Abstract
Changes in carbohydrate metabolism during grape berry development play a central role in shaping the final composition of the fruit. The present work aimed to identify metabolic switches during grape development and to provide insights into the timing of developmental regulation of carbohydrate metabolism. Metabolites from central carbon metabolism were measured using high-pressure anion-exchange chromatography coupled to tandem mass spectrometry and enzymatic assays during the development of grape berries from either field-grown vines or fruiting cuttings grown in the greenhouse. Principal component analysis readily discriminated the various stages of berry development, with similar trajectories for field-grown and greenhouse samples. This showed that each stage of fruit development had a characteristic metabolic profile and provided compelling evidence that the fruit-bearing cuttings are a useful model system to investigate regulation of central carbon metabolism in grape berry. The metabolites measured showed tight coordination within their respective pathways, clustering into sugars and sugar-phosphate metabolism, glycolysis, and the tricarboxylic acid cycle. In addition, there was a pronounced shift in metabolism around veraison, characterized by rapidly increasing sugar levels and decreasing organic acids. In contrast, glycolytic intermediates and sugar phosphates declined before veraison but remained fairly stable post-veraison. In summary, these detailed and comprehensive metabolite analyses revealed the timing of important switches in primary carbohydrate metabolism, which could be related to transcriptional and developmental changes within the berry to achieve an integrated understanding of grape berry development. The results are discussed in a meta-analysis comparing metabolic changes in climacteric versus non-climacteric fleshy fruits.
Key words: berry development, central carbon metabolism, fruit quality, grape ripening, metabolic coordination, metabolite profiling, Vitis vinifera.
Introduction
Grapes, in the form of fresh fruit or processed products (e.g. wine and dried fruit), are potential elements of a healthy diet because of their richness in chemical components that display antioxidant, cardioprotective, anti-aging, and anti-cancer activities (reviewed by Ali et al., 2010). Both the health benefits and organoleptic properties of grapes depend largely on a wide range of specific metabolites accumulated in the berries, such as sugars, organic acids, and polyphenols. The content of these metabolites changes over time as a result of varying rates of synthesis, degradation, and transport processes during fruit development and ripening. To date, our knowledge of the mechanisms underlying the developmental regulation of berry composition and metabolism remains fragmentary, and a better understanding is needed of the mechanisms that switch metabolic pathways on or off at different stages of grape development. This will provide insights into how the developmental regulation of berry metabolism is coordinated, and this information is of both scientific and economic interest.
The pathways of primary carbohydrate metabolism, such as sucrose synthesis and degradation, glycolysis, and the tricarboxylic acid (TCA) cycle, play a central role in the final composition of the grape berry. Sugars, especially sucrose, glucose, and fructose, determine both the sweetness of the fruit and the alcohol content of the wine produced by fermentation of the juice. Respiration of sugars via glycolysis, the oxidative pentose phosphate pathway, and the TCA cycle provides energy (ATP), reducing power [NAD(P)H] and precursors for the synthesis of organic acids, amino acids, anthocyanins, and many other secondary metabolites, including defence and aroma compounds. In addition to generating ATP and NADH, the TCA cycle also plays an important role in regulating fruit acidity, and some of the intermediates of the TCA cycle may act as signal compounds that regulate metabolic fluxes and gene expression (Centeno et al., 2011; Araújo et al., 2012). Climacteric fruits, such as tomato, banana, and peach, show a characteristic peak in respiration at maturity, which is linked to a burst of ethylene production. This behaviour has a profound impact on the fruit’s quality and its shelf life, and therefore the metabolic and gene expression changes associated with the climacteric have been investigated in detail in several economically important species (Ball et al., 1991; Moreno et al., 2008; Osorio et al., 2012). In contrast, there have been relatively few studies of respiratory metabolism in non-climacteric fruits, such as grape, strawberry, and citrus, which do not show a marked peak in respiration at maturity. One of the few reports on respiratory metabolism in grape berries indicated that there are massive changes in glycolytic intermediates during berry development in the seedless grape cultivar Thompson Seedless, despite the absence of a climacteric burst of respiration (Ruffner and Hawker, 1977).
Glycolysis and the TCA cycle are interconnected and under developmental regulation. In tomato, the γ-aminobutyric acid (GABA) shunt, which is connected to the TCA cycle, switches direction from net GABA accumulation during the early part of fruit development (up to the mature green stage) to net respiration of GABA during ripening, with some of the resulting organic acids possibly being converted back into sugars (Yin et al., 2010). Similarly, [14C]malate and [14C]fumarate are able to be incorporated into sugars when grape berries change colour and soften during ripening, indicating a gluconeogenic carbon flux from organic acids into sugars (Ruffner et al., 1975). A number of metabolomic analysis have been carried out to compare grape berry composition at various developmental stages (Ali et al., 2011), or looking at differences between cultivars and growing seasons (Pereira et al., 2006) or regions (Son et al., 2009). These studies have expanded our knowledge of the metabolite composition in relation to growth environment and grapevine genotypes. However, their coverage of primary metabolites and/or different developmental stages has been limited.
In addition to its economic importance, grape has become a valuable model species for studying fruit development, the control of ripening, and the factors that determine quality traits in non-climacteric fleshy fruits. However, research on grape is somewhat hampered by the perennial nature and large size of grapevines, which impose greater demands on time, growth space, and manpower compared with annual crops. As a result, fruit-bearing cuttings (i.e. only one primary shoot axis with a single cluster per plant) were developed as a simplified experimental model system to overcome these difficulties (Mullins, 1966). Since its introduction, this model system has been improved and applied to many aspects of grape research, including the effects of water stress (Antolín et al., 2010), climate-change scenarios (Salazar Parra et al., 2010), abscisic acid treatment (Giribaldi et al., 2010), source–sink ratio (Ollat and Gaudillere, 1998; Dai et al., 2009), and nitrogen supply (Geny and Broquedis, 2002) on grape physiology. In addition, factors affecting inflorescence development, fruit set (Aziz, 2003), carbon allocation (Vaillant-Gaveau et al., 2011), and transcriptome reprogramming (Lund et al., 2008) have also been investigated using fruit-bearing cuttings. In spite of their widespread use, few studies have been conducted to validate this experimental system by comparing results from fruiting cuttings with those from vineyard-grown grapevines. This lack of information is especially evident in terms of berry metabolism during berry development.
The present work was designed to fulfil two objectives. The first was to perform a comprehensive profiling of the intermediates in sucrose metabolism, glycolysis, and the TCA cycle in grape berries at different stages throughout development, in order to identify any coordinated switches in metabolism, and thus to shed light on the timing of developmental regulation of carbohydrate metabolism. The second objective was to check whether the fruit-bearing cuttings are a reliable model that accurately reproduces carbon metabolism in berries from field-grown vines.
