Abstract
Aging is associated with loss of muscle mass and strength, reduced satellite cell number, and lower regenerative potential. Testosterone increases muscle mass, strength, and satellite cell number in humans; however, the effects of testosterone on the regenerative potential of skeletal muscle are unclear. Here, we investigated the effect of testosterone on the skeletal muscle regeneration of young (2-month-old) and aged (24-month-old) male mice. We show that testosterone increases the number of proliferating satellite cells in regenerating “tibialis anterior” muscle of young and aged castrated mice 2 and 4 days postinjury. Testosterone supplementation increases the number and the cross-sectional area of regenerating fibers in both classes of age 4 days postinjury. Testosterone increases satellite cell activation and proliferation and the regeneration of both young and aged mouse muscle. These data suggest prospective application of androgens to improve the regenerating potential of the aged human skeletal muscle.
Keywords: Muscle regeneration, Testosterone
Muscle aging is characterized by reduced muscle mass, strength, and by lower muscle regenerative potential. The progressive loss of muscle mass and functional capacity with aging predisposes to increased risk of falls, disability, and dependency in the elderly person (1,2). The growing geriatric population has prompted efforts to develop therapeutic interventions to mitigate muscle wasting (3,4). Many factors contribute to the multifactorial pathophysiology of muscle loss and functional decline, such as reduced physical activity, nutritional deficits, oxidative stress, inflammation, as well as age-related decline in anabolic stimuli, like growth hormone (5), insulin-like growth factor-1 (IGF-1) (6), and testosterone (7).
Satellite cells are responsible for postnatal skeletal muscle growth and regeneration. Muscle repair recapitulates several aspects of the early steps of developmental myogenesis. There are two main stages of muscle regeneration: an initial degenerative phase, characterized by recruitment of inflammatory cells and necrosis of damaged muscle fibers, and the subsequent regenerative phase, characterized by the formation of new muscle fibers (8). Signals released either by necrotic fibers and inflammatory cells activate the satellite cells (8). Newly formed muscle fibers appear by 3–6 days postinjury, and by 10 days postinjury, most of the morphological appearance of damaged muscle is restored.
The early stage of muscle regeneration is characterized by the activation and proliferation of quiescent Pax7+ satellite cells that enter the cell cycle to generate new satellite cells and myoblasts that, in turn, migrate and fuse to form new myofibers (9,10). During muscle regeneration, satellite cells activation and proliferation are regulated by several extracellular stimuli, such as follistatin (11), IGF-1 (12), and hepatocyte growth factor (13).
Aged skeletal muscles show reduced satellite cell number due to the lower level of stimuli that sustain their self-renewal and activation and to the higher level of cell apoptosis (14,15); these limits partially explain the lower regenerative capacity of old muscle (16–19). However, aged skeletal muscles reacquire a regular regenerative capacity when exposed to a “young” environment or when Notch signaling is experimentally reactivated (16,20).
Testosterone induces skeletal muscle hypertrophy due to protein accumulation and myonuclear accretion (21–23). This suggests that androgens stimulate satellite cell proliferation and myoblast fusion to preexisting fibers to increase the myonuclear number due to the growing need for protein synthesis (23,24). This hypothesis is supported by studies showing that testosterone treatment increases satellite cell number in human (21,25), in the androgen-dependent “levator ani” muscle in rodents (26) and in vitro (27–29), and by the evidence that the androgen receptor (AR) plays a role on muscle cell proliferation and differentiation in vivo and in vitro (30–33). In addition, AR knockout mice showed more pronounced reduction of limb muscle mass compared with muscle fiber–specific AR knockout mice (34–36), further supporting the idea of a direct effect of testosterone on satellite cells. Androgen deprivation induces systemic loss of muscle mass, strength, and voluntary running in young and old rodents, whereas subsequent testosterone supplementation reverses these effects (37–39).
Few reports to date have examined the effects of androgen administration on the muscle regeneration. They show variable effects depending on the type of injured muscle and the extent of muscle regeneration of castrated and intact rodents (40,41). For example, a recent report showed the beneficial effect of nandrolone decanoate supplementation on the number and size of regenerating fibers and on the level of expression of MyoD and IGF-1 postinjury (42). Because testosterone modulates several pathways involved in satellite cell activation, proliferation and aging, we evaluated here the effect of testosterone supplementation on the early phases of the muscle regeneration of young and old mice.
METHOD S
Mice, Castration, and Testosterone Treatment
Sexually, mature 2- and 24-month-old male C57Bl/6J wild type mice, purchased from the Charles River Laboratories, Boston, MA, were acclimatized for at least 7 days before starting experiments. The mice were kept on a 12-hour light-dark cycle and given food and water ad libitum. The animals were handled according to the National Institute of Health guidelines for the care and use of laboratory animals, using protocols approved by the Committee for the Ethical Care and Use of Laboratory Animals of the Boston University.
The mice were randomly assigned to three experimental groups: eugonadal, sham-operated (Sham) implanted with empty silastic tubing; orchiectomized mice (Cast) implanted with empty silastic tubing; and orchiectomized mice implanted with silastic implants containing testosterone propionate (Tp) (Cast Tp). Surgical orchiectomy was performed under anesthesia through a midline scrotal incision allowing bilateral access to the hemiscrotal content.
Testosterone supplementation was administered through 1.0 cm silastic implants (1.47 mm inner diameter, 1.96 mm outer diameter; Dow Corning Corp. Midland, MI) containing 8 mg Tp (Sigma–Aldrich, St Louis, MO, T1875) and sealed with silicone glue (Dow Corning Corp.). The implants were inserted subcutaneously through a small incision at the nape of the neck 12 days after orchiectomy. Sham-operated eugonadal and Cast control mice were implanted with empty silastic tubing.
