Abstract
Aims
Catheter ablation of ventricular tachycardia (VT) can be limited by haemodynamic instability. In these cases, substrate-based ablation is typically performed. An alternative is to perform activation and entrainment mapping during VT supported by a percutaneous left ventricular assist device (pVAD). We sought to compare the complication and success rates of pVAD-assisted VT ablation with scar-based techniques.
Methods and results
Thirteen consecutive patients with haemodynamically unstable VT underwent pVAD-assisted ablation (pVAD group) and were retrospectively compared with 18-matched patients undergoing a substrate-based VT ablation (non-pVAD group). There was no significant difference in age or ejection fraction between the groups although pVAD patients tended to have more shocks in the preceding months. Procedure times were longer for the pVAD group. The number of monomorphic VTs induced was greater in the pVAD group (3.2 vs. 1.6, P= 0.04); however, after ablation, there was no difference in inducibility between the pVAD and non-pVAD group (10 of 13 vs. 12 of 18; 77 vs. 67%, P = 0.69). There was no difference in acute complications including stroke or death. At 9 ± 3 months, 1-year freedom from implantable cardioverter-defibrillator (ICD) shocks/therapies for sustained VT were similar (P= 0.96). In multivariable analysis, the absence of atrial fibrillation (hazard ratio=0.15, P= 0.04) was associated with a lower incidence of ICD shocks.
Conclusions
In high-risk patients, pVAD-assisted VT ablation guided by activation and entrainment mapping is a feasible alternative to substrate mapping and allows outcomes comparable to substrate mapping.
Keywords: Ventricular tachycardia, Catheter ablation, Left ventricular assist device
Introduction
Percutaneous catheter ablation of ventricular tachycardia (VT) is increasingly performed in patients with implantable cardioverter-defibrillator (ICD) shocks.1–3 Procedure results are suboptimal in part because VT is haemodynamically intolerable in 50–90%. This limits detailed activation and entrainment mapping.4,5 In these cases, substrate- and pace-mapping techniques are typically used to identify potential ablation targets in sinus rhythm.6–11 While such approaches are effective, these techniques require extensive scar mapping and ablation.
An alternative strategy is activation mapping during VT while maintaining perfusion with a mechanical circulatory support device. Such support devices include the intra-aortic balloon pump, cardiopulmonary support (CPS) with bypass pump, and percutaneous ventricular assist devices (pVADs) such as the TandemHeart™ (Cardiac Assist, Inc., Pittsburg, PA, USA) and Impella™ microcirculatory axial blood flow pump (Abiomed Inc., Danvers, MA, USA). Several small case series have reported ablation outcomes with such devices that establish the technique feasibility.12–15
However, no study has compared outcomes of pVAD-assisted activation mapping to substrate mapping alone in patients with haemodynamically intolerable VT. To this aim, we sought to compare the efficacy and safety of TandemHeart-assisted VT ablation to scar-based ablation alone in a consecutive series of patients with haemodynamically unstable VT who had failed medical therapy.
Methods
All 13 consecutive patients with drug refractory, haemodynamically unstable VT resulting in ICD shocks underwent pVAD-assisted VT ablation at two institutions (five at the University of Virginia and eight at the Intermountain Medical Center, Murray UT, USA) between October 2007 and January 2010 were included. The efficacy and safety of these ablation procedures were retrospectively compared with 18-matched patients who underwent purely substrate-based mapping and ablation for unstable VT during the same time period. By review of the electronic medical records of 417 VT ablation patients at the two study sites during the time period, the 18 patients who underwent ablation without pVAD were matched to the Tandem Heart group if they had similar age (within 5 years), left ventricular ejection fraction (LVEF) (within 5%), procedural New York Heart Association (NYHA) class (one class), presence of diabetes mellitus, use of irrigated catheter-tip technology, and medical therapy before and after the procedure including use of beta-blockers, angiotensin-converting enzyme inhibitors, and anti-arrhythmic drugs. The investigator doing the matching (AD) was blinded to the outcomes of patients in both groups.
