Abstract
Aims
Abnormal bone metabolism and progressive demineralization have been described in patients with heart failure (HF). We hypothesized that mechanical unloading through implantation of a ventricular assist device (VAD) with subsequent haemodynamic improvement would correct abnormal bone metabolism in patients with advanced HF.
Methods and results
Serum was collected from 14 controls, 20 patients with moderate HF, 34 patients with advanced HF undergoing VAD implantation, and 34 patients at the time of VAD explantation (mean duration: 169 ± 125 days). Bone metabolism markers were measured using enzyme-linked immunosorption assay (ELISA) or chemiluminescence immunoassay (CLIA). Compared with controls, HF patients showed increased parathyroid hormone (PTH: 42 ± 19 vs. 117 ± 117 pg/mL in HF; P < 0.02) with decreased 25-hydroxyvitamin D [25(OH)D: 29 ± 14 vs. 21 ± 11 ng/mL in HF; P = 0.05]. While procollagen-1 N-terminal peptide (P1NP) and osteocalcin were similar, cross-linked C- and N-telopeptides of type I collagen (CTX and NTX) were both higher in HF (NTX: 14 ± 6 vs. 20 ± 11 ng/mL; P < 0.05; CTX: 0.35 ± 0.13 vs. 1.05 ± 0.78 ng/mL; P < 0.01 for controls and HF, respectively). P1NP increased markedly after VAD implantation (49 ± 37 vs. 121 ± 62 ng/mL; P < 0.0001), with a mild decrease in CTX and NTX levels indicating a shift towards anabolic bone formation. Serum PTH correlated with estimated glomerular filtration rate (r = –0.245, P < 0.05).
Conclusion
Patients with advanced HF are characterized by increased levels of biochemical markers of bone resorption potentially as a result of secondary hyperparathyroidism and uncoupling of bone remodelling. Haemodynamic improvement and mechanical unloading after VAD implantation lead to correction of bone metabolism and increased levels of anabolic bone formation markers.
Keywords: Heart failure, Bone metabolism, Ventricular assist, Device
Introduction
Congestive heart failure (HF) is associated with metabolic disturbances which may affect bone metabolism and predispose the individual to exacerbated bone loss.1 Patients with advanced HF have reduced mobility and decreased outdoor physical activities which could influence vitamin D levels and bone metabolism.2 Reduced absorption due to intestinal congestion and urinary losses of Ca2+ and Mg2+ from diuretics and aldosteronism in this population may also play a role in decreased vitamin D levels. Advanced HF is also associated with progressive impairment of renal function which affects vitamin D metabolism, leading to secondary hyperparathyroidism and abnormal calcium (Ca2+) metabolism.3
At the cellular level, the skeleton undergoes continuous remodelling in which old bone is removed and new bone is deposited in its place. Osteoclasts first excavate small pits or resorption cavities on the surfaces of trabecular and cortical bone. Bone resorption is closely followed by bone formation, in which osteoblasts refill the cavities by first synthesizing and then mineralizing new bone matrix.4 These processes can be quantified directly on transiliac crest bone biopsies and indirectly by circulating markers of bone turnover. Serum measurements of procollagen-1 N-terminal peptide (P1NP) and osteocalcin reflect osteoblastic bone formation. Cross-linked N- and C-telopeptides of type I collagen (NTX and CTX) reflect bone resorption. As bone resorption and formation are tightly coupled in space and time, under normal circumstances, circulating bone formation and resorption markers reflect the overall tempo rather than the specific phase of remodelling. However, with ageing and disease, these processes can be uncoupled from each other and the biochemical markers may reflect this uncoupling. Of note, increased bone turnover markers are associated with increased risk of osteoporotic fractures.4
Cardiac transplantation has the greatest survival benefit and is the only curative treatment for patients with advanced HF, but it is only available to a small number of patients due to donor shortage.5 As an alternative, implantable ventricular assist devices (VADs) have been developed for patients with end-stage HF and are currently used as bridge-to-transplantation or destination therapy in patients ineligible for cardiac transplantation.6 VADs reverse many of the molecular, cellular, extracellular, ventricular, neurohormonal, and peripheral abnormalities that are present in patients with end-stage HF. This reverse remodelling includes myocardial changes in left ventricle myocyte diameter, sarcoplasmic reticulum Ca2+-ATPase isoform 2a levels, and force–frequency relationships of isolated superfused muscle fibres.7 VADs also lead to morphological changes including reversal of chamber enlargement, reduction in left ventricular mass, and improved global pump function. They provide volume and pressure unloading of the left ventricle and restore central and peripheral haemodynamics including systemic blood pressure and end-organ perfusion.8
The aim of this study was to assess the effects of haemodynamic improvement through VAD implantation on calciotropic hormones and markers of bone remodelling in patients with HF. We hypothesized that levels of calciotropic hormones are abnormal and bone formation and resorption are uncoupled in advanced HF. We further speculated that mechanical unloading with subsequent haemodynamic improvement through VAD implantation would correct abnormal bone and mineral metabolism in patients with advanced HF.
Methods
Study design
We retrospectively analysed 102 subjects; 88 patients were admitted to Columbia University Medical Center and 14 controls were recruited through the outpatient clinic at Columbia University Medical Center. Clinical and laboratory characteristics of all patients and haemodynamic conditions were taken from electronic medical records at the date closest to the blood draw. Blood was collected from 14 controls, 20 patients with moderate HF [New York Heart Association (NYHA) class II–III, brain natriuretic peptide (BNP) = 558 ± 753], 34 patients with severe HF (NYHA class III–IV, BNP = 1286 ± 1206) undergoing VAD implantation, and 34 patients at the time of VAD explantation (14 matched pairs) during cardiac transplantation. Peripheral venous blood samples were stored after centrifugation at −80°C until assays were performed. Estimated glomerular filtration rate (eGFR) was measured using the Modification of Diet in Renal Disease (MDRD) formula: eGFR = 175 ×(SCr)−1.154 × (Age)−0.203 × (0.742 if female) × (1.212 if African American). Patients with primary hyperparathyroidism were excluded from the study.
The investigation conforms with the principles outlined in the Declaration of Helsinki. The study was approved by the Institutional Review Board of Columbia University Medical Center. All patients provided written informed consent before inclusion in the study.