Serum total testosterone was measured using liquid chromatography-tandem mass spectrometry as described (43). For 24 month-old mice, serum T level (ng/dL; mean ± SD) was higher in Tp-treated mice (2931 ± 447.9) than in Sham (345.4 ± 132.9) and Cast mice (23.74 ± 8.32; mean values 4 days postinjury). Similar testosterone levels were found in 2-month-old mice: Sham (296.9 ± 114.1), Cast (19.69 ± 3.48), and Cast Tp mice (3589 ± 308.1; mean values 4 days postinjury).
Muscle Injury
After 12 days of testosterone delivery, the left “tibialis anterior” muscle was injured under anesthesia using four 5 μL injections of 10 μM Naja nigricollis cardiotoxin (Accurate Chemical & Scientific Corporation, Westbury, NY) administered along the longitudinal axis of the muscle using a Hamilton syringe (model 725, Hamilton Company, Reno, NV) with a 30½ gauge needle. Contralateral tibialis anterior muscle was left intact as control. At the end of the treatment, mice were injected intraperitoneally with 5-bromo-2′-deoxyuridine (BrdU; 50 mg/kg body weight; Sigma–Aldrich, B5002) dissolved in sterile saline solution 5 hours before sacrifice. The tibialis anterior muscle was isolated, freed of visible connective tissue, and snap frozen in liquid nitrogen–cooled isopentane.
Antibodies
Primary antibodies used: mouse monoclonal to embryonic myosin heavy chain (emb-MyHC; F1.652, 1:100); rat monoclonal to BrdU (Abcam, Cambridge, MA, ab6326, 1:25); rat monoclonal to laminin 2 alpha (Abcam, ab11576, 1:100); chicken polyclonal to laminin (Abcam ab14055, 1:200); and rabbit polyclonal to neural cell adhesion molecule (NCAM; Millipore, Billerica, MA, AB5032, 1:200). F1.652 hybridoma was developed by Dr Helen Blau and was obtained from the Developmental Studies Hybridoma Bank of the University of Iowa, Iowa City, IA. Secondary antibodies used: goat polyclonal to mouse Cy3 conjugated (Jackson Immunoresearch, West Grove, PA, 115-165-062, 1:400), goat polyclonal to rat FITC conjugated (Jackson Immunoresearch, 112-095-003, 1:200), goat polyclonal to rabbit Cy3 conjugated (Jackson Immunoresearch, 111-165-003, 1:200), and goat polyclonal to chicken fluorescein isothiocyanate (FITC) conjugated (Abcam, Cambridge, ab46969, 1:200).
Immunohistochemistry Analysis
Cryosections of the tibialis anterior, 6- to 8-μm-thick, tibialis anterior were fixed in 4% paraformaldehyde for 25 minutes at 4°C, permeabilized with 0.5% Triton X-100 for 15 minutes, and blocked in 5% normal goat serum (NGS) for 1 hour. Samples were incubated with primary antibodies overnight at 4°C in 1% bovine serum albumin (BSA)/1% NGS and then with secondary antibodies for 1 hour at room temperature. For the identification of BrdU+ nuclei, after fixation and permeabilization, muscle sections were incubated in 1 N HCl on ice for 10 minutes, 2 N HCl at 60°C for 5 minutes and then at room temperature for 15 minutes, washed with 0.1 M borate buffer for 12 minutes, incubated in 1% Triton X-100, 1 M glycine and 5% NGS for 45 minutes, in goat anti-mouse IgG (H + L) Fab fragment (Jackson Immunoresearch, 115-007-003, 1:100) in 5% NGS for 30 minutes, and then overnight at 4°C with primary antibodies diluted in 1% BSA and 1% NGS. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole. Haematoxylin and Eosin staining was performed using a standard protocol. Pictures were acquired using a Nikon Eclipse TE2000-E microscope (Nikon Instruments Inc., Melville, NY). Regenerating area was identified as the region of the section showing the infiltrate of inflammatory cells and the centro-nucleated/emb-MyHC+ fibers and was measured using the SPOT imaging analysis software (Diagnostic Instruments, Sterling Heights, MI).
Data Analysis
Results are means ± SEM. One-way analysis of variance (ANOVA) was used in experiments with more than two independent groups. If overall ANOVA revealed significant difference, Student’s t test was used to analyze differences between groups. Chi-square test was used to compare frequency distribution of fiber cross-sectional area (CSA) among groups. p values ≤.05 were considered statistically significant.
RESULTS
Testosterone Administration Is Associated With Improved Muscle Regeneration in 24-Month-Old Mice
To evaluate the effect of testosterone supplementation on the muscle regeneration of aged skeletal muscle, 24-month-old C57Bl/6J mice, described in Methods section, were sacrificed after BrdU injection 2, 4, and 9 days after cardiotoxin injury, to evaluate the rate and the extension of muscle regeneration and to capture the different phases of the regeneration process. Necrotic area and inflammatory infiltrates were found in the tibialis anterior 2 days after the cardiotoxin injury, indicating the induction of muscle regeneration in all groups of aged mice (Figure 1A). Double immunostaining for BrdU, an established marker of cell proliferation, and for NCAM, a recognized marker of satellite cells (44), revealed that 2 days after cardiotoxin injury, the control orchiectomized mice that were implanted with empty silastic implants had a lower number of proliferating BrdU+/NCAM+ satellite cells when compared with sham-operated mice (Figure 1B and C). However, the number of proliferating satellite cells was restored in orchiectomized mice treated with testosterone (Figure 1C). Similar data were obtained 4 days after cardiotoxin-induced injury in that the number of BrdU+/NCAM+ satellite cells in orchiectomized mice was still lower than in sham-operated mice, whereas testosterone rescued this deficit (Figure 1D and E).
