Abstract
Since the birth of the first IVF-conceived child in 1978, the use of assisted reproductive technologies (ART) has grown dramatically, contributing to the successful birth of 5 million individuals worldwide. However, there are several reported associations of ART with pregnancy complications, such as low birthweight (LBW), preterm birth, birth defects, epigenetic disorders, cancer and poor metabolic health. Whether this is attributed to ART procedures or to the subset of the population seeking ART remains a controversy, but the most relevant question today concerns the potential long-term implications of assisted conception. Recent evidence has emerged suggesting that ART-conceived children have distinct metabolic profiles that may predispose to cardiovascular pathologies in adulthood. Because the eldest IVF individuals are still too young to exhibit components of chronic middle-aged syndromes, the use of animal models has become particularly useful in describing the effects of unusual or stressful preimplantation experiences on adult fitness. Elucidating the molecular mechanisms by which embryos integrate environmental signals into development and metabolic gene expression programs will be essential for optimizing ART procedures such as in vitro culture conditions, embryo selection and transfer. In the future, additional animal studies to identify mechanisms underlying unfavorable ART outcomes, as well as more epidemiological reviews to monitor the long-term health of ART children are required, given that ART procedures have become routine medical practice.
Keywords: ART, DOHaD, barker hypothesis, reprogramming
Introduction
The use of assisted reproductive technologies (ART) such as IVF and ICSI has increased dramatically worldwide since the birth of the first IVF-conceived child in 1978. ART has been a source of incredible joy to a very large number of families, contributing to the birth of over 5 million babies (ICMART, 2012). Indeed, the great majority of children are healthy with normal development. At the same time, reports of an increased prevalence of birth defects, epigenetic disorders and adverse pregnancy outcomes such as low birth weight (LBW) shorter gestational age and multiple births suggest possible dangers associated with ART. Given that one of the primary goals of medical care is not to harm (the Hippocratic: ‘primum non nocere’), it is of paramount importance that studies are conducted to assess the health of ART children. Additionally, because the eldest IVF-conceived individuals are too young to exhibit signs of chronic middle-age syndromes or age-related diseases, long-term studies on the impact of ART on adult health need to be performed. This review will describe the risk of ART in human pregnancy and delineate the potential mechanisms responsible for the observed health outcomes using the rodent model. Excellent reviews on ART outcome in other species are available (McEvoy et al., 2000; Grace and Sinclair, 2009).
Confounding factors
ART procedures are used for the most part by individuals who failed to conceive spontaneously. Unfortunately, the majority of epidemiological studies compare ART outcomes with those of fertile populations, rather than to the results of a subfertile population conceiving spontaneously. This is important as infertility per se is a credible risk factor for multiple health complications. In fact, there is evidence that women with a history of infertility are at greater risk for antenatal health complications, such as miscarriage, LBW or malformation (Isaksson et al., 2002; Kovalevsky et al., 2003; Schieve et al., 2004; Chung et al., 2006; Davies et al., 2012). In addition, infertile women are known to have an increased prevalence of cardiovascular disease (Parikh et al., 2012), depression (Wilkins et al., 2010) and certain types of reproductive cancers, such as uterine cancer (Brinton et al., 2005). Investigations into perinatal effects of subfertility have revealed that compared with the general population, spontaneously conceiving subfertile women are at risk for hypertension, pre-eclampsia, antepartum hemorrhage and other labor complications, as well as bearing infants preterm or with LBWs (Thomson et al., 2005; Jaques et al., 2010). Additionally, one study revealed that the prevalence of congenital malformation is positively correlated with increased time to pregnancy (Zhu et al., 2006). Finally, comparison of naturally conceiving subfertile women with those treated with ART did not reveal any statistically significant differences in rate of Cesarean section, preterm birth, size for gestational age, requirement of neonatal intensive care or low Apgar score (Raatikainen et al., 2012). In contrast, there was a significantly increased risk of preterm delivery, LBW and need for neonatal intensive care if the untreated subfertile group was restricted to women naturally conceiving within 6 months, suggesting that prolonged infertility can affect perinatal outcome. It is unclear, however, whether particular etiologies of infertility pose greater risk than others (Schieve et al., 2007; Romundstad et al., 2008). A valuable study on this topic demonstrated that the correlation of ART conceptions with lower birthweight, shorter duration of gestation and perinatal death was not present among women who conceived singleton pregnancies both spontaneously and after ART, suggesting a greater effect of infertility on outcome than ART procedures (Romundstad et al., 2008).
A different caveat to investigations of ART outcomes is that the population using ART tends to be older. Unlike the average age of 23 for first (spontaneous) conception in the USA, the typical ART user is 36 years old (CDC; Martinez et al., 2012). By comparison, in 2009, the average maternal age of first child conceived spontaneously versus using ART was 28 versus 35.8 in Australia, and 30 versus 35.1 in the UK (OECD, 2011; Wang et al., 2011; Authority, 2012). Therefore, women who conceive with ART are more likely to enter pregnancy with pre-existing medical conditions, such as diabetes (Schieve et al., 2007).
Perinatal complications associated with ART use
A large number of studies have analyzed the outcome of ART pregnancies. While multiple adverse medical conditions have been linked to the use of assisted reproduction (Table I), it is clear that the most significant and consistent risk associated with the use of ART is the increased incidence of multiple pregnancies. The American Society of Assisted Reproductive Technology (SART) in 2010 (Technology, 2012) reported a 32.4% annual incidence of twins in women under the age of 35, indicating a relative risk (RR) 20-fold greater when compared with spontaneous conceptions (1.2% or 1/80 pregnancies; Martin et al., 2012). The incidence of higher-order multiple pregnancies (triplets or more) is 1.5% (Technology, 2012) or hundred fold greater than in natural conception (<27.3 per 100 000 births or ≤0.027%; Martin et al., 2009). In addition, the risk of monozygotic twins is increased at least 2-fold in ART gestations (Vitthala et al., 2009). Multiple gestations are strongly associated with increased risk of preterm deliveries (PTD; defined as less than 37 weeks gestation) and LBW (defined as a weight of <2500 g). LBW and PTD are both associated with severe negative neonatal outcomes (Jackson et al., 2004). This includes increased risks of necrotizing enterocolitis, cerebral palsy, neuromotor and cognitive dysfunctions and behavioral difficulties (Saigal et al., 1991; Lems et al., 1993; Holman et al., 1997; O'Shea et al., 1998; Buck et al., 2000; Saigal, 2000; Hille et al., 2001). Additionally, PTD is one of the predominant contributors to ART health care costs (Luke et al., 1996): charges for hospitalization increase as gestational age and birthweight decrease (Cuevas et al., 2005). In 2001, fees for PTD and LBW totaled $5.8 billion and represented 47% of all costs for infant hospitalizations in the USA (Russell et al., 2007).
Table I.
Perinatal risk associated with the use of ART.
| Complications | Fold changes with CI | References |
|---|---|---|
| Multiple pregnancy | 32.4% versus 1.25% in <35-year-olda | 2010 SART data |
| Martin et al. (2012) | ||
| Monozygotic twinning | RR 2.25 | Vitthala et al. (2009) |
| Preterm delivery singleton | OR 1.95 (1.73–2.20) | Jackson et al. (2004) |
| RR 2.04 (1.80–2.32) | Helmerhorst et al. (2004) | |
| Preterm delivery twins | RR 1.07 (1.02–1.13) | Helmerhorst et al. (2004) |
| RR 1.23 (1.09–1.41) | McDonald et al. (2010) | |
| LBW singleton | OR 1.77 (1.40–2.22) | Jackson et al. (2004) |
| SRR 1.8 (1.7–1.9) | Schieve et al. (2002) | |
| RR 1.70 (1.50–1.92) | Helmerhorst et al. (2004) | |
| LBW twins | RR 1.03 (0.99–1.08) | Helmerhorst et al. (2004) |
| RR 1.14 (1.06–1.22) | McDonald et al. (2010) | |
| Very LBW singleton | OR 2.70 (2.31–3.14) | Jackson et al. (2004) |
| SRR 1.8 (1.7–2.0) | Schieve et al. (2002) | |
| RR 3.00 (2.07–4.36) | Helmerhorst et al. (2004) | |
| Very LBW twins | RR 0.89 (0.74–1.07) | Helmerhorst et al. (2004) |
| RR 1.28 (0.73–2.24) | McDonald et al. (2010) | |
| Pre-eclampsia singleton | OR 1.55 (1.23–1.95) | Jackson et al. (2004) |
| Placenta previa singleton | OR 2.87 (1.54–5.37) | Jackson et al. (2004) |
| OR 5.6 (4.4–7.0) | Romundstad et al. (2006) | |
| Placenta previa twins | OR 2.9 (1.5–5.8) | Romundstad et al. (2006) |
| Placental abruption singleton | OR 2.4 (1.1–5.2) | Shevell et al. (2005) |
| Cesarean section singleton | OR 2.13 (1.72–2.63) | Jackson et al. (2004) |
| RR 1.54 (1.44–1.66) | Helmerhorst et al. (2004) | |
| Cesarean section twins | RR 1.21 (1.11–1.32) | Helmerhorst et al. (2004) |
aIndicates that OR or RR are not available.
OR, odds ratio; RR, relative risk; SRR, standardized risk ratio.
There is controversial evidence that the outcomes of ART twin gestations are worse than spontaneously conceived twin pregnancies. A meta-analysis of 14 studies determined that IVF twins had an increased risk of PTD and LBW (McDonald et al., 2010). However, others have reported that twins conceived with ART demonstrated perinatal risks similar (Schieve et al., 2002) or somewhat better (Boulet et al., 2008) compared with twins in the general population. Additional, well-controlled studies are necessary to fully elucidate the effects of ART on twin outcome.
Unfavorable obstetric outcomes after ART are often attributed to multiple gestations, but have also been observed in singleton pregnancies. This includes antepartum hemorrhaging (placenta previa, placenta abruption, uterine bleeding), preterm rupture of membranes Pandey et al., 2012), gestational diabetes, labor induction and pregnancy hypertensive disorders such as pregnancy-induced hypertension (Jackson et al., 2004; Romundstad et al., 2006; Shevell et al., 2005; Pandey et al., 2012). Increased hypertension in pregnancy is especially significant because these conditions are related to insufficient trophoblastic invasion into the myometrium (reviewed in Furuya et al., 2008). Prevalence of preterm delivery is greater (Helmerhorst et al., 2004; Jackson et al., 2004; McDonald et al., 2009), and ART singletons are at risk of low (<2500 g) and very low (<1500 g) birthweight, including in gestations lasting more than 37 weeks of length (i.e. gestation at term) (Schieve et al., 2002; Helmerhorst et al., 2004; Jackson et al., 2004; McDonald et al., 2009; Pandey et al., 2012; Camarano et al., 2012). Compared with spontaneous conceptions, perinatal mortality and admission into neonatal intensive care are higher in ART pregnancies (Helmerhorst et al., 2004; Jackson et al., 2004; Sutcliffe and Ludwig, 2007; Pandey et al., 2012). These risks persist after the removal of factors such as ovulation induction or transfer of multiple embryos (Pandey et al., 2012). Notably, these incidences continue to be observed in ART pregnancies today, demonstrating that in spite of advances in the field, there are still unfavorable consequences.
Overall an increase in Cesarean delivery as opposed to operative vaginal delivery following ART has been described (Pandey et al., 2012). This could be related to an increase in the severity of complications that would require a more aggressive surgical intervention. Conversely, the greater incidence of Cesarean section would suggest a more cautious management of ART pregnancies (the ‘precious pregnancy’ factor; Gillet et al., 2011).
The evidence available concerning whether or not ART is connected to an increased risk of miscarriage remains uncertain (Nayak et al., 2011). There is a high incidence of chromosomal irregularities in ART abortuses, with increasing frequency relevant to advanced maternal age, but these effects are also observed in the general population (Spandorfer et al., 2004). It is unclear if these results derive from a direct effect of ART or fertility. For example, there is no reported association of ICSI—which has previously been linked to a higher incidence of chromosomal abnormalities and aneuploidy—with miscarriage relative to other ART procedures (Bettio et al., 2008). However, ICSI pregnancies conceived after testicular sperm extraction do have a higher risk of miscarriage; this may be due in fact to inherent fertility defects, rather than the procedures themselves.
In summary, analysis of these complications demonstrates that reduction of multiple pregnancies is a primary goal in the field of infertility. Fortunately, the risk of multiple gestation can be managed by limiting the number of embryos transferred (ACOG Committee Opinion Number 324, 2005) and studies suggest that the overall rate of PTD and LBW has declined due to a reduction in the number of embryos transferred to the uterus (Practice Committee of the American Society for Reproductive Medicine, 2006). For example, the transfer of four or more fresh non-donor embryos has decreased from 34% in 2000 to 12% in 2009 (CDC). On a related note, since 2000, the percentage of multiple-infant live births has decreased by 14%. It will be interesting to observe if these decreases predispose an associated decline in the prevalence of unfavorable ART outcomes (CDC).
Pediatric complications following ART use
Several studies have reported an increased incidence of birth defects and other pediatric complications in ART-conceived children (Table II; Hansen et al., 2005; Reefhuis et al., 2009; Davies et al., 2012). Overall, the prevalence of major malformation in ART pregnancies is 4–5% as opposed to the risk in the general population of 3–4%, representing a 30% increase. Major birth defects associated with ART include cardiovascular, urogenital and gastrointestinal abnormalities (Reefhuis et al., 2009; Davies et al., 2012). As mentioned earlier, the mechanisms underlying these changes remain unclear, although spontaneously conceiving individuals in the subfertile population also display a greater occurrence of complications.
