Abstract
Aims
Interleukin-7 (IL-7) is a master regulator of T-cell development and homoeostasis. Increased IL-7 levels are associated with inflammatory diseases. The aims of this study were to determine whether IL-7 is a biomarker for inflammatory conditions or an active participant in atherogenesis.
Methods and results
Advanced atherosclerotic lesions in Apoe−/− mice were regressed by long-term cholesterol lowering through treatment with a helper-dependent adenovirus expressing apolipoprotein E (n= 6–10). Using this model, gene expression patterns in the aorta were analysed at an early phase of regression by microarray. After stringent statistical analysis, we found that IL-7 expression is significantly reduced in response to lowering of cholesterol (n= 6). To understand the importance of IL-7 down-regulation for atherosclerotic regression, we studied the effects and mechanisms of action of IL-7 on endothelial cells (ECs) in vitro as well as in vivo. Our major findings are: (i) IL-7 up-regulates cell adhesion molecules and monocyte chemoattractant protein-1 in ECs and promotes monocyte adhesion to ECs; (ii) this regulation is mediated by phosphatidylinositol 3-kinase (PI3K)/AKT-dependent and -independent activation of NF-κB; (iii) elevation of plasma IL-7 induces recruitment of monocytes/macrophages to endothelium without affecting plasma cholesterol (n= 5, 6); and (4) lack of IL-7 in bone marrow-derived cells reduces migration of monocytes/macrophages to the lesions (n= 5, 6).
Conclusion
These results suggest that IL-7 inflames endothelium via PI3K/AKT-dependent and -independent activation of NF-κB and recruits monocytes/macrophages to the endothelium, thus playing an active role in atherogenesis.
Keywords: Atherosclerosis, Cell adhesion molecules, Chemokine, Endothelium
Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in industrialized countries. This toll is expected to worsen because of a rising prevalence of CVD in developing countries and an increasing incidence of obesity and diabetes in industrialized countries.1 Atherosclerosis is characterized by the infiltration of immune cells and the presence of lipid-enriched macrophages in the arterial wall and is a major cause of CVD. Both innate and adaptive immunity have been suggested to modulate the rate of lesion progression.2 Despite its chronic progressive nature, this process is dynamic and can be reversed under some conditions.3 Understanding the mechanisms of atherosclerotic lesion regression should help identify targets for treatment. Although atherosclerotic lesion regression is complex and the key processes are not well understood, some mechanisms involving foam cell emigration have been proposed.4,5 Interleukin-7 (IL-7) is an essential cytokine for T-cell development and a master regulator for T-cell homoeostasis.6 There is suggestive evidence that IL-7-induced inflammation contributes to atherosclerosis; however, the pathways involved remain unclear. Interleukin-7 stimulates secretion of cytokines from peripheral blood mononuclear cells.7–9 Increased levels of serum IL-7 have been reported in patients with unstable angina9 or coronary heart disease,10 although others have reported IL-7 down-regulation in unstable angina.11 Patients with rheumatoid arthritis (RA) have increased prevalence of CVD,12 perhaps related to increased underlying inflammation, and IL-7 has been implicated in its pathogenesis.13 In hypercholesterolaemia, monocytes are recruited through the activation of endothelial cells (ECs), differentiate into macrophages and develop into foam cells.1 Interleukin-7 acts on ECs via its cognate receptor.14 However, the possibility that IL-7 plays a role in atherosclerosis by mediating EC dysfunction has not been directly studied.
We initiated this study to understand the mechanisms of atherosclerosis regression by long-term cholesterol lowering in Apoe−/− mice using a helper-dependent adenoviral vector (HDAd) expressing apolipoprotein E3 (apoE3). We next analysed the changes in gene expression patterns in the aorta by microarray using stringent statistical methods.15 Unexpectedly, we found that IL-7 was one of three major gene transcripts affected by cholesterol lowering. To determine whether IL-7 plays a role in atherogenesis, we studied the effects of IL-7 on human aortic endothelial cells (HAECs). Interleukin-7 stimulated expression of cell adhesion molecules (CAMs) and monocyte chemoattractant protein-1 (MCP-1). The response was dose- and time-dependent, leading to enhanced adhesion of human monocytic THP-1 cells to HAECs. This up-regulation was mediated by phosphatidylinositol 3-kinase (PI3K)-AKT-dependent and -independent activation of NF-κB. Furthermore, elevated plasma IL-7 increased macrophage-positive cells in aorta, while lack of IL-7 in bone marrow (BM)-derived cells reduced migration of macrophage-positive cells to atherosclerotic lesions. Collectively, these results provide strong evidence for an active role of IL-7 in atherosclerosis.
Methods
An expanded Methods section is available in the Supplementary material online.