Echocardiography
Echocardiography was used in order to provide measures of ventricular structure and function through both conventional echocardiography and tissue Doppler analysis (Philips Healthcare Corp., Andover, MA, USA). A standard comprehensive M-mode, 2D-echocardiogram and Doppler study were performed in each patient.
Analysis of markers of bone metabolism
Serum levels of the bone resorption markers NTX and CTX, and the bone formation marker carboxylated osteocalcin were determined using enzyme-linked immunosorbent assay (ELISA) kits (CTX, Immunodiagnostic Systems, Scottsdale, AZ, USA; and NTX, Inverness Medical, Princeton, NJ, USA). Serum levels of the calciotropic hormones 25(OH)D and intact parathyroid hormone (iPTH), and the bone formation marker trimeric (intact) P1NP were assessed by chemiluminescence immunoassays (all from Immuno Diagnostic Systems, Fountain Hills, A, USA). Tumour necrosis factor-α (TNF-α) was measured by ELISA (R&D Systems, Minneapolis, MN, USA). Procedures were performed according to instructions. The intra-assay variations were 2.5% for 25(OH)D, 9.1% for NTX, 6% for CTX, 4% for osteocalcin, 6.5% for P1NP, and 20% for PTH. The lower detection limit of the assays was 1.25 ng/mL for 25(OH)D, 0.01 ng/mL for NTX, 0.01 ng/mL for CTX, 0.5 ng/mL for osteocalcin, 2 µg/L for P1NP, and 1.23 pg/mL for PTH.
Statistics
Normality was assessed using the Kolmogorov–Smirnov test. Results were analysed using Student's unpaired t-test and the analysis of variance (ANOVA) Tukey post-hoc test for normal distributions. Samples with non-parametric distribution were assessed using the Mann–Whitney U-test and Kruskal–Wallis test. Paired samples were analysed using the paired Student's t-test. Data are presented as means ± SD. Correlations were determined using the Spearman's test. P-values < 0.05 were considered statistically significant.
Results
Baseline demographics
We retrospectively analysed 102 subjects who were admitted to Columbia University Medical Center. Blood was collected randomly throughout the day from 14 controls recruited from our outpatient clinic, 20 patients with moderate HF, and 34 patients with severe HF undergoing VAD implantation at Columbia University Medical Center between February 2003 and October 2010. Mean duration of HF was 5.6 ± 4.9 years, defined as the time from the patient's diagnosis of HF to the day of VAD implantation. Further, we analysed 34 patients at the time of VAD explantation during cardiac transplantation [age 54 ± 11 years, body mass index (BMI) 26 ± 4.3 kg/m2, mean VAD duration 169 ± 125 days]. Out of the 34 VAD-implanted and the 34 VAD-explanted subjects, 14 samples were paired.
Of the 34 patients undergoing VAD implantation, 17 patients were implanted with pulsatile flow devices and 17 patients were implanted with continuous flow devices. Pulsatile flow VADs included the HeartMate I, HeartMate XVE (Thoratec Corp., Pleasanton, CA, USA), and Abiomed BiVAD (Abiomed Inc., Danvers, MA, USA). Continuous flow VADs included the HeartMate II device, Thoratec Intracorporeal BiVAD (Thoratec Corp.), Duraheart VAD (Terumo Corp., Ann Arbor, MI, USA), and the DeBakey VAD (MicroMed Cardiovascular Inc., Houston, TX, USA). Clinical characteristics of all patients are summarized in Table 1.
Table 1.
Patient characteristics
| Control (n = 14) | HF NYHA class II–III (n = 20) | HF NYHA class III–IV pre-VAD (n = 34) | HF NYHA class III–IV post-VAD (n = 34) | |
|---|---|---|---|---|
| Age (years) | 48 ± 8 | 54 ± 8 | 53 ± 11 | 53 ± 11 |
| Gender (% male) | 11 (79) | 18 (90) | 28 (82) | 27 (79) |
| BMI (kg/m2) | 26.3 ± 4.9 | 29.6 ± 4.8 | 27 ± 5.5 | 26 ± 4.3 |
| Aetiology of HF (no of patients, %) | ||||
| Dilated cardiomyopathy | – | 15 (75) | 21 (62) | 19 (56) |
| Ischaemic cardiomyopathy | – | 5 (25) | 13 (38) | 13 (38) |
| Other | – | 0 (0) | 0 (0) | 2 (6) |
| Type of VAD (no. of patients, %) | ||||
| Pulsatile flow | – | 17 (50) | 17 (51.5) | |
| Continuous flow | – | 17 (50) | 16 (48.5) | |
| VAD duration (days) | – | – | – | 164 ± 123 |
| Medication (no. of patients, %) | ||||
| Diuretics | – | 15 (75) | 34 (100)* | 31 (94) |
| Beta-blockers | – | 17 (85) | 27 (79) | 25 (77) |
| ACE inhibitors/AII antagonists | – | 17 (85) | 23 (67) | 21 (62) |
| Calcium channel blockers | – | 1 (5) | 1 (3) | 5 (15) |
| Coumadin | – | 5 (25) | 19 (56) | 26 (84) |
| Aldosterone antagonists | – | 10 (20) | 21 (62) | 17 (55) |
| Vitamin D supplements | – | 0 (0) | 0 (0) | 2 (6) |
| Calcium supplements | – | 0 (0) | 1 (3) | 0 (0) |
| Bisphosphonates | – | 0 (0) | 0 (0) | 0 (0) |
| Multivitamins | 1 (11) | 1 (13) | 7 (21) | 21 (64)** |
Data show the means ± SD. Significance tested using analysis of variance (ANOVA) post-hoc Tukey analysis. Comparisons between class III–IV HF pre-VAD and post-VAD were performed by Student's t-test.
ACE, angiotensin-converting enzyme; AII, angiotensin II; BMI, body mass index; HF, heart failure; NYHA, New York Heart Association; VAD, ventricular assist device.
*P < 0.05 for NYHA HF class III–IV pre-VAD vs. NYHA HF class II–III.
**P < 0.05 for NYHA HF class III–IV pre-VAD vs. post-VAD.
Echocardiographic data
A comparison of echocardiographic parameters of controls, moderate HF patients, and patients with advanced HF before and after VAD implantation are listed in Table 2. Both moderate HF and advanced HF patients showed increased left ventricular end-diastolic dimension (LVEDD) and left ventricular end-systolic dimension (LVESD) compared with controls. Further, advanced HF was associated with decreased fractional shortening (FS), shorter deceleration time of E (DcT), reduced dP/dtmax (the maximum rate of LV pressure increase), and increased E/E' (ratio of early diastolic transmitral velocity to early diastolic mitral annular tissue velocity). These changes were partially reversed in patients studied after VAD implantation with decreased LVEDD and LVESD after mechanical unloading. Further, dP/dtmax and E'increased while E/E' decreased after VAD implantation. These data show that structural changes of the failing heart may be partially reversible after VAD implantation.