Table II.
Pediatric risks associated with ART.
| Complications | Fold changes | References |
|---|---|---|
| Malformation singleton | OR 1.35 (1.20–1.51) | Hansen et al. (2005) |
| OR 1.30 (1.16–1.45) | Davies et al. (2012) | |
| Malformation multiple birth | OR 1.16 (0.91–1.49) | Davies et al. (2012) |
| Chromosomal anomalies singleton | OR 0.87 (0.57–1.33) | Davies et al. (2012) |
| Chromosomal anomalies multiple birth | OR 1.34 (0.42–4.33) | Davies et al. (2012) |
| Chromosomal anomalies (post-ICSI): | Bonduelle et al. (1998) | |
| De novo chromosomal aberrations | 1.66% (1.0–2.7%) versus 0.44%a | |
| Sex–chromosomal | 0.83% (0.3–1.6%) versus 0.19%a | |
| Septal heart defects singleton | OR 2.1 (1.1–4.0) | Reefhuis et al. (2009) |
| Septal heart defects twins | OR 1.3 (0.6–2.8) | Reefhuis et al. (2009) |
| Esophageal atresia singleton | OR 4.5 (1.9–10.5) | Reefhuis et al. (2009) |
| Esophageal atresia twins | OR 2.2 (0.7–7.3) | Reefhuis et al. (2009) |
| Hypospadias singleton | OR 2.1 (0.9–5.2) | Reefhuis et al. (2009) |
| Hypospadias twins | OR 2.1 (0.7–6.4) | Reefhuis et al. (2009) |
| Cancer total: | OR 1.42 (1.09–1.87) | Kallen et al. (2010a, b, c) |
| Hepatoblastoma | RR 56.9 (24.0–130.7) | McLaughlin et al. (2006) |
| Retinoblastoma | RR 4·9 (1.6–11·3) | Moll et al. (2003) |
| Leukemia | OR 2.2 (1.2–3.85) | Petridou et al. (2012) |
| Metabolic disease: | Ceelen et al. (2008a, b) | |
| Hypertension | ||
| Systolic | OR 2.1 (1.4–3.3) | |
| Diastolic | OR 1.9 (1.2–3.0) | |
| Elevated fasting glucose | 5.0 versus 4.8 (mmol/l) (P=0.005)a | |
| Imprinting disorders: | ||
| AS | 2–3-fold increased riska | Manipalviratn et al. (2009) |
| Beckwith–Widemann syndrome | ||
| Cerebral palsy singleton | OR 2.8 (1.3–5.8) | Stromberg et al. (2002) |
| OR 1.82 (1.31–2.52) | Hvidtjorn (2006) | |
| Cerebral palsy twins | OR 0.9 (0.4–1.8) | Stromberg et al. (2002) |
| OR 1.00 (0.65–1.52) | Hvidtjorn (2006) |
aIndicates that OR or RR are not available.
Epigenetic alterations
One of the most studied complications of ART pregnancies is the incidence of imprinting disorders. Imprinted genes are genes in which a particular allele is inactivated in a parent-of-origin-dependent manner, and imprinting disorders arise when a maternal or paternal allele is inappropriately expressed because of abnormal DNA methylation [see these excellent reviews (Manipalviratn et al., 2009; Batcheller et al., 2011)]. Two conditions, Beckwith–Wiedemann syndrome (BWS) and Angelman syndrome (AS), have been described as more common in ART children. BWS is a congenital growth disorder arising from mutation or epimutation of certain imprinted genes on chromosome 11, with symptoms including enlargement of several parts of the body, abdominal wall defects, mild microcephaly, hypoglycemia and increased rate of tumor development, among others (Maher et al., 2003). The frequency of BWS is once every 13 700 pregnancies, but this increases 2–3-fold with ART. Overall, fewer than 50 ART children (out of 5 million total ART individuals) have been reported affected by BWS (Manipalviratn et al., 2009).
AS is a disorder characterized by functionally severe developmental delays, speech impairment, motor and behavioral abnormalities (Williams et al., 1995). Causes of AS include imprinting error, mutation, deletion or uniparental disomy of the gene UBE3A. Its prevalence in the general population is 1:12 000, although AS occurrence secondary to an imprinting error is found only once every 300 000 cases. To date, there are seven reports of children with AS born after IVF or ICSI, and 70% of these cases have imprinting defects as the etiologic factor. An additional seven cases have been identified following ovulation induction and/or intrauterine insemination (Sutcliffe et al., 2006; Doornbos et al., 2007). While the absolute risk of these conditions is low, their increased incidence is worrisome because it might indicate more widespread, as-of-yet unidentified epigenetic changes that could affect the life-long health of ART-conceived children (Maher et al., 2003).
The study published Katari et al. (2009) is useful toward understanding this concept. The authors examined DNA methylation at more than 700 genes in placenta and cord blood cells between newborns conceived in vitro (n= 13) versus in vivo (n= 10). The global changes in methylation were minimal and methylation patterns were not unique to form of conception, suggesting that IVF children do not have an obvious epigenetic fingerprint. However, placentae of IVF children had lower overall methylation, whereas cord blood had higher mean methylation at CpG sites. In addition, some individuals from the in vitro group displayed broader gene expression changes differing by more than two standard deviations from the in vivo group mean at select loci. In contrast, a recent study failed to observe global methylation differences after ART in placentae or cord blood samples (Rancourt et al., 2012). Interestingly, the authors correlated mode of conception with small but significant methylation changes at specific imprinted loci, yet did not observe a corresponding change in transcriptional activity of the related genes. These findings would suggest that there may be epigenetic differences in children born after IVF; whether these changes are secondary to ART procedures or attributable to infertility is unknown, and larger studies are required to address these questions.
Cancer
While older studies failed to observe a greater cancer risk (Klip et al., 2001; Källén et al., 2005), more recent analyses found a moderately enhanced risk for certain types of cancer in children conceived by IVF, which may be related to a growing IVF-conceived population (Kallen et al., 2010c). In particular, Kallen et al. (2010a) identified 53 cases of cancer in children who were born after IVF counter to an expected 38 cases. Among these were 18 instances of hematologic cancer (15 of which were acute lymphoblastic leukemia), 17 cases of eye or central nervous system tumors and 12 occurrences of other solid tumor cancers. The total cancer risk estimate was an odds ratio of 1.42. Other studies have confirmed a greater incidence of some of these cancers in the IVF population: Petridou et al. (2012) found an increase in early onset acute lymphoblastic leukemia (RR = 2.58), while Moll et al. (2003) reported an increased risk of retinoblastoma in IVF children. One study found a 9-fold increased risk of hepatoblastoma in children of parents who used infertility treatments (McLaughlin et al., 2006), although a more recent study did not confirm these findings (Puumala et al., 2012). Because of multiple confounding factors, it is unclear whether the reported increased risk is legitimate. As before, it remains uncertain whether the adverse health outcomes derive from the ART procedures, or are inherent characteristics of the ART patients themselves.
Long-term health
The long-term health of ART children is probably the most pressing question of the field today. Because the eldest IVF individual is in her early thirties, it is presently unknown whether ART children will have an increased incidence of age-related disorders, such as hypertension, metabolic syndrome or cardiovascular diseases (Rinaudo and Wang, 2012).
Thus far, there is no evidence of long-term behavioral or neurodevelopmental issues in ART individuals (Sutcliffe and Ludwig, 2007; Ludwig et al., 2009). A systematic review of neuromotor development, cognition, language and behavior did not observe any increased risk of neurodevelopmental disorders after ART (Middelburg et al., 2008). Similarly, IVF children demonstrate normal academic achievement and cognitive ability on measures of education level, school performance, rates of learning, general cognitive ability and developmental disorders (Wagenaar et al., 2008). There is neither a difference in cognitive development of singletons conceived by ICSI at 5–8 years of age, nor significant differences in their IQ distribution (Knoester et al., 2008). A Danish study showed that twins born after assisted conception had a similar risk for neurological sequelae as naturally conceived twins (Pinborg et al., 2004). In contrast, there are studies suggesting an increased risk in cerebral palsy following ART use (Stromberg et al., 2002; Zhu et al., 2010). While some reports indicate that the risk of cerebral palsy is secondary to the increased incidence of prematurity and multiple births associated with ART use (Hvidtjorn et al., 2006), other groups have concluded a direct role for the ART procedures (Zhu et al., 2010).
Research has also investigated a possible association between autism spectrum disorders (ASD) and assisted conception. A widespread Danish population-based study failed to observe greater risk of ASD in children born after assisted conception, after adjusting for variables such as parental age, parity, multiplicity and birthweight (Hvidtjorn et al., 2011). Similarly, a large case–control study in California found no evidence that a history of infertility or ART treatment was correlated with increased risk of ASD among singleton births (Grether et al., 2012). However, this group determined that for patients with a history of infertility or infertility treatment, multiples displayed an increased risk for ASD. A firmer interpretation of these results was precluded by the small sample size of multiple birth patients and the lack of detailed data on the type of ART treatment (Grether et al., 2012).
There are limited studies analyzing growth and metabolic characteristics after ART. Psychomotor, growth and general physical characteristics, as well as overall health are similar between 5-year-old ICSI and matched spontaneously conceived children (Bonduelle et al., 2004). However, ART children are more likely to have required health care resources by this age (Bonduelle et al., 2005). ART does not appear to affect the onset of puberty (Ceelen et al., 2008b; Beydoun et al., 2011), although breast development is reportedly delayed after ICSI (Belva et al., 2012b). In addition, pubertal girls conceived by IVF appear to have advanced bone age (Ceelen et al., 2008a, b). A case–control study comparing 69 IVF- with 71 spontaneously conceived children aged 4–10 years concluded that IVF children were taller, with slightly more favorable lipid profiles and higher insulin-like growth factor (IGF)-I and IGF-II levels (Miles et al., 2007). The authors speculated that IVF-conceived children may possess subtle alterations in DNA methylation patterns at imprinted loci associated with growth, such as IGF-II. In contrast, there are also reports that 3-month-old singletons girls have lower serum IGF, and that ICSI-conceived children have reduced testosterone levels (Mau Kai et al., 2007) and are smaller than their target height at 3 years of age (Mau Kai et al., 2006). Importantly, IGF and testosterone levels are related to infertility and are under genetic influence, suggesting that these characteristics may be inherited from ART-seeking parents rather than affiliated with the ICSI procedure (Mau Kai et al., 2006, 2007).
Probably the best-conducted metabolic studies available come from a group in the Netherlands who compared 225 ART children with 225 children conceived spontaneously by subfertile patients. Natural conception by subfertile parents is a particularly important control, and the optimal way to remove a potential infertility effect. ART children (mean age 12.6) displayed subtle, yet significant changes in blood pressure and glucose levels (Ceelen et al., 2008c), as well as altered fat deposition (Ceelen, 2007). Similar results were recently verified by Scherrer and colleagues (Scherrer et al., 2012) in a comparison of 65 prepubertal ART children with 57 naturally conceived children, including spontaneous conceptions after ovarian stimulation. There were significant differences in systemic circulation, artery structure and pulmonary vascular function after ART, all indicative of vascular dysfunction of the systemic and pulmonary circulation. These data were not related to ovulation induction, parental factors, perinatal complications, gestational age, nor correlated with specific ART variables such as IVF, ICSI or embryo freezing. These observations were confirmed in five ART children whose siblings were conceived spontaneously within the study cohort. Conversely, a different survey reported no significant changes to blood pressure in ICSI adolescents compared with spontaneously conceived controls after correction for growth and early life characteristics (Belva et al., 2012a). Although the clinical significance of these findings is modest, it is possible that the observed changes may amplify with age—a fact that deserves careful monitoring.
Overall, the studies describing perinatal outcomes of ART are reassuring, although often suffer from limitations such as insufficient power to detect statistically significant results in rare outcomes, or the lack of an appropriate comparison group (Buck Louis et al., 2005). Furthermore, most studies examined infants only, and some subtle developmental disorders may manifest at older ages.
Potential ART procedures influencing adverse perinatal outcomes
As we have seen, analysis of ART complications in human literature is compounded by the use of a fertile control group. Although the infertile population is predisposed to complications of pregnancy and additional health risks, there is also growing evidence of distinct effects of ART procedures on unfavorable health outcomes. Many aspects of ART could induce changes in gametes or tissues and predispose the embryos and mother to health problems. For example, preconceptual events such as use of gonadotrophins, and/or the manipulation of gametes and embryos in the laboratory, including the type of media used for embryo culture, have been suggested as potential mechanisms altering the genome or epigenome of embryos and therefore predisposing to the above-described conditions (Johnson et al., 1995; Hansen et al., 2002; Chung et al., 2006).
Most IVF protocols are conducted following the administration of exogenous gonadotrophins to stimulate the maturation of multiple oocytes. It is possible that the rescue of non-dominant follicles from atresia by excess gonadotrophins will permit use of developmentally incompetent oocytes, thus leading to poor outcome. A study investigating perinatal outcome in singleton pregnancies resulting from IVF in conjunction with either controlled ovarian stimulation or natural folliculogenesis revealed that natural cycle IVF infants have significantly higher birthweights (Pelinck et al., 2010). It remains unclear whether this outcome can be related to an effect of ovarian induction on oocyte quality or on endometrial receptivity, or both. There is evidence that exogenous gonadotrophins affect oocyte epigenetics and imprinted gene expression, which may in turn influence fetal growth and development (reviewed in Santos et al., 2010).