Materials and methods
Plant materials and growth conditions
Grape berries (Vitis vinifera cv. Cabernet Sauvignon, clone #337) were sampled at ten different developmental phases (from fruit set to maturity), from either vineyard-grown vines or fruit-bearing cuttings prepared as described by Mullins and Rajasekaran (1981). The vineyard-grown vines are part of a germplasm collection growing in level ground with no slope or geospatial variations located at Villenave d’Ornon, France (latitude 44° 46’ 46’’ N, longitude 00° 34’ 01’’ W). Berries were harvested from 20-year-old vines between 19 June and 18 September 2009. The fruit-bearing cuttings were grown in a naturally illuminated and semi-regulated greenhouse (mean seasonal temperature 20–35 °C). Five pre-veraison stages were sampled according to days after flowering (daf): P1, 10 daf; P2, 20 daf; P3, 30 daf; P4, 40 daf; and P5, 50% veraison (the time when 50% of berries change their colour). Five post-veraison stages were sampled according to berry density using the method described by Singleton et al. (1966). The selected berry densities correspond to five potential alcohol (PA) levels (%): P6, 7% PA; P7, 8% PA; P8, 9% PA; P9, 10% PA; and P10, 12% PA (maturity). These ten stages correspond to the developmental stages of EL29, -31, -32, -33, -35, -36, -36, -37, -37, and -38 according to the modified Eichhorn and Lorenz classification system (Coombe, 1995). Four biological replicates were sampled for each developmental phase and each replicate contained 15 berries from both the shaded and sun-exposed sides of the grape clusters of three individual plants. Berries were frozen in liquid nitrogen immediately after harvesting and stored at –80 °C until analysis. Temperatures were recorded for each experimental site and were used to calculate the accumulated thermal history (degree days) of the plants after flowering, which is a scaling method widely used to compare plants grown in different environments (Duchêne et al., 2012).
Biochemical analysis
Whole berries were ground to a fine powder in liquid nitrogen and aliquots (15–20mg) of frozen powder were extracted with chloroform/methanol as described by Lunn et al. (2006). Phosphorylated intermediates, organic acids, and trehalose-6-phosphate (T6P) were measured in chloroform/methanol extracts by anion-exchange high-performance liquid chromatography (LC) using an ICS 3000 chromatograph (Dionex Corporation, Sunnyvale, USA) coupled to a QTrap 5500 triple quadrupole mass spectrometer (MS; AB Sciex, Foster City, USA). Extracts were passed through a 9×75mm IonPack ATC-HC trap column (Dionex) before loading onto a 2×50mm AG11-HC guard column and 2×250mm IonPac AS11-HC column (Dionex) in series. Anionic compounds were eluted with the following gradient: 0–5min 5mM KOH, 5–25min linear gradient of 5–30mM KOH, 25–35min linear gradient of 30–50mM KOH; 32–34min linear gradient of 50–100mM KOH, 35–42min isocratic 100mM KOH. Aliquots of 25 µl (from a 1:10-diluted extract) were spiked with [6,6-2H]T6P as an internal standard to correct for ion suppression and matrix effects (Lunn et al., 2006), and injected with an autosampler AS50 (Dionex) working at 4 °C. The QTrap 5500 was operated in multiple reactions monitoring mode, with an electrospray ionization source in negative ionization mode, and centroid data acquisition. Nitrogen was used as curtain gas, ion source gas 1 and 2, and also as collision gas. Ion spray voltage was –4200V and the temperature was set to 480 °C. The declustering potential was –100V. Parent ions for metabolites of interest were selected in the first quadrupole using calculated m/z values. Suitable collision energies for fragmentation of the parent ion in the second quadrupole and m/z settings for detection of the three principal product ions in the third quadrupole were derived from authentic standards injected directly into the mass spectrometer. Metabolites were quantified by comparison of the integrated MS-Q3 signal peak area with a calibration curve obtained using authentic standards. The integration and calculation of chromatograms were done using Analyst software (AB Sciex).
Soluble sugars (glucose, fructose, and sucrose) were extracted with ethanol from aliquots [~20mg fresh weight (FW)] of frozen powdered berry material and assayed enzymatically according to the method of Jelitto et al. (1992).
Data analysis
Data were analysed with multivariate analysis methods using the R statistics environment (R Development Core Team, 2010). Principal component analysis (PCA) was performed on mean-centred and scaled data using the ade4 package in R (Dray and Dufour, 2007) in order to compare the alterations in metabolite levels during fruit development. PCA was first done using the field-grown berry dataset, and then the data from the fruit-bearing cuttings were projected as non-active variables. In this way, one can compare the prediction quality of the PCs identified from the active dataset in relation to the non-active dataset. K-means clustering and Pearson correlation were then employed to investigate the degree of similarity in accumulation patterns across development, and the results were visualized using the corrplot package (Wei, 2011). In order to evaluate these data further, we assessed the metabolite partial correlation network, which can provide insights into causal relationships between metabolite–metabolite associations (Opgen-Rhein and Strimmer, 2007). This was done using the GeneNet package (Schaefer et al., 2012) with log10-transformed data. The resulting network was visualized with Cytoscape version 2.8.2 (Shannon, 2003).
Results
Berries from field-grown vines and greenhouse-grown fruit-bearing cuttings have similar metabolite profiles
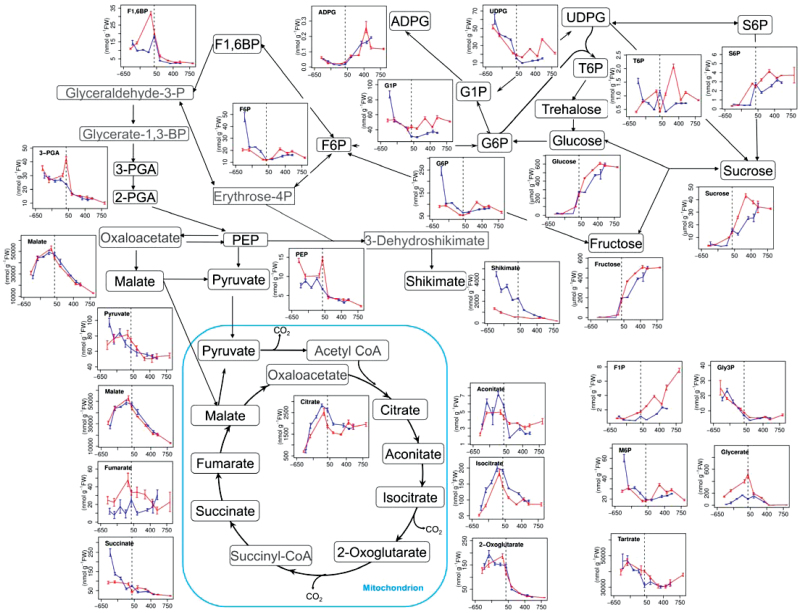
The 27 metabolites quantified (Supplementary Table S1 at JXB online for abbreviations) were mainly intermediates involved in sugar accumulation, glycolysis, or the TCA cycle. To put the measured metabolites into a metabolic context, a schematic representation of plant central carbon metabolism was created to display the metabolite profiles (Fig. 1). Most metabolites showed similar levels in berries from vines grown in the vineyard and from fruit-bearing cuttings grown in the greenhouse, when compared on a degree-day basis (thermal sums). In contrast, the profiles of sugar phosphates, including glucose 1-phosphate (G1P) and the sugar-signalling metabolite T6P, differed somewhat between greenhouse and vineyard plants during the late developmental phases. In vineyard plants, levels of these sugar phosphates decreased gradually throughout berry development, while they increased slightly after veraison in the greenhouse plants. This difference might be due to the higher temperature recorded in the greenhouse than in the vineyard during the corresponding period (Supplementary Fig. S1 at JXB online).