Table 2.
Echocardiographic parameters before and after ventricular assist device implantation
| Control (n = 14) | HF NYHA class II–III (n = 20) | HF NYHA class III–IV pre-VAD (n = 34) | HF NYHA class III–IV post-VAD (n = 34) | |
|---|---|---|---|---|
| LVEDD (mm) | 46 ± 3 | 64 ± 11* | 69 ± 12† | 52 ± 13§ |
| LVESD (mm) | 27 ± 2 | 57 ± 12* | 61 ± 12†,‡ | 44 ± 16§ |
| IVST (mm) | 7.8 ± 3.9 | 10.3 ± 2.6 | 9.8 ± 1.9 | 11.1 ± 1.9 |
| PWT (mm) | 8.8 ± 1.7 | 9.8 ± 2.6 | 10.1 ± 1.7 | 11.0 ± 1.8 |
| LVEF (%) | 70 ± 5 | 21 ± 11* | 22 ± 8† | 28 ± 14 |
| FS (%) | 39 ± 2 | 11 ± 6* | 11 ± 4† | 15 ± 9 |
| E (cm/s) | 69 ± 7 | 56 ± 10 | 69 ± 14‡ | 73 ± 10 |
| E/A | 1.5 ± 0.4 | 1.6 ± 0.7 | 2.1 ± 1.5 | 1.7 ± 0.9 |
| DcT (ms) | 149 ± 15 | 210 ± 34* | 174 ± 40‡ | 192 ± 26 |
| dP/dtmax (mmHg/s) | 1529 ± 197 | 733 ± 104* | 603 ± 124†,‡ | 813 ± 150§ |
| E' (cm/s) | 12.8 ± 1.7 | 4.6 ± 0.9* | 4.7 ± 1.3† | 7.0 ± 1.5§ |
| E/E' | 5.6 ± 0.9 | 12.4 ± 2.7* | 15.6 ± 5.1†,‡ | 11.2 ± 3.6§ |
Data show means ± SD. Significance tested using analysis of variance (ANOVA) post-hoc Tukey analysis. Comparisons between class IV HF pre-VAD and post-VAD were performed by Student's t-test.
DcT, deceleration time of E; dP/dtmax, the maximum rate of left ventricular pressure increase; E, early diastolic transmitral velocity; E', early diastolic mitral annular tissue velocity; E/A, ratio between early and late ventricular filling velocity; E/E', ratio of early diastolic transmitral velocity to early diastolic mitral annular tissue velocity; FS, fractional shortening; HF, heart failure; IVST, interventricular septum thickness; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, left ventricular end-systolic dimension; NYHA, New York Heart Association; PWT, posterior wall thickness; VAD, ventricular assist device.
*P < 0.05 for NYHA HF class I–III vs. control.
†P < 0.05 for NYHA HF class III–IV pre-VAD vs. control.
‡P <0.05 for NYHA HF class III–IV pre-VAD vs. NYHA HF class II–III.
§P < 0.05 for NYHA HF class III–IV pre-VAD vs. post-VAD.
Changes in laboratory parameters under a ventricular assist device
Laboratory examinations in controls, in moderate HF patients, and in patients before and after VAD implantation are compared in Table 3. In severe HF patients, blood urea nitrogen (BUN), creatinine (Creat), and direct bilirubin (D-Bili) concentrations were higher, whereas haematocrit (Hct), sodium (Na), calcium (Ca), and albumin (Alb) concentrations were lower than in controls. The eGFR was lower (60 ± 31 mL/min/1.73 m2) in severe HF patients compared with controls (85 ± 14 mL/min/1.73 m2).
Table 3.
Laboratory examinations
| Laboratory parameter | Control (n = 14) | HF NYHA class II–III (n = 20) | HF NYHA class III–IV pre-VAD (n = 34) | HF NYHA class III–IV post-VAD (n = 34) |
|---|---|---|---|---|
| White blood cell count (×103/μL) | 8.5 ± 1.9 | 7.0 ± 2.7 | 9.5 ± 4.2 | 9.3 ± 4.2 |
| Haematocrit (%) | 42.3 ± 4.0 | 40.0 ± 4.8 | 33.5 ± 5.4‡ | 33.4 ± 6.3 |
| Platelet count (×103/μL) | 242 ± 52 | 213 ± 47 | 200 ± 73 | 236 ± 90 |
| Sodium (mEq/L) | 144 ± 9 | 138 ± 4* | 132 ± 5†,‡ | 137 ± 3§ |
| Potassium (mEq/L) | 4.4 ± 0.3 | 4.3 ± 0.4 | 4.1 ± 0.4 | 4.2 ± 0.4 |
| Phosphorous (mg/dL) | 3.2 ± 0.1 | 3.6 ± 0.7 | 3.8 ± 0.8 | 3.6 ± 0.8 |
| Magnesium (mg/dL) | 2.1 ± 0.0 | 1.9 ± 0.2 | 2.1 ± 0.3 | 1.93 ± 0.2§ |
| Calcium (mg/dL) | 10.1 ± 0.8 | 9.2 ± 0.5* | 8.8 ± 0.7† | 9.2 ± 0.6 |
| Blood urea nitrogen (mg/dL) | 15 ± 4 | 20 ± 7 | 41 ± 26†,‡ | 28 ± 16§ |
| Creatinine (mg/dL) | 1.0 ± 0.2 | 1.2 ± 0.3 | 1.6 ± 0.6† | 1.3 ± 0.6 |
| Albumin (mg/dL) | 4.8 ± 0.4 | 4.2 ± 0.4* | 3.6 ± 0.5†,‡ | 3.9 ± 0.6§ |
| Total bilirubin (mg/dL) | 0.5 ± 0.2 | 0.9 ± 0.9* | 1.6 ± 1.4‡ | 0.9 ± 0.4§ |
| Direct bilirubin (mg/dL) | 0.1 ± 0.0 | 0.3 ± 0.5 | 0.6 ± 0.9† | 0.2 ± 0.1§ |
| Aspartate aminotransferase (U/L) | 25 ± 7 | 20 ± 7 | 64 ± 142 | 38 ± 24 |
| Alanine transaminase (U/L) | 29 ± 15 | 20 ± 7 | 37 ± 43 | 28 ± 16 |
| Alkaline phosphatase (U/L) | 65 ± 16 | 76 ± 39 | 96 ± 54 | 124 ± 82 |
| eGFR (mL/min/1.73 m2) | 85 ± 14 | 73 ± 21 | 60 ± 31† | 69 ± 27 |
Data show means ± SD. Significance tested using analysis of variance (ANOVA) post-hoc Tukey analysis. Comparisons between class IV HF pre-VAD and post-VAD were performed by Student's t-test.
eGFR, estimated glomerular filtration rate; HF, heart failure; NYHA New York Heart Association; VAD, ventricular assist device.