Figure 1.
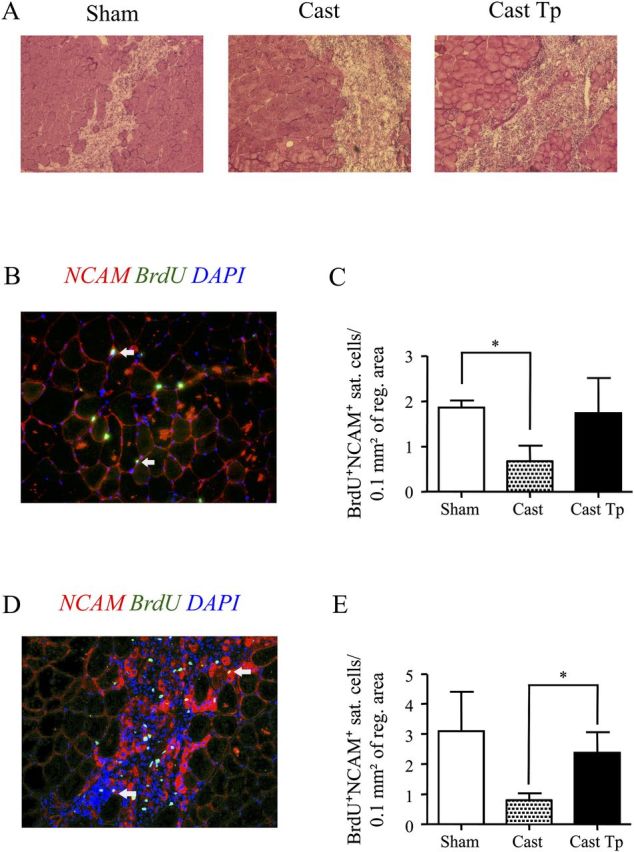
Effect of testosterone supplementation on satellite cell proliferation in aged skeletal muscle 2 and 4 days postinjury. Hematoxylin & Eosin staining of the tibialis anterior muscle 2 days postinjury; necrotic areas are present in damaged muscle (A). Representative image showing BrdU (green) and neural cell adhesion molecule (NCAM; red) staining 2 days postinjury (B). Testosterone supplementation in orchiectomized mice increased the number of the BrdU+/NCAM+ satellite cells when normalized for the extension of regenerating area, indicating a positive effect of androgens on satellite cell activation and/or proliferation in the aged skeletal muscle 2 days postinjury (C). Similar results are showed 4 days postinjury, when again castration is associated with reduced number of proliferating satellite cells, a deficit rescued by testosterone administration (D –E). Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (blue). In (B) and (D), arrows indicate BrdU+/NCAM+ satellite cells. Results are means ± S E M (n = 4 per group). *p < .05. Representative critical values for t test statistic: Figure C, Sham versus Cast: t = 3.117; df = 4.
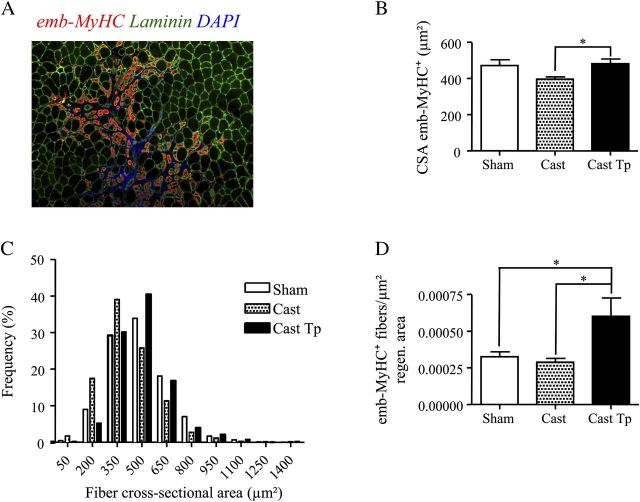
The orchiectomized mice exhibited a smaller CSA of regenerating fibers immunostained for emb-MyHC, a marker of regenerating muscle fibers 4 days after cardiotoxin-induced injury (Figure 2A and B). The control orchiectomized mice implanted with empty silastic implants had significantly lower mean CSA of emb-MyHC+ muscle fibers than testosterone-treated orchiectomized mice and intact sham-operated mice. Consistent with these findings, the distribution of the muscle fibers in testosterone-treated orchiectomized mice (Figure 2C) is shifted toward the right compared with control orchiectomized mice implanted with empty silastic implants (chi-square p value for trend <.001). Thus, testosterone supplementation in orchiectomized mice rescued the CSA of regenerating muscle fibers to that seen in sham-operated intact mice. However, even though testosterone-treated orchiectomized mice showed increased number of emb-MyHC+ muscle fibers, the number of emb-MyHC+ muscle fibers was not significantly different between orchiectomized placebo-control mice (implanted with empty drug delivery device) and intact sham-operated mice (Figure 2D). These data suggest that testosterone predominantly enhances the formation of new muscle fibers that have larger CSA than those in orchiectomized mice.
Figure 2.