Fig. 1.
Metabolite profiles of berries from vines grown in the vineyard (blue line) or fruit-bearing cuttings grown in the greenhouse (red line). Metabolites are shown in their respective metabolic pathways (sugar metabolism, glycolysis, and the TCA cycle) and their temporal profiles during berry development (µmol g–1 FW for sucrose, glucose, and fructose; and nmol g–1 FW for the others) are presented alongside. For each profile, the x-axis shows the accumulated thermal history of the plants in degree-days, normalized to zero at veraison (indicated by the dashed line) to facilitate comparison between the vineyard- and greenhouse-grown samples.
Metabolite signatures reflect developmental specificity
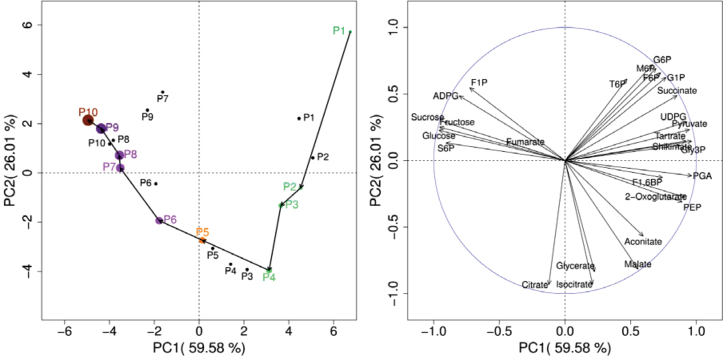
PCA readily discriminates the various stages of berry development, with similar trajectories for field-grown and greenhouse-grown samples (Fig. 2). The first two principal components (PC1 and PC2) explained about 86% of the total variance. PC1 separated the pre- and post-veraison stages, based largely on changes in sugars and glycolytic intermediates. PC2 resolved the individual stages of development within the pre- and post-veraison phases, related mainly to differences in TCA cycle intermediates, such as citrate, isocitrate, and malate.
Fig. 2.
PCA of metabolite profiles during berry development. Left: trajectories during development for berries grown in the vineyard (coloured points) and greenhouse (black points). Arrows indicate the order of development. Berry skin colour and size are represented by the colour and size of the symbols, respectively, for vineyard berries. Right: loading plots of metabolites for the first two principle components, PC1 and PC2. The PCA was first made using the field-grown berry dataset, and the data from the fruit-bearing cuttings were then projected as non-active variables. In this way, one can compare the prediction quality of the PCs identified from the active dataset in relation to the non-active dataset.
Coordinated changes in metabolically linked metabolites reveal the timing of switches in metabolism during fruit development
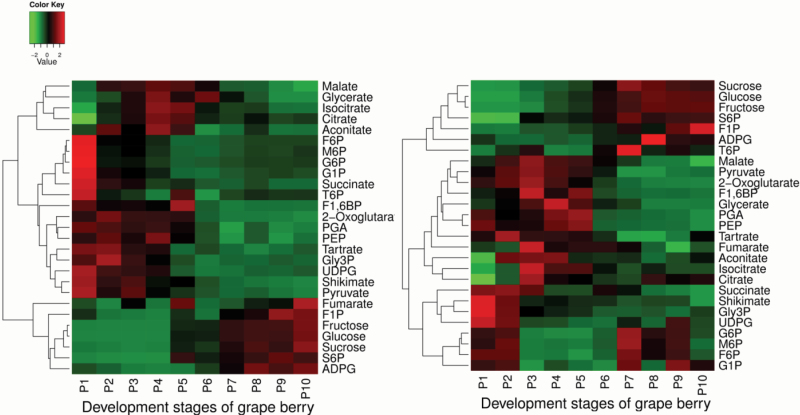
The temporal patterns of changes in the metabolites were dissected by K-means clustering (Fig. 3). The 27 measured metabolites could be clustered into three main groups representing: (i) sugars and sugar-phosphate metabolism, (ii) glycolysis, and (iii) the TCA cycle. Sugars (sucrose, fructose, and glucose) were present at very low levels before veraison but increased dramatically from veraison onwards. Similar profiles were observed for two sugar phosphates [fructose 1-phosphate (F1P) and sucrose 6-phosphate (S6P)]. Most of the TCA cycle intermediates (malate, citrate, aconitate, isocitrate, and 2-oxoglutarate) increased gradually with development up to a peak at veraison, and decreased gradually thereafter. The only exceptions were succinate, whose concentration was high in the early stages and then continually decreased throughout development, and fumarate, which showed a transient peak at veraison and then a gradual rise in the final stages of development. Glycolytic intermediates were less coordinated, with fructose 6-phosphate (F6P) and glucose 6-phosphate (G6P) levels declining during the early stages of berry development but then remaining relatively stable post-veraison, while fructose 1,6-bisphosphate (F1,6BP), 3-phospho-d-glycerate (PGA), phosphoenolpyruvate (PEP) and pyruvate showed a peak around veraison.
Fig. 3.
Heatmap and clustering of metabolite changes during berry development for berries from the vineyard (left panel) and greenhouse (right panel). Each row represents a metabolite and each column represents a developmental stage. Values were centred and scaled in the row direction to form virtual colours as presented in the colour key. Metabolites that showed a similar developmental profile were clustered together. Stage five corresponded to veraison.