All patients had significant structural heart disease (LVEF ≤ 40% or arrhythmogenic right ventricular cardiomyopathy), received at least one ICD shock after receiving at least one anti-arrhythmic drug. A decision to use haemodynamic support was based upon likely haemodynamic instability with VT presentation, underlying cardiovascular history, and characteristics, and if the device could be inserted in the peripheral vasculature. Haemodynamic instability was considered to potentially complicate the procedure if the patient had syncope prior to ICD discharge or documented hypotension (mean blood pressure <60 mmHg) during VT. In one patient the baseline blood pressure was low and the ejection fraction (EF) was ≤10% prompting the use of the TandemHeart device. In patients with severe peripheral vascular disease a TandemHeart may not be feasible, unless percutaneous intervention of the vessels could be performed to allow placement. In one patient this approach was used to place the TandemHeart cannula after a common iliac artery was dilated and stented. In one patient, due to extensive aortic arch disease, an epicardial only approach was used.
Haemodynamic support
After induction of VT to confirm inducibility, patients were cardioverted to sinus rhythm with pacing or shock. The pVAD was inserted by an interventional cardiologist as previously described.14 Briefly, the TandemHeart consists of a 21-French venous cannula placed transseptally with intra-cardiac echo and fluoroscopic guidance. A 17-French arterial cannula was placed femorally for return of blood from the extracorporeal pump. The device was then programmed to 3000–7500 r.p.m. to maintain a cardiac output of 4–5 L/min and heparin given for a goal ACT of 300 s. At Intermountain Medical Center, a double Perclose technique has been adopted. With this approach two vascular seals are placed orthogonally to each other in an effort to minimize complications and facilitated extubation and return to ambulation times.
Epicardial access
Epicardial mapping and ablation was performed in nine patients (three in pVAD group) as previously described.16,17 Pericardial access was obtained prior to pVAD placement to assure stability for the anti-coagulation required during use of the support device.
Mapping and ablation
A 12-lead electrocardiogram of the clinical VT (if available) and/or the induced VT were used to guide mapping. In all pVAD patients, a Thermocool 3.5 mm irrigated tip catheter (Biosense Webster, Diamond Bar, CA, USA) and the CARTO 3D mapping system (Biosense) were used for mapping, pacing, and ablation. Prior to activation and entrainment mapping, a detailed substrate map was created with left ventricular endocardial scar was defined as tissue with a bipolar electrogram voltage <0.5 mV, normal as >1.5 mV, and the border zone as 0.5–1.5 mV. Following this mapping, activation and entrainment mapping were performed as previously described.18,19 Ablation targets consisted of those in which criteria for concealed entrainment were met.
In the substrate group, scar mapping was the primary mapping modality. A 3D mapping system was also used to generate voltage maps of both ventricles and, in some cases, the epicardial space. The CARTO system was used in all but one case. In the remaining case EnSite NavX (St Jude, St Paul, MN, USA) was used. Substrate ablation was performed as previously described.1,6–11 Briefly, ablation targets included scar borders with good pace maps (identical morphology by visual comparison on 12/12 EKG leads); channels within scar with favourable pace maps and long stimulation to QRS times; and diastolic potentials in the scar and border zones.
Ablation was performed with an irrigated tip catheter in all patients with power settings up to 50 W power and up to 30 cc irrigation flow rate Each site was ablated for up to 60 s or until a significant reduction in the bipolar electrogram voltage was noted. If VT terminated during ablation, the lesion was continued for an additional 60–90 s with slight movement around the termination site.
In patients undergoing epicardial ablation, pacing at 20 mA was performed at all lateral epicardial sites to assess for phrenic nerve capture. In addition, intra-cardiac echocardiography and selective angiograms were used to visualize the coronary arteries. Ablation was not performed ≤5 mm of a coronary artery.
Endpoint of ablation
After ablation, programmed stimulation was repeated. The primary endpoint of ablation was the inability to induce the VT recorded at the beginning of the study. An additional endpoint was non-inducibility of any VT after ablation. A judgement to approach and ablate these additional VTs or persistence of the clinical VT was left to the electrophysiologist performing the procedure and based upon procedural length and patient stability.
Follow-up
All patients had ICDs and their devices were interrogated every 3 months to assess for recurrent VT or ventricular fibrillation (VF) and as needed for symptoms or ICD shocks. Patients were followed for survival, freedom from ICD therapies for the clinical VT, freedom for ICD therapies for any VT, and ICD shocks for VT or VF. The primary outcome was survival with freedom ICD shock. A single shock would be considered a failure with this outcome.