Effect of testosterone supplementation on muscle regeneration in aged skeletal muscle 4 days postinjury. Representative image showing the staining for embryonic myosin heavy chain (emb-MyHC; red) and for laminin (green) (A). Testosterone treatment restores the cross-sectional area (CSA) (B) of emb-MyHC+ muscle fibers to that of Sham when compared with Cast mice. Panel C shows the frequency distribution of muscle fibers by CSA: testosterone administration was associated with increased frequency of larger fibers (>/= 500 μm2) when compared with Cast mice (p value for chi-square analysis <.001). Testosterone treatment increases the number of emb-MyHC+ muscle fibers normalized for the regenerating area (D). Results are means ± SEM (n = 4 per group). *p < .05. Representative critical values for t test statistic: Figure B, Cast versus Cast Tp: t = 2.689; df = 10.
By 9 days after cardiotoxin-induced injury, the muscle regeneration was nearly complete. At this time, we quantitated the number of centro-nucleated fibers (CNFs) in the regenerating tibialis anterior muscle. We found that neither the number (Figure 3A) nor the CSA (Figure 3B) of CNFs differed significantly among the three groups of mice. These data suggest that neither orchiectomy nor testosterone supplementation affects the late steps of muscle regeneration.
Figure 3.
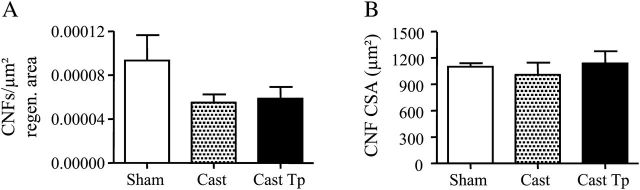
Effect of testosterone supplementation on muscle regeneration in aged skeletal muscle 9 days postinjury. No significant difference was found in the number of centro-nucleated fibers normalized for the regeneration area and in the cross-sectional area of regenerating fibers (A-B).
Testosterone Administration Is Associated With Improved Muscle Regeneration in 2-Month-Old Mice
To confirm the effect of testosterone on the muscle regeneration of old skeletal muscle we found, we repeated the same experimental protocol that was used for the aged mice in 2-month-old mice. Two days after cardiotoxin-induced injury, the mice of all experimental groups showed consistent muscle regeneration (Figure 4A). Consistent to what shown in aged mice, orchiectomized mice that received empty silastic implants had fewer proliferating satellite cells in comparison to sham-operated eugonadal controls and testosterone-treated orchiectomized mice (Figure 4B and C). Similarly, 4 days after injury, orchiectomized mice had a lower number of BrdU+/NCAM+ satellite cells than sham-operated eugonadal controls and testosterone-treated orchiectomized mice (Figure 4D and E). However, in both cases, the differences were not statistically significant.
Figure 4.
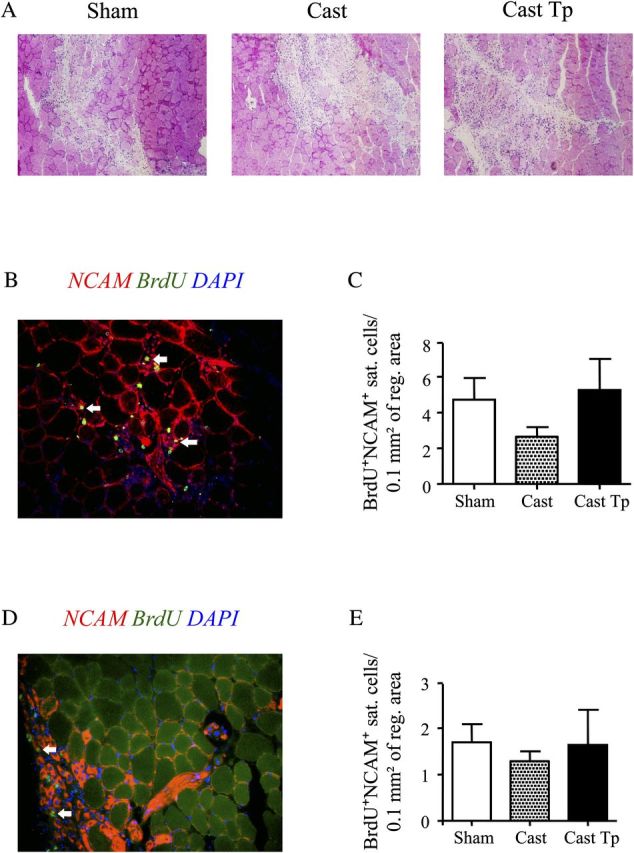
Effect of testosterone supplementation on satellite cell proliferation in young skeletal muscle 2 and 4 days postinjury. Hematoxylin & Eosin staining of the tibialis anterior muscle 2 days postinjury (A). Representative image showing BrdU (green) and neural cell adhesion molecule (NCAM; red) staining (B). Testosterone supplementation restored the number of BrdU+/NCAM+ satellite cells, indicating a positive effect of androgens on satellite cell activation and/or proliferation in the young skeletal muscle 2 days postinjury (C). As in aged mice, castrated mice showed a trend toward reduced number of proliferating satellite cells, a deficit rescued by testosterone administration 4 days postinjury (D-E). The number of BrdU+/NCAM+ satellite cells per unit area did not differ significantly between controls and testosterone-treated orchiectomized mice in figures C and E. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (blue). In (B) and (D), arrows indicate BrdU+/NCAM+ satellite cells. Results are means ± S E M (n = 4 per group).