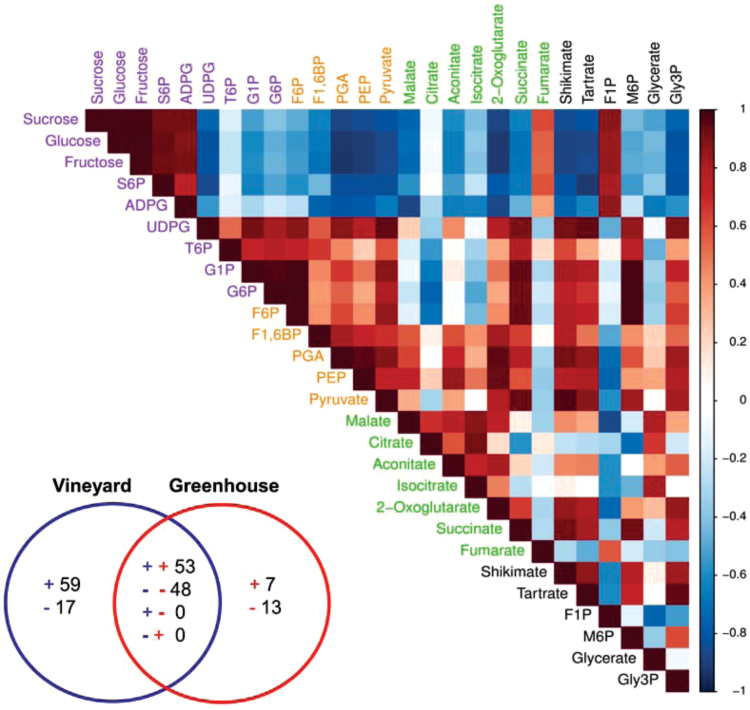
The coordinated shift in metabolites was further confirmed by pairwise correlation analysis (Fig. 4). Of the 351 metabolite pairs, there were 177 significant correlations (P <0.05 after Bonferroni false discovery rate correction) in berries from the vineyard-grown vines and 121 in those from greenhouse-grown cuttings. Of these, 112 and 60 were positive and 65 and 61 were negative, respectively. Most of the significant correlations (101) were observed in both sources of berries and shared the same sign in both conditions. Metabolites within a given pathway were often positively correlated, but negative correlations were observed between metabolites from different pathways. The main exception was the negative correlation between sugars and sugar phosphates.
Fig. 4.
Correlation matrix of all metabolites of berries grown in the vineyard. The Pearson correlation coefficient (positive or negative) between a pair of metabolites is represented by virtual colour as indicated in the colour key. Metabolites were grouped according to their metabolic pathways. A similar matrix was built for the greenhouse-grown berries (data not shown). Comparisons between samples from vineyard and greenhouse are summarized in the Venn diagram (inset), with ‘+’ and ‘–‘ indicating significant positive and negative correlations, respectively.
The metabolite correlation network reveals emerging metabolite co-regulation
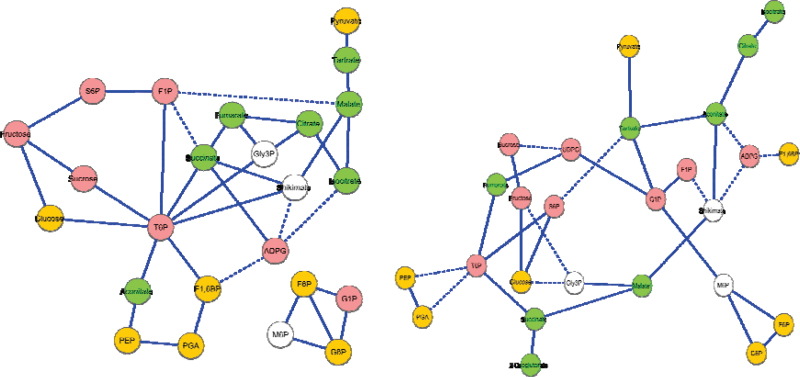
Partial correlation network analysis was used to identify metabolites that were functionally related or co-regulated in grape berry (Fig. 5). This method calculates the partial correlation between two given metabolites, after excluding the effect of all the other variables in a metabolite matrix, thus allowing the construction of a causation network among metabolites (Opgen-Rhein and Strimmer, 2007). The number of significant connections identified by this method was greatly reduced in comparison with the pairwise correlation described above, highlighting the most significant relations among metabolites. The network from vineyard-grown vines contained 36 edges, i.e. significant partial correlations (P <0.05), while the network from greenhouse-grown cuttings has 34 edges, with 12 edges in common between the two networks. These common edges generally reflected the close relationship between metabolites separated by only one or two enzymatic steps in their respective pathways, including PGA/PEP, citrate/isocitrate, and glucose/fructose/sucrose, or metabolites connected via a common precursor, e.g. malate/shikimate. Network analysis showed that each metabolite was connected on average to three neighbours in both networks. T6P was the most connected metabolite, with eight neighbours in vineyard berries and five in greenhouse berries. From these data, a novel connection between T6P and succinate was identified in both conditions. A module of sugar phosphates [G1P, G6P, F6P, and mannose 6-phosphate (M6P)] was evident, while S6P exhibited a different connection. In agreement with the observation from pairwise correlation, TCA intermediates were positively correlated with each other, while they were negatively correlated with intermediates of glycolysis. Glycolytic intermediates were less directly interconnected among themselves but were extensively connected with metabolites involved in other pathways. In particular, a strong, direct correlation between pyruvate and tartrate was found in both vineyard and greenhouse samples.
Fig. 5.
Metabolite–metabolite partial correlation network for berries from the vineyard (left) and greenhouse (right). Metabolites were grouped according to their metabolic pathways and highlighted by different colours: pink for intermediates of sugar metabolism, yellow for glycolysis, green for the TCA cycle, and white for the others. Significant positive partial correlations are indicated by a solid line between two metabolites, while significant negative partial correlations are represented by a dashed line.
Discussion
Monitoring the developmental profiles of metabolites is a valuable approach to gain a better understanding of the developmentally induced metabolic shifts that underlie and define the final fruit chemical composition. Developmental profiles of primary and secondary metabolites in grape have been reported in two studies (Ali et al., 2011; Toffali et al., 2011), but these covered a relatively small number of metabolites from primary metabolism (sugar accumulation, glycolysis, and the TCA cycle) and analysed a limited number of developmental stages. In the present study, we conducted a broader analysis, which, by means of an LC-MS/MS approach, covered a more comprehensive range of intermediates from these pathways and which also had denser sampling of the various stages of berry development. The resulting information provided novel insights into the developmental regulation of primary metabolism in grape.
Sugars and organic acids, which are the metabolites in grape berries of most interest to wine-growers, showed remarkably similar temporal profiles in berries from vineyard-grown plants and fruit-bearing cuttings. On the other hand, some of the phosphorylated intermediates had different profiles in berries from the two types of mother plant. These might reflect some fundamental differences between vines and cuttings in the balance or the fluxes through the various pathways of central carbon metabolism. However, it must be remembered that the pool sizes of many intermediates are relatively small, and these metabolites are often more responsive to short-term differences in environmental conditions than the larger pools of sugars and organic acids. Therefore, the differences observed for some of the phosphorylated intermediates may reflect short-term responses to differences in light, temperature, or other environmental conditions, which clearly did vary between the vines growing in the vineyard and the fruit-bearing cuttings.