Statistics
Differences between the PVAD and non-PVAD groups were determined utilizing the Student's t-test (or the Mann–Whitney U test when the data were non-normally distributed) and the Fisher's exact test for continuous and categorical variables, respectively. To evaluate differences in outcomes between the two groups, mixed-effect models and multivariable Logistic regression were utilized. Co-variables evaluated for model adjustment included age, sex, LVEF, NYHA, type of cardiomyopathy, AF, diabetes, sleep apnoea, prior cardiac surgery, medications, ICD shocks, ICD, and BiV pacing. The final model included significant and confounding co-variables. We estimated survival free rates using the Kaplan–Meier method for ICD shocks or therapies for recurrent VT. A P value of <0.05 was designated as nominally significant. All calculations were performed using SAS 9.1.3 (Cary, NC, USA).
Results
Patient demographics
There were no differences in demographics or therapies between the pVAD and non-pVAD VT ablation groups (Tables 1 and 2). In the 30 days prior to ablation, there was a non-significant trend towards pVAD patients having more ICD shocks (6.8 vs. 3.9, P = 0.07).
Table 1.
Baseline patient characteristics among the percutaneous left ventricular assist device and non-percutaneous left ventricular assist device groups
| Demographic | pVAD (n = 13) | Non-pVAD (n = 18) | P value |
|---|---|---|---|
| Agea | 59.7 | 62.5 | 0.83 |
| Male | 12 (92.3) | 15 (83.3) | 0.62 |
| LVEF (%)a | 20.0 | 25.0 | 0.57 |
| NYHA class | 2.7 ± 0.9 | 2.3 ± 0.7 | 0.14 |
| Ischaemic CM | 8 (61.5) | 12 (67.7) | 1.00 |
| Atrial fibrillation | 5 (38.5) | 5 (27.8) | 0.70 |
| Diabetes | 4 (30.8) | 4 (22.2) | 0.69 |
| Sleep apnoea | 6 (46.2) | 4 (22.2) | 0.25 |
| Prior cardiac surgery | 4 (30.8) | 6 (33.3) | 1.00 |
Proportions are presented as n (%) and continuous variables are presented as means ± standard deviation.
CM, cardiomyopathy; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; pVAD, percutaneous ventricular assist device.
aVariable (median) is non-normally distributed and comparisons between the two groups utilized the Mann–Whitney U test.
Table 2.
Medical and device therapies received among the percutaneous left ventricular assist device and non-percutaneous left ventricular assist device groups
| Therapy | pVAD (n = 13) | Non-pVAD (n = 18) | P value |
|---|---|---|---|
| ACE inhibitor or ARB (%) | 11 (84.6) | 14 (77.8) | 1.00 |
| Beta-blockers (%) | 12 (92.3) | 18 (100) | 0.42 |
| Failed AAD (%) | 13 (100) | 17 (94.4) | 1.00 |
| Mean number of failed AADsa | 2.0 | 2.0 | 0.49 |
| Receiving amiodarone (%) | 6 (46.2) | 15 (83.3) | 0.52 |
| Mean number of ICD shocks 30 days prior | 6.8 | 3.9 | 0.07 |
| ICD (%) | 11 (84.6) | 18 (100) | 0.17 |
| Biventricular pacing (%) | 2 (15.4) | 3 (16.7) | 1.00 |
| Prior catheter ablation | 6 (46.2) | 7 (38.9) | 0.73 |
Proportions are presented as n (%) and continuous variables are presented as means ± standard deviation.
AAD, anti-arrhythmic drug; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ICD, implantable cardioverter defibrillator; pVAD, percutaneous ventricular assist device.
aVariable (median) is non-normally distributed and comparisons between the two groups utilized the Mann–Whitney U test.
Ablation procedure
Haemodynamic support was used in all 13 pVAD patients to maintain a mean blood pressure >60 mmHg and allow activation and entrainment mapping. The pVAD maintained pressure >60 mmHg in VT in all patients in this series.
Three patients in the pVAD group underwent epicardial mapping and ablation compared with six in the substrate group (P = 1.00). The most frequent reason for pursuing epicardial mapping and ablation was a prior failed endocardial procedure (8). In one patient with non-ischaemic cardiomyopathy, an epicardial approach was performed during the initial ablation procedure due to endocardial signals suggestive of an epicardial source.
Important procedural differences between groups included a longer procedure time (400 vs. 274 min, P = 0.001) (Figure 1) and the induction of more VTs (3.2 vs. 1.6, P = 0.04) in the pVAD group (Table 3). More VTs were ablated per patient in the pVAD group (2.2 vs. 1.5, P = 0.04). However, despite this, the mean ablation time tended to lower in the pVAD compared with the non-pVAD groups (1656 ± 308 vs. 1992 ± 411, P= 0.38).