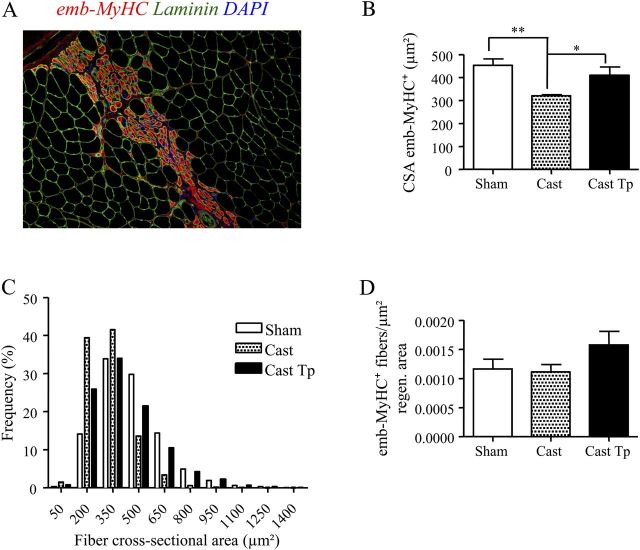
The CSA of emb-MyHC+ muscle fibers was significantly smaller in the control orchiectomized mice implanted with empty drug delivery device than in testosterone-treated orchiectomized mice and sham-operated eugonadal controls (Figure 5A and B). In addition, similar to aged mice, the distribution of the muscle fiber diameters was shifted toward right in testosterone-treated orchiectomized mice compared with control orchiectomized mice receiving empty silastic implants (Figure 5C). The number of emb-MyHC+ muscle fibers per unit regenerating area was greater in testosterone-treated orchiectomized mice than in castrated mice implanted with empty silastic implants (Figure 5D), although values do not reached statistical significance. As in the aged mice, CNFs number and mean CSA of CNFs were not significantly different among the three treatment groups 9 days after injury (Figure 6A and B).
Figure 5.
Effect of testosterone supplementation on muscle regeneration in young skeletal muscle 4 days postinjury. Representative image showing regenerating fibers positive for emb-MyHC (red) and for laminin (green) (A). Testosterone treatment increases the cross-sectional area (B) of emb-MyHC+ muscle fibers 4 days postinjury, when compared with Cast mice, as also corroborated by the right shift showed by the distribution by frequency classes of fiber diameter (C) (p value for chi-square analysis <.001). The number of emb-MyHC+ muscle fibers when normalized for the regenerating area did not differ significantly between testosterone-treated orchiectomized mice and Sham or Cast mice (D). Results are means ± SEM (n = 4/group). *p < .05; **p < .01. Representative critical values for t test statistic: Figure B, Sham versus Cast: t = 4.590; df = 6.
Figure 6.
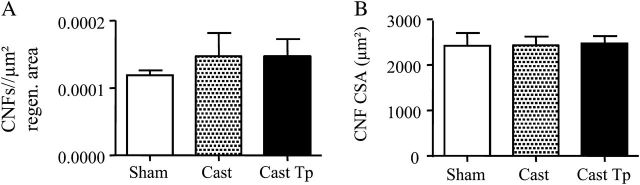
Effect of testosterone supplementation on muscle regeneration in young skeletal muscle 9 days postinjury. As for the aged mice, no significant difference was found in the centro-nucleated fibers number, normalized for regeneration area, and in the cross-sectional area of regenerating fibers (A–B).
DISCUSSION
We show here that testosterone supplementation modestly stimulated several early steps in the muscle regeneration that followed muscle injury created by the injection of cardiotoxin. Concordant changes were observed in the 2-month-old mice and the 24-month-old mice. Lowering of testosterone concentrations by surgical orchiectomy was associated with fewer BrdU+/NCAM+ proliferating myoblasts than in intact eugonadal age-matched controls. Conversely, testosterone-treated young and old mice had higher numbers of proliferating BrdU+/NCAM+ myoblasts 2 and 4 days after injury in comparison to control orchiectomized mice that received empty implants, although the difference in the number of BrdU+/NCAM+ cells of 2-month-old testosterone-treated mice did not reach statistical significance when compared with the corresponding values of age-matched orchiectomized mice treated with empty drug delivery devices, in part because the number of BrdU+/NCAM+ cells per unit area was small, consistent with the limited regenerating area. Similarly, we found an increased number of emb-MyHC+ regenerating fibers 4 days after injury in testosterone-treated old mice compared with orchiectomized mice treated with the empty silastic tubing, suggesting that the increased number of proliferating BrdU+/NCAM+ myoblasts resulted in greater differentiation rates of new myofibers in response to testosterone administration. Testosterone induced the formation of larger emb-MyHC+ fibers that had greater CSA than those in control orchiectomized mice. In addition, the rightward shift in fiber size distribution shown by testosterone-treated mice indicates that the treatment improved muscle regeneration. Taken together, our data suggest that testosterone administration contributes to the expansion of myoblasts during the early stages of muscle regeneration, allowing the formation of bigger regenerating myofibers. In addition, testosterone administration induced the hypertrophy of the early regenerating fibers, indicating a potential positive effect of testosterone on muscle protein accretion in this period of muscle regeneration. By 9 days after injury, neither the young and nor the old regenerating muscle revealed statistically significant differences in the indices of muscle regeneration at this late time point; the number and CSA of regenerated CNFs was similar in the three intervention groups. Moreover, the difference in the CSA of the regenerating fibers 9 days postinjury between young and old mice, for example, between the Sham mice of the two classes of age, is in line with the general agreement that a lower level of satellite cell activation and proliferation reduces and/or delays the overall rate of regeneration of the aged muscle. Thus, in this mouse model of cardiotoxin-induced muscle injury, the effects of testosterone appear limited to the early stages of muscle regeneration.
Previous studies have reported that aging is associated with a reduced intrinsic ability of the skeletal muscle to repair after an injury, presumably due to the reduced levels of circulating factors that regulate satellite cell activation and myoblast proliferation (17,20,45), the accumulation of cytokines that inhibit muscle differentiation, for example, TGFβ (18), as well as the intrinsic changes in the satellite cell niche (46,47). In this scenario, the aging generates a state of lower proliferation for muscle stem cells with the consequent reduced regenerative performance, maybe as an adaptation to reduce the risk of cell transformation.