In both plant materials, we observed an increase in sucrose from veraison to maturity, although the absolute concentrations were generally much lower than hexoses. S6P also increased from veraison to maturity. The presence of this metabolite indicates that some biosynthesis of sucrose was occurring in the developing berries, even though the berries are abundantly supplied with sucrose via the phloem (Swanson and El-Shishiny, 1958). There are several possible reasons why sucrose biosynthesis might be required in the berries. In grape, post-veraison phloem unloading occurs via an apoplastic route (Zhang et al., 2006), in which sucrose is hydrolysed by cell-wall invertase and the resulting glucose and fructose may be taken up via plasmalemma monosaccharide sugar transporters (Vignault et al., 2005). Consequently, resynthesis of sucrose would generate a diffusion gradient for the hexose sugars and so promote phloem unloading into the berry parenchyma cells. A futile cycle of sucrose hydrolysis and resynthesis could also provide greater sensitivity for regulating the net accumulation of sugars and the sucrose:hexose ratio in the cells. A third possibility is that there is conversion of organic acids, such as malate, to sucrose via a gluconeogenic pathway (Ruffner et al., 1975), which would fit with the observed accumulation of malate up to the time of veraison, followed by a decrease as sugars start to accumulate.
The nucleotide sugar, uridine diphosphate glucose (UDPG), is a substrate for both sucrose and cell-wall biosynthesis and is a product of sucrose cleavage via sucrose synthase. The level of UDPG decreased gradually over berry development, which is consistent with the previously described decrease in cell-wall polysaccharides during grape berry development (Nunan et al., 1998) and may result from the reported dramatic downregulation of sucrose synthase expression (Martinez-Esteso et al., 2011). The other nucleotide sugar detected in the berries, ADPG, is the substrate for starch biosynthesis. The role of starch and its contribution to grape quality has not been investigated fully. In the present study, the level of the starch precursor ADPG increased from veraison (Fig. 1), when starch levels were reported to decrease (Deluc et al., 2007; Fortes et al., 2011). This reverse accumulation pattern for ADPG and starch suggested that the decrease in starch did not result from substrate limitation but was most probably due to a decreased activity of the enzymes mediating starch synthesis or to increased starch degradation. Transcriptomic studies have revealed a complex pattern of changes in starch-related transcripts during grape berry development, with transcripts for some enzymes of starch synthesis increasing while others decreased, and those encoding starch degradation enzymes increasing over development (Deluc et al., 2007; Fortes et al., 2011).
Another metabolite of particular interest is T6P, which is proposed to be involved in sugar signalling (Lunn et al., 2006) and influences many aspects of plant metabolism and growth (Eastmond et al., 2002; Schluepmann et al., 2003; Schluepmann et al., 2004; Kolbe et al., 2005). A strong correlation between T6P and sugars, particularly sucrose, was reported in Arabidopsis thaliana seedlings and rosettes of soil-grown plants (Lunn et al., 2006). This strong correlation was also observed in the stems, shoots, and roots of grapevines cultured in vitro under non-chilling and chilling conditions (Fernandez et al., 2012). However, we did not find any significant correlation between T6P and sugars (hexose or sucrose) in grape berries, taking into account the whole time course of fruit development. This suggests that T6P might be responding to other factors in addition to, or instead of, sugars in grape berries. However, it is worth noting that trehalose-phosphate synthase, the enzyme that synthesises T6P, is most likely to be located in the cytosol (Van Dijck et al., 2002), suggesting that the level of T6P is specifically influenced by the metabolic status of this compartment. As most of the sugars in grape berries are accumulated in the vacuole, particularly in the later stages of development, the cytosolic pool of sugars will constitute only a small part of the total, and the proportion of the total sugar pool found in the cytosol probably changes during development. Therefore, whole-tissue sugar measurements are unlikely to be a reliable indicator of the size of the cytosolic sugar pool, masking any potential correlation with T6P. Although no correlation was observed with sugars, T6P appeared to be the most connected metabolite in the metabolite–metabolite network, with a significant correlation between T6P and succinate identified in both greenhouse and vineyard samples. T6P is reported to inhibit the sucrose non-fermenting 1-related kinase 1 (SnRK1) in A. thaliana (Zhang et al., 2009). The SnRK1 protein kinase is a central regulator of energy homeostasis and stress responses (Baena-Gonzalez et al., 2007); therefore, developmental changes in the level of T6P might be expected to affect fluxes and metabolite levels in multiple metabolic pathways. Further investigation will be required to understand the role of SnRK1 in grape berry, and whether T6P is a physiologically important regulator of SnRK1 activity in this tissue.
A decrease in glycolysis after veraison has been reported in a seedless grape cultivar (Ruffner and Hawker, 1977), based on the fact that the glycolytic intermediates PEP and pyruvate gradually decreased throughout development. Decreases in transcript or protein abundance of glycolytic enzymes have been reported in several transcriptomic and proteomic studies on wine grape cultivars (Famiani et al., 2000; Giribaldi et al., 2007; Martinez-Esteso et al., 2011). However, other reports have indicated that several glycolytic enzymes are upregulated after veraison (Terrier et al., 2005; Negri et al., 2008), or that there are differential changes with some enzymes being upregulated while others are downregulated (Fortes et al., 2011). Our data showed a general decrease in glycolytic intermediates during berry ripening. It is not clear whether this is linked to a change in flux through the pathway or a shift in the balance of the pathway, such that it operates with lower steady-state levels of intermediates without any change in flux. This can only be resolved by flux measurements using radioctive or stable isotope labelling experiments, which are outside the scope of the current study. The glycolytic intermediates can be further subdivided into two classes. The first encompasses G6P and F6P, which sharply decrease at a very early stage and then decline more gradually until veraison but then remain fairly stable. The second includes F1,6BP, PGA, PEP, and pyruvate, which show a similar pattern as the first class except that there is an additional peak around veraison. The opposite behaviour of F6P and F1, and 6BP suggests that changes in glycolytic flux may be occurring due to regulation of enzymes involved in the interconversion of F6P and F1,6BP. Phosphofructokinase (PFK) catalyses the irreversible phosphorylation of F6P to F1,6BP, while fructose-1,6-bisphosphatase catalyses the irreversible dephosphorylation of F1,6BP. A further enzyme, pyrophosphate:fructose-6-phosphate 1-phosphotransferase, catalyses the reversible interconversion of F6P and F1,6BP. These enzymes are known to have considerable regulatory potential (Plaxton, 1996).
The majority of the TCA intermediates showed coordinated changes during berry development, except that succinate, and to some extent fumarate, showed different profiles. Unlike the other enzymes of the TCA cycle, succinate dehydrogenase is membrane bound and an integral part of the mitochondrial electron transport chain (Bowsher and Tobin, 2001). Therefore, it is likely that the balance of this reaction, i.e. the ratio of succinate:fumarate, is influenced not only by the flux through the TCA cycle but also by other reactions feeding into the electron transport chain, and by the NAD:NADH ratio and the demand for ATP. These additional influences may explain the different profiles of succinate, and to some extent fumarate, in comparison with the other TCA cycle intermediates during development. The respiratory pathways provide not only ATP and reducing power but also carbon skeletons for biosynthesis of proteins, lipids, cell walls, secondary metabolites (e.g. anthocyanins and aroma compounds), and organic acids for pH homeostasis and osmoregulation. The quantitative importance of each of these processes will change during the development of the grape berry, placing different demands on the respiratory pathways at each stage of development (Conde et al., 2007). Therefore, changes in the abundance of TCA cycle intermediates probably reflect the varying demands for ATP, reducing power, and C-skeletons during grape berry development. However, metabolite data alone cannot tell us whether the fluctuating metabolite levels reflect altered fluxes through the pathways, or the metabolic network as a whole switching to a different poise to meet the sometimes competing demands on the respiratory pathways.