In vitro maturation (IVM), or the maturation of prophase I oocytes to metaphase II in vitro prior to fertilization, is a newer technology with limited follow-up of childhood health outcomes (Basatemur and Sutcliffe, 2011). Buckett et al. (2007) investigated obstetric outcome and congenital abnormalities in pregnancies conceived after IVM, IVF and ICSI compared with those in spontaneously conceived controls. They concluded that while all ART pregnancies were associated with an increased risk of multiple pregnancy, Cesarean delivery, and congenital abnormalities, IVM was not associated with any additional risk compared with IVF or ICSI. Interestingly, the mean birthweight of IVM infants (3482 g) was higher than in control (3260 g), IVF (3209 g) and ICSI infants (3163 g) suggestive of epigenetic differences in IVM children (Basatemur and Sutcliffe, 2011).
The use of ICSI has been regarded with suspicion, since it bypasses several events that occur during fertilization. In fact, ICSI-generated zygotes exhibit shorter, atypically patterned calcium oscillations and cleave at a slower rate, resulting in blastocysts with reduced cell number and reduced hatching rate (Giritharan et al., 2010). Several studies have identified an increased incidence of urogenital malformations and in particular hypospadias in boys (Wennerholm, 2000; Ericson and Kallen, 2001; Bonduelle et al., 2005). A study based on data from 5-year-old children has suggested that ICSI is associated with an increased risk of major congenital anomalies (Bonduelle et al., 2005). However, whether the association is due to the ICSI procedure itself, or to inherent sperm defects, could not be determined because the study did not distinguish between male factor conditions and other causes of infertility. Leunens et al. (2008) compared 10-year-old singletons born through ICSI with those born after spontaneous conception and found that the ICSI children obtained verbal and performance intelligence scores comparable to those of spontaneously conceived children. No significant differences were found between ICSI and spontaneously conceived children regarding overall motor or manual skills.
One of the most prevalent concerns of ICSI are its links to chromosomal aberrations, compared with those associated with conventional fertilization in IVF cycles. Gjerris et al. (2008) reported a significantly increased rate of chromosomal abnormalities after ICSI. They found that chromosome aberrations were more common in the ICSI-treated group compared with the IVF-treated group (1.3% versus 0.5%, P < 0.0001). If all chromosomal anomalies were excluded apart from those that were prenatally diagnosed, the results were still significant (4.3% ICSI versus 1.9% IVF, P < 0.01). Researchers in Belgium reported an increase in sex-chromosomal aberrations following ICSI (Bonduelle et al., 1998). The incidence of autosomal defects was due in part to the increase in trisomies, linked with higher maternal ages. There was also an increase in structural de novo aberrations (0.36% compared with 0.07% in the general newborn population).
Another important element with the potential to affect long-term health is the composition of the culture media used. The nutrient composition of culture media has varied tremendously throughout the history of IVF. A two-part study comparing embryo development, pregnancy rate and outcome of singleton IVF pregnancies from two commercially available sequential media culture systems suggested that culture conditions influence perinatal outcomes (Dumoulin et al., 2010; Nelissen et al., 2012). Oocytes retrieved after ovulation induction were fertilized and cultured either in G-IVF™ and G-1™ media available from the Vitrolife G-Series™, or K-SICM medium from Cook Medical before transfer at the 4–8-cell stage. Cycles using G-Series™ media resulted in better quality embryos, increased implantation and pregnancy rates, and higher birthweights, including a decreased incidence of LBW and LBW for gestations carried to term. Unfortunately, the complete composition of both media are unknown, so the authors were unable to speculate as to which components might be more beneficial or deleterious for development and competence. A different group showed that culture in Universal IVF Medium over ISM1™ medium (Origio) improves embryo quality, implantation and pregnancy rates (Xella et al., 2010). Although the concentrations are unknown, ISM1™ medium contains amino acids, including methionine, which would influence several metabolic and epigenetic pathways, leading to altered growth patterns.
There is also evidence that the length of in vitro culture could be important (Kallen et al., 2010b, Kalra et al., 2012). Currently, there is a tendency to culture embryos to the blastocyst stage (5 days in culture) to allow for better embryo selection in order to reduce the risk of multiple gestations, as opposed to transferring cleavage-stage embryos (3 days in culture). Kallen et al. examined pregnancy outcome following blastocyst versus cleavage-stage transfer, determining that after adjustment for confounding variables, the risk of preterm birth and congenital malformations among singletons was significantly greater after blastocyst-stage transfer. When the analysis was restricted to clinics where both types of transfer were conducted, the risk estimates associated with blastocyst transfer increased for preterm birth, LBW, low Apgar score and respiratory diagnoses, but did not change for congenital malformations (Kallen et al., 2010b). Prolonged culture has also been linked with increased rates of monozygotic twinning (Tarlatzis et al., 2002).
Because multiple embryos are often produced during ART procedures, remaining healthy embryos can be cryopreserved for future use. To this end, the cryopreservation and thawing processes could uniquely affect pregnancy outcomes. Overall, the evidence is reassuring. To date, no detectable differences in rates of malformations, imprinting disorders, neurological sequelae or other malignancies exist in children after IVF with fresh or frozen embryos (Pinborg et al., 2010). On the contrary, analysis of IVF singleton pregnancies conceived after cryopreserved versus fresh cycles showed a significantly decreased prevalence of LBW, preterm delivery, Cesarean section (Pinborg et al., 2010), as well as decreased rates of placenta previa (Sazonova et al., 2012) after frozen embryo transfer. One group additionally reported that transfer of cryopreserved embryos was associated with decreased perinatal morbidity (Kansal Kalra et al., 2011). However, frozen embryo transfers are associated with an increased incidence of pre-eclampsia, perinatal mortality, and macrosomia (>4500 g). The latter results are particularly significant because large offspring syndrome after IVF is commonly observed in sheep and cattle, and may derive from epigenetic mechanisms (Young et al., 1998; Sinclair et al., 2000). While the reason of improved outcome is unclear, it may be related to lower estrogen levels and therefore improved endometrium associated with frozen embryo transfer (Mitwally et al., 2006; Shih et al., 2008; Pelinck et al., 2010). However, until larger numbers of children have been born following freezing and thawing of embryos, it is not possible to ascertain that the rate of abnormalities after frozen embryo transfer deviates from fresh cycle rates.
Furthermore, it is possible that different outcomes present according to the stage at which embryos are cryopreserved. One group reported that cleavage-stage embryos are more likely to survive freeze/thaw cycles, without a significant effect on pregnancy or birth rates (Salumets et al., 2003). Finally, the existence of differing embryo freezing techniques (slow freezing versus vitrification), could play an important role. A randomized controlled study of human cleavage stage embryo cryopreservation by slow freezing or vitrification showed that vitrification was associated with higher survival after thawing, metabolic rate, and blastocyst formation (Balaban et al., 2008).
Insight provided by rodent studies
Animal models of ART offer an alternative way to develop and improve ART procedures, as well as investigate their effects on health. Importantly, the use of animal models removes the fertility factor, permitting in-depth analysis of potential effects of ART techniques without the variability caused by subfertility. A caveat is that requirements for fertilization and preimplantation development can vary across different mammalian species; caution must therefore be exercised because animal models might provide an incomplete or inaccurate view of the demands of the human embryo and fetus (He et al., 2010). For example, rodents carry large litters (n = 8–12 pups) for short (∼21 day) gestations, indicating energetic requirements distinct from human pregnancy. Rodents additionally exhibit unique physiology during development: mice have functional brown adipose tissue throughout life, and maturation of metabolic tissues and hormonal regulatory networks occurs at different times than in humans (Rinaudo and Wang, 2012). However, the studies conducted using mammalian models of fertilization and preimplantation development impart enormous promise for further optimizing ART techniques and minimizing potential hazards. Here, we explore the use of animal models to investigate the effects of fertilization method and culture conditions on developmental potential, gene expression and post-natal growth, with particular attention to insights provided from murine studies.
Embryonic development
Numerous reports have demonstrated an effect of fertilization method and preimplantation culture environment on a variety of embryo characteristics, including morphology, developmental potential, growth velocity, cell number and lineage ratio and gene expression (Ho et al., 1995; Zhao et al., 1995; Zhao and Baltz, 1996; Edwards et al., 1998a, b; Lane and Gardner, 2000, 2003; Rinaudo and Schultz, 2004; Rinaudo et al., 2006; Giritharan et al., 2007; Fernandez-Gonzalez et al., 2009; Smith et al., 2009; Delle Piane et al., 2010; Goovaerts et al., 2011; Hentemann et al., 2011). Components of the culture environment influencing development include composition of culture media, pH, oxygen tension, temperature and culture dish elasticity. Schwarzer et al. (2012) effectively demonstrated the importance of the preimplantation environment in a study comparing 13 human ART culture protocols on the developmental competence, cell lineage composition and global gene expression of mouse zygotes. They found culture medium-specific differences in blastocyst and fetal developmental rates, litter sizes and transcriptome profiles. Most surprisingly, rates of blastocyst and fetal potential were only moderately correlated (R2= 0.337), which questions the use of blastocyst formation and morphology as an accurate predictor of successful implantation and development to term.
Fertilization by IVF or direct penetration of the zona by ICSI also significantly impacts embryonic development: ICSI embryos have fewer ICM and TE cells, and display altered patterns of metabolic and developmental gene expression that is largely independent of culture conditions (Giritharan et al., 2010). IVF blastocysts have an increased ICM:TE cell ratio, with over 300 and 100 genes differentially expressed in each respective lineage after IVF (Giritharan et al., 2007, 2012).
Fetal and placental growth
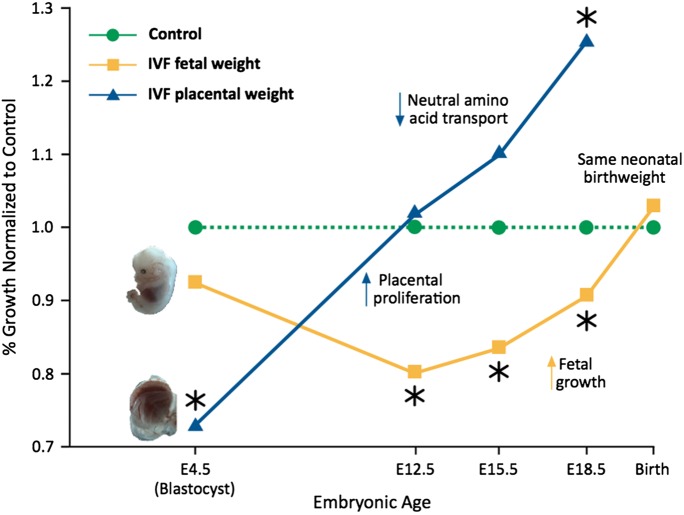
Animal studies show a clear effect of preimplantation embryo manipulation and culture on post-transfer viability and potential. Not only do IVF placentae originate from fewer TE cells, but IVF fetal and placental growth trajectories are significantly different throughout mouse fetal development. In one mouse study we examined the fetal effects of IVF after optimal or suboptimal culture conditions, compared with in vivo control embryos flushed from mouse oviducts and transferred into pseudo-pregnant recipient females (Delle Piane et al., 2010). The more severe culture environment resulted in higher abortion rates, delayed development, smaller fetuses and larger placental weights compared with optimal conditions or the in vivo group. Interestingly, fetuses displayed rapid catch-up growth during the second half of gestation and birthweights were not different between IVF and control animals, although the IVF placentae transported neutral amino acids less efficiently (Fig. 1; Bloise et al., 2012). The findings of normal birthweight in the IVF group in spite of the altered placental and fetal growth curves and modified placental transport suggest that birthweight is a weak indicator of fetal growth and nutrition (Bloise et al., 2012). Other groups have similarly reported impaired embryo viability after IVF or preimplantation embryo culture (Holm et al., 1996; Fernandez-Gonzalez et al., 2004; Block et al., 2010, 2011; Bermejo-Alvarez et al., 2012).
Figure 1.
IVF fetuses and placentae display unique growth trajectories during mouse prenatal development. Mouse IVF fetal (yellow) and placental (blue) weights are normalized to in vivo (green) control embryos (conceived in vivo and flushed from oviducts at the blastocyst stage). At implantation, IVF blastocysts contain fewer ICM and TE cells. Following implantation, there is rapid and continuous proliferation of placental tissue throughout prenatal development and the IVF placentae are 30% larger than controls at E18.5. Importantly, the IVF placentae are less efficient and transport a reduced amount of neutral amino acids. IVF fetuses on the contrary are slow-growing in early gestation; in later gestation they display rapid catch-up growth, possibly due to increased placental size. At birth, there is no significant difference in birthweight between IVF and control mice, suggesting that birthweight is not a reliable predictor of fetal health. (*) indicates significant difference, P < 0.05. Adapted from Bloise et al. (2012).
