Abstract
In a non-model staple crop like wheat (Triticum aestivumI L.), functional validation of potential drought stress responsive genes identified in Arabidopsis could provide gene targets for breeding. Virus-induced gene silencing (VIGS) of genes of interest can overcome the inherent problems of polyploidy and limited transformation potential that hamper functional validation studies in wheat. In this study, three potential candidate genes shown to be involved in abiotic stress response pathways in Arabidopsis thaliana were selected for VIGS experiments in wheat. These include Era1 (enhanced response to abscisic acid), Cyp707a (ABA 8’-hydroxylase), and Sal1 (inositol polyphosphate 1-phosphatase). Gene homologues for these three genes were identified in wheat and cloned in the viral vector barley stripe mosaic virus (BSMV) in the antisense direction, followed by rub inoculation of BSMV viral RNA transcripts onto wheat plants. Quantitative real-time PCR showed that VIGS-treated wheat plants had significant reductions in target gene transcripts. When VIGS-treated plants generated for Era1 and Sal1 were subjected to limiting water conditions, they showed increased relative water content, improved water use efficiency, reduced gas exchange, and better vigour compared to water-stressed control plants inoculated with RNA from the empty viral vector (BSMV0). In comparison, the Cyp707a-silenced plants showed no improvement over BSMV0-inoculated plants under limited water condition. These results indicate that Era1 and Sal1 play important roles in conferring drought tolerance in wheat. Other traits affected by Era1 silencing were also studied. Delayed seed germination in Era1-silenced plants suggests this gene may be a useful target for developing resistance to pre-harvest sprouting.
Key words: Arabidopsis, drought, Era1, Sal1, virus-induced gene silencing, wheat.
Introduction
Drought is a major problem that affects wheat production worldwide. Tolerance to water stress is a quantitative trait with a complex phenotype that is often confounded by plant phenology (Fleury et al., 2010). Because of the complexity of water stress tolerance and the effect of other harsh environmental factors in the field, improvement of this trait has largely relied on direct phenotypic selection for improved performance under drought conditions. On the other hand, the application of genomic tools in Arabidopsis has produced large datasets of gene expression profiles that result from exposure to water stress (Seki et al., 2001). Experimental validation of candidate genes identified from such transcript profiling studies has identified genes that appear to contribute to plant performance under limiting water conditions in greenhouse studies. Two such genes are Era1 and Cyp707a, which are both involved in abscisic acid (ABA) regulation in the abiotic stress pathway. Era1 is a gene that encodes the β-subunit of farnesyltransferase (Cutler et al., 1996), whose loss of function in Arabidopsis thaliana caused greater ABA-induced guard cell S-type anion-channel activation and a rapid increase in the cytosolic Ca2+ concentration. Era1 mutation resulted in significantly tighter stomatal closure at a wide range of physiologically relevant ABA concentrations (Pei et al., 1998; Allen et al., 2002). Transgenic Brassica napus silenced for Era1 showed enhanced ABA sensitivity as well as significant reduction in stomatal conductance and water transpiration under drought stress conditions (Wang et al., 2005). Cyp707a is a catabolic gene encoding ABA 8’-hydroxylase, an enzyme playing a key role in ABA catabolism and in controlling ABA levels in various aspects of the plant life cycle (Kushiro et al., 2004). The Cyp707a gene reportedly degrades ABA during seed imbibition and dehydration stress (Kushiro et al., 2004; Saito et al., 2004). A T-DNA insertion mutant of Cyp707a3, which is the most abundantly expressed gene among the four Cyp707a members under stress conditions, exhibited elevated drought tolerance with a concomitant reduction in transpiration rate (Umezawa et al., 2006). A third gene is Sal1, an inositol polyphosphate1-phosphatase-encoding gene. Sal1 was originally isolated from Arabidopsis for its ability to complement a salt-sensitive yeast strain (Quintero et al., 1996). In Arabidopsis, mutation of this gene has shown increased drought tolerance (Wilson et al., 2009). Sal1 acts as a negative regulator of predominantly ABA-independent and also ABA-dependent stress response pathways. As far as is known, none of these three genes have been experimentally proven to have a role in drought tolerance in wheat. Furthermore, wheat homologues for Sal1 have not been identified prior to this study.
Homologous genes in different species may not have the same function due to the different course of evolution that each species has taken. The high frequencies of duplication and deletion events in the wheat genome make it difficult to predict the function of a gene in one species based on the function of the homologous gene in the other species (Dubcovsky and Dvorak, 2007). Furthermore, it has been shown that while common regulatory mechanisms exist across species in response to abiotic stress, the conservation of the molecular response to dehydration across experiments (Mohammadi et al., 2007; Aprile et al., 2009) is low due to variation in stress dynamics, stage of development and tissue analysed.
Functional validation of the role of genes in stress can be studied either by overexpression or downregulation. For gene overexpression studies, full-length sequence information and an efficient genetic transformation protocol are required. However, wheat has been the most recalcitrant cereal species to culture in vitro (Shah et al., 2009). Transformation in wheat is still confined mainly to a few responsive varieties with quite different transformation frequencies such as the model spring genotype ‘Bobwhite’ (Cheng et al., 1997, 2003; Hu et al., 2003). For downregulation studies, knockout mutants are used. Not only will this require a transformation step, but the functional redundancy of homeologous genes present in the other wheat genomes could complement the absence of gene expression resulting from a single gene knockout (Lawrence and Pikaard, 2003). This limitation can be overcome by generating double and triple mutants, although this process is cumbersome and time consuming. Therefore, faster alternatives are required for functional gene analysis in polyploid wheat.
Virus-induced gene silencing (VIGS) provides an alternative strategy for gene functional analysis through the simultaneous knockdown of expression of multiple related gene copies. This is a technique that was first developed in dicots (Burch-Smith et al., 2006) and is based on post-transcriptional gene silencing of host sequences that are complementary to host-derived sequences contained in a recombinant viral vector inoculated on plants. VIGS was developed in barley using barley stripe mosaic virus (BSMV), a natural pathogen in this species and consisting of a tripartite genome (Holzberg et al., 2002; Lu et al., 2003). The usefulness of VIGS in wheat was first demonstrated by Scofield et al. (2005) and has now been used to study genes for resistance to pathogens and the Russian wheat aphid (Scofield et al., 2005; Cloutier et al., 2007; Zhou et al., 2007; Van Eck et al, 2010). VIGS has not been used for characterization of water stress response genes in wheat.
The main objective of this study was to evaluate the roles of Era1, Cyp707a, and Sal1 in the response of wheat to limiting water conditions using VIGS. Optimization of the VIGS protocol was required in order to accomplish this.
Materials and methods
Plant material
All experiments were conducted using hexaploid spring wheat (Triticum aestivum L.) cv UC1041, a breeding line (Yecora Rojo/’Tadinia’) from the University of California-Davis Wheat Breeding Program, which was kindly provided by Dr Jorge Dubcovsky.