Animals
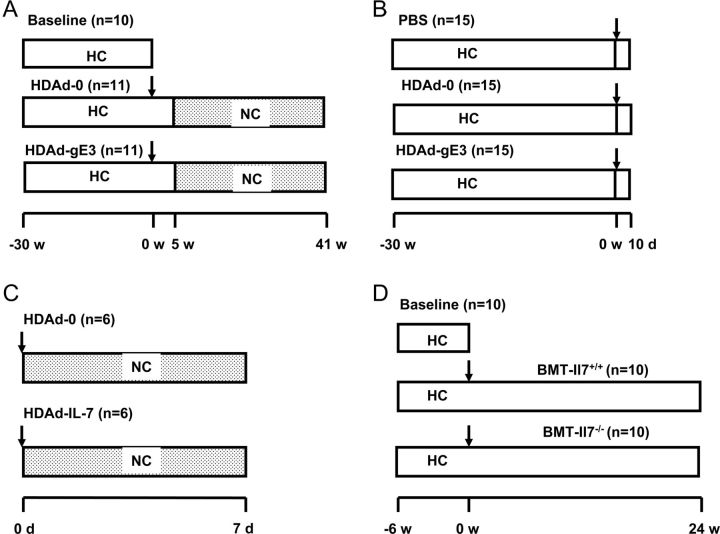
All animal protocols were performed according to the Guidelines of the Institutional Animal Care and Usage Committee at Baylor College of Medicine. The experimental designs for lesion regression16 and other animal studies are shown in Figure 1. In total, 32 female C57BL/6 Apoe−/− mice (6–8 weeks of age, The Jackson Laboratory, Stock No. 002052) were fed a diet containing 0.2% (w/w) cholesterol and 10% (v/w) coconut oil for 30 weeks to induce atherosclerosis.17 One group of mice (n= 10) was sacrificed to determine baseline atherosclerosis. The remaining mice were divided into two groups (n= 11) and treated with a tail vein injection of HDAd containing human apoE3 gene and liver control region18 (HDAd-gE3) or HDAd-0 (empty vector). For microarray analysis, 45 mice were fed a high-cholesterol diet for 30 weeks, divided into three groups (n= 15/group), treated with HDAd-0, HDAd-gE3, or phosphate-buffered saline (PBS) and sacrificed 10 days later. Three aortas extending from the sinus to the arch were pooled and RNA was extracted. For induction of IL-7 in the circulation, 9-week-old female Apoe−/− mice on normal chow diet were treated with HDAd expressing human IL-7 (HDAd-IL-7, n= 6) or HDAd-0 (n= 6) and sacrificed after seven days. For bone marrow transplantation (BMT), 30 female Ldlr−/− mice (The Jackson Laboratory, Stock No. 002207) were fed a high-cholesterol diet for 6 weeks to induce atherosclerosis, 10 mice were sacrificed for the baseline analysis and the remaining mice received 4 × 106 BM cells19 isolated from either male Il7−/− mice20 or Il7+/+ littermates (n= 10/group).
Figure 1.
Experimental design. (A) Athereoscelerosis legion regression. Female Apoe−/− mice were fed on high-cholesterol diet for 30 weeks and treated with either helper-dependent adenoviral vector-0 or helper-dependent adenoviral vectorg-E3. Five weeks after helper-dependent adenoviral vector treatment, diet was switched to normal chow. Mice were sacrificed 41 weeks after helper-dependent adenoviral vector treatment. Arrow indicates the injection of helper-dependent adenoviral vector. (B) Microarray analysis. Female Apoe−/− mice were fed a high-cholesterol diet for 30 weeks and then treated with PBS, helper-dependent adenoviral vector-0 or helper-dependent adenoviral vector-gE3. Mice were sacrificed ten days after helper-dependent adenoviral vector treatment. (C) Induction of circulating IL-7. Apoe−/− mice were fed normal chow and treated with either helper-dependent adenoviral vector-0 or helper-dependent adenoviral vector-IL-7. Animals were sacrificed seven days after helper-dependent adenoviral vector treatment. (D) Bone marrow transplantation. Female Ldlr−/− mice were fed a high-cholesterol diet for 6 weeks, and then received transplantation of bone marrow cells isolated from either male Il7−/− or littermate Il7+/+ mice. N, number of mice used.
Microarray analysis for gene expression profiling
The details for procedures and analyses are available in the Supplementary material online.
Cell culture
Human aortic endothelial cells were purchased from Lonza and maintained in endothelial cell growth medium (EGM) supplemented with EGM-2 BulletKit (Lonza Walkersville, Inc.). Endotoxin-free foetal bovine serum (FBS, Hyclone) was used for all cell cultures. For adhesion assay, HAECs were plated in a 96-well plate 1 day before the assay, and stimulated by IL-7 (R&D Systems) for 6 h before addition of 5 mM calcein-acetoxymethyl ester (calcAM, Molecular Probes)-labelled THP-1 cells. For inhibition of signalling pathways, HAECs were incubated with pathway-specific inhibitors for 1 h prior to stimulation by IL-7.
Other procedures
For immunoblot analyses, the membranes were incubated with mouse anti-intercellular adhesion molecule-1 (ICAM-1, 1.5 µg/mL, R&D Systems), rabbit anti-vascular cell adhesion molecule-1 (VCAM-1, 1:500, Santa Cruz), mouse anti-GAPDH (1:1000, Chemicon), rabbit anti-NF-κB p65 (1:300, Santa Cruz), rabbit anti-IκB-α (1:500, Santa Cruz), rabbit anti-phospho-AKT (Ser473) (1:1000, Cell Signaling), rabbit anti-AKT (1:1000, Cell Signaling), or rabbit anti-β-actin (1:1000, Chemicon), followed by incubation with anti-mouse or anti-rabbit antibody (1:3000) conjugated to horseradish peroxidase for detection by enhanced chemiluminescence (ECL) (GE Healthcare). For immunofluorescence, fluorophore-conjugated secondary antibodies (Invitrogen) were used. Interleukin-7 and MCP-1 protein levels were measured by ELISA (eBioscience).
Data analysis
Data are presented as mean ± SD unless specified. Differences were determined by t-test for two group comparisons or one-way ANOVA for multiple group comparisons using SIGMASTAT (Systat Software, Inc.). Rank sum test was used where necessary and data are presented as median, 25 and 75th percentile. Statistical significance was assigned at P< 0.05.
