or 1998 and its association with age-lagged mortality from 1999 to 2007. We also compared PSA testing in 1997 with a standardized prostate cancer mortality ratio compiled across 1998–2007 (23). This ratio compared the prostate cancer mortality rates at the county level with the rate for the entire United States. We also used a zero-inflated Poisson regression model to look at the association between PSA testing and counts of prostate cancer deaths from 1998 to 2007. In addition, we present data from the United States and European prospective trials of PSA testing for comparison (14–17). We used STATA version 11 (StataCorp, College Station, TX) for all analyses.
RESULTS
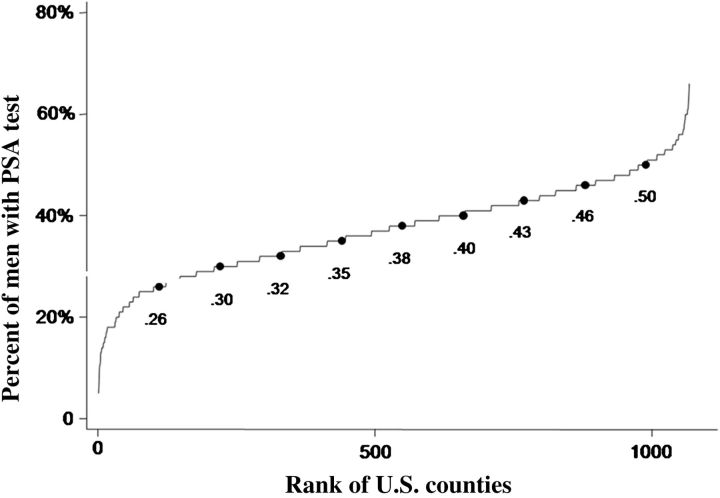
Figure 1 presents the cumulative distribution of rates of PSA testing in the 1,067 U.S. counties with at least 1,000 men aged 66–74 with parts A and B Medicare (allowing for at least 50 analyzable men in the 5% sample). In 1997, 10% of men lived in counties where 26% or fewer men received PSA testing and 10% lived in counties where 50% or greater received testing. Table 1 presents similar cumulative distributions of the rates of men receiving prostate biopsies, treatment for prostate cancer, and deaths from prostate cancer. There was substantial variation among counties in all measures.
Figure 1.
Cumulative distribution of county PSA testing rates for 1997 by rank order and decile of rank. Includes the 1,067 U.S. counties with 50 or more male beneficiaries in the 5% Medicare sample 66–74-years-old. The testing rates for each decile of U.S. counties are shown.
Table 1.
Cumulative Distribution of the Rates of Men Undergoing Prostate Cancer Biopsies, Men Receiving New Treatment for Prostate Cancer* and Prostate Cancer Mortality† in 1,067 U.S. Counties
| Decile of County | Prostate Biopsies | Prostate Cancer Treatment | Prostate Cancer Mortality |
| 1 | 0 | 0 | 99 |
| 2 | 1.33 | 0 | 118 |
| 3 | 1.72 | 0 | 133 |
| 4 | 2.06 | 0.75 | 145 |
| 5 | 2.54 | 0.99 | 155 |
| 6 | 3 | 1.23 | 165 |
| 7 | 3.45 | 1.47 | 177 |
| 8 | 3.92 | 1.77 | 198 |
| 9 | 4.79 | 2.22 | 233 |
Notes: *Biopsy and treatment rates were calculated for 1997 per 100 men aged 66–74.
Prostate cancer mortality rates per 100,000 men were calculated over 1998–2007 in a cohort that was aged-lagged for each year (see Methods for details).
We assessed the stability of county-level PSA testing rates by comparing the rates of PSA testing in 1997 to the rates 1 year and 11 years later, in 1998 and 2007. We ranked counties by PSA testing rate in each year and then categorized the ranking into quintiles. Of the counties in the top quintile in PSA testing in 1997, 73% were in the first or second quintile in 1998 and 59% in 2008. Of the counties in the lowest (fifth) quintile in 1997, 75% were in the fourth or fifth quintile in 1998 and 67% in 2008 (all p < .001).