Silencing construct development
PCR products used in the construction of silencing vectors were amplified from wheat cDNA using the VIGS primers listed in Table 1. Primers were designed using Primer3 (Rozen and Skaletsky, 2000) and used to amplify a 297-bp fragment of Cyp707a (accession EU430344.1) and a 316-bp fragment of Era1 (accession ABI74692). For identification of Sal1 sequences in wheat, annotated Arabidopsis Sal1 (TAIR accession 4010745380) sequences were screened against publicly available wheat expressed sequence tag sequences (http://wheat.pw.usda.gov/wEST/) which were then assembled into one contig using CAP3 at a stringency level of 95% similarity over a 20-bp overlap (Huang and Madan, 1999). The assembled sequences were validated by PCR amplification of wheat cDNA followed by sequencing, resulting in a 1077-bp region. A PCR product of 275bp was generated from this 1077bp using primers listed in Table1.
Table 1.
Wheat primers used in this study.
| Name/ Accession | Type | Target region | Primer (5’–3’) |
|---|---|---|---|
| Era1 | VIGS | 57–372 | AATGGCAGGGTCTGATGAAC |
| TGCGCTGTACTGGCTAACTG | |||
| qRT-PCR | 216–307 | TGAAGCTCATGGTGGGTACA | |
| AGCCAATCAAGCTAGGCAAA | |||
| Sal1 | VIGS | 219–494 | TCTATGGTGGCCGAAGAGGA |
| CCAACACGCCCAAAACAACT | |||
| qRT-PCR | 104–201 | ACAATTGGTTGTGGTGCTGA | |
| AAAAACGAGGCATTCACTGG | |||
| Cyp707a | VIGS | 368–664 | GTCCCCAGGCCATCTTCTTC |
| GTCCCCAGGCCATCTTCTTC | |||
| qRT-PCR | 375–469 | GGGTGATCCAGGAGACGAT | |
| GGGAATCAGGTACCCTTGGT |
Vector constructs were made by the method described by Cakir and Scofield (2008). The orientation of the cloned inserts was determined via PCR using a combination of vector-specific (Cakir and Scofield, 2008) and fragment-specific primers (Table 1). Clones putatively containing the fragments in the antisense orientation relative to the γ genes were sequenced to confirm their identity and subsequently used for gene silencing.
Viral inoculation
The α, β, and γ RNAs of the BSMV genome were synthesized from linearized plasmids containing cloned cDNA genome segments (Petty et al., 1989), using the mMessage mMachine T7 kit (Ambion, Austin, TX, USA). Capped in vitro transcripts of each RNA segment were combined in an equimolar ratio and added to an abrasive FES buffer (0.1 m glycine, 0.06 m K2HPO4, 1% w/v tetrasodium pyrophosphate, 1% w/v bentonite, 1% w/v celite, pH 8.5) according to the procedures of Scofield et al. (2005). Each silencing construct consisted of BSMV α, β, and γ with the target gene insert. The original BSMV, BSMV0, was used as the viral control, and was constituted from α, β, and γ RNA derived from the original empty pSL038-1 vector. A volume corresponding to 3 μg viral RNA was rub inoculated onto the second leaf of silenced seedlings at the 3–4 leaf stage. For Era1, another silencing experiment was done at the booting stage by rub inoculating the stem just underneath the growing spike. Seeds were collected at 23 days post-inoculation (dpi).
Quantitative PCR
Eleven days after viral inoculation of plants, leaf tissue was collected to determine the efficiency of silencing. Quantitative real-time PCR (qRT-PCR) was performed to determine changes in Era1, Sal1, and Cyp707a transcript abundance in each treatment group. All the qRT-PCR primers are listed in Table 1. The distal 5cm of the third leaf from four experimental plants per treatment was collected into liquid nitrogen. For Era1 inflorescence silencing, heads from silenced spikes were harvested on day 17 after viral inoculation. First-strand cDNA synthesis was conducted using the Retroscript reagent (Ambion) on purified RNA for each sample and primed with a mix of poly A and random decamers. All amplifications were performed on the iCycler iQ instrument (Biorad, Hercules, CA, USA) using the Perfecta SYBR Green Supermix (Quanta Biosciences, Gaithersburg, MD, USA) and input cDNA equivalent to 2.5ng total RNA. The following cycling parameters were used: initial denaturation at 95 °C for 2min, 30 cycles consisting of denaturation at 95 °C for 15 s and annealing and extension at 57 °C for 45 s. Single-fragment amplification was verified by dissociation curve analysis. Gene expression values were normalized as previously described (Willems et al., 2008). Relative transcript abundance was calibrated to the mean expression of the viral control (water-stressed BSMV0-treated plants) treatment group and normalized against the level of housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in each sample (Pfaffl, 2001).
Imposition of water stress
Each of the five treatment groups consisted of 10 plants. Plants were grown in plastic pots holding 500g of potting mixture. Plants were maintained in a temperature-controlled growth room at 22–25 °C and relative humidity of 60% with a 12h photoperiod with light intensity ranging from 300 to 400 μE m−2 s−1. Prophylactic measures were taken to maintain the plants disease and pest free. Samples from plants were not pooled, and each plant was observed as an independent biological replicate for a total of 10 biological replicates per treatment. For each experiment, two subsets of plants were maintained. One set of plants was maintained at 100% field capacity (FC) and stress was imposed on the other set of plants by withholding water until 50% FC, which was achieved by withholding water continuously for 3 days. Withholding water up to 50% FC was found to induce the water stress phenotype in the experimental plants placed in 500g of potting mix within the time frame of the experiment (24 days). The plants were maintained at 100% FC until imposition of moisture stress. Withholding of water started 3 days after rub inoculation of BSMV. Plants were maintained at 50% FC during the entire duration of the study. Soil moisture regimes were monitored gravimetrically by weighing the pots every day.
Estimation of plant water status
To assess the drought stress effects, leaf relative water content (RWC) was estimated according to the method of Ekanayake et al. (1993). Water use efficiency (WUE, seedling dry weight increment per weight of water used) was also estimated. The soil evaporation across all pots was assumed to be similar. Water used by the plant was estimated by the amount of water required to maintain the weight for each experimental pot with plants. At day 11 after initiation of water stress moisture, stress responses were assessed by taking leaf samples from the uppermost fully expanded leaves of both stressed and non-stressed plants. Samples were collected at midday, quickly sealed, and kept on ice. After determining the fresh weight, the leaf segments were floated on deionized water for 24h to determine their turgid weight. The dry weight was determined after oven drying at 70 °C to a constant weight. The RWC was calculated using the formula RWC (%) = [(W – DW) ÷ (TW – DW)] × 100, where W is sample fresh weight, TW is turgid weight after rehydrating plant sample for 24 hours, and DW is sample dry weight (Ekanayake et al., 1993).