We did not find major qualitative or significant quantitative differences in the temporal profile of muscle regeneration in response to testosterone administration between the 2-month-old and the 24-month-old mice. In both the young and the old mice, testosterone administration increased the number of proliferating BrdU+/NCAM+ satellite cells and promoted the formation of larger emb-MyHC+ regenerating fibers. As our experimental animals were healthy and living in a pathogen-free environment, these findings may not apply to the community-dwelling older humans who often have a cluster of comorbid conditions, which may directly or indirectly affect the regenerative response to injury, or to still older mice.
Only a few studies have investigated the role of androgens during muscle regeneration, using different types of muscle injury. In one study, nandrolone decanoate administration after a muscle contusion injury in intact male rats was associated with increased muscle twitch and tetanic force 14 days after injury (40) and improved growth of regenerating slow twitch muscle 25 days postinjury (41). However, the early stages of muscle regeneration were not investigated, as we have done in this study. In another study, nandrolone decanoate increased the expression of IGF-1, MyoD, and cyclin D1 after bupivacaine-induced muscle injury in the tibialis anterior muscle of castrated mice (42), leading the investigators to suggest that nandrolone accelerates healing processes through an IGF-1–mediated mechanism. Intramuscular IGF-1 signaling also plays an important role in mediating testosterone’s anabolic effect on the skeletal muscle (29,36,48). IGF-1 enhances myoblast proliferation and differentiation (49–52) and intramuscular IGF-1 receptor (IGF-1R) signaling mechanisms seem essential for mediating the effects of testosterone on the proliferation of muscle progenitor cells and the differentiation of myoblasts (42). Whether the observed effects of testosterone in the present study are also mediated through an IGF-1R–dependent mechanism requires further investigation.
Testosterone administration dose dependently induces skeletal muscle fiber hypertrophy and increases the number of satellite cells and myonuclei in young and older men. Muscle hypertrophy generated by testosterone administration seems to involve not only muscle protein accretion but also myoblast expansion and fusion with preexisting fibers (21–23,25). The studies in the AR knockout male mice (34–36,53) also support a direct role for androgens in inducing satellite cell activation and myoblast proliferation. We focused our investigation to the satellite cells and to the regenerating muscle fibers. However, it is possible that testosterone may also affect other cell types residing within the skeletal muscle such as the fibroblasts, blood vessels, and neural structures. For example, AR is expressed by muscle fibroblasts, vascular endothelial cells, and in nerve end plates. The role of AR signaling in these cell types should be investigated because, for example, fibroblasts regulate satellite cell activation and muscle regeneration (54). In addition, testosterone modulates the activity of immune cells that play an essential role in muscle regeneration (8). Upregulation of serum TGFβ1 and TGFβ family members in response to muscle injury have been reported contribute to decreased muscle regeneration in older animals (55–57). Testosterone has been shown to block TGFβ signaling through cross-communication of Wnt/β-catenin signaling through follistatin (58). Whether the effects of testosterone on muscle regeneration are also mediated through the modulation of the TGFβ and Wnt pathways needs further investigation.
The regenerative response to different modes of muscle injury and possibly the effects of testosterone on skeletal muscle regeneration in response to different modes of injury may vary (40–42). Our data indicate that testosterone stimulates myoblast proliferation and the formation of larger new fibers in the early phases of regenerative response to cardiotoxin-induced injury in both the young and the old mice. The clinical usefulness of androgens to promote muscle regeneration in humans and the underlying mechanisms by which testosterone modulates the early regenerative response need further investigation.
FUNDING
This work was supported by grants from the Department of Medicine, Evans Medical Foundation , and the Clinical and Translational Science Institute (grant UL1RR025771) of the Boston University to C.S. and R.J., by the National Institute of Health (grant 5R01DK070534-07 to S.B.), and by the Boston Claude D. Pepper Older Americans Independence Center (grant 5P30AG031679 to C.S. and S.B.).