Figure 1.
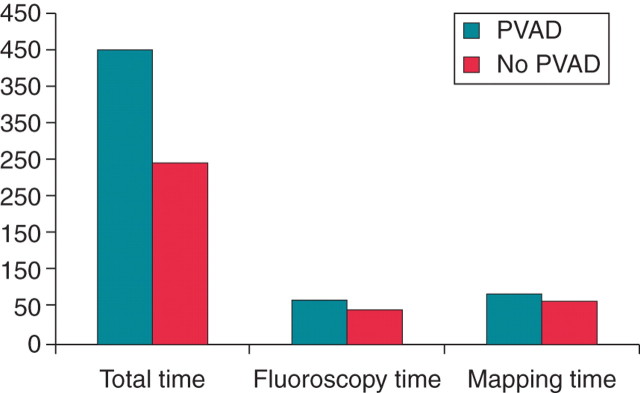
The mean procedure, fluoroscopy, and mapping times in the percutaneous left ventricular assist device and non-percutaneous left ventricular assist device groups. The y-axis indicates minutes. There was a significant difference in mean total procedure time between the groups (400 ± 90 vs. 246 ± 41 min, P = 0.001). Mean fluoroscopy and mapping times were also longer but without statistical significance (59 ± 14 vs. 47 ± 12 min, P = 0.10 and 68 ± 17 vs. 58 ± 29 min, P = 0.54, respectively).
Table 3.
Ablation procedure and outcomes in the percutaneous left ventricular assist device and non-percutaneous left ventricular assist device groups
| pVAD (n = 13) | Non-pVAD (18) | P value | |
|---|---|---|---|
| Procedure characteristics | |||
| VTs induced | 3.2 | 1.6 | 0.04 |
| Inducible VF | 1 | 2 | 1.00 |
| Emergent cardioversion/defibrillation | 4 | 7 | 0.71 |
| Peri-procedural IV amiodarone | 3 | 6 | 1.00 |
| Mean ablation time (s) | 1656 ± 308 | 1992 ± 411 | 0.38 |
| Ablation power delivery (W) | 44 ± 7 | 46 ± 6 | 0.54 |
| Procedure results | |||
| Acute procedural success (%) | 10 (77) | 12 (67) | 0.69 |
| VT non-inducibility (%) | 9 (69) | 10 (56) | 0.71 |
| Follow-upa | |||
| Freedom from clinical VT | 10 (83.3) | 11 (61.1) | 0.25 |
| Freedom from ICD shocks for VT (%) | 9 (75) | 10 (56) | 0.67 |
| Shock-free survival (%) | 7 (58) | 8 (44) | 0.74 |
Proportions are presented as n (%) and continuous variables are presented as means ± standard deviation. Median if non-normally distributed.
pVAD, percutaneous ventricular ventricular assist device; sec, seconds; VF, ventricular fibrillation; VT, ventricular tachycardia; W, Watts.
aOnly patients who survived the hospitalization surrounding the procedure were included in the follow-up analysis.
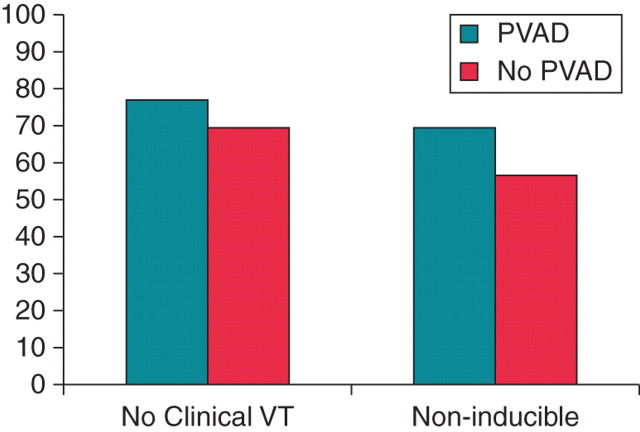
Acute procedural success, defined as the inability to induce all the VT(s) induced at the beginning of the procedure at the end of the procedure, was achieved in 10 of 13 patients (77%) in the pVAD group compared with 67% of the substrate-based ablation patients (P = 0.69, Figure 2; Table 3). Two patients in the pVAD group clearly failed ablation, as one had persistent spontaneous VT despite ablation, while another patient's initial clinical VT was persistently inducible with programmed stimulation. The patient with persistent spontaneous VT was originally intended to undergo a combined endocardial/epicardial procedure which was unable to be completed as significant vascular disease precluded retrograde access to the left ventricle and transeptal access was not possible with simultaneous use of the pVAD. Only epicardial ablation was attempted. In a third patient, the primary VT was rendered non-inducible but two other VTs that were found prior to ablation were still inducible. The secondary endpoint of complete non-inducibility for all VTs was reached in 69% of pVAD patients compared with 56% of the non-pVAD patients (P = 0.71).