Table 2 presents the results of regression models examining the association of county PSA testing rates in men aged 66–74 with the rate of prostate cancer biopsies and new prostate cancer treatments in 1997 and with prostate cancer mortality and mortality from all other causes from 1998 through 2007. These multivariable models controlled for percent poverty, percent black, percent uninsured, housing density, and HMO penetration in each county. PSA testing rates are significantly associated with biopsy rates, treatment rates, and prostate cancer mortality (all p < .001) but not with nonprostate cancer mortality (p = .60). For every 100,000 men screened in 1997, there were 4,894 additional men undergoing prostate biopsies and 1,597 additional men undergoing treatment for prostate cancer in 1997. There were also 61 fewer deaths from prostate cancer in 1998–2007 for every 100,000 men receiving PSA testing in 1997. Of the 1,597 additional men receiving treatment for prostate cancer, 1,138 received parenteral GnRH agonists, 222 underwent prostatectomy, and 778 received radiation treatment.
Table 2.
Linear Regression Models Estimating the Number of Additional Men Who Undergo Prostate Biopsies or Prostate Cancer Treatment and the Reduction in Deaths from Prostate Cancer, Per 100,000 Men Who Receive PSA Testing*
| Additional Men Receiving Prostate Biopsies | (95% CI) | Additional Men Treated for Prostate Cancer | (95% CI) | Reduction in Prostate Cancer Deaths in 1998–2008 | (95% CI) | Change in Deaths from Causes Other Than Prostate Cancer | (95% CI) | |
| PSA test | 4894 | (4029, 5760) | 1597 | (1087, 2106) | −61 | (−94, −29) | −448 | (−1483, 587) |
| Age 70–74 (vs 66–69) | 17 | (−140, 173) | 214 | (126, 303) | 91 | (88, 94) | 1963 | (1907, 2019) |
| % in poverty (each 1% increase) | 16 | (−19, 51) | 10 | (−11, 32) | 2 | (−0, 3) | 142 | (101, 183) |
| % Black (each 1% increase) | 13 | (6, 20) | 3 | (−1, 7) | 2 | (2, 2) | 37 | (26, 47) |
| % Uninsured (each 1% increase) | −29 | (−66, 8) | −14 | (−35, 7) | −2 | (−3, −0) | −141 | (−180, −102) |
| Housing density (each 1000 house increase) | 4 | (−8, 16) | −8 | (−18, 1) | 1 | (−1, 2) | −40 | (−91, 10) |
| HMO penetration (each 1% increase) | 1 | (−6, 7) | −2 | (−6, 1) | 0 | (−0, 0) | 5 | (−11, 1) |
| Latitude (>40° N vs 40°N) | −153 | (−333, 28) | −12 | (−118, 95) | −1 | (−10, 7) | −494 | (−729, −260) |
| Intercept | 1014 | (544, 1483) | 428 | (153, 703) | 123 | (99, 148) | 4275 | (3659, 4890) |
| R-squared | 0.076† | 0.035§ | 0.550** | 0.450 |
Notes: HMO = health maintenance organization; PSA = prostate-specific antigen.
These models examine the association of the rate PSA testing in men aged 65–74 in the 1067 U.S. counties with at least 50 relevant beneficiaries in the 5% sample of Medicare data. PSA testing rate was assessed in 1997. Biopsy and treatment rates were assessed for 1997. Prostate cancer mortality was assessed for 1998–2007. All models were weighted by the number of men aged 67–74 with parts A and B Medicare and no HMO in each county in 1997.
r-squared for PSA testing rate and biopsy rate, without the other covariates included in the regression model, was 0.06.
r-squared for PSA testing rate and treatment, without the other covariates included in the regression model, was 0.02.
r-squared for PSA testing rate and prostate cancer mortality, without the other covariates included in the regression model, was <0.002.
We also asked how much of the variation among U.S. counties in mortality from prostate cancer was explained by the variation in rates of PSA testing and also by variations among counties in all the variables included in Table 2. This is expressed by the square of the correlation coefficient, or R2, in Table 2. The variation among counties in all the variables included in Table 1 explained 55% of the variation in prostate cancer mortality. However, variation in the PSA testing rate by itself explained less than 0.2% of the variation in prostate cancer mortality.
Table 3 presents the results of analyses similar to those presented in Table 2, with the population stratified by age and race. The number of counties included in each analysis varies because of the requirement for 50+ men per county in the 5% Medicare sample with the relevant characteristic. The number of black men receiving treatment per 100,000 black men undergoing PSA testing was significantly higher than in white men. There were also trends for more biopsies and fewer prostate cancer deaths in black men, but these differences were not significant, perhaps due to the small number of observations (120 counties) included in the analysis for black men. There were also higher treatment rates associated with PSA testing in men aged 70–74 than in those 66–69, but this difference did not reach statistical significance (p = .06).