Gas exchange
Plants in all treatment groups (water-stressed silenced plants, water-stressed viral control, non-silenced well-watered (WW), and non-silenced water-stressed (WS) plants) were tested for variation in photosynthetic gas exchange since the genes under consideration may have direct or indirect effects on stomatal opening/closure (Wang et al., 2009). The level of water stress used was selected to cause immediate visible phenotypes to accommodate the transient nature of the VIGS assay. This led to all the water-stressed wheat seedlings looking wilted and chlorotic compared to well-watered plants. To carry out the gas exchange studies, this study used a higher light intensity compared to normal greenhouse light conditions to ensure that photosynthesis and transpiration were not light limited and that the difference in gas exchange among treatment plants is due to the effect of silencing stomatal conductance-regulating genes. The light intensity was set at 800 μmol m–2 s–1 with a red/blue light source and the CO2 level was set at 400 μmol mol–1. A portable, open flow, gas-exchange system (LCpro+, Opti-Sciences, Hudson, NH, USA) was used to record the gas exchange on the fifth fully expanded leaf. One day prior to the gas exchange reading, the pots were watered to maintain the exact 50 or 100% FC depending on the treatment. Leaf gas-exchange rates were measured at 7, 14, and 20 dpi.
Germination study
Seeds from plants inoculated with BSMVEra1 during the booting stage were collected 23 days post- inoculation. Seeds from the control BSMV0-inoculated plants were also collected. Seeds from silenced and control plants of similar size were surface sterilized with 5% (w/v) calcium hypochlorite for 15min and washed four times with sterile deionized water. These seeds were then transferred to sterile Petri dishes (five seeds per dish) containing moist Whatman No. 1 filter paper. Four replicates of five seeds per dish were used in each treatment. Seeds were allowed to germinate at room temperature in the dark and germination percentages were recorded daily up to 6 days using radicle extrusion as a criterion. A seed was considered germinated if the radicle length was ≥2mm.
Bacterial pathogen study on Era1-silenced plants under drought
Xanthomonas translucens pv. undulosa (denoted as strain B75) and Xanthomonas translucens pv. translucens (denoted as strain B74) were incubated at 28 °C for 72h on modified Wilbrink’s media and a single colony was selected and grown in nutrient broth for 16 hours at 28 °C. An aqueous suspension of 109 colony-forming units ml–1 were inoculated by syringe infiltration on the flag leaf at the 3-leaf stage, and the plants were incubated in the greenhouse. Sterilized water was used as the negative control for inoculation treatments. The bacterial pathogen inoculation was done at 7 dpi with BSMVEra1 or BSMV0. In a pilot study, it was determined that the strains used exhibit a compatible interaction with wheat cv. UC1041 (data not shown). Seven days after inoculation with X. translucens, the leaf lesion area was quantified using digital image analysis using ASSESS software (American Phytopathological Society, St. Paul, MN, USA). The experiment was arranged in a completely random design. There were 24 individual treatments (3 bacterial inoculants × 2 water stress conditions × 3 silencing types) and each of these treatments was replicated 24 times. Statistical analysis was carried out using the general linear model of the SAS 9.1 statistical package (SAS Institute, Cary, NC, USA). Data were subjected to two-way analysis of variance (ANOVA) for finding the effect of drought and Era1 silencing on disease, followed by a comparison of the means according to a Duncan’s multiple range test at P < 0.05.
Results
Reduction in the transcript levels of silenced genes
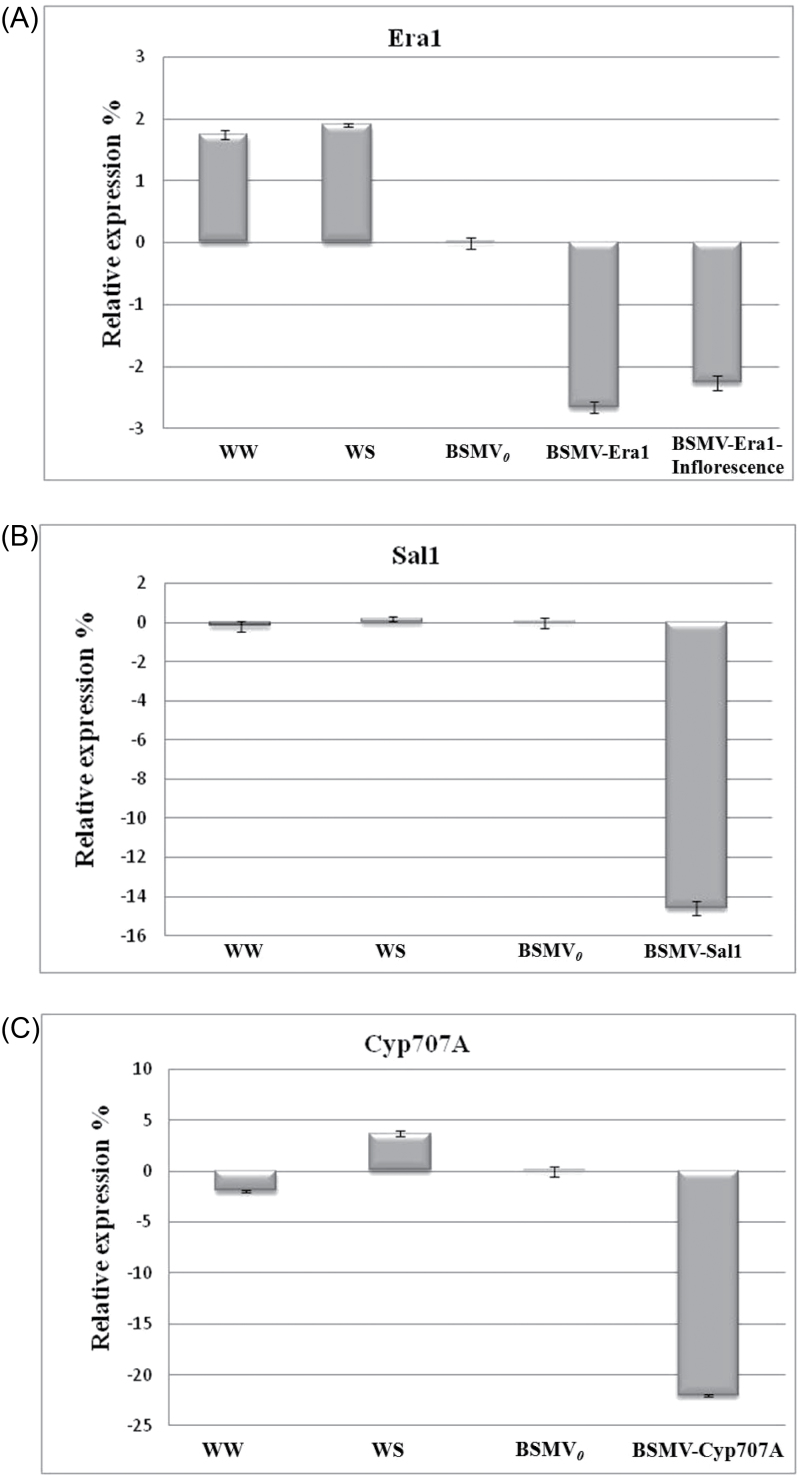
qRT-PCR was performed to determine the transcript levels of Era1, Sal1, and Cyp707a in water-stressed silenced plants relative to the water-stressed viral controls at 11 dpi. As Fig. 1 shows, the transcript levels of the genes were reduced in silenced plants compared to transcript levels of the genes in plants inoculated only with BSMV0. Plants that were inoculated with BSMVEra1 showed average 2.3-fold and 2.6-fold reductions in Era1 at the inflorescence and seedling stages, respectively, while the mean Sal1 transcript level was reduced 14.2-fold in BSMVSal1-inoculated plants (P < 0.0001, Fig. 1A and B). The largest reduction was observed in BSMVCyp707a plants with an average 22-fold reduction in transcript level compared to water-stressed BSMV0-treated plants (P < 0.0001, Fig. 1C).