In addition to the point-by-point analysis, a meta-analysis was conducted to compare our data with the available developmental profiles of primary metabolites in both climacteric and non-climacteric fleshy fruits (Supplementary Table S2 at JXB online). We collected metabolomic data from fruit development studies in tomato (Carrari et al., 2006; Osorio et al., 2011; Yin et al., 2010), peach (Lombardo et al., 2011), strawberry (Fait et al., 2008; Zhang et al., 2011), citrus (Katz et al., 2011), and grape (Ruffner and Hawker, 1977; Ali et al., 2011). The developmental changes in glycolytic and TCA cycle intermediates were extracted from the combined dataset and their temporal patterns compared. This revealed some general patterns of developmental changes in metabolites that are common to both grape and other fleshy fruits. Firstly each stage of fruit development was characterized by its own unique metabolic profiles, and the most evident metabolic shift occurred at the transition from a hard and acidic fruit to a soft and sugar-rich fruit. Secondly, the levels of metabolites involved in the same metabolic pathway generally exhibited coordinated changes throughout fruit development. Finally, the developmental profiles of glycolytic and TCA cycle intermediates did not discriminate between the climacteric and non-climacteric fruits. This indicates that the regulatory mechanisms controlling these pathways may be common to both climacteric and non-climacteric fruits (Fait et al., 2008).
In conclusion, it is clear that glycolytic and TCA cycle intermediates are tightly coordinated with metabolites of their respective pathways during berry development. In addition, there is a pronounced shift in metabolism around veraison, characterized by rapidly increasing sugar levels and decreasing organic acids. In contrast, glycolytic intermediates and sugar phosphates declined during the early stages of development but then remained relatively stable post-veraison. The detailed and comprehensive metabolite data presented here reveal the timing of important switches in primary carbohydrate metabolism in grape berry, which can be related to transcriptional and developmental changes within the berry to reach an integrated understanding of grape berry development. Moreover, our data showed that the temporal profiles of most of the measured metabolites (especially sugars and organic acids, which are major determinants of fruit and wine quality) were remarkably similar in berries from vineyard-grown vines and greenhouse-grown fruit-bearing cuttings, demonstrating that fruit-bearing cuttings are a meaningful and useful model system to study the pathways and regulation of central carbon metabolism in grape berries. Meanwhile, it is worth noting that several phosphorylated intermediates accumulations exhibited differences, and further efforts are needed to assess the reliability of this model system for studying metabolic pathways not analysed in the present work, especially the downstream pathways related to the phosphorylated intermediates and the secondary metabolism. In the future, the metabolite profiling will be integrated with enzyme activity measurements and kinetic model analysis to evaluate the regulation of metabolic flux as described for other plant species (Stitt et al., 2010).
Supplementary data
Supplementary data are available at JXB online.
Table S1. List of the 27 measured metabolites and their abbreviations.
Table S2. Comparison of the temporal profiling of primary metabolites across the development of different climacteric and non-climacteric fleshy fruits.
Fig. S1. Daily temperature and sum of temperature (degree-days) from flowering to maturity in the vineyard and greenhouse.
Acknowledgements
We thank Thierry Robert for his excellent assistance in taking care of the fruit-bearing cuttings in the greenhouse. This study was partially supported by the Pari Scientifique grant from the department Environnement et Agronomie of Institut National de la Recherche Agronomique, France.
Glossary
Abbreviations:
- daf
days after flowering
- F1,6BP
fructose 1,6-bisphosphate
- F1P
fructose 1-phosphate
- GABA
γ-aminobutyric acid
- G1P
glucose 1-phosphate
- G6P
glucose 6-phosphate
- PEP
phosphoenolpyruvate
- PGA
3-phospho-d-glycerate
- S6P
sucrose 6-phosphate
- T6P
trehalose-6-phosphate
- TCA
tricarboxylic acid
- UDPG
uridine diphosphate glucose.
References
- Ali K, Maltese F, Choi YH, Verpoorte R. 2010. Metabolic constituents of grapevine and grape-derived products. Phytochemistry Review 9, 357–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali K, Maltese F, Fortes AM, Pais MS, Choi YH, Verpoorte R. 2011. Monitoring biochemical changes during grape berry development in Portuguese cultivars by NMR spectroscopy. Food Chemistry 124, 1760–1769 [Google Scholar]
- Antolín MC, Santesteban H, Ayari M, Aguirreolea J, Sánchez-Díaz M. 2010. Grapevine fruiting cuttings: an experimental system to study grapevine physiology under water deficit conditions. In: Delrot S, Medrano H, Or E, Bavaresco L, Grando S, eds. Methodologies and results in grapevine research The Netherlands: Springer, 151–163 [Google Scholar]
- Araújo WL, Nunes-Nesi A, Nikoloski Z, Sweetlove LJ, Fernie AR. 2012. Metabolic control and regulation of the tricarboxylic acid cycle in photosynthetic and heterotrophic plant tissues. Plant, Cell & Environment 35, 1–21 [DOI] [PubMed] [Google Scholar]
- Aziz A. 2003. Spermidine and related metabolic inhibitors modulate sugar and amino acid levels in Vitis vinifera L.: possible relationships with initial fruitlet abscission. Journal of Experimental Botany 54, 355–363 [DOI] [PubMed] [Google Scholar]
- Baena-Gonzalez E, Rolland F, Thevelein JM, Sheen J. 2007. A central integrator of transcription networks in plant stress and energy signalling. Nature 448, 938–942 [DOI] [PubMed] [Google Scholar]
- Ball KL, Green JH, ap Rees T. 1991. Glycolysis at the climacteric of bananas. European Journal of Biochemistry 197, 265–269 [DOI] [PubMed] [Google Scholar]
- Bowsher CG, Tobin AK. (2001). Compartmentation of metabolism within mitochondria and plastids. Journal of Experimental Botany 52, 513–527 [PubMed] [Google Scholar]
- Carrari F, Baxter C, Usadel B, et al. 2006. Integrated analysis of metabolite and transcript levels reveals the metabolic shifts that underlie tomato fruit development and highlight regulatory aspects of metabolic network behavior. Plant Physiology 142, 1380–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centeno DC, Osorio S, Nunes-Nesi A, et al. 2011. Malate plays a crucial role in starch metabolism, ripening, and soluble solid content of tomato fruit and affects postharvest softening. Plant Cell 23, 162–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde BC, Silva P, Fontes N, Dias ACP, Tavares RM, Sousa MJ, Agasse A, Delrot S, Geros H. 2007. Biochemical changes throughout grape berry development and fruit and wine quality. Food 1, 1–22 [Google Scholar]