*P < 0.05 for NYHA HF class II–III vs. control.
†P < 0.05 for NYHA HF class III–IV pre-VAD vs. control.
‡P < 0.05 for NYHA HF class III-IV pre-VAD vs. NYHA HF class II–III
§P < 0.05 for NYHA HF class III–IV pre-VAD vs. post-VAD.
The Na and Alb concentrations were higher in patients studied after VAD implantation, while Mg, BUN, total bilirubin, and D-Bili, concentrations were lower. eGFR was also higher after VAD implantation, but this difference did not reach statistical significance [pre-VAD 60 ± 31 vs. post-VAD 69 ± 27 mL/min/1.73 m2, P = non-significant (NS)].
Serum levels of bone metabolism markers
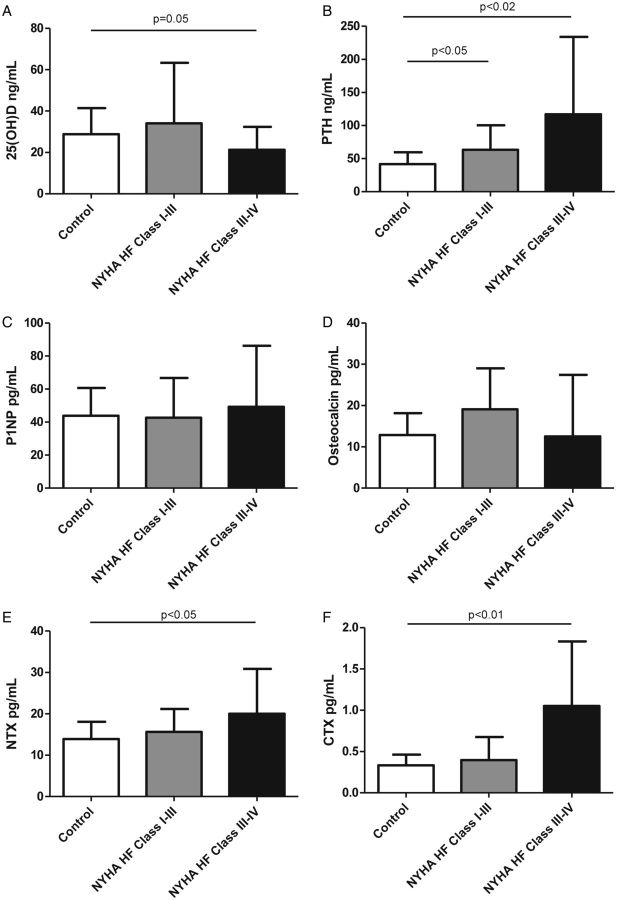
In order to determine the impact of HF on bone metabolism, we compared serum levels of calciotropic hormones [25(OH)D and PTH] and bone turnover markers (P1NP, NTX, CTX, and osteocalcin) in controls, patients with moderate HF, and those with severe HF (Figure 1). Compared with controls, patients with moderate or severe HF had significantly higher serum PTH (control, 42 ± 19 pg/mL; moderate HF, 63 ± 34 pg/mL; P < 0.05; advanced HF, 117 ± 117 pg/mL; P < 0.02). As expected, those with more advanced HF also showed marginally lower serum 25(OH)D levels than controls, while patients with moderate HF did not differ from controls (control, 29 ± 14 ng/mL; moderate HF, 34 ± 29 ng/mL; P = NS; advanced HF, 21 ± 11 ng/mL; P = 0.05). While serum levels of P1NP and osteocalcin were similar, NTX and CTX were higher in patients with more advanced HF (NTX: control, 14 ± 6 ng/mL; moderate HF, 16 ± 5 ng/mL; P = NS; advanced HF, 20 ± 11 ng/mL; P < 0.05; CTX: control, 0.35 ± 0.13 ng/mL; moderate HF, 0.40 ± 0.28 ng/mL; P = NS; advanced HF, 1.05 ± 0.78 ng/mL; P < 0.01).
Figure 1.
Comparison of bone metabolism markers between controls and patients with moderate and advanced heart failure. (A) 25-Hydroxyvitamin D [25(OH)D], (B) parathyroid hormone (PTH), (C) procollagen-1 N-terminal peptide (P1NP), (D) osteocalcin, (E) cross-linked N-terminal telopeptide of type I collagen (NTX), and (F) cross-linked C-terminal telopeptide of type I collagen (CTX).