Table 3.
Linear Regression Models* Showing the Number of Biopsies, Treatments, and Prostate Cancer Deaths per 100,000 Men Who Underwent PSA Testing by Race and Age Group
| Additional Men Biopsied per 100,000 Men Tested for PSA | (95% CI) | Additional Men Treated per 100,000 Men Tested for PSA | (95% CI) | Reduction in Prostate Cancer Deaths in 1998–2008 per 100,000 Men Tested for PSA | (95% CI) | |
| Whites† (n = 1992) | 4852 | (3980, 5723) | 1451 | (932, 1971) | −54 | (−83, −25) |
| Blacks† (n = 120) | 9148 | (4268, 14028) | 5307§ | (3019, 7595) | −277 | (−690, 136) |
| Age 66–69‡ (n = 509) | 5748 | (4196, 7301) | 919 | (116, 1723) | −55 | (−89, −21) |
| Age 70–74‡ (n = 569) | 4378 | (2869, 5887) | 2081‖ | (1158, 3004) | −96 | (−152, −40) |
Notes: HMO = health maintenance organization.
All models weighted by the number of men in the relevant race and age stratum.
Models include age group, percent poverty, percent uninsured, housing density, and HMO penetration; mortality rates are age-lagged rates. The number of counties included in each analysis is given in parentheses.
Models included percent poverty, percent black, percent uninsured, housing density, and HMO penetration; mortality rates are age-lagged rates, similar to Table 1.
Different from white, p < .01.
p = .06, compared with results for age 66–69.
We also performed supplemental analyses estimating the association between PSA testing and treatment and mortality from prostate cancer to examine the robustness of the results shown in Table 2 (results not shown). One model included all U.S. counties in the analysis—not just those with 50 or more men in the appropriate age range in the 5% Medicare sample. In another analysis, we used the rate of men receiving PSA testing in either 1997 or 1998 rather than the 1997 rate. We also looked at the cross-sectional relationship of PSA testing and prostate cancer mortality in the same year. In addition, we used standardized mortality ratios for prostate cancer rather than rates in some analyses. Finally, we used a zero-inflated Poisson regression of PSA testing in 1997 with the number of prostate cancer deaths in 1998–2007.
All of the different analyses found a significant association of PSA testing rates with both new prostate cancer treatments and mortality from prostate cancer. The range of estimates of additional men treated for prostate cancer per 100,000 men receiving PSA testing was 1,597–2,714, and the range for prostate cancer deaths avoided was 22–104. These estimates are similar to the results of the European trial of PSA testing published in 2012: 2,799 additional cancer cases treated (/100,000) and 107 deaths (/100,000) prevented. This is in contrast to the U.S. trial which found 901 additional men treated for prostate cancer (/100,000) and a nonsignificant increase of 34 additional prostate cancer deaths (/100,000) associated with PSA testing.
DISCUSSION
There has been a growing consensus that the introduction of PSA testing has contributed to some decreases in prostate cancer mortality but at a cost of greater numbers of men diagnosed with and treated for prostate cancer (1, 9–11). This has led to a reexamination of recommendations on PSA screening. For example, the American Cancer Society in 2010 changed from a recommendation of yearly PSA screening for men aged 50 and older to one emphasizing individual decision making based on the patients’ individual risks and their values (24). In 2011, the U.S. Preventive Services Task Force signaled its intention to change its guideline on prostate cancer screening to recommending against routine PSA testing in asymptomatic men at normal risk, regardless of age. This has generated objections from various quarters (25, 26). Because of this controversy, it becomes important to furnish patients and their physicians with accurate data on which to base discussions about PSA screening. Typically, the most valid information comes from prospective trials. However, the two large prospective trials published in 2009 and with extended follow-up published in 2012 produced conflicting results (14–17) and appeared to have minimal impact on PSA screening in the community (27). PSA screening is highly prevalent, even among men aged 80 and older, where no organization has ever recommended testing (28). In addition to their contradictory results, other aspects of the two large trials may limit generalizability of their results. A major limitation of the American trial was the high PSA testing rate in the control group (14, 15). In addition, the benefit of screening in the European trial was restricted to participants aged 55–69 years old (16, 17). It is possible, however, that there was an insufficient number of men older than 69 enrolled to detect potential benefit in the European study. Our study examined beneficiaries aged 66–75 years old, somewhat older than those studied by either trial. We found that an increase of PSA testing at the county level was associated with an increase in diagnosis and treatment of prostate cancer and a decrease in prostate cancer mortality. As a control, we also examined mortality from all causes other than prostate cancer and found no association with PSA testing. This finding suggests that the association of PSA testing and lower prostate cancer mortality is specific; that is, PSA testing was not a marker for better overall health or better access to and receipt of health care.