Figure 2.
Acute success rates of ablation in the percutaneous left ventricular assist device and substrate groups. The y-axis represents percent of patients. The two strategies resulted in similar acute success rates in terms of successful ablation of the clinical ventricular tachycardia (77 vs. 67%, P = 0.69) and non-inducibility for any ventricular tachycardia (69 vs. 56%, P = 0.71).
There were no statistically significant differences in the complication rates between the two groups. There was one in-hospital death among the pVAD patients (peri-procedural stroke with withdrawal of care) and no deaths in the substrate group (P = 0.42). There was one stroke in each group (P= 1.00) The pVAD patient who suffered a stroke was felt to have stroke due to severe aorto-vascular disease and is the same patient who suffered an in-hospital death. There was also one cerebrovascular event in the substrate group resulting in right-sided hemiparesis which has persisted in follow-up. There was no difference in pericardial effusions requiring intervention (1 vs. 0, P = 0.42), ST segment elevation (0 vs. 1, P= 1.00), phrenic nerve injury (0 vs. 0, P= 1.00), or acute congestive heart failure requiring ventilation >24 h (1 vs. 1, P= 1.00) between the pVAD and non-pVAD groups.
Over a mean follow-up of 9 ± 3 months (range 6–24 months), among the 12 surviving pVAD patients, there was one death from progressive heart failure 3 months after the ablation procedure. One patient in the pVAD group received an orthotopic heart transplant (OHT) based upon heart failure status with an available donor, and a second patient received an LVAD in anticipation of transplant. There were four deaths in the substrate group, all secondary to progressive, drug-refractory heart failure. In addition, one patient in the substrate group has had an OHT, and a second patient is on the transplant list with advanced heart failure and recurrent, drug-refractory ICD shocks.
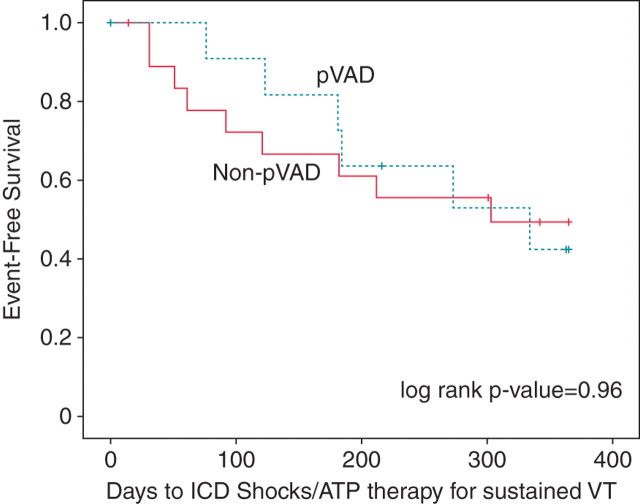
Overall, survival free of ICD shocks/recurrence of sustained VT in the two groups was not significantly different between the pVAD and non-pVAD groups (55 vs. 48%, P= 0.96.) (Figure 3). In multivariable analysis, the absence of AF (hazard ratio=0.15, P= 0.04) was associated with a lower incidence of ICD shocks.
Figure 3.
One-year Kaplan–Meier survival estimates for survival free of recurrent implantable cardioverter defibrillator shocks/ATP therapies for sustained ventricular tachycardia. There was no significant differences noted in survival-free event rates (log rank P value=0.96).
Discussion
Our results suggest that pVAD-assisted VT ablation is a reasonable alternative to substrate ablation and may allow for targeting more inducible VTs.