Post-natal and long-term consequences
The successes of ART procedures are defined by the birth of a live, healthy baby. To this end, post-natal effects occurring outside of the neonatal period are often overlooked. Because the eldest IVF individual has just reached her mid-30s, animal models become especially important in determining potential consequences of ART in adulthood. In 2004, Ecker et al. (2004) demonstrated that adult mice cultured in vitro from the 2-cell to blastocyst-stage exhibit decreased anxiety and impaired spatial memory, without a significant effect of culture or embryo transfer on development to term. Alterations in anxiety levels and memory based upon different preimplantation culture conditions were similarly observed by Fernandez-Gonzalez et al. (2004), and these authors extended their analyses to show that in vitro culture can affect organ size, as well as the development of pneumonia, steatosis and kidney inflammation pathologies. Embryo culture additionally has a significant role in the increased incidence of large offspring syndrome in cattle (reviewed in Sinclair et al., 2000).
Beyond behavioral changes, there is evidence of affected metabolism after ART. Embryo culture increases systolic blood pressure in 21 week mice independent of litter size, maternal origin or body weight, as well as modifies expression of genes regulating cardiovascular and metabolic physiology (Watkins et al., 2007). Our laboratory has evidence that conditions of fertilization and preimplantation development of mouse embryos are linked with long-term growth and glucose homeostasis in adulthood (Rinaudo et al., 2009). Global metabolomic analyses of adult liver and serum demonstrate that IVF-derived mice have unique metabolic profiles, with changes in several pathways affecting glucose metabolism (Rinaudo et al., 2012). However, there is no evidence that embryo culture affects longevity in mice (Sommovilla et al., 2005).
Transgenerational effects have also been described. The progeny of in vitro cultured (IVC) mouse embryos (F1 generation) have lower body weights at weaning, and exhibit organomegaly of the brain, pituitary and kidney (Mahsoudi et al., 2007). One group observed that male IVC mice transmit glucose intolerance and organomegaly into the F1 and F2 generations, suggesting an irreversible effect of preimplantation environment on adult phenotype (Calle et al., 2012).
As described in this section, an important lesson from animal models is that different fertilization methods and preimplantation environments consistently establish distinct developmental profiles. Varying ART conditions may affect the cell number, lineage ratio, growth velocity or pattern of gene expression compared with in vivo-derived control embryos, but each outcome is unique to that condition. Consequently, changes to the preimplantation environment can result in embryos that are comparable or dissimilar to in vivo-derived embryos, but not necessarily ‘better’ or ‘worse’. This implies that the circumstances surrounding preimplantation development affect specific regulatory pathways, or exert an influence on development through a defined mechanism.
Molecular mechanisms
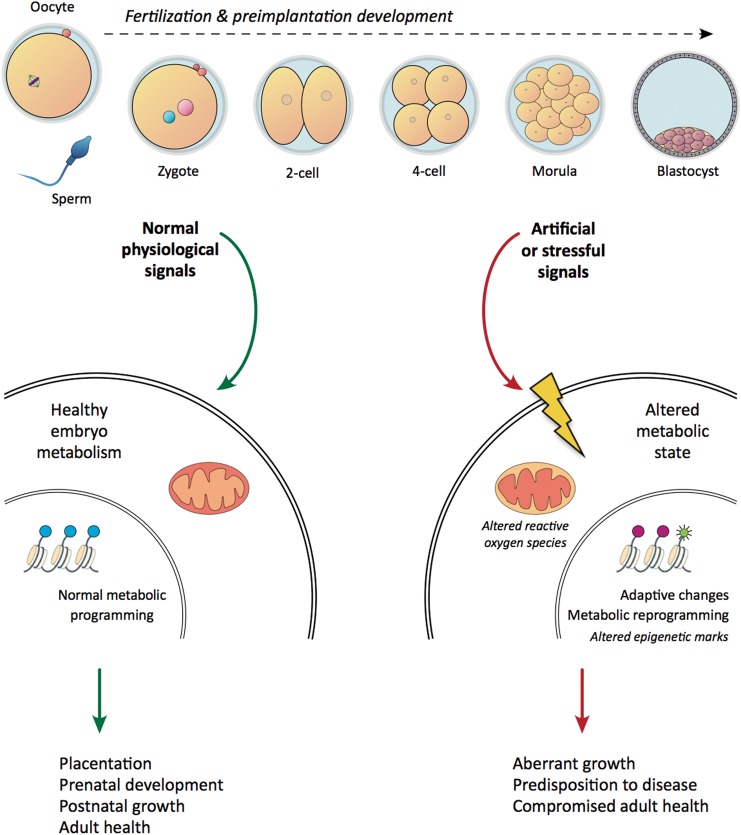
Reports of adverse fetal growth and pregnancy outcome in animal models not only corroborate the similar findings in humans, but also indicate that these consequences are a result of ART procedures, rather than the infertility condition per se. It is therefore essential to understand the potential mechanisms responsible for the observed findings, so that improved culture conditions may be developed to minimize negative health effects. A validated conceptual framework is provided by the Developmental Origins of Health and Disease (DOHaD) hypothesis. This hypothesis states that different environmental conditions occurring at critical points during development have the potential to inform developmental trajectories (Barker, 1998). This concept relies on the understanding of developmental plasticity, which provides one genotype the potential to engender a variety of morphological, physiological or metabolic phenotypes. In particular, if the developing individual is exposed to suboptimal or stressful circumstances that do not accurately represent the post-natal environment, the embryo may reprogram patterns of gene expression or metabolic pathways in a manner conferring an immediate survival advantage, yet rendering it ill-equipped to cope with future events, with consequences for adult fitness (Fig. 2; Bateson et al., 2004; Wadhwa et al., 2009; Low et al., 2011).
Figure 2.
Suboptimal preimplantation environments may irreversibly alter developmental competence. During fertilization and preimplantation development, embryos respond to the environment (growth factors, nutrient availability) by fine-tuning gene expression networks, metabolic pathways and epigenetic marks. Aberrant or suboptimal signals may alter the intracellular metabolic states, leading to permanent adaptations to gene expression and the establishment of altered metabolic pathways and epigenetic marks. This will affect prenatal development and post-natal growth, with deleterious consequences for adult fitness.
More recently, it has been established that developmental periods susceptible to predictive adaptive responses include the fertilization and preimplantation stages. For example, maternal undernutrition specifically during preimplantation stages of rat development affects blastocyst viability, and results in different birthweight, post-natal growth rate, organ sizes and hypertension (Kwong et al., 2000). This implies that maternal or in utero stress can trigger adaption to the developmental program. It follows that reprogramming has profound significance for ART, which rely on procedures of gamete manipulation, preimplantation culture, cryopreservation and embryo transfer. That these techniques may be perceived by the embryo as stressors—and ultimately facilitate permanent changes to cell fates or metabolism—demands further exploration.
Epigenetic
Despite the majority of our cells containing the identical genomic blueprints, most cells adopt unique tissue-specific identities conferred by stable patterns of gene expression. Cell fates are determined largely by epigenetic profiles established during development, such that mechanisms of developmental plasticity may depend on a balance between flexible and permanent adjustment to expression profiles influenced by stochastic environments. Epigenetic regulation occurs at the DNA level via methylation of cytosine bases residing in CpG dinucleotides (Ooi et al., 2009), or by modification of histone proteins through methylation, acetylation, ubiquitination, sumoylation or phosphorylation (Zhang and Reinberg, 2001). These covalent moieties combine into epigenetic signatures that affect gene expression through a variety of mechanisms, including (but not limited to) control of gene accessibility by relaxation or condensation of chromatin, the recruitment of chromatin remodeling enzymes, and occlusion of transcriptional machinery (Fischer et al., 2008; Burdge and Lillycrop, 2010).
Disruptions of epigenetic processes occurring during embryonic and fetal development may contribute to disease-related outcomes, which is relevant in particular to many ART procedures. Preimplantation development is a period of significant epigenetic reorganization, during which the complementary parental genomes undergo dramatic global demethylation and remethylation events (reviewed in Reik, 2007). Shortly after fertilization, rapid demethylation of the male pronucleus occurs by an active, though incompletely understood mechanism. By comparison, methylation marks in the maternal genome are passively removed over the course of several cell divisions by consecutive cycles of chromatin replication and segregation (Saitou et al., 2012). Imprinted genes escape demethylation and are involved in governing normal embryonic and placental development. Novel methylation signatures are largely re-established by the peri-implantation stage, and changes in methylation status are believed to control pluripotency, permitting cells to adopt different fates. Because of the extensive reorganization of chromatin during preimplantation development, the embryo may be particularly vulnerable to perturbation at this time: early environmental signals could elicit epigenetic modifications to permanently shape metabolism, and disease risk (Haaf, 2006).
It is possible that suboptimal conditions or inappropriate nutritional cues can disturb epigenetic programming of metabolic gene networks, such that certain adaptations conferring immediate survival advantages in culture may be somatically maintained and contribute to a variety of post-natal consequences. This could account not only for the increased observation of epigenetic disorders in IVF-conceived individuals, but also for long-term changes to metabolic profiles. Preimplantation culture has been associated with global DNA methylation changes in mice, rats, pigs and humans (Shi and Haaf, 2002; Zaitseva et al., 2007; Katari et al., 2009; Deshmukh et al., 2011), as well as several locus-specific regions (see below).
Although imprinted genes encompass 0.1–0.5% of the genome, they play a pivotal role in early development by controlling processes such as nutrient consumption and the cell cycle (Fowden et al., 2006). Inappropriate regulation of imprinted genes—inherited, sporadic or environmentally induced—has been associated with diseases of growth and development (Miozzo and Simoni, 2002). Different forms of ART affect several imprinting control regions in mice (Doherty et al., 2000; Tremblay et al., 2000; Mann et al., 2004; Rivera et al., 2008; Giritharan et al., 2010, 2012; Bloise et al., 2012). One very interesting study published this year compared embryo growth rates with imprinting regulation. Although counterintuitive, the authors observed that slower developing embryos were most similar to in vivo controls; faster embryos displayed greater embryo volume and cell number, yet imprinted and metabolic gene expression was dysregulated (Market Velker et al., 2012). Because embryo culture creates an 18–24 h delay in development (Bowman and McLaren, 1970; Harlow and Quinn, 1982), faster embryos were historically regarded as optimal. This new evidence that slower developing embryos may in fact best achieve ‘normal’ development patterns suggests a need to reevaluate embryo selection criteria, utilizing biochemical markers over morphological or growth rate assessments.
Metabolism
Excellent reviews already exist describing the fluctuating metabolic needs of preimplantation embryos. Analysis of metabolic physiology in preimplantation embryos indicates diverse nutritional requirements during development from zygote to blastocyst (Brinster, 1965; Biggers et al., 1967). Consequently, preimplantation development occurs over a dynamic range of conditions, demonstrating strong metabolic plasticity and the ability of embryos to compensate for nutrient variability at this time (Edwards et al., 1998a; Gardner and Leese, 1988, 1990; Horsthemke and Ludwig, 2005). Importantly, the dynamic metabolic environment characteristic of the female reproductive tract is lost with in vitro culture. Furthermore, culture conditions considered the most optimal for development affect the expression of several nutrient transporter genes, suggesting that the intracellular metabolic state of IVF-derived embryos is unique (Rinaudo and Schultz, 2004).
New evidence has emerged revealing that the expression of particular metabolic enzymes can affect chromatin remodeling to regulate gene expression (Rathmell and Newgard, 2009). Wellen et al. elegantly linked metabolism and epigenetics by demonstrating that ATP citrate lyase, which catalyzes the production of acetyl-coA from citrate, localizes to the nucleus and is the chief provider of acetyl moieties for histone acetylation (Wellen et al., 2009). The presence of ATP citrate lyase and resulting acetylation events affect cell cycle progression and adipocyte differentiation in a glucose-dependent manner, indicating a key role of cellular metabolic state in crucial developmental processes. If the availability of different metabolites affects chromatin organization, and the critical preimplantation stages of permanent gene reprogramming are disturbed due to unusual developmental circumstances imposed by ART, this offers a reasonable mechanism by which embryo conditions influence metabolic programming and change the future metabolic potential of cells irreversibly.
Conclusions and future perspectives
Recent reports have identified the fertilization and preimplantation stages as critical periods of developmental plasticity. As a result, gamete manipulation and in vitro culture procedures associated with various ART procedures potentially profound consequences for developmental trajectories. While the majority of IVF children are healthy, the evidence presented in this review indicates that ART children possess unique developmental programs. Animal models have broadened these observations, and suggest that there exist additional, longer-term effects on adult metabolic and cardiovascular health. These effects may remain latent until adulthood or the presentation of an additional stressor, but the evidence that preimplantation conditions have a measurable effect that persists beyond the embryonic period is undeniable. This warrants increased research efforts toward investigating the developmental, growth and metabolic requirements of an embryo prior to implantation, as well as improving identification strategies for distinguishing favorable outcomes of ART. Although blastocyst morphology and birthweight are the most ubiquitous, non-invasive means of measuring embryonic and fetal health (respectively) today, they may not be reliable predictors of future health. To this end, there is a need for better markers of ART ‘success’, which would not only affect embryo selection protocols before transfer, but also aid the identification of increased risk for different pathologies that might originate during periods of early developmental sensitivity (Bromer and Seli, 2008).
Authors' roles
P.F.R. conceptualized and designed the article. L.C. contributed to the research and drafting of the clinical topics and tables. S.K.F. researched and drafted the animal studies and molecular mechanisms sections, contributed to the research and drafting of the clinical topics and created the figures. P.R. and S.F. critically revised the manuscript. All the authors approved the final version of this article.
Funding
This work was supported by a National Institute of Child Health and Human Development (NICHD) grant (R01:HD 062803–01A1) to P.F.R. S.K.F. was supported by a National Institute of Health (NIH) training fellowship (5T32DK007418-32).