Results
Gene expression profiling reveals that cholesterol lowering down-regulates interleukin-7 expression
A single i.v. injection of HDAd-gE3 into Apoe−/− mice led to stable restoration of plasma apoE3 levels (Supplementary material online, Figure S1B) and normalization of plasma cholesterol (Supplementary material online, Figure S1C). This was associated with a marked reduction in VLDL and chylomicron remnants and an increase in HDL fractions (Supplementary material online, Figure S1D). En face morphometric analysis of the descending aortas at 41 weeks after HDAd treatment showed a progressive 3.4-fold increase in lesion area in the empty vector (HDAd-0, n= 6) group, but a total arrest of atherosclerosis progression in the HDAd-gE3-treated group (n= 8) compared with baseline (n= 10) (Supplementary material online, Figure S1E). Quantification of lesion size by cross-sectional analysis of the aortic sinuses indicated an 80% increase in lesion size in the HDAd-0 group, compared with a significant decrease of 28% in the HDAd-gE3 group compared to baseline values (Supplementary material online, Figure S1F). Immunocytochemical and histological analyses revealed a stabilized plaque phenotype in the HDAd-gE3 group, compared with the baseline and HDAd-0 groups as indicated by decreased macrophage-positive cells detected by anti-Mac3 antibody, enhancement of collagen-rich extracellular matrix, and a thickened α-actin layer in the tunica media (Supplementary material online, Figure S2A-L). Vascular cell adhesion molecule-1 positive area was reduced in the HDAd-gE3 group, while relative calcium deposits were increased in both treatment groups (Supplementary material online, Figure S2M-T).
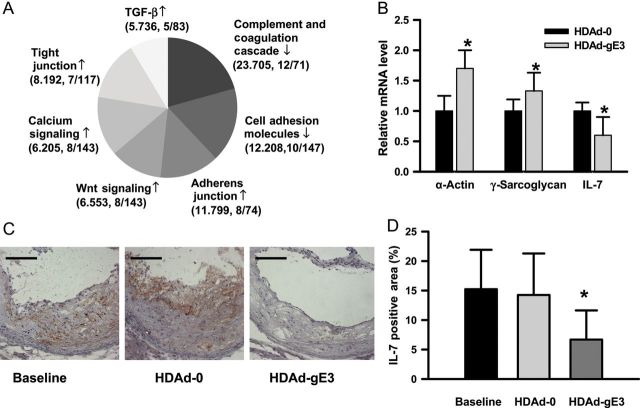
We next performed gene expression profiling of RNA isolated from the aorta by microarray analysis. The changes in gene expression during treatments leading to lesion regression have been reported to be rapid.4 Apoe−/− mice fed a high-cholesterol diet for 30 weeks were treated with HDAd vectors and then sacrificed 10 days after vector treatment. Cholesterol levels in the HDAd-gE3 group (n= 15) decreased from 16.3 ± 3.30 mmol/L at day 7 to 4.80 ± 1.70 mmol/L. The microarray data have been deposited in NCBI's Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE15914). A total of 339 genes were found to be significantly up-regulated and 121 genes down-regulated by the HDAd-gE3 treatment. To determine which pathways or gene networks were impacted, we performed functional profiling using Pathway Express and Ontology Express, and found that complement and coagulation cascades as well as CAMs were down-regulated, while adherens junction, tight junction, Wnt signalling, calcium signalling, and TGF-β signalling pathways were up-regulated (Figure 2A). However, after stringent statistical analysis15 we found three gene transcripts that were influenced with the highest significance; α-actin and γ-sarcoglycan were up-regulated, whereas IL-7 was down-regulated. The results were verified by qRT-PCR (n= 5/group, Figure 2B). The aortas used for microarray analysis were not available for further immunocytochemical characterization. We therefore analysed aortas used for regression study. Interleukin-7 immunoreactive protein in the lesion was significantly reduced in the HDAd-gE3 group (n= 4) compared with the HDAd-0 (n= 4) or baseline group (n= 4) (Figure 2C and D). In contrast, α-actin immunoreactivity was increased in the HDAd-gE3 group (Supplementary material online, Figure S2I-L). The γ-sarcoglycan immunoreactivity was not significantly different among groups (data not shown), suggesting that up-regulation of γ-sarcoglycan is transient or not associated with changes in the protein level.
Figure 2.
Interleukin-7 in aortas is down-regulated with cholesterol lowering in Apoe−/− mice. (A) Pathways influenced by cholesterol lowering. Microarray analysis was performed on RNA isolated from aortas harvested 10 days after helper-dependent adenoviral vector injection. Pathway Express and Ontology Express were used for functional profiling. ↓, down-regulation; ↑, up-regulation. Parenthesis: novel impact factor, number of genes affected/number of genes in each pathway. (B) Relative mRNA expression. *P< 0.05. n = 5. (C) Representative interleukin-7 immunoreactivity in cross-sections of aorta 41 weeks after treatment. Bar, 50 µm. (D) Interleukin-7 immunoreactive areas in the lesions. *P< 0.01 (vs. baseline and helper-dependent adenoviral vector-0 group; n = 4/group.