- Coombe B. 1995. Growth stages of the grapevine: adoption of a system for identifying grapevine growth stages. Australian Journal of Grape and Wine Research 1, 104–110 [Google Scholar]
- Dai ZW, Vivin P, Robert T, Milin S, Li SH, Génard M. 2009. Model-based analysis of sugar accumulation in response to source-sink ratio and water supply in grape (Vitis vinifera) berries. Functional Plant Biology 36, 527–540 [DOI] [PubMed] [Google Scholar]
- Deluc LG, Grimplet J, Wheatley MD, Tillett RL, Quilici DR, Osborne C, Schooley DA, Schlauch KA, Cushman JC, Cramer GR. 2007. Transcriptomic and metabolite analyses of Cabernet Sauvignon grape berry development. BMC Genomics 8, 429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray S, Dufour AB. 2007. The ade4 package: implementing the duality diagram for ecologists. Journal of Statistical Software 22, 1–20 [Google Scholar]
- Duchêne E, Dumas V, Jaegli N, Merdinoglu D. 2012. Deciphering the ability of different grapevine genotypes to accumulate sugar in berries. Australian Journal of Grape and Wine Research 18, 319–328 [Google Scholar]
- Eastmond PJ, Van Dijken AJH, Spielman M, Kerr A, Tissier AF, Dickinson HG, Jones JDG, Smeekens SC, Graham IA. 2002. Trehalose-6-phosphate synthase 1, which catalyses the first step in trehalose synthesis, is essential for Arabidopsis embryo maturation. The Plant Journal 29, 225–235 [DOI] [PubMed] [Google Scholar]
- Fait A, Hanhineva K, Beleggia R, Dai N, Rogachev I, Nikiforova VJ, Fernie AR, Aharoni A. 2008. Reconfiguration of the achene and receptacle metabolic networks during strawberry fruit development. Plant Physiology 148, 730–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famiani F, Walker RP, Técsi L, Chen ZH, Proietti P, Leegood RC. 2000. An immunohistochemical study of the compartmentation of metabolism during the development of grape (Vitis vinifera L.) berries. Journal of Experimental Botany 51, 675–683 [PubMed] [Google Scholar]
- Fernandez O, Vandesteene L, Feil R, Baillieul F, Lunn J, Clément C. 2012. Trehalose metabolism is activated upon chilling in grapevine and might participate in Burkholderia phytofirmans induced chilling tolerance. Planta 236, 355–369 [DOI] [PubMed] [Google Scholar]
- Fortes A, Agudelo-Romero P, Silva M, et al. 2011. Transcript and metabolite analysis in Trincadeira cultivar reveals novel information regarding the dynamics of grape ripening. BMC Plant Biology 11, 149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geny L, Broquedis M. 2002. Developmental processes, polyamine composition and content of fruiting cuttings of Vitis vinifera L.: Responses to nitrogen deficiency. Vitis 41, 123–127 [Google Scholar]
- Giribaldi M, Gény L, Delrot S, Schubert A. 2010. Proteomic analysis of the effects of ABA treatments on ripening Vitis vinifera berries. Journal of Experimental Botany 61, 2447–2458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giribaldi M, Perugini I, Sauvage FX, Schubert A. 2007. Analysis of protein changes during grape berry ripening by 2-DE and MALDI-TOF. Proteomics 7, 3154–3170 [DOI] [PubMed] [Google Scholar]
- Jelitto T, Sonnewald U, Willmitzer L, Hajirezeai M, Stitt M. 1992. Inorganic pyrophosphate content and metabolites in potato and tobacco plants expressing E. coli pyrophosphatase in their cytosol. Planta 188, 238–244 [DOI] [PubMed] [Google Scholar]
- Katz E, Boo KH, Kim HY, Eigenheer RA, Phinney BS, Shulaev V, Negre-Zakharov F, Sadka A, Blumwald E. 2011. Label-free shotgun proteomics and metabolite analysis reveal a significant metabolic shift during citrus fruit development. Journal of Experimental Botany 62, 5367–5384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolbe A, Tiessen A, Schluepmann H, Paul M, Ulrich S, Geigenberger P. 2005. Trehalose 6-phosphate regulates starch synthesis via posttranslational redox activation of ADP-glucose pyrophosphorylase. Proceedings of the National Academy of Sciences, USA 102, 11118–11123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo VA, Osorio S, Borsani J, Lauxmann MA, Bustamante CA, Budde CO, Andreo CS, Lara MV, Fernie AR, Drincovich MF. 2011. Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage. Plant Physiology 157, 1696–1710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund ST, Peng FY, Nayar T, Reid KE, Schlosser J. 2008. Gene expression analyses in individual grape (Vitis vinifera L.) berries during ripening initiation reveal that pigmentation intensity is a valid indicator of developmental staging within the cluster. Plant Molecular Biology 68, 301–315 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Feil R, Hendriks JHM, Gibon Y, Morcuende R, Osuna D, Scheible WR, Carillo P, Hajirezaei MR, Stitt M. 2006. Sugar-induced increases in trehalose 6-phosphate are correlated with redox activation of ADPglucose pyrophosphorylase and higher rates of starch synthesis in Arabidopsis thaliana . Biochemical Journal 397, 139–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Esteso MJ, Sellés-Marchart S, Lijavetzky D, Pedreño MA, Bru-Martinez R. 2011. A DIGE-based quantitative proteomic analysis of grape berry flesh development and ripening reveals key events in sugar and organic acid metabolism. Journal of Experimental Botany 62, 2521–2569 [DOI] [PubMed] [Google Scholar]
- Moreno E, Obando JM, Dos-Santos N, Fernández-Trujillo JP, Monforte AJ, Garcia-Mas J. 2008. Candidate genes and QTLs for fruit ripening and softening in melon. Theoretical and Applied Genetics 116, 589–602 [DOI] [PubMed] [Google Scholar]
- Mullins MG. 1966. Test-plants for investigations of the physiology of fruiting in Vitis vinifera L. Nature 209, 419–420 [Google Scholar]
- Mullins MG, Rajasekaran K. 1981. Fruiting cuttings:revised method for producing test plants of grapevine cultivars. American Journal of Enology and Viticulture 32, 35–40 [Google Scholar]
- Negri A, Prinsi B, Rossoni M, Failla O, Scienza A, Cocucci M, Espen L. 2008. Proteome changes in the skin of the grape cultivar Barbera among different stages of ripening. BMC Genomics 9, 378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunan KJ, Sims IM, Bacic A, Robinson SP, Fincher GB. 1998. Changes in cell wall composition during ripening of grape berries. Plant Physiology 118, 783–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ollat N, Gaudillere JP. 1998. The effect of limiting leaf area during stage I of berry growth on development and composition of berries of Vitis vinifera L. cv. Cabernet Sauvignon. American Journal of Enology and Viticulture 49, 251–258 [Google Scholar]