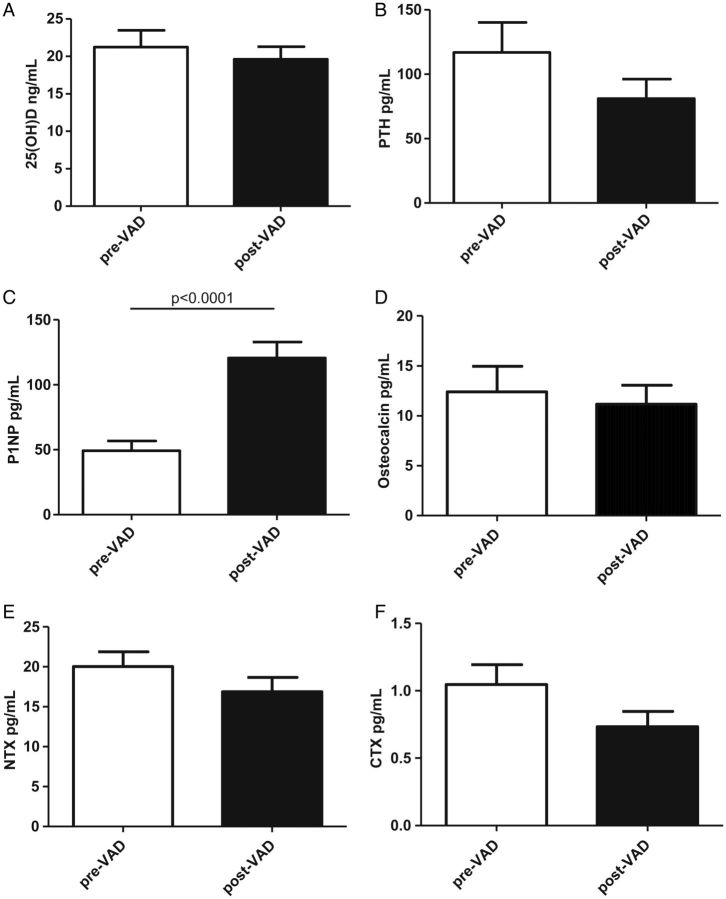
In order to determine the impact of haemodynamic correction through VAD implantation on bone metabolism, we also compared serum levels of 25(OH)D, PTH, P1NP, NTX, osteocalcin, and CTX in patients with advanced HF before (n = 34) and after VAD implantation (n = 34) (Figure 2). Individual trends were analysed in a subset of 14 patients with paired samples available. P1NP was markedly higher in those who had undergone VAD placement compared with those who had not (49 ± 37 vs. 121 ± 62 ng/mL; P < 0.0001). Serum osteocalcin did not differ between the two groups. Serum CTX was slightly but not significantly lower in the group studied after than before VAD placement (1.05 ± 0.78 vs. 0.73 ± 0.44 ng/mL; P = 0.24), as were NTX levels (20 ± 11 vs. 17 ± 10 ng/mL; P = 0.16), indicating a shift towards anabolic bone collagen formation. Serum 25(OH)D and osteocalcin showed no changes, while PTH was slightly but not significantly lower in patients studied after VAD implantation (117 ± 117 vs. 81 ± 74.7 pg/mL; P = 0.18).
Figure 2.
Comparison of bone metabolism markers between pre-ventricular assist device (VAD) implantation and post-VAD implantation. (A) 25-Hydroxyvitamin D [25(OH)D], (B) parathyroid hormone (PTH), (C) procollagen-1 N-terminal peptide (P1NP), (D) osteocalcin, (E) cross-linked N-terminal telopeptide of type I collagen (NTX), and (F) cross-linked C-terminal telopeptide of type I collagen (CTX).
Analysis of paired samples revealed individual trends showing that P1NP increased significantly following VAD implantation (54 ± 39 vs. 116 ± 61 ng/mL; P < 0.05), whereas all other markers followed the above-described group differences without reaching statistical significance in this subset of patients.
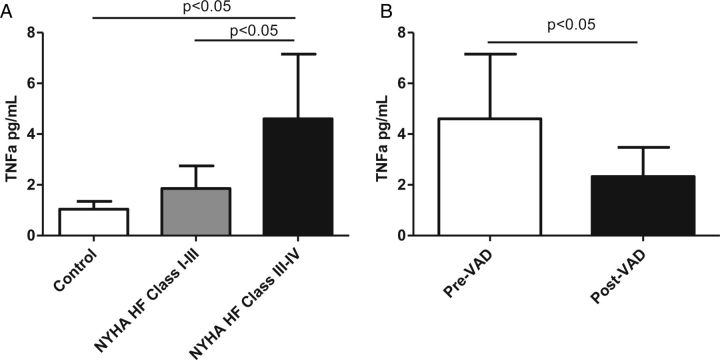
Levels of the proinflammatory cytokine TNF-α were elevated in patients with moderate stable HF and further increased in patients with stage IV advanced HF. Patients had lower levels of TNF-α after VAD implantation (Figure 3). TNF-α levels correlated with NTX (r = 0.52, P < 0.0001) and CTX (r = 0.54, P < 0.0001), and marginally correlated with osteocalcin (r = 0.25, P = 0.08).
Figure 3.
Levels of the proinflammatory cytokine tumour necrosis factor-α (TNF-α) in patients with heart failure (HF) and following ventricular assist device (VAD) placement. (A) Comparison of TNF-α between controls and patients with moderate and advanced HF. (B) Comparison of TNF-α between pre-VAD implantation and post-VAD implantation. NYHA, New York Heart Association.
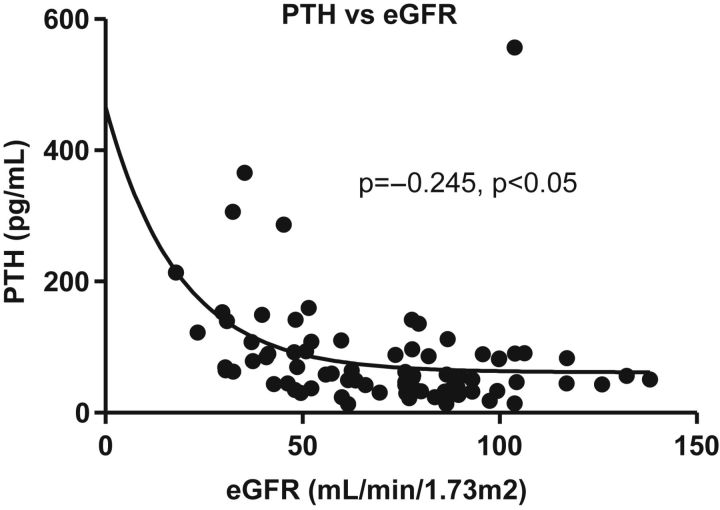
Correlation of bone metabolism and renal function
Patients with HF had progressive impairment of renal function that non-significantly improved with VAD implantation. Serum PTH correlated with eGFR (r = –0.245, P < 0.05) (Figure 4). While eGFR did not show any correlation with 25(OH)D and osteocalcin, both NTX and CTX correlated inversely with eGFR (NTX, r = –0.37, P = 0.0002; CTX, r = –0.42, P = 0.0009). Levels of PTH showed no relationship to 25(OH)D (r = –0.16, P = 0.14), marginally positive correlations with CTX (r = 0.2, P = 0.07), and positive correlations with NTX (r = 0.43, P < 0.0001). Patients with eGFR <50 mL/min/1.73 m2 had significantly higher NTX, CTX, and PTH levels than patients with higher eGFR values (Table 4). No interaction was found between eGFR and markers of hepatic function.