Fig. 1.
Silencing efficiency as revealed by quantitative real-time PCR. Gene expression values were standardized across six independent biological replicates with three technical replicates. Expression of Era1 (A), Sal1 (B), and Cyp707a (C) in well-watered non-silenced (WW), water-stressed non-silenced (WS), and silenced plants (denoted by the gene name) at 11 dpi were calibrated to the mean level of expression of the respective gene in the water-stressed BSMV0-treated plants.
Transcript levels were also determined in non-silenced WW and WS plants. The transcript levels of Sal1 and Cyp707a in WW and WS plants were not statistically different from water-stressed viral control plants (P = 0.1550 and P = 0.2700, for Sal1 in WW and WS, respectively; P = 0.3250 and P = 0.3300 for Cyp707a in WW and WS, respectively, Fig. 1B and C). Interestingly, the mean transcript levels of Era1 in WW and WS plants were statistically higher compared to the viral controls (P < 0.0350 and P < 0.0175, Fig. 1A). This suggests that Era1 was downregulated as a result of virus infection.
Silencing alters the rate of gas exchange
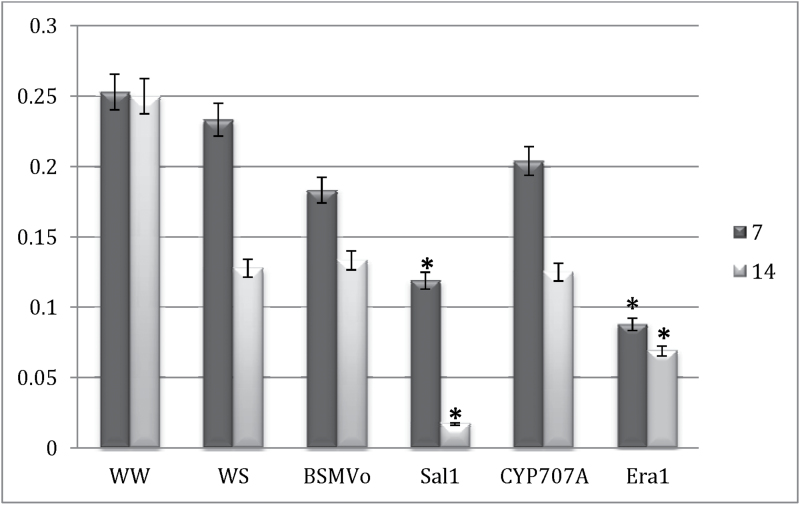
Era1 and Sal1 function to modulate a plant’s ABA-mediated stomatal response to control the rate of transpiration (Pei et al., 1998; Wilson et al., 2009). Cyp707a is involved in the catabolism of ABA and also has the potential to regulate stomatal response under drought (Umezawa et al., 2006). The present study therefore tested whether and how the rate of gas exchange would be affected by silencing of these genes. Water-stressed BSMV0-treated plants and non-silenced WS plants did not show statistically significant differences in stomatal conductance and transpiration rate (Fig. 2). The water-stressed BSMVEra1- and BSMVSal1-treated lines showed statistically significant reductions in stomatal conductance and transpiration rate under limiting water conditions compared to the water-stressed viral control plants at both 7 and 14 dpi. This is consistent with previous reports of reduced stomatal conductance in loss-of-function mutants in Arabidopsis (Pei et al., 1998; Rossel et al., 2004; Wilson et al., 2009). These loss-of-function mutant plants also showed increased tolerance in drought stress conditions in greenhouse experiments. At 14 dpi, the transpiration level of BSMVSal1-treated plants showed further reduction in stomatal conductance relative to that at 7 dpi. A slight reduction was also observed in BSMVEra1-treated plants at 14 dpi compared to 7 dpi (Fig. 2). On the other hand, BSMVCyp707a-treated plants did not show a significant reduction in stomatal conductance compared to viral control plants. Photosynthesis measurements taken during this study were inconclusive due to the total shut down of photosynthesis across all water-stressed treatment groups.
Fig. 2.
Mean stomatal conductance (mmol m–2 s–1) at 7 and 14 dpi. Measurements were taken daily from each treatment group for 14 days. Water-stressed BSMV0-inoculated plants served as control. Values are means of six observations. Asterisks denote significant difference from the control WS (P = 0.05). WW, non-silenced well-watered; WS, non-silenced water-stressed; all others plants were water-stressed and inoculated with BSMV targeted to specific genes.