Catheter ablation of VT is performed with increasing frequency. The increase in ablation is driven in part by data suggesting that shocks are associated with increased mortality as well that more patients are surviving longer with advanced heart disease.20
Despite multiple technological advances, the success rate for VT ablation in structurally abnormal hearts is suboptimal. A recent large series found that VT recurrence 1 month after ablation in patients with ischaemic and non-ischaemic cardiomyopathy was 29 and 39%, respectively.21 Freedom from shock at 1 year is typically 55%.22
Haemodynamic instability during VT may limit the efficacy of the procedure by preventing detailed activation and entrainment mapping. Even in patients with tolerated VTs, anaesthesia can produce severe hypotension with potential deleterious effects. As such, as few as 10% of monomorphic VTs are sufficiently haemodynamically stable to allow comprehensive activation and entrainment mapping.5 Multiple options have been advocated to target unstable VTs including non-contact mapping and substrate mapping.
Substrate mapping, the most frequently used technique, allows modification of the arrhythmogenic substrate, but has limitations. Since the critical isthmus of the clinical VT is often not known, a broad ablative approach is required to homogenize scar borders, find and treat all late potentials within scar boundaries, or perform extensive linear ablation of critical regions between scar borders and/or electrically inert structures. This approach may miss small VT circuits and may impact on cardiac function if extensive ablation is performed. Furthermore, if VT is induced without support, the haemodynamic instability may impact on coronary flow and thus cause cardiac injury.
An alternative is placement of a pVAD to ensure haemodynamic stability during VT, thereby permitting detailed activation and entrainment mapping during the clinical tachycardia. Mapping in tachycardia allows identification of regions critical to the tachycardia circuit to minimize unnecessary ablation and also allows a dynamic understanding of the arrhythmia in context to the underlying substrate. Not only does the pVAD maintain mean haemodynamic stability during VT, but it can also enhance the stability of the patient throughout the procedure and in the peri-operative period. The latter aspect of the device is important in these patients as most have significant structural heart disease, heart failure, and are exposed to large volumes of fluid from the irrigated tip catheter. The enhanced stability was likely behind the observation that more VTs were targeted in those patients with a pVAD-assisted procedure.
In examination of long-term outcomes, these data show two groups with similar freedoms from long-term ICD shocks. This is interesting because the pVAD group was not randomly selected for this invasive therapy and may have been sicker despite having similar demographics. These patients were selected for pVAD support either due to the instability of the VT or some other indicator that they would be unstable during the procedure. Furthermore, the pVAD group tended to have lower EFs, more atrial fibrillation (AF), diabetes, and higher heart failure classes although this was not statistically significant. Nonetheless, we see the similar outcomes, despite the use of a pVAD in a selected population. It should also be noted that the operators in this series have extensive experience with scar-based VT ablation, but less experience with pVAD-assisted VT ablation. It is possible that with more experience, success rates will improve with the novel pVAD technique but will remain the same for the scar-based technique. If this occurred, the pVAD technique may have better outcomes.
Nonetheless, these potential benefits must be balanced against the hazards associated with the use of pVADs which include the use of large venous and arterial cannulae, increased procedure time to insert the device, increased cost, and the inability to use transseptal access for mapping and ablation. In addition, pVAD support will not maintain blood pressure during VF and may not maintain blood pressure even during VT if the right ventricle fails to deliver volume to the left ventricle. Finally, because haemodynamic support devices require a skilled multidisciplinary team, they will likely be used only in specialized centres with high VT volumes. At this point the use of pVAD-assisted ablation will likely be limited to these experienced teams when substrate ablation either fails or in cases where patients are haemodynamically unstable.
Our findings also demonstrate that an epicardial ablation approach should not preclude pVAD use. With use of a pVAD, systemic heparinization is required. However, epicardial access is often required as many have had prior failed ablations or have non-ischaemic cardiomyopathies. We observed no complications with pericardial access followed by pVAD with heparinization in this study cohort.
Percutaneous alternatives to the TandemHeart include a balloon pump or the Impella™ which are less invasive but do not provide the same level of haemodynamic support. On the other extreme, complete CPS is more invasive but provides complete right ventricle and left ventricle support with the ability to still use transseptal or retrograde LV access. A retrospective study of 19 patients using CPS showed, at 42 months, 28% of patients were VT free and 39% had significant reduction in ICD therapy.15 A potential disadvantage of CPS is the inability to use it for >15 consecutive minutes due to concern for myocardial ischaemia. Also, CPS provides only 2–3 L/min of flow vs. 4–5 L/min with the TandemHeart device, and CPS may require more personnel to operate. With both techniques success may be limited. The limited success rates in both arms in our series likely reflect the overall poor substrate of these patients.