Figure 4.
Correlation between parathyroid hormone (PTH) and estimated glomerular filtration rate (eGFR).
Table 4.
Impact of renal dysfunction on markers of bone turnover
| eGFR <50 mL/min/1.73 m2 | eGFR >50 mL/min/1.73 m2 | P-value | |
|---|---|---|---|
| 25(OH)D (ng/mL) | 21 ± 10 | 27 ± 20 | 0.12 |
| PTH (pg/mL) | 129 ± 98 | 66 ± 72 | 0.003 |
| NTX (pg/mL) | 24 ± 13 | 15 ± 6 | <0.0001 |
| CTX (pg/mL) | 1.19 ± 0.89 | 0.57 ± 0.39 | 0.0004 |
| P1NP (pg/mL) | 74 ± 62 | 67 ± 50 | 0.6 |
| Osteocalcin (pg/mL) | 16 ± 18 | 12 ± 8 | 0.15 |
Data show means ± SD. Significance tested using Student's t-test.
CTX, C-terminal telopeptide; eGFR, estimated glomerular filtration rate; NTX, N-terminal telopeptide; 25(OH)D, 25-hydroxyvitamin D; PTH, parathyroid hormone; P1NP, procollagen 1 N-terminal peptide.
Discussion
In this study, we evaluated bone metabolism in patients with various degrees of HF and in controls, as well as the impact of VAD implantation in patients with advanced HF. Patients with advanced HF showed lower levels of 25(OH)D, a marker of body stores of vitamin D. Patients with moderate and advanced HF had higher levels of PTH and the bone resorption markers NTX and CTX, while levels of osteocalcin and P1NP did not differ from those of controls. These differences appeared to be related to impaired renal function and subsequent secondary hyperparathyroidism. Circulating levels of these bone metabolism markers were partially corrected after implantation of a VAD, with an increase in the early bone formation marker P1NP while the bone resorption markers NTX and CTX were decreased. There were no changes in 25(OH)D and osteocalcin in response to VAD placement.
Several previous studies have described abnormal bone metabolism and progressive demineralization in patients with advanced HF. It has been well established that progressive renal dysfunction in advanced HF with decreased eGFR9 and pre-renal azotaemia10 is related to abnormal vitamin D metabolism, deranged Ca2+ homeostasis, and secondary hyperparathyroidism. Patients with advanced HF show decreased 25(OH)D levels and increased PTH levels that correlate with renal function parameters such as eGFR, as confirmed in the current study.3,11,12 In addition to lower levels of 25(OH)D, changes in circulating levels of bone metabolic markers in our study may also reflect changes in renal clearance. NTX, CTX, PTH, osteocalcin, and small percentages of 25(OH)D are cleared by the kidney,13–16 whereas intact P1NP is cleared from blood by scavenger receptors on liver endothelial cells and not affected by the eGFR.17 However, serum CTX, P1NP, and osteocalcin levels do not increase until the eGFR becomes <50 mL/min, which is less than the average eGFR of our patients.
Secondary hyperparathyroidism is a compensatory mechanism to counteract decreased serum ionized calcium concentrations that may occur in a wide variety of conditions, including renal insufficiency and vitamin D deficiency. Our finding of an inverse correlation between PTH and eGFR, but not between PTH and 25(OH)D, suggests that the secondary hyperparathyroidism in our HF patients is related to renal insufficiency. It is likely that the elevated PTH concentrations, in turn, stimulate osteoclast activity, as evidenced by increased NTX and CTX levels in our patients. Previous studies have reported conflicting results on collagen markers of bone metabolism in HF patients. While one study found increased serum NTX levels in HF,18 another reported no significant differences in CTX and NTX levels in advanced HF.19 PTH also plays a role in homeostatic activation of 1α-hydroxylase in the kidney and bone.20 Of note, it has been shown that excess PTH increases blood pressure and cardiac contractility.21 PTH in HF patients could also increase intracellular calcium overload, leading to increased inflammation, necrosis, and fibrosis.22 We observed increased inflammation as measured by TNF-α in our HF population.
It has been demonstrated that secondary hyperparathyroidism23 and vitamin D deficiency24 occur in HF patients, and both are associated with disease severity and adverse outcome. Further, it has been shown that HF patients have increased risk of fractures.25 However, vitamin D supplementation has been shown to be independently associated with reduced mortality in HF patients.24
Less is known regarding bone formation markers and metabolism in patients with HF. However, we did not find lower P1NP levels in our patients with advanced HF. P1NP reflects an early phase of bone formation, namely synthesis of procollagen type 1. Later stages of bone formation that reflect mineralization of previously synthesized type 1 collagen are reflected by osteocalcin. In our study, there were no differences in osteocalcin in advanced HF patients and only one previous study showed significant differences in this marker in HF patients.26 We speculate that differences in bone catabolism in patients in our study did not reach a sufficient degree of severity to detect differences in this marker.