Impact of silencing on plant water status under water limitation
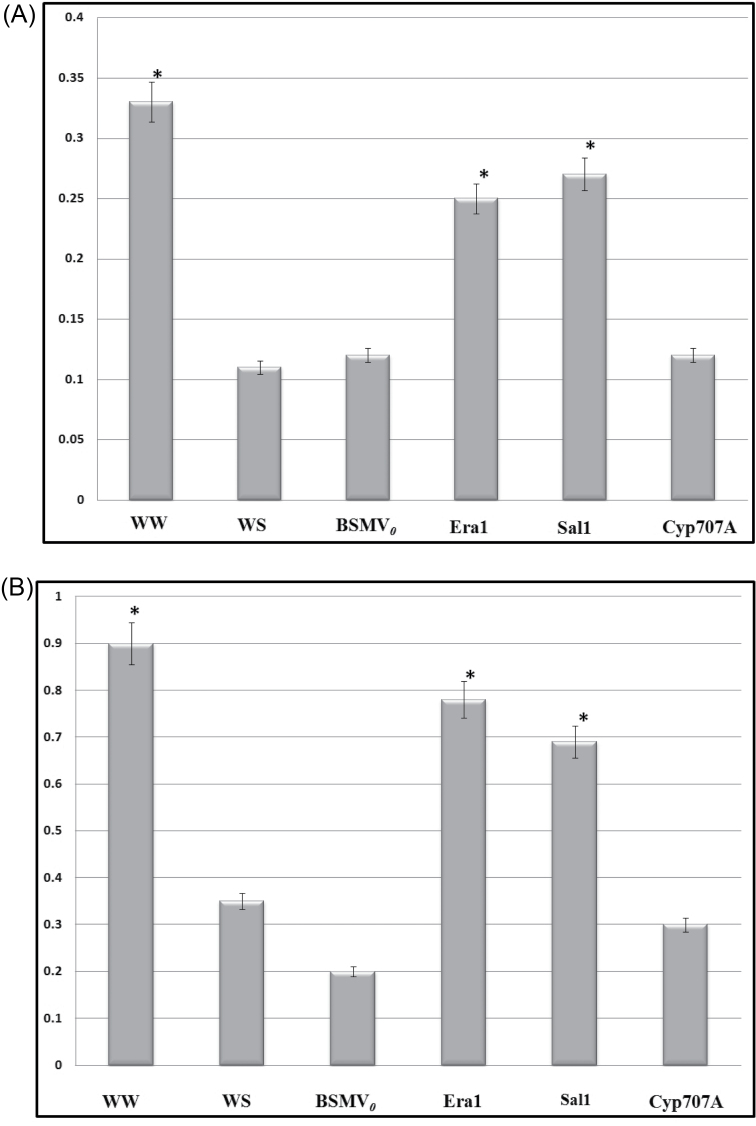
Water stress was examined in terms of RWC and WUE at 14 dpi in all the treatment groups (Fig. 3). The RWC and WUE measurements indicate potential photosynthetic gain during a water stress period. The WUE values obtained were lower than typically observed in potted plants (usually in the range 0.6–0.9g kg–1). This is most likely a result of the plants not getting sufficient light in the greenhouse during the winter when these experiments were carried out. This would have resulted in reduced photosynthesis and consequently low biomass gain. However, since all plants were grown under the same conditions, it was possible to compare plants in the different treatments. The non-silenced plants showed a drastic reduction in both WUE and RWC in the WS plants relative to the WW plants, which was expected. The WS plants also did not differ significantly from the water-stressed viral control, indicating that virus inoculation had no effect on these two measurements. Similarly, plants silenced for Cyp707a did not differ significantly from the viral control in both WUE and RWC. On the other hand, plants silenced for Era1 and Sal1 showed significant gains in RWC and WUE over viral control and WS plants.
Fig. 3.
(A) Water use efficiency by gravimetric determination (g kg–1) at the end of treatment (24 dpi). (B) Mean relative water content (g) per treatment at 14 dpi. Water-stressed BSMV0-inoculated plants served as control. Values are means of six observations. Asterisks denote significant difference from the control WS (P = 0.05). WW, non-silenced well-watered; WS, non-silenced water-stressed; all others plants were water-stressed and inoculated with BSMV targeted to specific genes.
Plants silenced for Era1 and Sal1 showed improved vigour under water stress
Phenotypes of the plants were observed daily during the entire course of the experiment (27 days). Slight chlorosis was observed in all the silenced and viral control plants immediately after rub inoculation of the BSMV constructs. This has been reported in previous VIGS studies in wheat (Scofield et al., 2005; Van Eck et al., 2010) and is due to the plant’s response to virus infection. Typical wilting symptoms and poor vigour were observed in all water-stressed plants, whether silenced for a gene or not (Fig. 4). The BSMV0-treated control plants were visibly similar to the non-silenced WS plants under water stress. The water-stressed BSMVCyp707a-treated plants showed similar symptoms as the water-stressed viral control plants. On the other hand, water-stressed BSMVEra1- and BSMVSal1-treated plants showed distinctly better turgidity and appeared more vigourous compared to plants in all the other water-stressed treatments. This improved vigour held up during the entire 24 day-stress period. Interestingly, more tillers were observed in BSMVSal1-treated plants compared to plants in all other treatments, including BSMVEra1-treated plants. However, the BSMVSal1-treated plants were smaller compared to BSMVEra1-treated plants under limiting water conditions during the initial 5–7 days of water stress. By day 24 of water stress, both BSMVEra1 and BSMVSal1-treated plants were of similar size.
Fig. 4.
Phenotypes of wheat plants at 24 dpi with BSMV RNA transcripts containing one of the following wheat genes: Sal1, Cyp707A, or Era1. Water stress was imposed on these plants by withholding water until 50% of field capacity. Water-stressed BSMV0-inoculated plants served as control. Non-silenced well-watered (100%), non-silenced water-stressed (50%) plants, and water-stressed (50%) plants were included for comparison of phenotypes. Note the improved vigour of plants silenced for Sal1 and Era1 compared to the viral control plants.
All in all, silencing of Era1 and Sal1 had similar effects on the plant. This includes reduced stomatal conductance, increased WUE, and increased RWC. These physiological measurements matched the observed improved vigour in BSMVEra1- and BSMVSal1-treated plants under water stress. Decreased stomatal conductance under limiting water conditions may have contributed to improved WUE and RWC. Stomatal conductance was significantly and negatively correlated with the level of expression of Era1 (r = –0.80, P ≤ 0.01) and Sal1 (r = −0.89, P ≤ 0.01). In comparison, BSMVCyp707a-treated plants did not show significant changes in any of the physiological measurements or phenotypes relative to non-silenced or BSMV0-treated plants, despite a significant reduction in expression level of Cyp707a.
Effects of Era1 silencing on seed germination and response to fungal pathogens
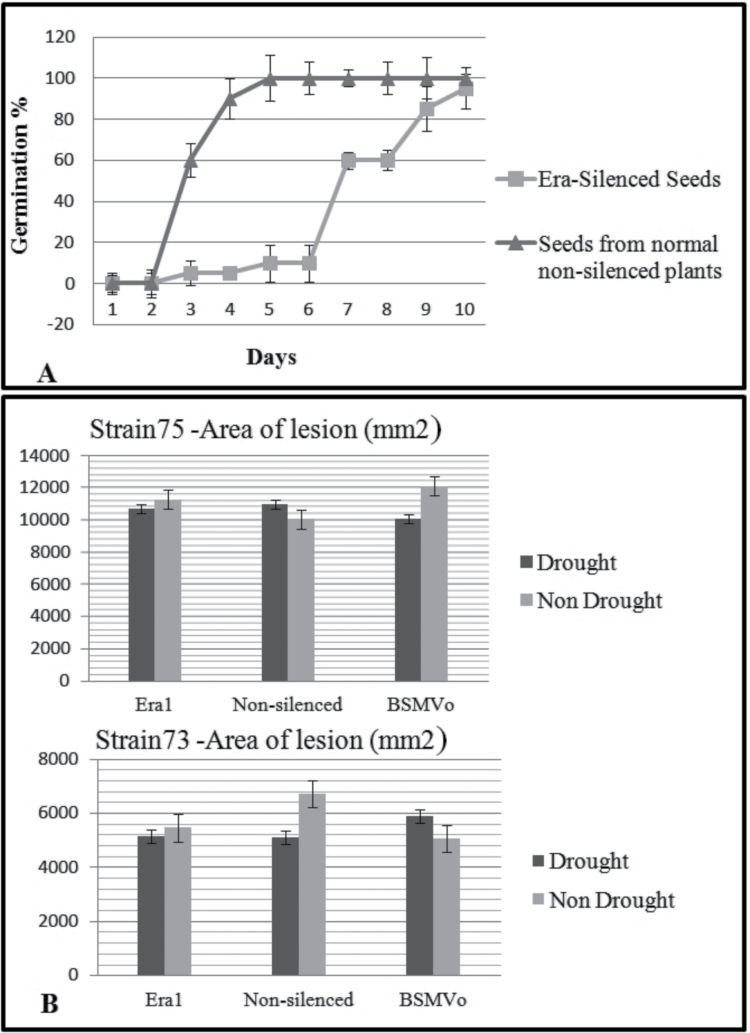
Silencing of Era1 potentially makes plants hypersensitive to ABA. Since ABA is involved in seed germination, a germination study was conducted on BSMVEra1-treated plants. Seeds harvested from plants inoculated with BSMVEra1 at the booting stage were tested for germination capacity. As Fig. 5A shows, the earliest germination of Era1-silenced seeds occurred 6 days later than those of control (BSMV0 treated) plants, 6–8 days after 100% of seeds from control plants have germinated, only 60% of seeds from Era1-silenced plants had germinated, and 100% germination was achieved 10 days later than those of control plants.