Future studies will define the role of each of these tools in VT ablation.
Limitations
The primary limitation of this study is that it is a retrospective review rather than a randomized, controlled trial. In addition, the relatively small number of patients in the two groups limits the conclusions which may be drawn, and the study is too small to identify a mortality difference between the two groups. This is a small series and thus may not be powered to detect differences in complications in the two groups. A final potential limitation is that fewer VTs were ablated per patient than in some prior studies. This may be due to our patients having advanced cardiac disease with haemodynamic instability in VT. This instability, particularly in the non-pVAD group, impaired the targeting of multiple VTs. Despite these limitations, our experience suggests that pVAD-assisted ablation is feasible and produces results comparable with those achieved with substrate-based ablation techniques.
Conclusions
Percutaneous left ventricular assist device-assisted VT ablation is a reasonable alternative to substrate mapping for haemodyanamically unstable, medically refractory VT in high-risk patients. Future randomized studies should further define the role of pVADs during VT ablation.
Conflict of interest: S.M. has in the last year received consulting fees from JNJ and STJ, lecture honoria from Cardiac Assist, is a founder of EpiEP and is now an employee of St Jude Medical. T.J.B. has received consulting fees from JNJ and STJ. D.S.L. has received consulting fees from Cardiac Assist.
References
- 1.Aliot EM, Stevenson WG, Almendral-Garrote JM, Bogun F, Calkins CH, Delacretaz E, et al. EHRA/HRS expert consensus on catheter ablation of ventricular arrhythmias. Europace. 2009;11:771–817. doi: 10.1093/europace/eup098. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson WG, Friedman PL, Kocovic D, Sager PT, Saxon LA, Pavri B. Radiofrequency catheter ablation of ventricular tachycardia after myocardial infarction. Circulation. 1998;98:308–14. doi: 10.1161/01.cir.98.4.308. [DOI] [PubMed] [Google Scholar]
- 3.Stevenson WG, Soejima K. Catheter ablation for ventricular tachycardia. Circulation. 2007;115:2750–60. doi: 10.1161/CIRCULATIONAHA.106.655720. [DOI] [PubMed] [Google Scholar]
- 4.Callans DJ, Zado E, Sarter BH, Schwartzman D, Gottlieb CD, Marchlinski F. Efficacy of radiofrequency catheter ablation for ventricular tachycardia in healed myocardial infarction. Am J Cardiol. 1998;82:429–32. doi: 10.1016/s0002-9149(98)00353-1. [DOI] [PubMed] [Google Scholar]
- 5.Morady F, Harvey M, Kalbfleisch SJ, el-Atassi R, Calkins H, Langberg JJ. Radiofrequency ablation of ventricular tachycardia in patients with coronary artery disease. Circulation. 1993;87:363–72. doi: 10.1161/01.cir.87.2.363. [DOI] [PubMed] [Google Scholar]
- 6.Marchlinski FE, Callans DJ, Gottlieb CD, Zado E. Linear ablation lesions for control of unmappable ventricular tachycardia in patients with ischemic and nonischemic cardiomyopathy. Circulation. 2000;101:1288–96. doi: 10.1161/01.cir.101.11.1288. [DOI] [PubMed] [Google Scholar]
- 7.Ellison KE, Stevenson WG, Sweeney MO, Lefroy DC, Delacretaz E, Friedman PL. Catheter ablation for hemodynamically unstable monomorphic ventricular tachycardia. J Cardiovasc Electrophysiol. 2000;1:41–4. doi: 10.1111/j.1540-8167.2000.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 8.Soejima K, Suzuki M, Maisel WH, Brunckhorst CB, Delacretaz E, Blier L, et al. Catheter ablation in patients with multiple and unstable ventricular tachycardias after myocardial infarction: short ablation lines guided by reentry circuit isthmuses and sinus rhythm mapping. Circulation. 2001;104:664–9. doi: 10.1161/hc3101.093764. [DOI] [PubMed] [Google Scholar]