Interleukin-7 stimulates expression of adhesion molecules and monocyte chemoattractant protein-1 in human aortic endothelial cells
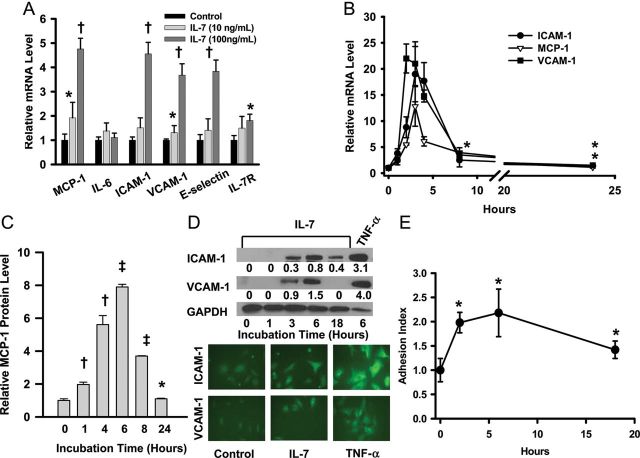
α-Actin and γ-sarcoglycan21 are intracellular proteins expressed in smooth muscle cells (SMCs), while IL-7 is a cytokine that signals through the IL-7 receptor α-chain (IL-7Rα) and common γc receptor complex. Since the down-regulation of IL-7 was paralleled by the down-regulation of the CAM pathway (Figure 2A and B), we hypothesized that IL-7 could act on ECs and impact the atherosclerotic process. Incubation of HAECs with IL-7 markedly increased the mRNA levels of E-selectin, ICAM-1, MCP-1, and VCAM-1, while IL-6 expression was not affected (n= 4, Figure 3A). In contrast to down-regulation of IL-7Rα in T-cells in response to IL-7,22 IL-7Rα mRNA in HAECs was up-regulated.
Figure 3.
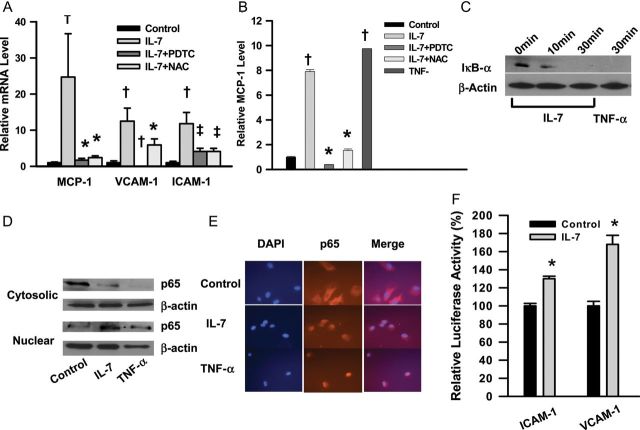
Interleukin-7 up-regulates monocyte chemoattractant protein-1 and adhesion molecules, and promotes adhesion of THP-1 cells to human aortic endothelial cells. (A) Relative expression of monocyte chemoattractant protein-1, interleukin-6, intercellular adhesion molecule-1, vascular cell adhesion molecule-1, E-selectin, and interleukin-7Rα in human aortic endothelial cells 6 h after incubation with interleukin-7. *P< 0.05, †P< 0.001; n = 4. (B) Time course of up-regulation of intercellular adhesion molecule-1, monocyte chemoattractant protein-1, and vascular cell adhesion molecule-1 by interleukin-7 (100 ng/mL). All time points were statistically significant (P< 0.001 vs. 0 h), except intercellular adhesion molecule-1 (at 8 and 24 h, *P< 0.05), and vascular cell adhesion molecule-1 (at 24 h, *P< 0.05). Monocyte chemoattractant protein-1 at 24 h was not significantly different from that at 0 h; n = 4. (C) Monocyte chemoattractant protein-1 levels in medium. *P< 0.05(vs. 0 h, n= 3), †P< 0.001, ‡P< 0.0001. Monocyte chemoattractant protein-1 concentration at 0 h was 95 ± 10 pg/mL; n = 3. (D) Immunocytochemical analyses. Immunofluorescence was performed after 6 h incubation with IL-7 (100 ng/mL). Tumor necrosis factor (TNF)-α (10 ng/mL) was used as control. The numbers below the intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 panel indicate band volume relative to that of GAPDH. (E) THP-1 cells adhere to human aortic endothelial cells stimulated by interleukin-7. *P< 0.05; n = 6. All experiments were repeated two to three times.
ICAM-1, MCP-1, and VCAM-1 have been implicated in the recruitment of monocytes to ECs.23 We characterized IL-7's action on these three genes in greater detail and found that up-regulation was dose- and time-dependent (n= 4, Figure 3A and B). Interleukin-7 significantly up-regulated MCP-1 at 1 ng/mL, but higher concentrations were required to up-regulate VCAM-1 and ICAM-1 (Supplementary material online, Figure S3). One hour exposure of the ECs to IL-7 was sufficient to up-regulate the expression of these genes and maximal mRNA levels were observed after 3 h (Figure 3B). The increase in protein level was confirmed by immunochemical methods (n= 3, Figure 3C and D). The HAEC responses to IL-7 at the protein level took longer than those at the mRNA level, peaking at 6 h. The time course closely paralleled that of adhesion of calcAM-labelled THP-1 cells to HAECs (n= 6, Figure 3E).