Fig. 5.
Effect of Era1 silencing on wheat seed germination and response to pathogens. (A) Number of germinated seeds in Era1-silenced and non-silenced plants, determined according to the 2-mm radicle extrusion criterion. (B) Cumulative lesion area observed after 7 days post infection with Xanthomonas translucens B74 and B75 on Era1-silenced or non-silenced plants under drought or non-drought conditions. Era1, plants silenced in Era1; BSMV0, plants inoculated with empty BSMV viral vector; non-silenced, plants not inoculated with BSMV.
Goritschnig et al. (2008) recently showed that loss of function in Arabidopsis results in enhanced susceptibility to virulent bacterial and oomycete pathogens. This led the authors to suggest that farnesylation is involved in basal defence. To study the potential for increased susceptibility in Era1-silenced wheat plants, two known wheat pathogenic bacterial strains (X. translucens B74 and B75) were tested for their increase or decrease in virulence against Era1-silenced wheat plants compared to non-silenced wheat plants under drought and well-watered conditions (Fig. 5B). Two-way ANOVA showed no significant difference in the lesion area of plants under drought and silencing treatments compared to control plants in both bacterial strains (P D75 = 0.52, P s75 = 0.46 and P D73 = 0.51, P s73 = 0.304). Also, there was no significant interaction between two treatments (drought X silencing) for both strains’ lesion area (P D75*S75 = 0.12 and P D73*S73 = 0.28), suggesting that a combination of drought and Era1 silencing does not affect bacterial growth in wheat individually and interactively.
Discussion
This study was undertaken to test whether single genes that have been shown to enhance drought tolerance in Arabidopsis when mutated would have similar effects in wheat. Three genes, namely Era1, Cyp707a, and Sal1, that represent different abiotic stress pathways were selected. The VIGS technique offered the best approach for testing the function of these genes in wheat for several reasons. The most important reason is that VIGS does not require a transformation step, which is still technically challenging in wheat. The potential of homologous copies of a gene to compensate for the absence of a homologue that has been silenced can be overcome in VIGS. All endogenous mRNA with at least 80% similarity to the host-derived sequence contained in the viral vector are targets for degradation. While this is an advantage of VIGS, it is also a disadvantage in that the specific gene responsible for the effect is not known and would require further experiments.
One concern of using VIGS for evaluating putative drought tolerance genes is the fact that environmental conditions used for stress induction might not favour viral replication leading to poor silencing. The stress protocol used in the present study involved initial exposure of silenced plants to an acclimation stress prior to exposure to severe stress. The viral inoculation was done at late evening on well-watered healthy plants and water stress was imposed gradually over 4 dpi by gradually withholding water. The initial adjustment time was important as the virus also imparts some level of stress on the plants. The protocol to characterize genes involved in the stress-recovery response involved inoculation of VIGS vector 4 days prior to water stress induction to coincide with higher transcript downregulation of targeted genes. The levels of silencing (from 2.6- to 22-fold) achieved for the targeted genes were comparable to other VIGS wheat studies (Scofield et al., 2005; Bruun-Rasmussen et al., 2007; Van Eck et al., 2010). The consistent phenotype across the silenced plants also neutralized the concern of the stress response to BSMV masking the effect of water stress effects, suggesting that drought trait functional studies can be carried out by VIGS in wheat.
Of the three genes tested, only Era1 and Sal1 showed similar phenotypes in wheat upon loss of function as has been reported in Arabidopsis (Wang et al., 2005; Wilson et al., 2009). A possible role of regulation of stomatal conductance was indicated for the improved performance of Era1- and Sal1-silenced plants. Closed stomata has been suggested to lead to decreased photosynthesis and therefore decreased productivity for the plant in well-watered conditions (Farquhar and Sharkey, 1982; Schulze, 1986). The present experiment observed improved WUE compared to the viral control water-stressed BSMV0-inoculated plants, indicating better dry weight production under limiting water condition for BSMVEra1- and BSMVSal1-treated plants. A likely explanation is that plants which regulate the stomatal opening ‘efficiently’ and conserve water can achieve larger biomass per gram of water used (Farquhar and Richards 1984; Araus et al., 2008).
In the case of Sal1, loss of function was reported to yield shorter and rounder leaves in Arabidopsis with an enhanced drought tolerance (Umezawa et al., 2006). Down-regulation of Sal1 leads to constitutively increased ABA content in A. thaliana (Wilson et al., 2009). Sal1 acts as a negative regulator of predominantly ABA-independent as well as ABA-dependent stress response pathways, such that its inactivation results in altered osmoprotectants, higher leaf RWC, and maintenance of viable tissues during prolonged water stress (Wilson et al., 2009). This study also showed that reduced expression of Sal1 in wheat resulted in increased RWC, and increased WUE, as well as improved vigour, suggesting a similar function of Sal1 in wheat. No other unusual phenotype was observed except increased/accelerated tillering, which is difficult to interpret at this point due to limited understanding of the mode of action of Sal1.