PSA testing appeared to have a bigger impact on black patients, with more men biopsied and treated for prostate cancer and more deaths from prostate cancer prevented. However, only the difference in men treated for prostate cancer was statistically significant, and the analyses of black men were limited to the 120 counties with 50 or more relevant black patients in the 5% sample, so the results might not generalize to counties with lower densities of older black residents.
Most of the excess treatment for prostate cancer associated with increasing PSA rates was due to men receiving GnRH agonists and/or radiation. Though there is no evidence to support the practice, it became very common in the 1990s for older men with low-grade prostate cancer to receive GnRH agonists (29, 30). Indeed, some physicians were administering GnRH agonists to older men with elevated PSA levels even without a biopsy-proven diagnosis of prostate cancer (31). This is concerning, given the substantial late toxicities associated with GnRH agonist use (32, 33).
The interpretation of our analyses rests on several assumptions. First, we are assuming that the variation in PSA testing rates is driving the variation in prostate cancer treatments and mortality. An alternative explanation is that some unmeasured factor that varies among counties might be responsible for both PSA screening and prostate cancer diagnoses and mortality. This is a limitation of all ecological analyses and cannot be directly refuted. On the other hand, it is difficult to envision what this unmeasured variable would be. The factor would have to be specific for prostate cancer mortality, not overall mortality. Also, the known risk factors for prostate cancer mortality would tend to be associated with higher PSA testing rates, not lower, and we controlled for the most obvious risk factors for prostate cancer mortality such as age, racial composition, and measures of socioeconomic status.
Another assumption in our ecological analyses is that populations and PSA testing rates within counties are stable over time. We found that counties with high or low rates in 1997 tended to maintain those high and low rates up to 11 years later. We assume that the association of prostate cancer mortality in 1998–2007 with PSA testing rates in 1997 was driven by the fact that the 1997 rates were generally reflective of the rates in prior and subsequent years. We explored a number of alternative analyses, all ecological, in order to test the robustness of the results. All of these alternative analyses found significant associations between PSA testing and new treatments and between PSA testing and prostate cancer mortality. Most of the point estimates produced by these analyses were close to those produced by the main analysis and also to the results of the European study.
There are several limitations to this study. First, our measure of mortality from prostate cancer is derived from vital statistics. Studies comparing prostate cancer deaths in vital statistics data to medical record review have found more than 90% agreement (34–36) and no effect of different levels of PSA testing on coding of prostate cancer deaths (35). Another limitation is Medicare data provide no information on stage of cancer at diagnosis or level of PSA. In addition, we limited our sample to beneficiaries who were enrolled in Medicare parts A and B and who had not been enrolled in an HMO. The results may not be applicable to younger men. Not all PSA testing is for screening. We eliminated men with a diagnosis on any medical claim in the prior year suggestive of a history of prostate cancer before we calculated the rate of PSA testing. Any residual PSA testing for surveillance should have only a minor impact on the estimates of PSA screening rates by county.
In conclusion, our results show that although PSA testing at the county level is associated with modest reductions in mortality from prostate cancer, it is also associated with large increases in the number of men diagnosed and treated for prostate cancer. This supports the generalizability of the results from the European trial of PSA testing and should lead to a more selective use of the PSA test in the future.
FUNDING
This work was supported by the Cancer Prevention and Research Institute of Texas (grant number RP101207) and the National Institutes of Health (T32-AG00270, K05-CA134923, and P30 AG024832).
CONFLICT OF INTEREST
None of the authors have any financial or personal conflicts of interest. All authors had full access to all the data in the study and take full responsibility for the integrity of the data and accuracy of the data analysis.
Acknowledgments
Authors contributions: All authors have made substantial contributions to the intellectual content of the paper as described later: B.T.H. (conception and design, drafting of the manuscript, and statistical analysis); Y.-F.K. (conception and design, revision of the manuscript for important intellectual content, statistical analysis, and interpretation of data); Y.-L.L. (analysis and interpretation of data, critical revision of the manuscript for important intellectual content, and statistical analysis); and J.S.G. (conception and design, acquisition of data, planning and interpretation of analyses, drafting of the manuscript, obtaining funding, and supervision).