Interleukin-7 up-regulates monocyte chemoattractant protein-1 and vascular cell adhesion molecule-1 but not intercellular adhesion molecule-1 via the phosphatidylinositol 3-kinase pathway
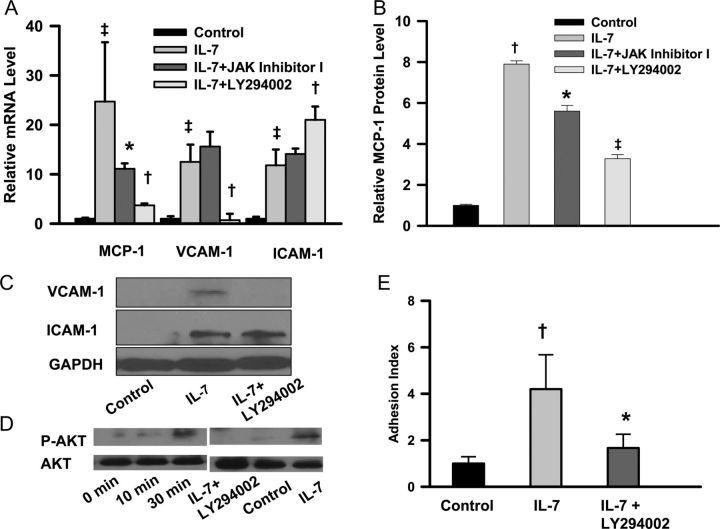
Interleukin-7Rα activation initiates at least two separate signalling cascades, the JAK/STAT and PI3K-AKT pathways.6 To understand which downstream signalling pathways are involved in IL-7 induced up-regulation of CAMs and MCP-1, we examined the effects of specific inhibitors. Preincubation of HAECs with the JAK inhibitor I reduced IL-7-mediated up-regulation of MCP-1, but not VCAM-1 or ICAM-1, while the PI3K inhibitor LY294002 reduced IL-7 induced up-regulation of MCP-1 and VCAM-1, but not ICAM-1 (n= 5, Figure 4A). These inhibitors had no effects on mRNA expression of MCP-1 or CAM's in the absence of IL-7 (data not shown). The effect was corroborated at the protein level by immunochemical analyses (Figure 4B and C). The PI3K is upstream of AKT, and AKT kinase activity is induced by phosphorylation upon activation of PI3K. Phospho-AKT was detectable 10 min after incubation with IL-7 and further increased up to at least 30 min. This AKT phosphorylation was blocked by pretreatment with LY294002 (Figure 4D). Moreover, inhibition of the PI3K pathway significantly reduced the adhesion of THP-1 cells to HAECs (n= 6, Figure 4E).
Figure 4.
Phosphatidylinositol 3-kinase inhibition blunts interleukin-7 induced up-regulation of monocyte chemoattractant protein-1 and vascular cell adhesion molecule-1, but not intercellular adhesion molecule-1. Cellular RNA was extracted for qRT-PCR 4 h after incubation with interleukin-7. Cellular proteins were extracted 6 h after incubation for immunoblot analysis. (A) Effects of inhibitors on mRNA levels. *P< 0.05 (vs. interleukin-7), †P< 0.01 (vs. interleukin-7), ‡P< 0.001 (vs. control); n = 5. (B) Effects of inhibitors on monocyte chemoattractant protein-1 level. *P< 0.001 (vs. interleukin-7), †P< 0.0001 (vs. control), ‡P< 0.00001 (vs. interleukin-7). Monocyte chemoattractant protein-1 levels in control were 191 ± 10 pg/mL; n = 3. (C) Effects of inhibitors on cell adhesion molecule protein levels. (D) Phosphatidylinositol 3-kinase inhibitor blocks interleukin-7 stimulated AKT phosphorylation. (E) Inhibition of THP-1 cell adhesion to human aortic endothelial cells by LY294002. *P< 0.05 (vs. interleukin-7), †P< 0.01 (vs. control). n = 6. All experiments were repeated two to three times.
Up-regulation of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 is dependent on NF-κB activation
To investigate whether NF-κB activation is involved in IL-7-mediated up-regulation of CAMs, we incubated HAECs with NF-κB inhibitors. These inhibitors blunted the induction of CAMs and MCP-1 by IL-7 (n= 5, Figure 5A and B). NF-κB activation requires a sequential cascade with IκB kinase (IKK-α/β)-dependent IκB phosphorylation, ubiquitination, degradation, and translocation of the cytosolic NF-κB complex to the nucleus to affect target gene expression. We examined the effect of IL-7 on IκB-α degradation by immunoblot analysis. Degradation of IκB-α in HAECs was observed as early as 10 min after addition of IL-7 and was essentially complete at 30 min (Figure 5C). When IκB-α is degraded, the NF-κB complex, including p65, translocates to the nucleus. We incubated HAECs with IL-7 for 30 min and performed subcellular fractionation. After incubation with IL-7, immunoreactive p65 decreased in the cytosolic fraction and increased in the nuclear fraction (Figure 5D). The relocation of p65 was further confirmed by immunofluorescence microscopy (Figure 5E). To determine whether IL-7 activates transcription of genes for CAMs via the NF-κB pathway, we constructed lentiviral vectors expressing luciferase driven by ICAM-1 or VCAM-1 proximal promoter. Interleukin-7 significantly stimulated ICAM-1 and VCAM-1 promoter activities (n= 3, Figure 5F). Therefore, IL-7 directly stimulates the promoter activities of these genes.
Figure 5.
NF-κB inhibitors suppress up-regulation of cell adhesion molecules and monocyte chemoattractant protein-1. (A) Effects of NF-κB inhibitors on up-regulation of cell adhesion molecules and monocyte chemoattractant protein-1. Human aortic endothelial cells were incubated with NF-κB inhibitors, pyrrolidine dithiocarbamate or N-acetyl-l-cysteine. *P< 0.01 (vs. interleukin-7), †P< 0.001 (vs. control), ‡P< 0.001 (vs. interleukin-7); n = 5. (B) Effects on monocyte chemoattractant protein-1 protein levels. *P< 0.001 (vs. interleukin-7), †P< 0.0001 (vs. control). TNF-α (10 ng/mL) was used as positive control; n = 3. (C) Interleukin-7 induces degradation of IκB-α. (D) P65 immunoreactive protein in cytosolic fraction was reduced while it was increased in nuclear fraction after incubation with interleukin-7 for 1 h. (E) Nuclear translocation of the P65 subunit of NF-κB upon incubation with IL-7. (F) Interleukin-7 increases activities of luciferase reporter driven by intercellular adhesion molecule-1 or vascular cell adhesion molecule-1 promoter. *P< 0.05 (vs. control); n = 3. All experiments were repeated two to three times.