- 9.Brunckhorst CB, Delacretaz E, Soejima K, Maisel WH, Friedman PL, Stevenson WG. Identification of the ventricular tachycardia isthmus after infarction by pace mapping. Circulation. 2004;110:652–9. doi: 10.1161/01.CIR.0000138107.11518.AF. [DOI] [PubMed] [Google Scholar]
- 10.Hsia HH, Callans DJ, Marchlinski FE. Characterization of endocardial electrophysiological substrate in patients with nonischemic cardiomyopathy and monomorphic ventricular tachycardia. Circulation. 2003;108:704–10. doi: 10.1161/01.CIR.0000083725.72693.EA. [DOI] [PubMed] [Google Scholar]
- 11.Arenal A, Glez-Torrecilla E, Ortiz M, Villacastin J, Fdez-Portales J, Sousa E, et al. Ablation of electrograms with an isolated, delayed component as treatment of unmappable monomorphic ventricular tachycardias in patients with structural heart disease. J Am Coll Cardiol. 2003;41:81–92. doi: 10.1016/s0735-1097(02)02623-2. [DOI] [PubMed] [Google Scholar]
- 12.Friedman PA, Munger TM, Torres N, Rihal C. Percutaneous endocardial and epicardial ablation of hypotensive ventricular tachycardia with percutaneous left ventricular assist in the electrophysiology laboratory. J Cardiovasc Electrophysiol. 2007;18:106–9. doi: 10.1111/j.1540-8167.2006.00619.x. [DOI] [PubMed] [Google Scholar]
- 13.Zanobini M, Rossi F, Bertera A, Sandano S, Costa C, Fabrizi R, et al. Cardiopulmonary support during electrophysiological procedures for ventricular tachycardias not hemodynamically tolerated. Perfusion. 2003;18:79–82. doi: 10.1191/0267659103pf651oa. [DOI] [PubMed] [Google Scholar]
- 14.Hussam A, Roshan J, Lim B, Asirvatham SJ. Use of the Impella™ microaxial blood pump for ablation of hemodynamically unstable ventricular tachycardia. J Cardiovasc Electrophysiol. 2010;21:458–464. doi: 10.1111/j.1540-8167.2009.01673.x. [DOI] [PubMed] [Google Scholar]
- 15.Carbucicchio C, Della Bella P, Fassini G, Trevisi N, Riva S, Giraldi F, et al. Percutaneous cardiopulmonary support for catheter ablation of unstable ventricular arrhythmias in high-risk patients. Herz. 2009;34:545–52. doi: 10.1007/s00059-009-3289-3. [DOI] [PubMed] [Google Scholar]
- 16.Sosa E, Scanavacca M, d'Avila A, Oliviera F, Ramires J. Nonsurgical transthoracic epicardial catheter ablation to treat recurrent ventricular tachycardia occurring late after myocardial infarction. J Am Coll Cardiol. 2000;35:1442–9. doi: 10.1016/s0735-1097(00)00606-9. [DOI] [PubMed] [Google Scholar]
- 17.Mahapatra S, Tucker-Schwartz J, Wiggins D, Gillies G, Mason P, McDaniel G, et al. Pressure frequency characteristics of the pericardial space and thorax during subxiphoid access for epicardial ventricular tachycardia ablation. Heart Rhythm. 2010;7:604–9. doi: 10.1016/j.hrthm.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevenson WG, Khan H, Sager P, Saxon LA, Middlekauff HR, Natterson PD, et al. Identification of reentry circuit sites during catheter mapping and radiofrequency ablation of ventricular tachycardia late after myocardial infarction. Circulation. 1993;88:1647–70. doi: 10.1161/01.cir.88.4.1647. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson WG, Friedman PL, Sager PT, Saxon LA, Kocovic D, Harada T, et al. Exploring postinfarction reentrant ventricular tachycardia with entrainment mapping. J Am Coll Cardiol. 1997;29:1180–9. doi: 10.1016/s0735-1097(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 20.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–17. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sacher F, Tedrow U, Field ME, Raymond JM, Koplan BA, Epstein LM, et al. Ventricular tachycardia ablation: evolution of patients and procedures over 8 years. Circ Arrhythmia Electrophysiol. 2008;1:153–61. doi: 10.1161/CIRCEP.108.769471. [DOI] [PubMed] [Google Scholar]
- 22.Natale A, Raviele A, Al-Ahmad A, Alfieri O, Aliot E, Almendral J, et al. Venice chart international consensus document on ventricular tachycardia/ventricular fibrillation ablation. J Cardiovasc Electrophysiol. 2010;21:339–79. doi: 10.1111/j.1540-8167.2009.01686.x. [DOI] [PubMed] [Google Scholar]