References
- 1.Hicks GE, Shardell M, Alley DE, et al. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2012;67:66–73. doi: 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark DJ, Fielding RA. Neuromuscular contributions to age-related weakness. J Gerontol A Biol Sci Med Sci. 2012;67:41–47. doi: 10.1093/gerona/glr041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carter CS, Marzetti E, Leeuwenburgh C, et al. Usefulness of preclinical models for assessing the efficacy of late-life interventions for sarcopenia. J Gerontol A Biol Sci Med Sci. 2012;67:17–27. doi: 10.1093/gerona/glr042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Couteur DG, McLachlan AJ, Quinn RJ, Simpson SJ, de Cabo R. Aging biology and novel targets for drug discovery. J Gerontol A Biol Sci Med Sci. 2012;67:168–174. doi: 10.1093/gerona/glr095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corpas E, Harman SM, Blackman MR. Human growth hormone and human aging. Endocr Rev. 1993;14:20–39. doi: 10.1210/edrv-14-1-20. [DOI] [PubMed] [Google Scholar]
- 6.Abbasi AA, Drinka PJ, Mattson DE, Rudman D. Low circulating levels of insulin-like growth factors and testosterone in chronically institutionalized elderly men. J Am Geriatr Soc. 1993;41:975–982. doi: 10.1111/j.1532-5415.1993.tb06764.x. [DOI] [PubMed] [Google Scholar]
- 7.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metab. 2001;86:724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 8.Ten Broek RW, Grefte S, Von den Hoff JW. Regulatory factors and cell populations involved in skeletal muscle regeneration. J Cell Physiol. 2010;224:7–16. doi: 10.1002/jcp.22127. [DOI] [PubMed] [Google Scholar]
- 9.Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne GS. In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle. J Cell Sci. 1999;112:2895–2901. doi: 10.1242/jcs.112.17.2895. [DOI] [PubMed] [Google Scholar]
- 10.Collins CA, Olsen I, Zammit PS, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122:289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 11.Armand AS, Della Gaspera B, Launay T, Charbonnier F, Gallien CL, Chanoine C. Expression and neural control of follistatin versus myostatin genes during regeneration of mouse soleus. Dev Dyn. 2003;227:256–265. doi: 10.1002/dvdy.10306. [DOI] [PubMed] [Google Scholar]
- 12.Sato K, Li Y, Foster W, et al. Improvement of muscle healing through enhancement of muscle regeneration and prevention of fibrosis. Muscle Nerve. 2003;28:365–372. doi: 10.1002/mus.10436. [DOI] [PubMed] [Google Scholar]
- 13.Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- 14.Renault V, Thornell LE, Eriksson PO, Butler-Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell. 2002;1:132–139. doi: 10.1046/j.1474-9728.2002.00017.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldspink G, Harridge SD. Growth factors and muscle ageing. Exp Gerontol. 2004;39:1433–1438. doi: 10.1016/j.exger.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 16.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 17.Carlson ME, Hsu M, Conboy IM. Imbalance between pSmad3 and Notch induces CDK inhibitors in old muscle stem cells. Nature. 2008;454:528–532. doi: 10.1038/nature07034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlson ME, Conboy MJ, Hsu M, et al. Relative roles of TGF-beta1 and Wnt in the systemic regulation and aging of satellite cell responses. Aging Cell. 2009;8:676–689. doi: 10.1111/j.1474-9726.2009.00517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Foster W, Deasy BM, et al. Transforming growth factor-beta1 induces the differentiation of myogenic cells into fibrotic cells in injured skeletal muscle: a key event in muscle fibrogenesis. Am J Pathol. 2004;164:1007–1019. doi: 10.1016/s0002-9440(10)63188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 21.Sinha-Hikim I, Cornford M, Gaytan H, Lee ML, Bhasin S. Effects of testosterone supplementation on skeletal muscle fiber hypertrophy and satellite cells in community-dwelling older men. J Clin Endocrinol Metab. 2006;91:3024–3033. doi: 10.1210/jc.2006-0357. [DOI] [PubMed] [Google Scholar]
- 22.Kadi F, Bonnerud P, Eriksson A, Thornell LE. The expression of androgen receptors in human neck and limb muscles: effects of training and self-administration of androgenic-anabolic steroids. Histochem Cell Biol. 2000;113:25–29. doi: 10.1007/s004180050003. [DOI] [PubMed] [Google Scholar]
- 23.Sinha-Hikim I, Artaza J, Woodhouse L, et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- 24.Kadi F, Eriksson A, Holmner S, Thornell LE. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc. 1999;31:1528–1534. doi: 10.1097/00005768-199911000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Sinha-Hikim I, Roth SM, Lee MI, Bhasin S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am J Physiol Endocrinol Metab. 2003;285:E197–205. doi: 10.1152/ajpendo.00370.2002. [DOI] [PubMed] [Google Scholar]
- 26.Joubert Y, Tobin C. Testosterone treatment results in quiescent satellite cells being activated and recruited into cell cycle in rat levator ani muscle. Dev Biol. 1995;169:286–294. doi: 10.1006/dbio.1995.1144. [DOI] [PubMed] [Google Scholar]
- 27.Powers ML, Florini JR. A direct effect of testosterone on muscle cells in tissue culture. Endocrinology. 1975;97:1043–1047. doi: 10.1210/endo-97-4-1043. [DOI] [PubMed] [Google Scholar]
- 28.Doumit ME, Cook DR, Merkel RA. Testosterone up-regulates androgen receptors and decreases differentiation of porcine myogenic satellite cells in vitro. Endocrinology. 1996;137:1385–1394. doi: 10.1210/endo.137.4.8625915. [DOI] [PubMed] [Google Scholar]
- 29.Serra C, Bhasin S, Tangherlini F, et al. The role of GH and IGF-I in mediating anabolic effects of testosterone on androgen-responsive muscle. Endocrinology. 2011;152:193–206. doi: 10.1210/en.2010-0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DK. Androgen receptor enhances myogenin expression and accelerates differentiation. Biochem Biophys Res Commun. 2002;294:408–413. doi: 10.1016/S0006-291X(02)00504-1. [DOI] [PubMed] [Google Scholar]