Sponsor's role: The sponsors played no role in the design, methods, participant recruitment, data collection, analysis, or preparation of this manuscript.
References
- 1.Welch HG, Albertsen PC. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening: 1986–2005. J Natl Cancer Inst. 2009;101:1325–1329. doi: 10.1093/jnci/djp278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kilpelainen TP, Tammela TL, Maattanen L, et al. False-positive screening results in the Finnish prostate cancer screening trial. Br J Cancer. 2010;102:469–474. doi: 10.1038/sj.bjc.6605512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vickers AJ, Cronin AM, Bjork T, et al. Prostate specific antigen concentration at age 60 and death or metastasis from prostate cancer: case-control study. BMJ. 2010;341 doi: 10.1136/bmj.c4521. c4521. doi: 10.1136/bmj.c4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Concato J, Wells CK, Horwitz RI, et al. The effectiveness of screening for prostate cancer: a nested case-control study. Arch Intern Med. 2006;166:38–43. doi: 10.1001/archinte.166.1.38. [DOI] [PubMed] [Google Scholar]
- 5.Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery E, Barry MJ. Screening, treatment, and prostate cancer mortality in the Seattle area and Connecticut: fifteen-year follow-up. J Gen Intern Med. 2008;23:1809–1814. doi: 10.1007/s11606-008-0785-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stattin P, Holmberg E, Johansson JE, Holmberg L, Adolfsson J, Hugosson J. Outcomes in localized prostate cancer: National Prostate Cancer Register of Sweden follow-up study. J Natl Cancer Inst. 2010;102:950–958. doi: 10.1093/jnci/djq154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hugosson J, Carlsson S, Aus G, et al. Mortality results from the Goteborg randomised population-based prostate-cancer screening trial. Lancet Oncol. 2010;11:725–732. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ilic D, O’Connor D, Green S, Wilt T. Screening for prostate cancer: a Cochrane systematic review. Cancer Causes Control. 2007;18:279–285. doi: 10.1007/s10552-006-0087-6. [DOI] [PubMed] [Google Scholar]
- 9.Djulbegovic M, Beyth RJ, Neuberger MM, et al. Screening for prostate cancer: systematic review and meta-analysis of randomized controlled trials. BMJ. 2010;341:c4543. doi: 10.1136/bmj.c4543. doi: 10.1136/bmj.c4543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Etzioni R, Penson DF, Legler JM, et al. Overdiagnosis due to prostate-specific antigen screening: lessons from U.S. prostate cancer incidence trends. J Natl Cancer Inst. 2002;94:981–990. doi: 10.1093/jnci/94.13.981. [DOI] [PubMed] [Google Scholar]
- 11.Brawley OW. Prostate cancer screening: is this a teachable moment? J Natl Cancer Inst. 2009;101:1295–1297. doi: 10.1093/jnci/djp310. [DOI] [PubMed] [Google Scholar]
- 12.Draisma G, Etzioni R, Tsodikov A, et al. Lead time and overdiagnosis in prostate-specific antigen screening: importance of methods and context. J Natl Cancer Inst. 2009;101:374–383. doi: 10.1093/jnci/djp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ketchandji M, Kuo YF, Shahinian VB, Goodwin JS. Cause of death in older men after diagnosis of prostate cancer. J Am Geriatr Soc. 2008;57:24–30. doi: 10.1111/j.1532-5415.2008.02091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andriole GL, Crawford ED, Grubb RL, et al. Mortality results from a randomized prostate-cancer screening trial. N Eng J Med. 2009;360:1310–1319. doi: 10.1056/NEJMoa0810696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andriole GL, Crawford ED, Grubb RL, et al. Prostate cancer screening in the randomized prostate, lung, colorectal, and ovarian cancer screening trial: mortality results after 13 years of follow-up. J Natl Cancer Inst. 2012;104:125–132. doi: 10.1093/jnci/djr500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360:1320–1328. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 17.Schroder FH, Hugosson J, Roobol MJ, et al. Prostate-cancer mortality at 11 years of follow-up. N Engl J Med. 2012;366:981–990. doi: 10.1056/NEJMoa1113135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu-Yao G, Albertsen PC, Stanford JL, Stukel TA, Walker-Corkery ES, Barry MJ. Natural experiment examining impact of aggressive screening and treatment on prostate cancer mortality in two fixed cohorts from Seattle area and Connecticut. BMJ. 2002;325:740–745. doi: 10.1136/bmj.325.7367.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shibata A, Ma J, Whittemore AS. Prostate cancer incidence and mortality in the United States and the United Kingdom. J Natl Cancer Inst. 1998;90:1230–1231. doi: 10.1093/jnci/90.16.1230. [DOI] [PubMed] [Google Scholar]