In addition to the systematic characterization of markers of bone metabolism in patients with various degrees of HF and controls, our study for the first time analysed the impact of haemodynamic differences according to VAD placement on bone metabolism in patients with advanced HF. Mechanical unloading of the failing myocardium is reflected by decreased LVEDD and LVESD and increased IVST (interventricular septum thickness), PWT (posterior wall thickness), and FS% in our patient's echocardiographic data during VAD support. Our findings indicate that the haemodynamic improvement and mechanical unloading after VAD implantation are associated with a trend towards lower serum PTH levels and lower bone resorption markers and significantly higher bone formation markers in HF patients. As shown before, VAD implantation improves renal and hepatic function in HF patients.27 Improved renal function with increased eGFR would be expected to decrease PTH levels, therefore reducing the degree of secondary hyperparathyroidism. Further, lower serum NTX and CTX levels suggest that osteoclast activity is decreased, and higher serum P1NP levels suggest that osteoblast activity and early bone formation are increased. However, the changes in NTX and CTX following VAD were not significant, which may be caused by the short duration of VAD support (average of 169 days) that might have not affected bone catabolism. It should be noted that P1NP is also a marker of cardiac fibrosis, which could also account for the association with VAD implantation we observed in this study.28 While there were differences in P1NP between patients who had or had not received a VAD, we did not detect changes in osteocalcin. P1NP levels reflect collagen synthesis while osteocalcin reflects collagen mineralization; these markers therefore represent two different stages of osteoblastic bone formation. In the bone formation process, osteoblasts synthesize P1NP before osteocalcin, and the half-life of P1NP is shorter than that of osteocalcin; thus increases in P1NP precede increases in osteocalcin. The absence of changes in both 25(OH)D and osteocalcin levels could be related to the median duration of VAD support, which was only 124 days, which is enough time to see changes in the collagen synthesis marker P1NP but may not be enough time to see changes in 25(OH)D and the collagen mineralization marker, osteocalcin. Also, 46% of patients with severe HF undergoing VAD implantation were on coumadin which plays a role in the activation and carboxylation of osteocalcin. Vitamin K is required for the carboxylation of osteocalcin, and it has been shown that individuals supplemented with vitamin K had increased serum levels of carboxylated osteocalcin and decreased undercarboxylated osteocalcin.29 Therefore, vitamin K antagonists may have influenced the measurements of osteocalcin. Epidemiological studies show that this abnormal vitamin K metabolism may increase osteoporotic fracture risk.30
Our study has several limitations. The analysis is limited by its retrospective nature, and our cohort is relatively small for a cross-sectional study. Our comparison is restricted to different time intervals for the duration of the VAD implantation. We also did not have direct imaging of bone morphology such as DEXA (dual-energy X-ray absorptiometry) scanning, computed tomography, or magnetic resonance imaging. These techniques would allow us to quantify bone mineral density and assess bone health more completely in our patients. Our samples were collected randomly throughout the day; both food intake and considerable circadian variability affect bone turnover markers. Because our patients were recruited only from Columbia University Medical Center, this decreases the generalizability of our results. Further studies evaluating patients from various hospitals would be beneficial.
In conclusion, our current study shows biochemical evidence of increased bone resorption in patients with advanced HF associated with impaired renal function. Patients who had VAD implantation had lower bone resorption markers and higher bone formation markers, suggesting reversal of these changes following haemodynamic improvement through VAD implantation. These findings support close monitoring and potential therapeutic correction of impaired bone metabolism in patients with advanced HF, as well as a role for future prospective studies to confirm our findings and elucidate their mechanistic underpinnings.
Funding
The National Institutes of Health Heart, Lung, and Blood Institute (grant nos HL095742-01 and HL101272-01 to P.C.S.); the Thomas Kempner Foundation; Columbia University's Clinical and Translational Science Awards (grant no. UL1RR024156).
Conflict of interest: None declared.
Acknowledgements
We thank Mrs Elzbieta Dworakowski for her technical expertise in running the assays.
References
- 1.Jankowska EA, Jakubaszko J, Cwynar A, Majda J, Ponikowska B, Kustrzycka-Kratochwil D, Reczuch K, Borodulin-Nadzieja L, Banasiak W, Poole-Wilson PA, Ponikowski P. Bone mineral status and bone loss over time in men with chronic systolic heart failure and their clinical and hormonal determinants. Eur J Heart Fail. 2009;11:28–38. doi: 10.1093/eurjhf/hfn004. [DOI] [PubMed] [Google Scholar]
- 2.Zittermann A, Schleithoff SS, Koerfer R. Markers of bone metabolism in congestive heart failure. Clin Chim Acta. 2006;366:27–36. doi: 10.1016/j.cca.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 3.Shane MDE, Mancini MDD, Aaronson MDK, Silverberg MDSJ, Seibel MDMJ, Addesso V, McMahon MSDJ. Bone mass vitamin D deficiency hyperparathyroidism in congestive heart failure. Am J Med. 1997;103:197–207. doi: 10.1016/s0002-9343(97)00142-3. [DOI] [PubMed] [Google Scholar]
- 4.Christenson RH. Biochemical markers of bone metabolism: an overview. Clin Biochem. 1997;30:573–593. doi: 10.1016/s0009-9120(97)00113-6. [DOI] [PubMed] [Google Scholar]
- 5.Stehlik J, Edwards LB, Kucheryavaya AY, Aurora P, Christie JD, Kirk R, Dobbels F, Rahmel AO, Hertz MI. The Registry of the International Society for Heart and Lung Transplantation: Twenty-Seventh Official Adult Heart Transplant Report—2010. J Heart Lung Transplant. 2010;29:1089–1103. doi: 10.1016/j.healun.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Lietz K, Long JW, Kfoury AG, Slaughter MS, Silver MA, Milano CA, Rogers JG, Naka Y, Mancini D, Miller LW. Outcomes of left ventricular assist device implantation as destination therapy in the post-rematch era: implications for patient selection. Circulation. 2007;116:497–505. doi: 10.1161/CIRCULATIONAHA.107.691972. [DOI] [PubMed] [Google Scholar]