Interestingly, the effect of silencing Cyp707a homologues in wheat was different from previous findings in Arabidopsis. Loss of function of Cyp707a was reported to enhance drought tolerance in A. thaliana (Umezawa et al., 2006). In contrast, Cyp707a-silenced wheat plants did not show any improvements in terms of dry weight, WUE, or RWC of silenced plants compared to water-stressed BSMV0-treated control plants under water stress. The differences in response between Arabidopsis and wheat plants to loss of function of Cyp707a may be due to differences in stress adaptation pathways between wheat and Arabidopsis. Another possible reason for the observed difference may have to do with the structure of the Cyp707a gene family in the two species. In A. thaliana, the Cyp707a gene family consists of Cyp707a1–Cyp707a4, all of which are upregulated in response to dehydration and subsequent rehydration, suggesting that the function of Cyp707a genes is redundant (Kushiro et al., 2004). Although there is only one Cyp707a cDNA sequence reported for wheat (accession EU430344.1), with three copies amplified by PCR in hexaploid wheat in this study, it is possible that other copies of Cyp707a exist in wheat. The DNA sequences of these other copies could potentially be different enough to cause ineffective silencing using the VIGS construct used in this study, leading to compensation of gene function by the remaining active genes. This emphasizes the importance of having the wheat genome sequence information in attempts to functionally test Arabidopsis gene homologues in wheat.
Out of the three genes tested in this study, Era1 appeared to be the most promising as a potential target for drought tolerance breeding in wheat. Mutations in Era1 could be readily identified through TILLING, taking advantage of resources that already exist in wheat (Slade and Knauf, 2005; Uauy et al., 2009). Because of the role of ABA in germination and dormancy, the present study tested Era1-silenced plants for germination rates. Indeed, Era1-silencing done at the booting stage resulted in a 6–10-day delay in germination compared to non-silenced plants. This trait may provide a solution for tackling pre-harvest sprouting in wheat. Pre-harvest sprouting is a consequence of ABA deficiency and leads to loss of grain weight and a reduction in end-use quality of wheat (Fang and Chu, 2008). While Era1 has also been suggested to play a role in basal defence against bacteria and oomycytes (Goritschnig et al., 2008), the Era1-silenced wheat plants showed similar phenotypes in response to bacterial infection as non-silenced wheat plants under both water stress and well-watered conditions. This implies that there is no fitness cost to Era1-silenced plants under favourable conditions. It would be interesting to further study the differences in the function of Era1 in pathogen susceptibility between wheat and Arabidopsis.
In conclusion, this study showed the involvement of Era1 and Sal1 in response to water deficit stress tolerance in wheat. This is the first report of VIGS being successfully used in wheat to functionally characterize genes thought to be involved in drought tolerance. The transient nature of VIGS did not deter from generating valuable observations for screening potential drought tolerant genes in wheat. One major strategy adopted by plants during drought tolerance is avoiding dehydration (Serraj and Atlin, 2009). Results from the present study indicate that downregulation of genes which reduce stomatal conductance may be a useful strategy for enhancing drought tolerance in wheat, if the same results hold in the field.
Acknowledgements
This study was supported by the US Department of Agriculture (Cooperative Agreements USDA contract nos. 2009-34205-19960 and 2010-34205-21350 and Hatch Funds project no. 644, to NL). The authors thank Leon Van Eck (University of Stellenbosch, South Africa) and Hong Wang (Colorado State University) for helping with the VIGS experiment.
References
- Allen GJ, Murata Y, Chu SP, Nafisi M, Schroeder JI. 2002. Hypersensitivity of abscisic acid-induced cytosolic calcium increases in the Arabidopsis farnesyltransferase mutant era1-2. The Plant Cell 14, 1649–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprile A, Mastrangelo AM, De Leonardis AM, Galiba G, Roncaglia E, Ferrari F, De Bellis L, Turchi L, Giulliano G, Cattivelli L. 2009. Transcriptional profiling in response to terminal drought stress reveals differential responses along the wheat genome. BioMed Central Genomics 10, 279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araus JL, Slafer GA, Royo C, Dolores SM. 2008. Breeding for yield potential and stress adaptation in cereals. Critical Reviews in Plant Science 27, 377–412 [Google Scholar]
- Bruun-Rasmussen M, Madsen CT, Jessing S, Albrechtsen M. 2007. Stability of barley stripe mosaic virus-induced gene silencing in barley. Molecular Plant–Microbe Interactions 20, 1323–1331 [DOI] [PubMed] [Google Scholar]
- Burch-Smith TM, Schiff M, Liu YL, Dinesh-Kumar SP. 2006. Efficient virus-induced gene silencing in Arabidopsis. Plant Physiology 142, 21–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakir C, Scofield SR. 2008. Evaluating the ability of the barley stripe mosaic virus-induced gene silencing system to simultaneously silence two wheat genes. Cereal Research Communications 36, 217–222 [Google Scholar]
- Cheng M, Fry JE, Pang S, Zhou H, Hironaka CM, Duncan DR, Conner TW, Wan Y. 1997. Genetic transformation of wheat mediated by Agrobacterium tumefaciens. Plant Physiology 115, 971–980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng M, Hu TC, Layton J, Liu CN, Fry JE. 2003. Desiccation of plant tissues post-Agrobacterium infection enhances T-DNA delivery and increases stable transformation efficiency in wheat. In Vitro Cellular and Developmental Biology – Plant 39, 595–604 [Google Scholar]
- Cloutier S, McCallum BD, Loutre C, Banks TW, Wicker T, Feuillet C, Keller B, Jordan MC. 2007. Leaf rust resistance gene Lr1, isolated from bread wheat (Triticum aestivum L.) is a member of the large psr567 gene family. Plant Molecular Biology 65, 93–106 [DOI] [PubMed] [Google Scholar]
- Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P. 1996. A protein farnesyl transferase involved in abscisic acid signal transduction in Arabidopsis. Science 273, 1239–1241 [DOI] [PubMed] [Google Scholar]
- Dubcovsky J, Dvorak J. 2007. Genome plasticity a key factor in the success of polyploid wheat under domestication. Science 316, 1862–1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekanayake IJ, De Datta SK, Steponkus PL. 1993. Effect of water deficit stress on diffusive resistance, transpiration, and spikelet dessication of rice (Oryza sativa L.). Annals of Botany 72, 73–80 [Google Scholar]
- Fang J, Chu C. 2008. Abscisic acid and the pre-harvest sprouting in cereals. Plant Signaling and Behavior 3, 1046–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar GD, Richards RA. 1984. Isotopic composition of plant carbon correlates with water-use efficiency of wheat genotypes. Australian Journal of Plant Physiology 11, 539–552 [Google Scholar]