- 20.Crocetti E, Ciatto S, Zappa M. Prostate cancer: different incidence but not mortality trends within two areas of Tuscany, Italy. J Natl Cancer Instit. 2001;93:876–877. doi: 10.1093/jnci/93.11.876-a. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz G, Hanchette C. UV, latitude, and spatial trends in prostate cancer mortality: all sunlight is not the same (United States) Cancer Causes Control. 2006;17:1091–1101. doi: 10.1007/s10552-006-0050-6. [DOI] [PubMed] [Google Scholar]
- 22.Bray F. Age-standardization. In: Parkin D, Whelan S, Ferlay J, et al., editors. Cancer Incidence in Five Continents. Lyon, France: International Agency for Research on Cancer; 2002. pp. 87–89. [Google Scholar]
- 23.Selvin S. Epidemiologic Analysis: A Case-oriented Approach. New York, NY: Oxford University Press; 2001. [Google Scholar]
- 24.Wolf AMD, Wender RC, Etzioni RB, et al. American Cancer Society guideline for the early detection of prostate cancer: update 2010. CA Cancer J Clin. 2010;60:70–98. doi: 10.3322/caac.20066. [DOI] [PubMed] [Google Scholar]
- 25.Miller DC, Hollenbeck BK. Missing the mark on prostate-specific antigen screening. JAMA. 2011;306(24):2719–2720. doi: 10.1001/jama.2011.1879. [DOI] [PubMed] [Google Scholar]
- 26.Volk RJ, Wolf A. Grading the new US preventive services task force prostate cancer screening recommendation. JAMA. 2011;306(24):2715–2716. doi: 10.1001/jama.2011.1893. [DOI] [PubMed] [Google Scholar]
- 27.Zeliadt SB, Hoffman RM, Etzioni R, Gore JL, Kessler LG, Lin DW. Influence of publication of US and European prostate cancer screening trials on PSA testing practices. J Natl Cancer Inst. 2011;103:520–523. doi: 10.1093/jnci/djr007. [DOI] [PubMed] [Google Scholar]
- 28.Bynum J, Song Y, Fisher E. Variation in prostate-specific antigen screening in men aged 80 and older in fee-for-service Medicare. J Am Geriatr Soc. 2010;58:674–680. doi: 10.1111/j.1532-5415.2010.02761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shahinian VB, Kuo YF, Freeman JL, Orihuela E, Goodwin JS. Increasing use of gonadotropin-releasing hormone agonists for the treatment of localized prostate carcinoma. Cancer. 2005;103:1615–1624. doi: 10.1002/cncr.20955. [DOI] [PubMed] [Google Scholar]
- 30.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Determinants of androgen deprivation use for prostate cancer: role of the urologist. J Natl Cancer Instit. 2006;98:839–845. doi: 10.1093/jnci/djj230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuo YF, Shahinian VB, Goodwin JS. Gonadotropin-releasing hormone agonist use in men without cancer registry diagnoses of prostate cancer. BMC Health Serv Res. 2008;8:146. doi: 10.1186/1472-6963-8-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Eng J Med. 2005;352:154–164. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 33.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of the “Androgen Deprivation Syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166:465–471. doi: 10.1001/.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Percy C, Stanek E, Gloeckler L. Accuracy of cancer deaths certificates and its effect on cancer mortality statistics. Am J Pub Health. 1981;71:242–250. doi: 10.2105/ajph.71.3.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Albertsen P, Walters S, Hanley J. A comparison of cause of death determination in men previously diagnosed with prostate cancer who died in 1985 or 1995. J Urol. 2000;163:519–523. [PubMed] [Google Scholar]
- 36.Penson DF, Albertsen PC, Nelson PS, Barry M, Stanford JL. Determining cause of death in prostate cancer: are death certificates valid? J Natl Cancer Inst. 2001;93:1822–1823. doi: 10.1093/jnci/93.23.1822. [DOI] [PubMed] [Google Scholar]