Conflict of interest
None declared.
References
- ACOG Committee Opinion Number 324. Perinatal risks associated with assisted reproductive technology. Obstet Gynecol. 2005;106:1143–1146. doi: 10.1097/00006250-200511000-00061. [DOI] [PubMed] [Google Scholar]
- Authority HFE. Fertility Treatment in 2012. Trends and Figures. Danvers, MA: Authority HFE; 2012. [Google Scholar]
- Balaban B, Urman B, Ata B, Isiklar A, Larman MG, Hamilton R, Gardner DK. A randomized controlled study of human Day 3 embryo cryopreservation by slow freezing or vitrification: vitrification is associated with higher survival, metabolism and blastocyst formation. Hum Reprod. 2008;23:1976–1982. doi: 10.1093/humrep/den222. [DOI] [PubMed] [Google Scholar]
- Barker DJ. Mothers, Babies and Health in Later Life. 2nd edn. Glasgow: Churchill Livingstone; 1998. [Google Scholar]
- Basatemur E, Sutcliffe A. Health of IVM children. J Assist Reprod Genet. 2011;28:489–493. doi: 10.1007/s10815-011-9561-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batcheller A, Cardozo E, Maguire M, DeCherney AH, Segars JH. Are there subtle genome-wide epigenetic alterations in normal offspring conceived by assisted reproductive technologies? Fertil Steril. 2011;96:1306–1311. doi: 10.1016/j.fertnstert.2011.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, et al. Developmental plasticity and human health. Nature. 2004;430:419–421. doi: 10.1038/nature02725. [DOI] [PubMed] [Google Scholar]
- Belva F, Roelants M, De Schepper J, Roseboom TJ, Bonduelle M, Devroey P, Painter RC. Blood pressure in ICSI-conceived adolescents. Hum Reprod. 2012a;27:3100–3108. doi: 10.1093/humrep/des259. [DOI] [PubMed] [Google Scholar]
- Belva F, Roelants M, Painter R, Bonduelle M, Devroey P, De Schepper J. Pubertal development in ICSI children. Hum Reprod. 2012b;27:1156–1161. doi: 10.1093/humrep/des001. [DOI] [PubMed] [Google Scholar]
- Bermejo-Alvarez P, Roberts RM, Rosenfeld CS. Effect of glucose concentration during in vitro culture of mouse embryos on development to blastocyst, success of embryo transfer, and litter sex ratio. Mol Reprod Dev. 2012;79:329–336. doi: 10.1002/mrd.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettio D, Venci A, Levi Setti PE. Chromosomal abnormalities in miscarriages after different assisted reproduction procedures. Placenta. 2008;29(Suppl. B):126–128. doi: 10.1016/j.placenta.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Beydoun HA, Sicignano N, Beydoun MA, Bocca S, Stadtmauer L, Oehninger S. Pubertal development of the first cohort of young adults conceived by in vitro fertilization in the United States. Fertil Steril. 2011;95:528–533. doi: 10.1016/j.fertnstert.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggers JD, Whittingham DG, Donahue RP. The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci USA. 1967;58:560–567. doi: 10.1073/pnas.58.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block J, Bonilla L, Hansen PJ. Efficacy of in vitro embryo transfer in lactating dairy cows using fresh or vitrified embryos produced in a novel embryo culture medium. J Dairy Sci. 2010;93:5234–5242. doi: 10.3168/jds.2010-3443. [DOI] [PubMed] [Google Scholar]
- Block J, Hansen PJ, Loureiro B, Bonilla L. Improving post-transfer survival of bovine embryos produced in vitro: actions of insulin-like growth factor-1, colony stimulating factor-2 and hyaluronan. Theriogenology. 2011;76:1602–1609. doi: 10.1016/j.theriogenology.2011.07.025. [DOI] [PubMed] [Google Scholar]
- Bloise E, Lin W, Liu X, Simbulan R, Kolahi KS, Petraglia F, Maltepe E, Donjacour A, Rinaudo P. Impaired placental nutrient transport in mice generated by in vitro fertilization. Endocrinology. 2012;153:3457–3467. doi: 10.1210/en.2011-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonduelle M, Aytoz A, Van Assche E, Devroey P, Liebaers I, Van Steirteghem A. Incidence of chromosomal aberrations in children born after assisted reproduction through intracytoplasmic sperm injection. Hum Reprod. 1998;13:781–782. doi: 10.1093/humrep/13.4.781. [DOI] [PubMed] [Google Scholar]
- Bonduelle M, Bergh C, Niklasson A, Palermo GD, Wennerholm UB. Medical follow-up study of 5-year-old ICSI children. Reprod Biomed Online. 2004;9:91–101. doi: 10.1016/s1472-6483(10)62116-5. [DOI] [PubMed] [Google Scholar]
- Bonduelle M, Wennerholm UB, Loft A, Tarlatzis BC, Peters C, Henriet S, Mau C, Victorin-Cederquist A, Van Steirteghem A, Balaska A, et al. A multi-centre cohort study of the physical health of 5-year-old children conceived after intracytoplasmic sperm injection, in vitro fertilization and natural conception. Hum Reprod. 2005;20:413–419. doi: 10.1093/humrep/deh592. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Schieve LA, Nannini A, Ferre C, Devine O, Cohen B, Zhang Z, Wright V, Macaluso M. Perinatal outcomes of twin births conceived using assisted reproduction technology: a population-based study. Hum Reprod. 2008;23:1941–1948. doi: 10.1093/humrep/den169. [DOI] [PubMed] [Google Scholar]
- Bowman P, McLaren A. Viability and growth of mouse embryos after in vitro culture and fusion. J Embryol Exp Morphol. 1970;23:693–704. [PubMed] [Google Scholar]
- Brinster RL. Studies on the development of mouse embryos in vitro. IV. Interaction of energy sources. J Reprod Fertil. 1965;10:227–240. doi: 10.1530/jrf.0.0100227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinton LA, Westhoff CL, Scoccia B, Lamb EJ, Althuis MD, Mabie JE, Moghissi KS. Causes of infertility as predictors of subsequent cancer risk. Epidemiology. 2005;16:500–507. doi: 10.1097/01.ede.0000164812.02181.d5. [DOI] [PubMed] [Google Scholar]
- Bromer JG, Seli E. Assessment of embryo viability in assisted reproductive technology: shortcomings of current approaches and the emerging role of metabolomics. Curr Opin Obstet Gynecol. 2008;20:234–241. doi: 10.1097/GCO.0b013e3282fe723d. [DOI] [PubMed] [Google Scholar]
- Buck GM, Msall ME, Schisterman EF, Lyon NR, Rogers BT. Extreme prematurity and school outcomes. Paediatr Perinat Epidemiol. 2000;14:324–331. doi: 10.1046/j.1365-3016.2000.00276.x. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Schisterman EF, Dukic VM, Schieve LA. Research hurdles complicating the analysis of infertility treatment and child health. Hum Reprod. 2005;20:12–18. doi: 10.1093/humrep/deh542. [DOI] [PubMed] [Google Scholar]
- Buckett WM, Chian R-C, Holzer H, Dean N, Usher R, Tan SL. Obstetric outcomes and congenital abnormalities after in vitro maturation, in vitro fertilization, and intracytoplasmic sperm injection. Obstet Gynecol. 2007;110:885–891. doi: 10.1097/01.AOG.0000284627.38540.80. 10.1097/01.AOG.0000284627.38540.80. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Lillycrop KA. Nutrition, epigenetics, and developmental plasticity: implications for understanding human disease. Annu Rev Nutr. 2010;30:315–339. doi: 10.1146/annurev.nutr.012809.104751. [DOI] [PubMed] [Google Scholar]
- Calle A, Miranda A, Fernandez-Gonzalez R, Pericuesta E, Laguna R, Gutierrez-Adan A. Male mice produced by in vitro culture have reduced fertility and transmit organomegaly and glucose intolerance to their male offspring. Biol Reprod. 2012;87:34. doi: 10.1095/biolreprod.112.100743. [DOI] [PubMed] [Google Scholar]
- Camarano L, Alkon A, Nachtigall R, Schembri M, Weiss S, Croughan M. Preterm delivery and low birth weight in singleton pregnancies conceived by women with and without a history of infertility. Fertil Steril. 2012;98:681–686. doi: 10.1016/j.fertnstert.2012.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Centers for Disease Control and Prevention; Assisted Reproductive Technology Report 2009. [Google Scholar]
- Ceelen M. Body composition in children and adolescents born after in vitro fertilization or spontaneous conception. J Clin Endocrinol Metab. 2007;92:3417–3423. doi: 10.1210/jc.2006-2896. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Growth and development of children born after in vitro fertilization. Fertil Steril. 2008a;90:1662–1673. doi: 10.1016/j.fertnstert.2007.09.005. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JP, van Leeuwen FE, Delemarre-van de Waal HA. Pubertal development in children and adolescents born after IVF and spontaneous conception. Hum Reprod. 2008b;23:2791–2798. doi: 10.1093/humrep/den309. [DOI] [PubMed] [Google Scholar]
- Ceelen M, van Weissenbruch MM, Vermeiden JPW, van Leeuwen FE, Delemarre-van de Waal HA. Cardiometabolic differences in children born after in vitro fertilization: follow-up study. J Clin Endocrinol Metab. 2008c;93:1682–1688. doi: 10.1210/jc.2007-2432. [DOI] [PubMed] [Google Scholar]
- Chung K, Coutifaris C, Chalian R, Lin K, Ratcliffe SJ, Castelbaum AJ, Freedman MF, Barnhart KT. Factors influencing adverse perinatal outcomes in pregnancies achieved through use of in vitro fertilization. Fertil Steril. 2006;86:1634–1641. doi: 10.1016/j.fertnstert.2006.04.038. [DOI] [PubMed] [Google Scholar]
- Cuevas KD, Silver DR, Brooten D, Youngblut JM, Bobo CM. The cost of prematurity: hospital charges at birth and frequency of rehospitalizations and acute care visits over the first year of life: a comparison by gestational age and birth weight. Am J Nurs. 2005;105:56–64. doi: 10.1097/00000446-200507000-00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MJ, Moore VM, Willson KJ, Van Essen P, Priest K, Scott H, Haan EA, Chan A. Reproductive technologies and the risk of birth defects. N Engl J Med. 2012;366:1803–1813. doi: 10.1056/NEJMoa1008095. [DOI] [PubMed] [Google Scholar]
- Delle Piane L, Lin W, Liu X, Donjacour A, Minasi P, Revelli A, Maltepe E, Rinaudo PF. Effect of the method of conception and embryo transfer procedure on mid-gestation placenta and fetal development in an IVF mouse model. Hum Reprod. 2010;25:2039–2046. doi: 10.1093/humrep/deq165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh RS, Ostrup O, Ostrup E, Vejlsted M, Niemann H, Lucas-Hahn A, Petersen B, Li J, Callesen H, Hyttel P. DNA methylation in porcine preimplantation embryos developed in vivo and produced by in vitro fertilization, parthenogenetic activation and somatic cell nuclear transfer. Epigenetics. 2011;6:177–187. doi: 10.4161/epi.6.2.13519. [DOI] [PubMed] [Google Scholar]
- Doherty AS, Mann MR, Tremblay KD, Bartolomei MS, Schultz RM. Differential effects of culture on imprinted H19 expression in the preimplantation mouse embryo. Biol Reprod. 2000;62:1526–1535. doi: 10.1095/biolreprod62.6.1526. [DOI] [PubMed] [Google Scholar]
- Doornbos ME, Maas SM, McDonnell J, Vermeiden JP, Hennekam RC. Infertility, assisted reproduction technologies and imprinting disturbances: a Dutch study. Hum Reprod. 2007;22:2476–2480. doi: 10.1093/humrep/dem172. [DOI] [PubMed] [Google Scholar]
- Dumoulin JC, Land JA, Van Montfoort AP, Nelissen EC, Coonen E, Derhaag JG, Schreurs IL, Dunselman GA, Kester AD, Geraedts JP, et al. Effect of in vitro culture of human embryos on birthweight of newborns. Hum Reprod. 2010;25:605–612. doi: 10.1093/humrep/dep456. [DOI] [PubMed] [Google Scholar]