- Farquhar GD, Sharkey TD. 1982. Stomatal conductance and photosynthesis. Annual Review of Plant Physiology 33, 317–345 [Google Scholar]
- Fleury D, Jefferies S, Kuchel H, Langridge P. 2010. Genetic and genomic tools to improve drought tolerance in wheat. Journal Experimental Botany 61, 3211–3222 [DOI] [PubMed] [Google Scholar]
- Goritschnig S, Weihmann T, Zhang Y, Fobert P, McCourt P, Li X. 2008. A novel role for protein farnesylation in plant innate immunity. Plant Physiology 148, 348–357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzberg S, Brosio P, Gross C, Pogue GP. 2002. Barley stripe mosaic virus-induced gene silencing in a monocot plant. The Plant Journal 30, 315–327 [DOI] [PubMed] [Google Scholar]
- Hu T, Metz S, Chay C, et al. 2003. Agrobacterium-mediated large-scale transformation of wheat (Triticum aestivum L.) using glyphosate selection. Plant Cell Reports 21, 1010–1019 [DOI] [PubMed] [Google Scholar]
- Huang X, Madan A. 1999. CAP3: a DNA sequence assembly program. Genome Research 9, 868–877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushiro T, Okamoto M, Nakabayashi K, Yamagishi K, Kitamura S, Asami T, Hirai N, Koshiba T, Kamiya Y, Nambara E. 2004. The Arabidopsis cytochrome P450 CYP707A encodes ABA 8’-hydroxylases: key enzymes in ABA catabolism. EMBO Journal 23, 1647–1656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence RJ, Pikaard CS. 2003. Transgene-induced RNA interference: a strategy for overcoming gene redundancy in polyploids to generate loss-of-function mutations. The Plant Journal 36, 114–121 [DOI] [PubMed] [Google Scholar]
- Lu R, Martin-Hernandez AM, Peart JR, Malcuit I, Baulcombe DC. 2003. Virus-induced gene silencing in plants. Methods 30, 296–303 [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Kav NNV, Deyholos MK. 2007. Transcriptional profiling of hexaploid wheat (Triticum aestivum L.) roots identifies novel, dehydration-responsive genes. Plant, Cell and Environment 30, 630–645 [DOI] [PubMed] [Google Scholar]
- Pei Z-M, Ghassemian M, Kwak CM, McCourt P, Schroeder JI. 1998. Role of farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss. Science 282, 287–290 [DOI] [PubMed] [Google Scholar]
- Petty IT, Hunter BG, Wei N, Jackson AO. 1989. Infectious barley stripe mosaic virus RNA transcribed in vitro from full-length genomic cDNA clones. Virology 171, 342–349 [DOI] [PubMed] [Google Scholar]
- Pfaffl MW. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Research 29, e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quintero FJ, Garciadeblas B, Rodriguez-Navarro A. 1996. The SAL1 gene of Arabidopsis, encoding an enzyme with 3’(2’), 5’-bisphosphate nucleotide and inositol polyphosphate 1-phosphatase activities, increases salt tolerance in yeast. The Plant Cell 8, 529–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossel JB, Cuttriss AJ, Pogson BJ. 2004. Identifying photoprotection mutants in Arabidopsis . In: Carpentier R, ed, Photosynthesis research protocols. Totowa: Humana Press; pp 287–300 [DOI] [PubMed] [Google Scholar]
- Rozen S, Skaletsky HJ. 2000. Primer3 on the WWW for general users and for biologist programmers. In: Krawetz S, Misener S, eds, Bioinformatics methods and protocols: methods in molecular biology. Totowa, NJ: Humana Press, 365–386 [DOI] [PubMed] [Google Scholar]
- Saito S, Hirai N, Matsumoto C, Ohigashi H, Ohta D, Sakata K, Mizutani M. 2004. Arabidopsis CYP707As encode (+)-abscisic acid 80-hydroxylase, a key enzyme in the oxidative catabolism of abscisic acid. Plant Physiology 134, 1439–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze ED. 1986. Carbon dioxide and water vapor exchange in response to drought in the atmosphere and in the soil. Annual Reviews in Plant Physiology and Plant Molecular Biology 37, 247–274 [Google Scholar]
- Scofield SR, Huang L, Brandt AS, Gill BS. 2005. Development of a virus-induced gene-silencing system for hexaploid wheat and its use in functional analysis of the lr21-mediated leaf rust resistance pathway. Plant Physiology 138, 2165–2173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seki M, Narusaka M, Abe H, Kasuga M, Yamaguchi-Shinozaki K, Carninci P, Hayashizaki Y, Shinozaki K. 2001. Monitoring the expression pattern of 1300 Arabidopsis genes under drought and cold stresses by using a full-length cDNA microarray. The Plant Cell 13, 61–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serraj R, Atlin GN. 2009. Drought-resistant rice for increased rainfed production and poverty alleviation: a concept note. In: Serraj J, Bennett J, Hardy B, eds, Drought frontiers in rice: crop improvement for increased rainfed production. Singapore: World Scientific Publishing; pp 385–400 [Google Scholar]
- Shah MM, Khalid Q, Khan UW, Shah SAH, Shah SH, Hassan A, Pervez A. 2009. Variation in genotypic responses and biochemical analysis of callus induction in cultivated wheat. Genetics and Molecular Research 8, 783–793 [DOI] [PubMed] [Google Scholar]
- Slade AJ, Knauf VC. 2005. TILLING moves beyond functional genomics into crop improvement. Transgenic Research 14, 109–115 [DOI] [PubMed] [Google Scholar]
- Uauy C, Paraiso F, Colasuonno P, Tran RK, Tsai H, Berardi S, Comai L, Dubcovsky J. 2009. A modified TILLING approach to detect induced mutations in tetraploid and hexaploid wheat. BMC Plant Biology 9, 115–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezawa T, Okamoto M, Kushiro T, Nambara E, Oono Y, Seki M, Kobayashi M, Koshiba T, Kamiya Y, Shinozaki K. 2006. CYP707A3, a major ABA 8’-hydroxylase involved in dehydration and rehydration response in Arabidopsis thaliana. The Plant Journal 46, 171–182 [DOI] [PubMed] [Google Scholar]
- Van Eck L, Schultz T, Leach JE, Scofield SR, Peairs FB, Botha AM, Lapitan NL. 2010. Virus-induced gene silencing of WRKY53 and an inducible phenylalanine ammonia-lyase in wheat reduces aphid resistance. Plant Biotechnology Journal 8, 1023–1032 [DOI] [PubMed] [Google Scholar]
- Wang Y, Beaith M, Chalifoux M, Ying J, Uchacz T, Sarvas C, Griffiths R, Kuzma M, Wan J, Huang Y. 2009. Shoot-specific down-regulation of protein farnesyltransferase (α-subunit) for yield protection against drought in canola. Molecular Plant 2, 191–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ying J, Kuzma M, et al. 2005. Molecular tailoring of farnesylation for plant drought tolerance and yield protection. The Plant Journal 43, 413–424 [DOI] [PubMed] [Google Scholar]
- Willems E, Leyns L, Vandesompele J. 2008. Standardization of real-time PCR gene expression data from independent biological replicates. Analytical Biochemistry 379, 127–129 [DOI] [PubMed] [Google Scholar]
- Wilson PB, Estavillo GM, Field KJ, et al. 2009. The nucleotidase/phosphatase SAL1 is a negative regulator of drought tolerance in Arabidopsis. The Plant Journal 58, 299–317 [DOI] [PubMed] [Google Scholar]
- Zhou H, Li S, Deng Z, et al. 2007. Molecular analysis of three new receptor-like kinase genes from hexaploid wheat and evidence for their participation in the wheat hypersensitive response to stripe rust fungus infection. The Plant Journal 52, 420–434 [DOI] [PubMed] [Google Scholar]