- 31.Diel P, Baadners D, Schlupmann K, Velders M, Schwarz JP. C2C12 myoblastoma cell differentiation and proliferation is stimulated by androgens and associated with a modulation of myostatin and Pax7 expression. J Mol Endocrinol. 2008;40:231–241. doi: 10.1677/JME-07-0175. [DOI] [PubMed] [Google Scholar]
- 32.Sinha-Hikim I, Taylor WE, Gonzalez-Cadavid NF, Zheng W, Bhasin S. Androgen receptor in human skeletal muscle and cultured muscle satellite cells: up-regulation by androgen treatment. J Clin Endocrinol Metab. 2004;89:5245–5255. doi: 10.1210/jc.2004-0084. [DOI] [PubMed] [Google Scholar]
- 33.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 34.MacLean HE, Chiu WS, Notini AJ, et al. Impaired skeletal muscle development and function in male, but not female, genomic androgen receptor knockout mice. FASEB J. 2008;22:2676–2689. doi: 10.1096/fj.08-105726. [DOI] [PubMed] [Google Scholar]
- 35.Ophoff J, Van Proeyen K, Callewaert F, et al. Androgen signaling in myocytes contributes to the maintenance of muscle mass and fiber type regulation but not to muscle strength or fatigue. Endocrinology. 2009;150:3558–3566. doi: 10.1210/en.2008-1509. [DOI] [PubMed] [Google Scholar]
- 36.Chambon C, Duteil D, Vignaud A, et al. Myocytic androgen receptor controls the strength but not the mass of limb muscles. Proc Natl Acad Sci U S A. 2010;107:14327–14332. doi: 10.1073/pnas.1009536107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Axell AM, MacLean HE, Plant DR, et al. Continuous testosterone administration prevents skeletal muscle atrophy and enhances resistance to fatigue in orchidectomized male mice. Am J Physiol Endocrinol Metab. 2006;291:E506–E516. doi: 10.1152/ajpendo.00058.2006. [DOI] [PubMed] [Google Scholar]
- 38.Kovacheva EL, Hikim AP, Shen R, Sinha I, Sinha-Hikim I. Testosterone supplementation reverses sarcopenia in aging through regulation of myostatin, c-Jun NH2-terminal kinase, Notch, and Akt signaling pathways. Endocrinology. 2010;151:628–638. doi: 10.1210/en.2009-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibebunjo C, Eash JK, Li C, Ma Q, Glass DJ. Voluntary running, skeletal muscle gene expression and signaling inversely regulated by orchidectomy and testosterone replacement. Am J Physiol Endocrinol Metab. 2011;300:E327–E340. doi: 10.1152/ajpendo.00402.2010. [DOI] [PubMed] [Google Scholar]
- 40.Beiner JM, Jokl P, Cholewicki J, Panjabi MM. The effect of anabolic steroids and corticosteroids on healing of muscle contusion injury. Am J Sports Med. 1999;27:2–9. doi: 10.1177/03635465990270011101. [DOI] [PubMed] [Google Scholar]
- 41.Ferry A, Noirez P, Page CL, Salah IB, Daegelen D, Rieu M. Effects of anabolic/androgenic steroids on regenerating skeletal muscles in the rat. Acta Physiol Scand. 1999;166:105–110. doi: 10.1046/j.1365-201x.1999.00549.x. [DOI] [PubMed] [Google Scholar]
- 42.White JP, Baltgalvis KA, Sato S, Wilson LB, Carson JA. Effect of nandrolone decanoate administration on recovery from bupivacaine-induced muscle injury. J Appl Physiol. 2009;107:1420–1430. doi: 10.1152/japplphysiol.00668.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sir-Petermann T, Codner E, Perez V, et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1923–1930. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Illa I, Leon-Monzon M, Dalakas MC. Regenerating and denervated human muscle fibers and satellite cells express neural cell adhesion molecule recognized by monoclonal antibodies to natural killer cells. Ann Neurol. 1992;31:46–52. doi: 10.1002/ana.410310109. [DOI] [PubMed] [Google Scholar]
- 45.Carlson ME, Suetta C, Conboy MJ, et al. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med. 2009;1:381–391. doi: 10.1002/emmm.200900045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carlson ME, Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6:371–382. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells. 2007;25:885–894. doi: 10.1634/stemcells.2006-0372. [DOI] [PubMed] [Google Scholar]
- 48.Lewis MI, Horvitz GD, Clemmons DR, Fournier M. Role of IGF-I and IGF-binding proteins within diaphragm muscle in modulating the effects of nandrolone. Am J Physiol Endocrinol Metab. 2002;282:E483–490. doi: 10.1152/ajpendo.00191.2001. [DOI] [PubMed] [Google Scholar]
- 49.Coolican SA, Samuel DS, Ewton DZ, McWade FJ, Florini JR. The mitogenic and myogenic actions of insulin-like growth factors utilize distinct signaling pathways. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- 50.Rommel C, Clarke BA, Zimmermann S, et al. Differentiation stage-specific inhibition of the Raf-MEK-ERK pathway by Akt. Science. 1999;286:1738–1741. doi: 10.1126/science.286.5445.1738. [DOI] [PubMed] [Google Scholar]
- 51.Rommel C, Bodine SC, Clarke BA. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 52.Jacquemin V, Butler-Browne GS, Furling D, Mouly V. IL-13 mediates the recruitment of reserve cells for fusion during IGF-1-induced hypertrophy of human myotubes. J Cell Sci. 2007;120:670–681. doi: 10.1242/jcs.03371. [DOI] [PubMed] [Google Scholar]
- 53.Callewaert F, Venken K, Ophoff J. Differential regulation of bone and body composition in male mice with combined inactivation of androgen and estrogen receptor-alpha. FASEB J. 2009;23:232–240. doi: 10.1096/fj.08-113456. [DOI] [PubMed] [Google Scholar]
- 54.Murphy MM, Lawson JA, Mathew SJ, Hutcheson DA, Kardon G. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration. Development. 2011;138:3625–3637. doi: 10.1242/dev.064162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Massague J, Cheifetz S, Endo T, Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986;83:8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. doi: 10.1083/jcb.200207056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu D, Kang JS, Derynck R. TGF-beta-activated Smad3 represses MEF2-dependent transcription in myogenic differentiation. EMBO J. 2004;23:1557–1566. doi: 10.1038/sj.emboj.7600179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Singh R, Bhasin S, Braga M, et al. Regulation of myogenic differentiation by androgens: cross talk between androgen receptor/ beta-catenin and follistatin/transforming growth factor-beta signaling pathways. Endocrinology. 2009;150:1259–1268. doi: 10.1210/en.2008-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]