- Opgen-Rhein R, Strimmer K. 2007. From correlation to causation networks: a simple approximate learning algorithm and its application to high-dimensional plant gene expression data. BMC Systems Biology 1, 37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S, Alba R, Damasceno CMB, et al. 2011. Systems biology of tomato fruit development: combined transcript, protein, and metabolite analysis of tomato transcription factor (nor, rin) and ethylene receptor (Nr) mutants reveals novel regulatory interactions. Plant Physiology 157, 405–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osorio S, Alba R, Nikoloski Z, Kochevenko A, Fernie AR, Giovannoni JJ. 2012. Integrative comparative analyses of transcript and metabolite profiles from pepper and tomato ripening and development stages uncovers species-specific patterns of network regulatory behavior. Plant Physiology 159, 1713–1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira GE, Gaudillere J-P, Van Leeuwen C, Hilbert G, Maucourt M, Deborde C, Moing A, Rolin D. 2006. 1H NMR metabolite fingerprints of grape berry: comparison of vintage and soil effects in Bordeaux grapevine growing areas. Analytica Chimica Acta 563, 346–352 [Google Scholar]
- Plaxton WC. 1996. The organization and regulation of plant glycolysis. Annual Review of Plant Physiology and Plant Molecular Biology 47, 185–214 [DOI] [PubMed] [Google Scholar]
- R Development Core Team 2010. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing [Google Scholar]
- Ruffner HP, Hawker JS. 1977. Control of glycolysis in ripening berries of Vitis vinifera. Phytochemistry 16, 1171–1175 [Google Scholar]
- Ruffner HP, Koblet W, Rast D. 1975. Gluconeogenesis in the ripening fruit of Vitis vinifera . Vitis 13, 319–328 [Google Scholar]
- Salazar Parra C, Aguirreolea J, Sánchez-Díaz M, Irigoyen J, Morales F. 2010. Effects of climate change scenarios on Tempranillo grapevine (Vitis vinifera L.) ripening: response to a combination of elevated CO2 and temperature, and moderate drought. Plant and Soil 337, 179–191 [Google Scholar]
- Schaefer J, Opgen-Rhein R, Strimmer K. 2012. GeneNet: modeling and inferring gene networks R package version 1.2.5. http://CRAN.R-project.org/package=GeneNet. Last access: monday the 21st of January 2013.
- Schluepmann H, Pellny T, van Dijken A, Smeekens S, Paul M. 2003. Trehalose 6-phosphate is indispensable for carbohydrate utilization and growth in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, USA 100, 6849–6854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schluepmann H, van Dijken A, Aghdasi M, Wobbes B, Paul M, Smeekens S. 2004. Trehalose mediated growth inhibition of Arabidopsis seedlings is due to trehalose-6-phosphate accumulation. Plant Physiology 135, 879–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon P. 2003. Cytoscape: a software environment for integrated models of biomolecular interaction networks. Genome Research 13, 2498–2504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton VL, Ough CS, Nelson KE. 1966. Density separations of wine grape berries and ripeness distribution. American Journal of Enology and Viticulture 17, 95–105 [Google Scholar]
- Son HS, Hwang GS, Kim KM, Ahn HJ, Park WM, Van Den Berg F, Hong YS, Lee CH. 2009. Metabolomic studies on geographical grapes and their wines using 1H NMR analysis coupled with multivariate statistics. Journal of Agricultural and Food Chemistry 57, 1481–1490 [DOI] [PubMed] [Google Scholar]
- Stitt M, Sulpice R, Keurentjes J. 2010. Metabolic networks: how to identify key components in the regulation of metabolism and growth. Plant Physiology 152, 428–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CA, El-Shishiny EDH. 1958. Translocation of sugars in the Concord grape. Plant Physiology 33, 33–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrier N, Glissant D, Grimplet J, et al. 2005. Isogene specific oligo arrays reveal multifaceted changes in gene expression during grape berry (Vitis vinifera L.) development. Planta 222, 832–847 [DOI] [PubMed] [Google Scholar]
- Toffali K, Zamboni A, Anesi A, Stocchero M, Pezzotti M, Levi M, Guzzo F. 2011. Novel aspects of grape berry ripening and post-harvest withering revealed by untargeted LC-ESI-MS metabolomics analysis. Metabolomics 7, 424–436 [Google Scholar]
- Vaillant-Gaveau N, Maillard P, Wojnarowiez G, Gross P, Clément C, Fontaine F. 2011. Inflorescence of grapevine (Vitis vinifera L.): a high ability to distribute its own assimilates. Journal of Experimental Botany 62, 4183–4190 [DOI] [PubMed] [Google Scholar]
- Van Dijck P, Mascorro-Gallardo JO, De Bus M, Royackers K, Iturriaga G, Thevelein JM. 2002. Truncation of Arabidopsis thaliana and Selaginella lepidophylla trehalose-6-phosphate synthase unlocks high catalytic activity and supports high trehalose levels on expression in yeast. Biochemical Journal 366, 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignault C, Vachaud M, Cakir B, Glissant D, Dedaldechamp F, Buttner M, Atanassova R, Fleurat-Lessard P, Lemoine R, Delrot S. 2005. VvHT1 encodes a monosaccharide transporter expressed in the conducting complex of the grape berry phloem. Journal of Experimental Botany 56, 1409–1418 [DOI] [PubMed] [Google Scholar]
- Wei T. 2011. corrplot: visualization of a correlation matrix. R package version 0.60. http://CRAN.R-project.org/package=corrplot. Last access: monday the 21st of January 2013
- Yin YG, Tominaga T, Iijima Y, Aoki K, Shibata D, Ashihara H, Nishimura S, Ezura H, Matsukura C. 2010. Metabolic alterations in organic acids and γ-aminobutyric acid in developing tomato (Solanum lycopersicum L.) fruits. Plant and Cell Physiology 51, 1300–1314 [DOI] [PubMed] [Google Scholar]
- Zhang J, Wang X, Yu O, Tang J, Gu X, Wan X, Fang C. 2011. Metabolic profiling of strawberry (Fragaria×ananassa Duch.) during fruit development and maturation. Journal of Experimental Botany 62, 1103–1118 [DOI] [PubMed] [Google Scholar]
- Zhang XY, Wang XL, Wang XF, Xia GH, Pan QH, Fan RC, Wu FQ, Wu XC, Zhang DP. 2006. A shift of phloem unloading from symplasmic to apoplasmic pathway is involved in developmental onset of ripening in grape berry. Plant Physiology 142, 220–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A, Paul MJ. 2009. Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiology 149, 1860–1871 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.