- Ecker DJ, Stein P, Xu Z, Williams CJ, Kopf GS, Bilker WB, Abel T, Schultz RM. Long-term effects of culture of preimplantation mouse embryos on behavior. Proc Natl Acad Sci USA. 2004;101:1595–1600. doi: 10.1073/pnas.0306846101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the mouse preimplantation embryo: amino acids act as buffers of intracellular pH. Hum Reprod. 1998a;13:3441–3448. doi: 10.1093/humrep/13.12.3441. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, Williams DA, Gardner DK. Intracellular pH of the preimplantation mouse embryo: effects of extracellular pH and weak acids. Mol Reprod Dev. 1998b;50:434–442. doi: 10.1002/(SICI)1098-2795(199808)50:4<434::AID-MRD7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ericson A, Kallen B. Congenital malformations in infants born after IVF: a population-based study. Hum Reprod. 2001;16:504–509. doi: 10.1093/humrep/16.3.504. [DOI] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, Moreira P, Bilbao A, Jimenez A, Perez-Crespo M, Ramirez MA, Rodriguez De Fonseca F, Pintado B, Gutierrez-Adan A. Long-term effect of in vitro culture of mouse embryos with serum on mRNA expression of imprinting genes, development, and behavior. Proc Natl Acad Sci USA. 2004;101:5880–5885. doi: 10.1073/pnas.0308560101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Gonzalez R, de Dios Hourcade J, Lopez-Vidriero I, Benguria A, De Fonseca FR, Gutierrez-Adan A. Analysis of gene transcription alterations at the blastocyst stage related to the long-term consequences of in vitro culture in mice. Reproduction. 2009;137:271–283. doi: 10.1530/REP-08-0265. [DOI] [PubMed] [Google Scholar]
- Fischer JJ, Toedling J, Krueger T, Schueler M, Huber W, Sperling S. Combinatorial effects of four histone modifications in transcription and differentiation. Genomics. 2008;91:41–51. doi: 10.1016/j.ygeno.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Sibley C, Reik W, Constancia M. Imprinted genes, placental development and fetal growth. Horm Res. 2006;65(Suppl. 3):50–58. doi: 10.1159/000091506. [DOI] [PubMed] [Google Scholar]
- Furuya M, Ishida J, Aoki I, Fukamizu A. Pathophysiology of placentation abnormalities in pregnancy-induced hypertension. Vasc Health Risk Manag. 2008;4:1301–1313. doi: 10.2147/vhrm.s4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner DK, Leese HJ. The role of glucose and pyruvate transport in regulating nutrient utilization by preimplantation mouse embryos. Development. 1988;104:423–429. doi: 10.1242/dev.104.3.423. [DOI] [PubMed] [Google Scholar]
- Gardner DK, Leese HJ. Concentrations of nutrients in mouse oviduct fluid and their effects on embryo development and metabolism in vitro. J Reprod Fertil. 1990;88:361–368. doi: 10.1530/jrf.0.0880361. [DOI] [PubMed] [Google Scholar]
- Gillet E, Martens E, Martens G, Cammu H. Prelabour caesarean section following IVF/ICSI in older-term nulliparous women: too precious to push? J Pregnancy. 2011;2011:362518. doi: 10.1155/2011/362518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giritharan G, Talbi S, Donjacour A, Di Sebastiano F, Dobson AT, Rinaudo PF. Effect of in vitro fertilization on gene expression and development of mouse preimplantation embryos. Reproduction. 2007;134:63–72. doi: 10.1530/REP-06-0247. [DOI] [PubMed] [Google Scholar]
- Giritharan G, Li MW, De Sebastiano F, Esteban FJ, Horcajadas JA, Lloyd KC, Donjacour A, Maltepe E, Rinaudo PF. Effect of ICSI on gene expression and development of mouse preimplantation embryos. Hum Reprod. 2010;25:3012–3024. doi: 10.1093/humrep/deq266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giritharan G, Delle Piane L, Donjacour A, Esteban FJ, Horcajadas JA, Maltepe E, Rinaudo P. In vitro culture of mouse embryos reduces differential gene expression between inner cell mass and trophectoderm. Reprod Sci. 2012;19:243–252. doi: 10.1177/1933719111428522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjerris AC, Loft A, Pinborg A, Christiansen M, Tabor A. Prenatal testing among women pregnant after assisted reproductive techniques in Denmark 1995–2000: a national cohort study. Hum Reprod. 2008;23:1545–1552. doi: 10.1093/humrep/den103. [DOI] [PubMed] [Google Scholar]
- Goovaerts IG, Leroy JL, Rizos D, Bermejo-Alvarez P, Gutierrez-Adan A, Jorssen EP, Bols PE. Single in vitro bovine embryo production: coculture with autologous cumulus cells, developmental competence, embryo quality and gene expression profiles. Theriogenology. 2011;76:1293–1303. doi: 10.1016/j.theriogenology.2011.05.036. [DOI] [PubMed] [Google Scholar]
- Grace KS, Sinclair KD. Assisted reproductive technology, epigenetics, and long-term health: a developmental time bomb still ticking. Semin Reprod Med. 2009;27:409–416. doi: 10.1055/s-0029-1237429. [DOI] [PubMed] [Google Scholar]
- Grether JK, Qian Y, Croughan MS, Wu YW, Schembri M, Camarano L, Croen LA. Is Infertility Associated with Childhood Autism? J Autism Dev Disord. 2012;42 doi: 10.1007/s10803-012-1598-5. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Haaf T. Methylation dynamics in the early mammalian embryo: implications of genome reprogramming defects for development. Curr Top Microbiol Immunol. 2006;310:13–22. doi: 10.1007/3-540-31181-5_2. [DOI] [PubMed] [Google Scholar]
- Hansen M, Kurinczuk JJ, Bower C, Webb S. The risk of major birth defects after intracytoplasmic sperm injection and in vitro fertilization. N Engl J Med. 2002;346:725–730. doi: 10.1056/NEJMoa010035. [DOI] [PubMed] [Google Scholar]
- Hansen Ml, Bower C, Milne E, de Klerk N, Kurinczuk J. Assisted reproductive technologies and the risk of birth defects—a systematic review. Human Reproduction. 2005;20:328–338. doi: 10.1093/humrep/deh593. [DOI] [PubMed] [Google Scholar]
- Harlow GM, Quinn P. Development of preimplantation mouse embryos in vivo and in vitro. Aust J Biol Sci. 1982;35:187–193. doi: 10.1071/bi9820187. [DOI] [PubMed] [Google Scholar]
- He K, Zhao H, Wang Q, Pan Y. A comparative genome analysis of gene expression reveals different regulatory mechanisms between mouse and human embryo pre-implantation development. Reprod Biol Endocrinol. 2010;8:41. doi: 10.1186/1477-7827-8-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmerhorst FM, Perquin DA, Donker D, Keirse MJ. Perinatal outcome of singletons and twins after assisted conception: a systematic review of controlled studies. Br Med J. 2004;328:261. doi: 10.1136/bmj.37957.560278.EE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentemann M, Mousavi K, Bertheussen K. Differential pH in embryo culture. Fertil Steril. 2011;95:1291–1294. doi: 10.1016/j.fertnstert.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Hille ET, den Ouden AL, Saigal S, Wolke D, Lambert M, Whitaker A, Pinto-Martin JA, Hoult L, Meyer R, Feldman JF, et al. Behavioural problems in children who weigh 1000 g or less at birth in four countries. Lancet. 2001;357:1641–1643. doi: 10.1016/S0140-6736(00)04818-2. [DOI] [PubMed] [Google Scholar]
- Ho Y, Wigglesworth K, Eppig JJ, Schultz RM. Preimplantation development of mouse embryos in KSOM: augmentation by amino acids and analysis of gene expression. Mol Reprod Dev. 1995;41:232–238. doi: 10.1002/mrd.1080410214. [DOI] [PubMed] [Google Scholar]
- Holm P, Walker SK, Seamark RF. Embryo viability, duration of gestation and birth weight in sheep after transfer of in vitro matured and in vitro fertilized zygotes cultured in vitro or in vivo. J Reprod Fertil. 1996;107:175–181. doi: 10.1530/jrf.0.1070175. [DOI] [PubMed] [Google Scholar]
- Holman RC, Stoll BJ, Clarke MJ, Glass RI. The epidemiology of necrotizing enterocolitis infant mortality in the United States. Am J Public Health. 1997;87:2026–2031. doi: 10.2105/ajph.87.12.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horsthemke B, Ludwig M. Assisted reproduction: the epigenetic perspective. Hum Reprod Update. 2005;11:473–482. doi: 10.1093/humupd/dmi022. [DOI] [PubMed] [Google Scholar]
- Hvidtjorn D, Grove J, Schendel DE, Vaeth M, Ernst E, Nielsen LF, Thorsen P. Cerebral palsy among children born after in vitro fertilization: the role of preterm delivery—a population-based, cohort study. Pediatrics. 2006;118:475–482. doi: 10.1542/peds.2005-2585. [DOI] [PubMed] [Google Scholar]
- Hvidtjorn D, Grove J, Schendel D, Schieve LA, Svaerke C, Ernst E, Thorsen P. Risk of autism spectrum disorders in children born after assisted conception: a population-based follow-up study. J Epidemiol Community Health. 2011;65:497–502. doi: 10.1136/jech.2009.093823. [DOI] [PubMed] [Google Scholar]
- ICMART. ICMART World Report: Preliminary 2008 data. ESHRE Annual Meeting; Istanbul, Turkey. 2012. [Google Scholar]
- Isaksson R, Gissler M, Tiitinen A. Obstetric outcome among women with unexplained infertility after IVF: a matched case–control study. Hum Reprod. 2002;17:1755–1761. doi: 10.1093/humrep/17.7.1755. [DOI] [PubMed] [Google Scholar]
- Jackson RA, Gibson KA, Wu YW, Croughan MS. Perinatal outcomes in singletons following in vitro fertilization: a meta-analysis. Obstet Gynecol. 2004;103:551–563. doi: 10.1097/01.AOG.0000114989.84822.51. [DOI] [PubMed] [Google Scholar]
- Jaques AM, Amor DJ, Baker HW, Healy DL, Ukoumunne OC, Breheny S, Garrett C, Halliday JL. Adverse obstetric and perinatal outcomes in subfertile women conceiving without assisted reproductive technologies. Fertil Steril. 2010;94:2674–2679. doi: 10.1016/j.fertnstert.2010.02.043. [DOI] [PubMed] [Google Scholar]
- Johnson MR, Irvine R, Hills F, Bolton VN, Abbas AA, Brooks AA, Allman AC, Chard T, Nicolaides KH. Superovulation, IGFBP-1 and birth weight. Eur J Obstet Gynecol Reprod Biol. 1995;59:193–195. doi: 10.1016/0028-2243(95)02040-y. [DOI] [PubMed] [Google Scholar]
- Källén B, Finnström O, Nygren K-G, Otterblad Olausson P. In vitro fertilization in Sweden: child morbidity including cancer risk. Fertil Steril. 2005;84:605–610. doi: 10.1016/j.fertnstert.2005.03.035. [DOI] [PubMed] [Google Scholar]
- Kallen B, Finnström O, Lindam A, Nilsson E, Nygren K-Gs, Olausson PO. Cancer risk in children and young adults conceived by in vitro fertilization. Pediatrics. 2010a;126:270–276. doi: 10.1542/peds.2009-3225. [DOI] [PubMed] [Google Scholar]
- Kallen B, Finnström O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Blastocyst versus cleavage stage transfer in in vitro fertilization: differences in neonatal outcome? Fertil Steril. 2010b;94:1680–1683. doi: 10.1016/j.fertnstert.2009.12.027. [DOI] [PubMed] [Google Scholar]
- Kallen B, Finnström O, Lindam A, Nilsson E, Nygren KG, Olausson PO. Cancer risk in children and young adults conceived by in vitro fertilization. Pediatrics. 2010c;126:270–276. doi: 10.1542/peds.2009-3225. [DOI] [PubMed] [Google Scholar]
- Kalra SK, Ratcliffe SJ, Barnhart KT, Coutifaris C. Extended embryo culture and an increased risk of preterm delivery. Obstet Gynecol. 2012;120:69–75. doi: 10.1097/AOG.0b013e31825b88fc. [DOI] [PubMed] [Google Scholar]
- Kansal Kalra S, Ratcliffe SJ, Milman L, Gracia CR, Coutifaris C, Barnhart KT. Perinatal morbidity after in vitro fertilization is lower with frozen embryo transfer. Fertil Steril. 2011;95:548–553. doi: 10.1016/j.fertnstert.2010.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katari S, Turan N, Bibikova M, Erinle O, Chalian R, Foster M, Gaughan JP, Coutifaris C, Sapienza C. DNA methylation and gene expression differences in children conceived in vitro or in vivo. Hum Mol Genet. 2009;18:3769–3778. doi: 10.1093/hmg/ddp319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klip H, Burger CW, de Kraker J, van Leeuwen FE for the O-pg. Risk of cancer in the offspring of women who underwent ovarian stimulation for IVF. Hum Reprod. 2001;16:2451–2458. doi: 10.1093/humrep/16.11.2451. [DOI] [PubMed] [Google Scholar]
- Knoester M, Helmerhorst FM, Vandenbroucke JP, van der Westerlaken LAJ, Walther FJ, Veen S. Cognitive development of singletons born after intracytoplasmic sperm injection compared with in vitro fertilization and natural conception. Fertil Steril. 2008;90:289–296. doi: 10.1016/j.fertnstert.2007.06.090. [DOI] [PubMed] [Google Scholar]
- Kovalevsky G, Rinaudo P, Coutifaris C. Do assisted reproductive technologies cause adverse fetal outcomes? Fertil Steril. 2003;79:1270–1272. doi: 10.1016/s0015-0282(03)00397-2. [DOI] [PubMed] [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Lactate regulates pyruvate uptake and metabolism in the preimplantation mouse embryo. Biol Reprod. 2000;62:16–22. doi: 10.1095/biolreprod62.1.16. [DOI] [PubMed] [Google Scholar]