Increased plasma level of interleukin-7 facilitates monocyte/macrophage migration to endothelium
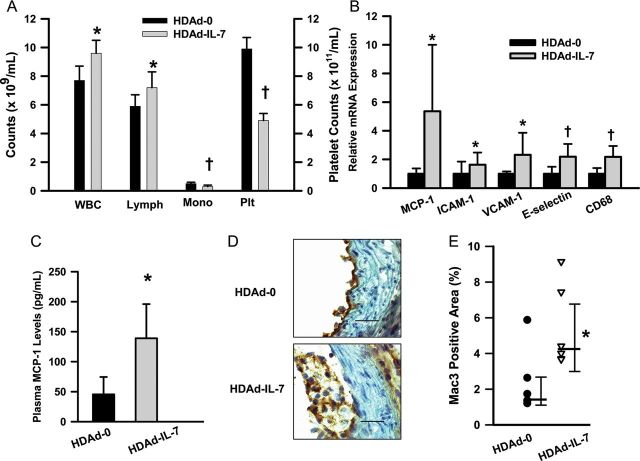
To determine whether IL-7 impacts recruitment of monocyte/macrophages to aortic endothelium in vivo, we produced HDAd-IL-7 expressing human IL-7 and injected it i.v. into Apoe−/− mice. It is known that human IL-7 is active in mice.24 The plasma level of human IL-7 increased to 30.0 ± 11.9 ng/mL in the HDAd-IL-7 group (n= 6), whereas those in the HDAd-0 group (n= 6) and in mice before treatment were undetectable (<0.8 pg/mL). Plasma cholesterol level remained unchanged in both groups; however, lymphocyte counts were increased in the HDAd-IL-7 group (Figure 6A). Aortas were harvested at day 7 for the analysis. The mRNAs for CAMs, MCP-1, and CD68 were significantly increased in the HDAd-IL-7 group (n= 6/group, Figure 6B). Circulating MCP-1 levels were correlated with the mRNA levels (n= 5/group, Figure 6C). Mac3 positive areas were also significantly increased in the HDAd-IL-7 group (n= 6) compared with the HDAd-0 group (n= 5) (Figure 6D and E) signifying that more macrophages migrated to the aorta. We also analysed for T cells and tumor necrosis factor (TNF)-α, a marker for inflammation, in the aorta. T cells detected by anti-CD3 positive cells are increased in the HDAd-IL-7-treated mice (Supplementary material online, Figure S4). TNF-α mRNA expression and protein levels were significantly increased in the HDAd-IL-7 group (n= 6/group, Supplementary material online, Figure S5). Interestingly, α-actin mRNA and immunoreactive protein were markedly reduced in the HDAd-IL-7 group (n= 5/group, Supplementary material online, Figure S6).
Figure 6.
Interleukin-7 promotes macrophage recruitment to the endothelium. Apoe−/− mice on normal chow were treated with either helper-dependent adenoviral vector-0 (n= 6) or helper-dependent adenoviral vector-interleukin-7 (n= 6) and aortas were harvested after 1 week. (A) Blood cell counts 1 week after treatment. *P< 0.05, †P< 0.001. WBC, whole blood cell counts; lymph, lymphocytes; Mono, mononuclear cells; Plt, platelets; n = 6. (B) Up-regulation of monocyte chemoattractant protein-1 and cell adhesion molecules in aorta in response to increased levels of circulating interleukin-7. *P< 0.05, †P< 0.01; n = 6. (C) Plasma monocyte chemoattractant protein-1 levels. *P< 0.01; n= 5. (D) Mac3 immunostaining. ×630. Bar, 20 μm. (E) Mac3 positive areas in aorta. *P< 0.01 (Mann–Whitney rank sum test); n = 5 (helper-dependent adenoviral vector-0) and 6 (helper-dependent adenoviral vector-interleukin-7). Median, 25 and 75th percentiles are shown.
Lack of interleukin-7 in bone marrow-derived cells reduces recruitment of monocyte/macrophage to atherosclerotic lesions
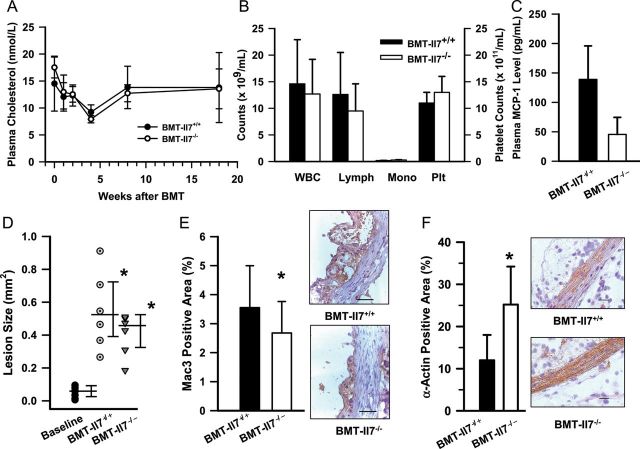
Oxidized LDL (oxLDL) may play a key role in the proatherogenic effect of elevated plasma cholesterol.23 To determine whether oxLDL can influence IL-7 production in any of the cell types present in lesions, we examined the effect of oxLDL exposure of HAECs, rat vascular SMCs, mouse peritoneal macrophages (PMs), and RAW264.1 macrophage cells in culture. Oxidized LDL up-regulated IL-7 in PMs and RAW264.1, but not vascular SMCs or HAECs (n= 3, Supplementary material online, Figure S7). Therefore, part of the atherogenic effect of hypercholesterolaemia may be mediated by macrophage-derived IL-7. To examine the role of macrophage IL-7 in lesion regression, Ldlr−/− mice were fed a high-cholesterol diet for 6 weeks to induce atherosclerosis and then BM cells isolated from Il7−/− mice or wild-type littermates were transplanted. We used Ldlr−/− mice because the donor Il7−/− mice are wild-type for the Apoe and Ldlr alleles and transplantation of wild-type BM cells would markedly reduce plasma cholesterol in Apoe−/− mice but not in Ldlr−/− mice. A high-cholesterol diet was maintained for the rest of the experiment and mice were sacrificed for the analysis 20 weeks after BMT. The plasma cholesterol levels and blood cell counts were not different between the two groups (Figure 7A and B). Plasma MCP-1 levels were lower in the BMT-Il7−/− group than the BMT-Il7+/+ group, though the difference was not significant (n= 5/group, Figure 7C). Although the lesion area in the BMT-Il7−/− group (n= 6) was 26% smaller than that in the BMT-Il7+/+ group (n= 6), atherosclerosis progressed in both groups (Figure 7D). Therefore, we further characterized the BMT groups. Interleukin-7 positive areas (Supplementary material online, Figure S8) and the relative Mac3-positive areas (Figure 7E) in the BMT-Il7−/− group (n= 6) were significantly smaller than those in the BMT-Il7+/+ group (n= 6). In contrast, α-actin positive areas were markedly increased in the BMT-Il7−/− group (n= 6/group, Figure 7F). There was no difference in CD3 or TNF-α immunoreactivity between the groups (Supplementary material online, Figure S9).