- 7.Klotz S, Barbone A, Reiken S, Holmes JW, Naka Y, Oz MC, Marks AR, Burkhoff D. Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. J Am Coll Cardiol. 2005;45:668–676. doi: 10.1016/j.jacc.2004.11.042. [DOI] [PubMed] [Google Scholar]
- 8.Klotz S, Jan Danser AH, Burkhoff D. Impact of left ventricular assist device (LVAD) support on the cardiac reverse remodeling process. Prog Biophys Mol Biol. 2008;97:479–496. doi: 10.1016/j.pbiomolbio.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 9.Packer M, Lee WH, Kessler PD. Preservation of glomerular filtration rate in human heart failure by activation of the renin–angiotensin system. Circulation. 1986;74:766–774. doi: 10.1161/01.cir.74.4.766. [DOI] [PubMed] [Google Scholar]
- 10.Cannon PJ. The kidney in heart failure. N Engl J Med. 1977;296:26–32. doi: 10.1056/NEJM197701062960108. [DOI] [PubMed] [Google Scholar]
- 11.Khouzam RN, Dishmon DA, Farah V, Flax SD, Carbone LD, Weber KT. Secondary hyperparathyroidism in patients with untreated and treated congestive heart failure. Am J Med Sci. 2006;331:30–34. doi: 10.1097/00000441-200601000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Lee AH, Mull RL, Keenan GF, Callegari PE, Dalinka MK, Eisen HJ, Mancini DM, DiSesa VJ, Attie MF. Osteoporosis and bone morbidity in cardiac transplant recipients. Am J Med. 1994;96:35–41. doi: 10.1016/0002-9343(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 13.Dusso AS, Tokumoto M. Defective renal maintenance of the vitamin d endocrine system impairs vitamin D renoprotection: a downward spiral in kidney disease. Kidney Int. 2011;79:715–729. doi: 10.1038/ki.2010.543. [DOI] [PubMed] [Google Scholar]
- 14.Tolouian R, Hernandez GT, Chiang W-Y, Gupta A. A new approach for evaluating bone turnover in chronic kidney disease. Eur J Intern Med. 2010;21:230–232. doi: 10.1016/j.ejim.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Magnusson P, Sharp CA, Magnusson M, Risteli J, Davie MWJ, Larsson L. Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int. 2001;60:257–265. doi: 10.1046/j.1523-1755.2001.00794.x. [DOI] [PubMed] [Google Scholar]
- 16.Alvarez L, Torregrosa J-V, Peris P, Monegal A, Bedini J-L, De Osaba M-JM, Filella X, Martin G, Ricos C, Oppenheimer F, Ballesta A-M. Effect of hemodialysis and renal failure on serum biochemical markers of bone turnover. J Bone Miner Metab. 2004;22:254–259. doi: 10.1007/s00774-003-0476-9. [DOI] [PubMed] [Google Scholar]
- 17.Koivula M-K, Richardson J, Leino A, Valleala H, Griffiths K, Barnes A, Konttinen YT, Garrity M, Risteli J. Validation of an automated intact N-terminal propeptide of type I procollagen (PINP) assay. Clin Biochem. 2010;43:1453–1457. doi: 10.1016/j.clinbiochem.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 18.Kerschan-Schindl K, Strametz-Juranek J, Heinze G, Grampp S, Bieglmayer C, Pacher R, Maurer G, Fialka-Moser V, Pietschmann P. Pathogenesis of bone loss in heart transplant candidates and recipients. J Heart Lung Transplant. 2003;22:843–850. doi: 10.1016/s1053-2498(02)00806-9. [DOI] [PubMed] [Google Scholar]
- 19.Schleithoff SS, Zittermann A, Stüttgen B, Tenderich G, Berthold HK, Körfer R, Stehle P. Low serum levels of intact osteocalcin in patients with congestive heart failure. J Bone Min Metab. 2003;21:247–252. doi: 10.1007/s00774-003-0417-7. [DOI] [PubMed] [Google Scholar]
- 20.Haug CJ, Aukrust P, Haug E, Morkrid L, Muller F, Froland SS. Severe deficiency of 1,25-dihydroxyvitamin D3 in human immunodeficiency virus infection: association with immunological hyperactivity and only minor changes in calcium homeostasis. J Clin Endocrinol Metab. 1998;83:3832–3838. doi: 10.1210/jcem.83.11.5270. [DOI] [PubMed] [Google Scholar]
- 21.Rostand SG, Drueke TB. Parathyroid hormone vitamin D cardiovascular disease in chronic renal failure. Kidney Int. 1999;56:383–392. doi: 10.1046/j.1523-1755.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 22.Kamalov G, Bhattacharya SK, Weber KT. Congestive heart failure: where homeostasis begets dyshomeostasis. J Cardiovasc Pharmacol. 2010;56:320–328. doi: 10.1097/FJC.0b013e3181ed064f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terrovitis J, Zotos P, Kaldara E, Diakos N, Tseliou E, Vakrou S, Kapelios C, Chalazonitis A, Nanas S, Toumanidis S, Kontoyannis D, Karga E, Nanas J. Bone mass loss in chronic heart failure is associated with secondary hyperparathyroidism and has prognostic significance. Eur J Heart Fail. 2012;14:326–332. doi: 10.1093/eurjhf/hfs002. [DOI] [PubMed] [Google Scholar]
- 24.Gotsman I, Shauer A, Zwas DR, Hellman Y, Keren A, Lotan C, Admon D. Vitamin D deficiency is a predictor of reduced survival in patients with heart failure; vitamin D supplementation improves outcome. Eur J Heart Fail. 2012;14:357–366. doi: 10.1093/eurjhf/hfr175. [DOI] [PubMed] [Google Scholar]
- 25.Majumdar SR, Ezekowitz JA, Lix LM, Leslie WD. Heart failure is a clinically and densitometrically independent risk factor for osteoporotic fractures: population-based cohort study of 45,509 subjects. J Clin Endocrinol Metab. 2012;97:1179–1186. doi: 10.1210/jc.2011-3055. [DOI] [PubMed] [Google Scholar]
- 26.Bozic B, Loncar G, Prodanovic N, Radojicic Z, Cvorovic V, Dimkovic S, Popovic-Brkic V. Relationship between high circulating adiponectin with bone mineral density and bone metabolism in elderly males with chronic heart failure. J Card Fail. 2010;16:301–307. doi: 10.1016/j.cardfail.2009.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Russell SD, Rogers JG, Milano CA, Dyke DB, Pagani FD, Aranda JM, Klodell CT, Jr, Boyle AJ, John R, Chen L, Massey HT, Farrar DJ, Conte JV for the HeartMate II Clinical Investigators. Renal and hepatic function improve in advanced heart failure patients during continuous-flow support with the heartmate II left ventricular assist device. Circulation. 2009;120:2352–2357. doi: 10.1161/CIRCULATIONAHA.108.814863. [DOI] [PubMed] [Google Scholar]
- 28.Cavallari LH, Groo VL, Momary KM, Stamos TD, Vaitkus PT. Markers of cardiac collagen turnover are similar in patients with mild and more severe symptoms of heart failure. Congest Heart Fail. 2007;13:275–279. doi: 10.1111/j.1527-5299.2007.07217.x. [DOI] [PubMed] [Google Scholar]
- 29.Emaus N, Gjesdal C, Almås B, Christensen M, Grimsgaard A, Berntsen G, Salomonsen L, Fønnebø V. Vitamin K2 supplementation does not influence bone loss in early menopausal women: a randomised double-blind placebo-controlled trial. Osteoporos Int. 2010;21:1731–1740. doi: 10.1007/s00198-009-1126-4. [DOI] [PubMed] [Google Scholar]
- 30.Zittermann A. Effects of vitamin k on calcium and bone metabolism. Curr Opin Clin Nutr Metab Care. 2001;4:483–487. doi: 10.1097/00075197-200111000-00003. [DOI] [PubMed] [Google Scholar]