- Lane M, Gardner DK. Ammonium induces aberrant blastocyst differentiation, metabolism, pH regulation, gene expression and subsequently alters fetal development in the mouse. Biol Reprod. 2003;69:1109–1117. doi: 10.1095/biolreprod.103.018093. [DOI] [PubMed] [Google Scholar]
- Lems W, Hopkins B, Samson JF. Mental and motor development in preterm infants: the issue of corrected age. Early Hum Dev. 1993;34:113–123. doi: 10.1016/0378-3782(93)90046-w. [DOI] [PubMed] [Google Scholar]
- Leunens L, Celestin-Westreich S, Bonduelle M, Liebaers I, Ponjaert-Kristoffersen I. Follow-up of cognitive and motor development of 10-year-old singleton children born after ICSI compared with spontaneously conceived children. Hum Reprod. 2008;23:105–111. doi: 10.1093/humrep/dem257. [DOI] [PubMed] [Google Scholar]
- Low FM, Gluckman PD, Hanson MA. Developmental plasticity and epigenetic mechanisms underpinning metabolic and cardiovascular diseases. Epigenomics. 2011;3:279–294. doi: 10.2217/epi.11.17. [DOI] [PubMed] [Google Scholar]
- Ludwig AK, Katalinic A, Thyen U, Sutcliffe AG, Diedrich K, Ludwig M. Physical health at 5.5 years of age of term-born singletons after intracytoplasmic sperm injection: results of a prospective, controlled, single-blinded study. Fertil Steril. 2009;91:115–124. doi: 10.1016/j.fertnstert.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Luke B, Bigger HR, Leurgans S, Sietsema D. The cost of prematurity: a case–control study of twins vs singletons. Am J Public Health. 1996;86:809–814. doi: 10.2105/ajph.86.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher ER, Afnan M, Barratt CL. Epigenetic risks related to assisted reproductive technologies: epigenetics, imprinting, ART and icebergs? Hum Reprod. 2003;18:2508–2511. doi: 10.1093/humrep/deg486. [DOI] [PubMed] [Google Scholar]
- Mahsoudi B, Li A, O'Neill C. Assessment of the long-term and transgenerational consequences of perturbing preimplantation embryo development in mice. Biol Reprod. 2007;77:889–896. doi: 10.1095/biolreprod.106.057885. [DOI] [PubMed] [Google Scholar]
- Manipalviratn S, DeCherney A, Segars J. Imprinting disorders and assisted reproductive technology. Fertil Steril. 2009;91:305–315. doi: 10.1016/j.fertnstert.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann MR, Lee SS, Doherty AS, Verona RI, Nolen LD, Schultz RM, Bartolomei MS. Selective loss of imprinting in the placenta following preimplantation development in culture. Development. 2004;131:3727–3735. doi: 10.1242/dev.01241. [DOI] [PubMed] [Google Scholar]
- Market Velker BA, Denomme MM, Mann MR. Loss of genomic imprinting in mouse embryos with fast rates of preimplantation development in culture. Biol Reprod. 2012;86:143. doi: 10.1095/biolreprod.111.096602. 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin JA, Hamilton BE, Sutton PD, Ventura SJ, Menacker F, Kirmeye S, Mathews TJ. Births: final data for 2006. 2009. p. 57. National Vital Statistics Report. Atlanta, GA: The Center for Disease Control (CDC),
- Martin JA, Hamilton BE, Ventura SJ, Osterman MJK, Wilson EC, Mathews TJ. Births: final data for 2010. 2012. p. 61. National Vital Statistics Report. Atlanta, GA: The Center for Disease Control (CDC) [PubMed]
- Martinez G, Danniels K, Chandra A. National Health Report: fertility of men and women aged 15–44 years in the United States: National Survey of Family Growth, 2006–2010. 2012. . Atlanta, GA: The Center for Disease Control (CDC), [PubMed]
- Mau Kai C, Main KM, Andersen AN, Loft A, Chellakooty M, Skakkebaek NE, Juul A. Serum insulin-like growth factor-I (IGF-I) and growth in children born after assisted reproduction. J Clin Endocrinol Metab. 2006;91:4352–4360. doi: 10.1210/jc.2006-0701. [DOI] [PubMed] [Google Scholar]
- Mau Kai C, Main KM, Andersen AN, Loft A, Skakkebaek NE, Juul A. Reduced serum testosterone levels in infant boys conceived by intracytoplasmic sperm injection. J Clin Endocrinol Metab. 2007;92:2598–2603. doi: 10.1210/jc.2007-0095. [DOI] [PubMed] [Google Scholar]
- McDonald SD, Han Z, Mulla S, Murphy KE, Beyene J, Ohlsson A. Preterm birth and low birth weight among in vitro fertilization singletons: a systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2009;146:138–148. doi: 10.1016/j.ejogrb.2009.05.035. [DOI] [PubMed] [Google Scholar]
- McDonald SD, Han Z, Mulla S, Ohlsson A, Beyene J, Murphy KE. Preterm birth and low birth weight among in vitro fertilization twins: A systematic review and meta-analyses. Eur J Obstet Gynecol Reprod Biol. 2010;148:105–113. doi: 10.1016/j.ejogrb.2009.09.019. [DOI] [PubMed] [Google Scholar]
- McEvoy TG, Sinclair KD, Young LE, Wilmut I, Robinson JJ. Large offspring syndrome and other consequences of ruminant embryo culture in vitro: relevance to blastocyst culture in human ART. Hum Fertil (Camb) 2000;3:238–246. doi: 10.1080/1464727002000199061. [DOI] [PubMed] [Google Scholar]
- McLaughlin CC, Baptiste MS, Schymura MJ, Nasca PC, Zdeb MS. Maternal and infant birth characteristics and hepatoblastoma. Am J Epidemiol. 2006;163:818–828. doi: 10.1093/aje/kwj104. [DOI] [PubMed] [Google Scholar]
- Middelburg KJ, Heineman MJ, Bos AF, Hadders-Algra M. Neuromotor, cognitive, language and behavioural outcome in children born following IVF or ICSI a systematic review. Hum Reprod Update. 2008;14:219–231. doi: 10.1093/humupd/dmn005. [DOI] [PubMed] [Google Scholar]
- Miles HL, Hofman PL, Peek J, Harris M, Wilson D, Robinson EM, Gluckman PD, Cutfield WS. In vitro fertilization improves childhood growth and metabolism. J Clin Endocrinol Metab. 2007;92:3441–3445. doi: 10.1210/jc.2006-2465. [DOI] [PubMed] [Google Scholar]
- Miozzo M, Simoni G. The role of imprinted genes in fetal growth. Biol Neonate. 2002;81:217–228. doi: 10.1159/000056752. [DOI] [PubMed] [Google Scholar]
- Mitwally MF, Bhakoo HS, Crickard K, Sullivan MW, Batt RE, Yeh J. Estradiol production during controlled ovarian hyperstimulation correlates with treatment outcome in women undergoing in vitro fertilization-embryo transfer. Fertil Steril. 2006;86:588–596. doi: 10.1016/j.fertnstert.2006.02.086. [DOI] [PubMed] [Google Scholar]
- Moll A, Imhof S, Cruysberg J, Schouten-van Meeteren A, Boers M, van Leeuwen F. Incidence of retinoblastoma in children born after in-vitro fertilization. Lancet. 2003;361:309–310. doi: 10.1016/S0140-6736(03)12332-X. [DOI] [PubMed] [Google Scholar]
- Nayak S, Pavone ME, Milad M, Kazer R. Aneuploidy rates in failed pregnancies following assisted reproductive technology. J Womens Health (Larchmt) 2011;20:1239–1243. doi: 10.1089/jwh.2010.2648. [DOI] [PubMed] [Google Scholar]
- Nelissen EC, Van Montfoort AP, Coonen E, Derhaag JG, Geraedts JP, Smits LJ, Land JA, Evers JL, Dumoulin JC. Further evidence that culture media affect perinatal outcome: findings after transfer of fresh and cryopreserved embryos. Hum Reprod. 2012;27:1966–1976. doi: 10.1093/humrep/des145. [DOI] [PubMed] [Google Scholar]
- OECD. OECD Family Database. Paris: OECD; 2011. [Google Scholar]
- Ooi SK, O'Donnell AH, Bestor TH. Mammalian cytosine methylation at a glance. J Cell Sci. 2009;122:2787–2791. doi: 10.1242/jcs.015123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea TM, Klinepeter KL, Dillard RG. Prenatal events and the risk of cerebral palsy in very low birth weight infants. Am J Epidemiol. 1998;147:362–369. doi: 10.1093/oxfordjournals.aje.a009458. [DOI] [PubMed] [Google Scholar]
- Pandey S, Shetty A, Hamilton M, Bhattacharya S, Maheshwari A. Obstetric and perinatal outcomes in singleton pregnancies resulting from IVF/ICSI: a systematic review and meta-analysis. Hum Reprod Update. 2012;18:485–503. doi: 10.1093/humupd/dms018. [DOI] [PubMed] [Google Scholar]
- Parikh NI, Cnattingius S, Mittleman MA, Ludvigsson JF, Ingelsson E. Subfertility and risk of later life maternal cardiovascular disease. Hum Reprod. 2012;27:568–575. doi: 10.1093/humrep/der400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelinck MJ, Keizer MH, Hoek A, Simons AH, Schelling K, Middelburg K, Heineman MJ. Perinatal outcome in singletons after modified natural cycle IVF and standard IVF with ovarian stimulation. Eur J Obstet Gynecol Reprod Biol. 2010;148:56–61. doi: 10.1016/j.ejogrb.2009.09.015. [DOI] [PubMed] [Google Scholar]
- Petridou ET, Sergentanis TN, Panagopoulou P, Moschovi M, Polychronopoulou S, Baka M, Pourtsidis A, Athanassiadou F, Kalmanti M, Sidi V, et al. In vitro fertilization and risk of childhood leukemia in Greece and Sweden. Pediatr Blood Cancer. 2012;58:930–936. doi: 10.1002/pbc.23194. [DOI] [PubMed] [Google Scholar]
- Pinborg A, Loft A, Schmidt L, Greisen G, Rasmussen S, Andersen AN. Neurological sequelae in twins born after assisted conception: controlled national cohort study. Br Med J. 2004;329:311. doi: 10.1136/bmj.38156.715694.3A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinborg A, Loft A, Aaris Henningsen AK, Rasmussen S, Andersen AN. Infant outcome of 957 singletons born after frozen embryo replacement: the Danish National Cohort Study 1995–2006. Fertil Steril. 2010;94:1320–1327. doi: 10.1016/j.fertnstert.2009.05.091. [DOI] [PubMed] [Google Scholar]
- Practice Committee of the American Society for Reproductive Medicine. Multiple pregnancy associated with infertility therapy. Fertil Steril. 2006;86:S106–SS10. doi: 10.1016/j.fertnstert.2006.08.073. [DOI] [PubMed] [Google Scholar]
- Puumala SE, Ross JA, Feusner JH, Tomlinson GE, Malogolowkin MH, Krailo MD, Spector LG. Parental infertility, infertility treatment and hepatoblastoma: a report from the Children's Oncology Group. Hum Reprod. 2012;27:1649–1656. doi: 10.1093/humrep/des109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raatikainen K, Kuivasaari-Pirinen P, Hippelainen M, Heinonen S. Comparison of the pregnancy outcomes of subfertile women after infertility treatment and in naturally conceived pregnancies. Hum Reprod. 2012;27:1162–1169. doi: 10.1093/humrep/des015. [DOI] [PubMed] [Google Scholar]
- Rancourt RC, Harris HR, Michels KB. Methylation levels at imprinting control regions are not altered with ovulation induction or in vitro fertilization in a birth cohort. Hum Reprod. 2012;27:2208–2216. doi: 10.1093/humrep/des151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathmell JC, Newgard CB. Biochemistry. A glucose-to-gene link. Science. 2009;324:1021–1022. doi: 10.1126/science.1174665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reefhuis J, Honein MA, Schieve LA, Correa A, Hobbs CA, Rasmussen SA the National Birth Defects Prevention S. Assisted reproductive technology and major structural birth defects in the United Statesâ€. Hum Reprod. 2009;24:360–366. doi: 10.1093/humrep/den387. [DOI] [PubMed] [Google Scholar]
- Reik W. Stability and flexibility of epigenetic gene regulation in mammalian development. Nature. 2007;447:425–432. doi: 10.1038/nature05918. [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Schultz RM. Effects of embryo culture on global pattern of gene expression in preimplantation mouse embryos. Reproduction. 2004;128:301–311. doi: 10.1530/rep.1.00297. [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Wang E. Fetal programming and metabolic syndrome. Annu Rev Physiol. 2012;74:107–130. doi: 10.1146/annurev-physiol-020911-153245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaudo PF, Giritharan G, Talbi S, Dobson AT, Schultz RM. Effects of oxygen tension on gene expression in preimplantation mouse embryos. Fertil Steril. 2006;86:1252–1265. doi: 10.1016/j.fertnstert.2006.05.017. 65 e1–36. [DOI] [PubMed] [Google Scholar]
- Rinaudo P, Giritharan G, Delle Piane L. Mice conceived by in vitro fertilization (IVF) display reduced growth curve and glucose intolerance. 56th Annual Meeting of the Society for Gynecologic Investigation; Glasgow, Scotland. 2009. [Google Scholar]