Figure 7.
Interleukin-7 deficiency attenuates migration of monocyte/macrophages to atherosclerotic lesions. (A) Plasma cholesterol levels; n = 6/group. (B) Blood cell counts in recipient mice; n = 6/group. Abbreviations are listed in Figure 6B legend. (C) Plasma monocyte chemoattractant protein-1 levels; n = 6/group. (D) Atherosclerotic lesion size on cross-sections. *P< 0.0001 (vs. baseline, one-way ANOVA on Ranks); n = 8 (baseline), 6 (bone marrow transplantation-Il7+/+), and 6 (bone marrow transplantation-Il7−/−). Median, 25 and 75th percentiles are shown. (E) Lack of IL-7 in BM-derived cells attenuates macrophage migration to the atherosclerotic lesions. *P< 0.05; n = 6/group. (F) α-Actin positive area. *P= 0.001; n = 6/group. ×400. Bar, 20 μm.
Discussion
The most prominent features of regressing lesions are reduced macrophages and lipid content and preservation of collagen-rich extracellular matrix and a thick smooth muscle layer. Potential mechanisms by which macrophages disappear from the lesion include increased cholesterol efflux, reduced monocyte recruitment and emigration,4 and reduced trapping of foam cells.5 However, other cells may also be involved by creating an environment that favours lesion regression. To understand the process of regression, we established a mouse model of regression of advanced lesions and used it to identify genes involved in the early stages of lesion regression. We applied microarray analysis to whole aortas. This approach identified several pathways impacted by cholesterol lowering. The most prominent effect was found in the down-regulation of complement and coagulation cascades. Genes involved in cell–cell junction (adherens and tight junctions) were up-regulated, while CAMs were down-regulated. These results suggest that cholesterol lowering induces changes in gene expression, which in turn down-regulates a procoagulation state and reduces the recruitment and/or invasion of immune cells to atherosclerotic lesions. We did not find changes in expression of CCR74 or CD36,5 two genes previously reported to be involved in foam cell emigration. The most likely explanation is that foam cells, whose expression of CCR7 or CD36 was impacted, might have already emigrated, or that our approach did not have the power to detect these changes. We also applied a mathematical method to minimize false discovery.15 By this analysis, we found three genes most significantly responding to cholesterol lowering. Of these, IL-7 is a cytokine and thus could have a penetrating effect on many cells through the IL-7Rα.
With pathway analysis, we considered the possibility of IL-7's action via ECs. Interleukin-7 induced the expression of CAMs and MCP-1, which are involved in atherogenesis because they facilitate the adherence of monocytes to ECs and their recruitment to the intima.23 Upon exposure of HAECs to IL-7, the change in expression of CAMs and MCP-1 genes occurred as early as 1 h, a time course that correlates with the IL-7-induced adhesion of THP-1 cells to HAECs. We next demonstrated that up-regulation of MCP-1 and VCAM-1 by IL-7 involves the PI3K/AKT-dependent activation of NF-κB, while ICAM-1 is regulated by PI3K/AKT-independent NF-κB activation. Therefore, the JAK/STAT mediated IL-7 signalling pathway, critical for T-cell survival or proliferation,6 apparently does not mediate the IL-7-induced up-regulation of CAMs in ECs. Our results are in agreement with other reports that indicate the involvement of PI3K/AKT-dependent and -independent activation of NF-κB in regulation of MCP-1 and CAMs in macrophages and ECs.25–27 In support of our interpretation that IL-7 leads to an inflammatory state, we found that HDAd-IL-7-induced circulating IL-7 stimulated CAMs and MCP-1 expression and enhanced monocyte/macrophage recruitment to the endothelium in vivo.