- Rinaudo P, Lin W, Liu X, Simbulan R, F S donjacour. Metabolic differences in serum and liver can explain the impaired glucose tolerance found in adult mice conceived by IVF. 45th Annual Meeting of Society for the Study of Reproduction; State College, PA. 2012. [Google Scholar]
- Rivera RM, Stein P, Weaver JR, Mager J, Schultz RM, Bartolomei MS. Manipulations of mouse embryos prior to implantation result in aberrant expression of imprinted genes on day 9.5 of development. Hum Mol Genet. 2008;17:1–14. doi: 10.1093/hmg/ddm280. [DOI] [PubMed] [Google Scholar]
- Romundstad LB, Romundstad PR, Sunde A, von During V, Skjaerven R, Vatten LJ. Increased risk of placenta previa in pregnancies following IVF/ICSI; a comparison of ART and non-ART pregnancies in the same mother. Hum Reprod. 2006;21:2353–2358. doi: 10.1093/humrep/del153. [DOI] [PubMed] [Google Scholar]
- Romundstad LB, Romundstad PR, Sunde A, von During V, Skjaerven R, Gunnell D, Vatten LJ. Effects of technology or maternal factors on perinatal outcome after assisted fertilisation: a population-based cohort study. Lancet. 2008;372:737–743. doi: 10.1016/S0140-6736(08)61041-7. [DOI] [PubMed] [Google Scholar]
- Russell RB, Green NS, Steiner CA, Meikle S, Howse JL, Poschman K, Dias T, Potetz L, Davidoff MJ, Damus K, et al. Cost of hospitalization for preterm and low birth weight infants in the United States. Pediatrics. 2007;120:e1–e9. doi: 10.1542/peds.2006-2386. [DOI] [PubMed] [Google Scholar]
- Saigal S. Follow-up of very low birthweight babies to adolescence. Semin Neonatol. 2000;5:107–118. doi: 10.1053/siny.1999.0003. [DOI] [PubMed] [Google Scholar]
- Saigal S, Rosenbaum P, Szatmari P, Campbell D. Learning disabilities and school problems in a regional cohort of extremely low birth weight (less than 1000 G) children: a comparison with term controls. J Dev Behav Pediatr. 1991;12:294–300. [PubMed] [Google Scholar]
- Saitou M, Kagiwada S, Kurimoto K. Epigenetic reprogramming in mouse pre-implantation development and primordial germ cells. Development. 2012;139:15–31. doi: 10.1242/dev.050849. [DOI] [PubMed] [Google Scholar]
- Salumets A, Tuuri T, Makinen S, Vilska S, Husu L, Tainio R, Suikkari AM. Effect of developmental stage of embryo at freezing on pregnancy outcome of frozen-thawed embryo transfer. Hum Reprod. 2003;18:1890–1895. doi: 10.1093/humrep/deg339. [DOI] [PubMed] [Google Scholar]
- Santos MA, Kuijk EW, Macklon NS. The impact of ovarian stimulation for IVF on the developing embryo. Reproduction. 2010;139:23–34. doi: 10.1530/REP-09-0187. [DOI] [PubMed] [Google Scholar]
- Sazonova A, Kallen K, Thurin-Kjellberg A, Wennerholm UB, Bergh C. Obstetric outcome in singletons after in vitro fertilization with cryopreserved/thawed embryos. Hum Reprod. 2012;27:1343–1350. doi: 10.1093/humrep/des036. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Rimoldi SF, Rexhaj E, Stuber T, Duplain H, Garcin S, de Marchi SF, Nicod P, Germond M, Allemann Y, et al. Systemic and pulmonary vascular dysfunction in children conceived by assisted reproductive technologies. Circulation. 2012;125:1890–1896. doi: 10.1161/CIRCULATIONAHA.111.071183. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Meikle SF, Ferre C, Peterson HB, Jeng G, Wilcox LS. Low and very low birth weight in infants conceived with use of assisted reproductive technology. N Engl J Med. 2002;346:731–737. doi: 10.1056/NEJMoa010806. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Ferre C, Peterson HB, Macaluso M, Reynolds MA, Wright VC. Perinatal outcome among singleton infants conceived through assisted reproductive technology in the United States. Obstet Gynecol. 2004;103:1144–1153. doi: 10.1097/01.AOG.0000127037.12652.76. [DOI] [PubMed] [Google Scholar]
- Schieve LA, Cohen B, Nannini A, Ferre C, Reynolds MA, Zhang Z, Jeng G, Macaluso M, Wright VC. A population-based study of maternal and perinatal outcomes associated with assisted reproductive technology in Massachusetts. Maternal Child Health J. 2007;11:517–525. doi: 10.1007/s10995-007-0202-7. [DOI] [PubMed] [Google Scholar]
- Schwarzer C, Esteves TC, Arauzo-Bravo MJ, Le Gac S, Nordhoff V, Schlatt S, Boiani M. ART culture conditions change the probability of mouse embryo gestation through defined cellular and molecular responses. Hum Reprod. 2012;27:2627–2640. doi: 10.1093/humrep/des223. [DOI] [PubMed] [Google Scholar]
- Shevell T, Malone FD, Vidaver J, Porter TF, Luthy DA, Comstock CH, Hankins GD, Eddleman K, Dolan S, Dugoff L, et al. Assisted reproductive technology and pregnancy outcome. Obstet Gynecol. 2005;106:1039–1045. doi: 10.1097/01.AOG.0000183593.24583.7c. [DOI] [PubMed] [Google Scholar]
- Shi W, Haaf T. Aberrant methylation patterns at the two-cell stage as an indicator of early developmental failure. Mol Reprod Dev. 2002;63:329–334. doi: 10.1002/mrd.90016. [DOI] [PubMed] [Google Scholar]
- Shih W, Rushford DD, Bourne H, Garrett C, McBain JC, Healy DL, Baker HW. Factors affecting low birthweight after assisted reproduction technology: difference between transfer of fresh and cryopreserved embryos suggests an adverse effect of oocyte collection. Hum Reprod. 2008;23:1644–1653. doi: 10.1093/humrep/den150. [DOI] [PubMed] [Google Scholar]
- Sinclair KD, Young LE, Wilmut I, McEvoy TG. In-utero overgrowth in ruminants following embryo culture: lessons from mice and a warning to men. Hum Reprod. 2000;15(Suppl. 5):68–86. doi: 10.1093/humrep/15.suppl_5.68. [DOI] [PubMed] [Google Scholar]
- Smith SL, Everts RE, Sung LY, Du F, Page RL, Henderson B, Rodriguez-Zas SL, Nedambale TL, Renard JP, Lewin HA, et al. Gene expression profiling of single bovine embryos uncovers significant effects of in vitro maturation, fertilization and culture. Mol Reprod Dev. 2009;76:38–47. doi: 10.1002/mrd.20927. [DOI] [PubMed] [Google Scholar]
- Sommovilla J, Bilker WB, Abel T, Schultz RM. Embryo culture does not affect the longevity of offspring in mice. Reproduction. 2005;130:599–601. doi: 10.1530/rep.1.00872. [DOI] [PubMed] [Google Scholar]
- Spandorfer SD, Davis OK, Barmat LI, Chung PH, Rosenwaks Z. Relationship between maternal age and aneuploidy in in vitro fertilization pregnancy loss. Fertil Steril. 2004;81:1265–1269. doi: 10.1016/j.fertnstert.2003.09.057. [DOI] [PubMed] [Google Scholar]
- Stromberg B, Dahlquist G, Ericson A, Finnstrom O, Koster M, Stjernqvist K. Neurological sequelae in children born after in-vitro fertilisation: a population-based study. Lancet. 2002;359:461–465. doi: 10.1016/S0140-6736(02)07674-2. [DOI] [PubMed] [Google Scholar]
- Sutcliffe AG, Ludwig M. Outcome of assisted reproduction. Lancet. 2007;370:351–359. doi: 10.1016/S0140-6736(07)60456-5. [DOI] [PubMed] [Google Scholar]
- Sutcliffe AG, Peters CJ, Bowdin S, Temple K, Reardon W, Wilson L, Clayton-Smith J, Brueton LA, Bannister W, Maher ER. Assisted reproductive therapies and imprinting disorders–a preliminary British survey. Hum Reprod. 2006;21:1009–1011. doi: 10.1093/humrep/dei405. [DOI] [PubMed] [Google Scholar]
- Tarlatzis BC, Qublan HS, Sanopoulou T, Zepiridis L, Grimbizis G, Bontis J. Increase in the monozygotic twinning rate after intracytoplasmic sperm injection and blastocyst stage embryo transfer. Fertil Steril. 2002;77:196–198. doi: 10.1016/s0015-0282(01)02958-2. [DOI] [PubMed] [Google Scholar]
- Technology SoAR. SART 2010 National Clinic Summary Report. 2012. . Atlanta, GA: The Center for Disease Control (CDC),
- Thomson F, Shanbhag S, Templeton A, Bhattacharya S. Obstetric outcome in women with subfertility. BJOG. 2005;112:632–637. doi: 10.1111/j.1471-0528.2004.00489.x. [DOI] [PubMed] [Google Scholar]
- Tremblay KD, Schultz RM, Doherty AS, Bartolomei MS. Effect of embryo culture on imprinted gene expression in the preimplantation mouse embryo. In: Goldberg E, editor. The Testis: From Stem Cell to Sperm Function. New York: Springer; 2000. [Google Scholar]
- Vitthala S, Gelbaya TA, Brison DR, Fitzgerald CT, Nardo LG. The risk of monozygotic twins after assisted reproductive technology: a systematic review and meta-analysis. Hum Reprod Update. 2009;15:45–55. doi: 10.1093/humupd/dmn045. [DOI] [PubMed] [Google Scholar]
- Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27:358–368. doi: 10.1055/s-0029-1237424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar K, Ceelen M, van Weissenbruch MM, Knol DL, Delemarre-van de Waal HA, Huisman J. School functioning in 8- to 18-year-old children born after in vitro fertilization. Eur J Pediatr. 2008;167:1289–1295. doi: 10.1007/s00431-008-0677-2. [DOI] [PubMed] [Google Scholar]
- Wang YA, Macaldowie A, Hayward I, Chambers GM, Sullivan EA. Assisted reproductive technology in Australia and New Zealand 2009. Assist Reprod Ser. 2011;15:1–67. [Google Scholar]
- Watkins AJ, Platt D, Papenbrock T, Wilkins A, Eckert JJ, Kwong WY, Osmond C, Hanson M, Fleming TP. Mouse embryo culture induces changes in postnatal phenotype including raised systolic blood pressure. Proc Natl Acad Sci USA. 2007;104:5449–5454. doi: 10.1073/pnas.0610317104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellen KE, Hatzivassiliou G, Sachdeva UM, Bui TV, Cross JR, Thompson CB. ATP-citrate lyase links cellular metabolism to histone acetylation. Science. 2009;324:1076–1080. doi: 10.1126/science.1164097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerholm WB. Cryopreservation of embryos and oocytes: obstetric outcome and health in children. Hum Reprod. 2000;15(Suppl. 5):18–25. doi: 10.1093/humrep/15.suppl_5.18. [DOI] [PubMed] [Google Scholar]
- Wilkins KM, Warnock JK, Serrano E. Depressive symptoms related to infertility and infertility treatments. Psychiatr Clin North Am. 2010;33:309–321. doi: 10.1016/j.psc.2010.01.009. [DOI] [PubMed] [Google Scholar]
- Williams CA, Angelman H, Clayton-Smith J, Driscoll DJ, Hendrickson JE, Knoll JH, Magenis RE, Schinzel A, Wagstaff J, Whidden EM, et al. Angelman syndrome: consensus for diagnostic criteria. Angelman Syndrome Foundation. Am J Med Genet. 1995;56:237–238. doi: 10.1002/ajmg.1320560224. [DOI] [PubMed] [Google Scholar]
- Xella S, Marsella T, Tagliasacchi D, Giulini S, La Marca A, Tirelli A, Volpe A. Embryo quality and implantation rate in two different culture media: ISM1 versus Universal IVF Medium. Fertil Steril. 2010;93:1859–1863. doi: 10.1016/j.fertnstert.2008.12.030. [DOI] [PubMed] [Google Scholar]
- Young LE, Sinclair KD, Wilmut I. Large offspring syndrome in cattle and sheep. Rev Reprod. 1998;3:155–163. doi: 10.1530/ror.0.0030155. [DOI] [PubMed] [Google Scholar]
- Zaitseva I, Zaitsev S, Alenina N, Bader M, Krivokharchenko A. Dynamics of DNA-demethylation in early mouse and rat embryos developed in vivo and in vitro. Mol Reprod Dev. 2007;74:1255–1261. doi: 10.1002/mrd.20704. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Reinberg D. Transcription regulation by histone methylation: interplay between different covalent modifications of the core histone tails. Genes Dev. 2001;15:2343–2360. doi: 10.1101/gad.927301. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Baltz JM. Bicarbonate/chloride exchange and intracellular pH throughout preimplantation mouse embryo development. Am J Physiol. 1996;271:C1512–C1520. doi: 10.1152/ajpcell.1996.271.5.C1512. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Chauvet PJ, Alper SL, Baltz JM. Expression and function of bicarbonate/chloride exchangers in the preimplantation mouse embryo. J Biol Chem. 1995;270:24428–24434. doi: 10.1074/jbc.270.41.24428. [DOI] [PubMed] [Google Scholar]
- Zhu JL, Basso O, Obel C, Bille C, Olsen J. Infertility, infertility treatment, and congenital malformations: Danish national birth cohort. Br Med J. 2006;333:679. doi: 10.1136/bmj.38919.495718.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JL, Hvidtjorn D, Basso O, Obel C, Thorsen P, Uldall P, Olsen J. Parental infertility and cerebral palsy in children. Hum Reprod. 2010;25:3142–3145. doi: 10.1093/humrep/deq206. [DOI] [PMC free article] [PubMed] [Google Scholar]