Which cells are responding to cholesterol lowering with down-regulation of IL-7? Patients with RA experience increased premature mortality as a consequence of CVD.12 Intraarticular IL-7 is increased in RA patients and monocyte/macrophages have been implicated as a major cellular source of IL-7.28 Moreover, our results with oxLDL suggested that macrophages may be important sources of IL-7 in atherosclerotic lesions. To examine the potential role of macrophage-derived IL-7 in atherogenesis, we performed BMT and found that lack of IL-7 expression in BM-derived cells significantly attenuates monocyte/macrophage migration to atherosclerotic lesions, consistent with this cytokine playing a role in monocyte/macrophage recruitment to endothelium. However, atherosclerotic lesion size increased in both BMT groups, suggesting that lack of IL-7 expression in BM-derived cells per se does not induce lesion regression. Interestingly, an increase in circulating IL-7 reduced α-actin expression, while loss of IL-7 in BM-derived cells increased α-actin content in atherosclerotic lesions, indicating that IL-7 affects α-actin expression in aortas.
Despite the evidence presented in this study, there are limitations to consider in interpreting the present findings. It cannot be ruled out that IL-7 expression in BM-derived cells has no effect on atherosclerosis because the sample size was relatively small and mice were fed a high-cholesterol diet throughout the experiment, which could have blunted the effect of IL-7 deficiency. Furthermore, we did not study the effect of increased IL-7 expression in BM-derived cells. On the other hand, a role of IL-7 in recruiting monocytes/macrophages to ECs is supported by the fact that IL-7 stimulates expression of mRNAs for MCP-1 and CAMs in ECs. Nevertheless, our results expand our understanding of a role of IL-7 in atherogenesis and support the hypothesis that IL-7 is not a biomarker for inflammatory conditions but actively involved in atherogenesis via ECs.
Supplementary material
Supplementaryterial is available at European Heart Journal online.
Funding
This work was supported by HL73144 (to K.O.), HL51586 (to L.C.), and AHA0535118N (to A.P.) and partly by P0DK079638.
Conflict of interest: none declared.
Supplementary Material
Acknowledgements
We thank A.M. Antar and S.L. Samson for valuable discussion and L.A.M. Liles and L. White for their technical assistance.
Appendix
Rana Razook, Baylor College of Medicine, participated in this study.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. doi:10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. doi:10.1161/01.RES.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- 3.Williams KJ, Feig JE, Fisher EA. Rapid regression of atherosclerosis: insights from the clinical and experimental literature. Nat Clin Pract Cardiovasc Med. 2008;5:91–102. doi: 10.1038/ncpcardio1086. doi:10.1038/ncpcardio1086. [DOI] [PubMed] [Google Scholar]
- 4.Trogan E, Feig JE, Dogan S, Rothblat GH, Angeli V, Tacke F, Randolph GJ, Fisher EA. Gene expression changes in foam cells and the role of chemokine receptor CCR7 during atherosclerosis regression in ApoE-deficient mice. Proc Natl Acad Sci U S A. 2006;103:3781–3786. doi: 10.1073/pnas.0511043103. doi:10.1073/pnas.0511043103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park YM, Febbraio M, Silverstein RL. CD36 modulates migration of mouse and human macrophages in response to oxidized LDL and may contribute to macrophage trapping in the arterial intima. J Clin Invest. 2009;119:136–145. doi: 10.1172/JCI35535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kittipatarin C, Khaled AR. Interlinking interleukin-7. Cytokine. 2007;39:75–83. doi: 10.1016/j.cyto.2007.07.183. doi:10.1016/j.cyto.2007.07.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ziegler SF, Tough TW, Franklin TL, Armitage RJ, Alderson MR. Induction of macrophage inflammatory protein-1 beta gene expression in human monocytes by lipopolysaccharide and IL-7. J Immunol. 1991;147:2234–2239. [PubMed] [Google Scholar]
- 8.Standiford TJ, Strieter RM, Allen RM, Burdick MD, Kunkel SL. IL-7 up-regulates the expression of IL-8 from resting and stimulated human blood monocytes. J Immunol. 1992;149:2035–2039. [PubMed] [Google Scholar]
- 9.Damas JK, Waehre T, Yndestad A, Otterdal K, Hognestad A, Solum NO, Gullestad L, Froland SS, Aukrust P. Interleukin-7-mediated inflammation in unstable angina: possible role of chemokines and platelets. Circulation. 2003;107:2670–2676. doi: 10.1161/01.CIR.0000070542.18001.87. doi:10.1161/01.CIR.0000070542.18001.87. [DOI] [PubMed] [Google Scholar]
- 10.Romano Carratelli C, Nuzzo I, Cozzolino D, Bentivoglio C, Paolillo R, Rizzo A. Relationship between Chlamydia pneumoniae infection, inflammatory markers, and coronary heart diseases. Int Immunopharmacol. 2006;6:848–853. doi: 10.1016/j.intimp.2005.10.012. doi:10.1016/j.intimp.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 11.Randi AM, Biguzzi E, Falciani F, Merlini P, Blakemore S, Bramucci E, Lucreziotti S, Lennon M, Faioni EM, Ardissino D, Mannucci PM. Identification of differentially expressed genes in coronary atherosclerotic plaques from patients with stable or unstable angina by cDNA array analysis. J Thromb Haemost. 2003;1:829–835. doi: 10.1046/j.1538-7836.2003.00113.x. doi:10.1046/j.1538-7836.2003.00113.x. [DOI] [PubMed] [Google Scholar]
- 12.Tyrrell PN, Beyene J, Feldman BM, McCrindle BW, Silverman ED, Bradley TJ. Rheumatic disease and carotid intima-media thickness: a systematic review and meta-analysis. Arterioscler Thromb Vasc Biol. 2010;30:1014–1026. doi: 10.1161/ATVBAHA.109.198424. doi:10.1161/ATVBAHA.109.198424. [DOI] [PubMed] [Google Scholar]
- 13.Hartgring SA, Bijlsma JW, Lafeber FP, van Roon JA. Interleukin-7 induced immunopathology in arthritis. Ann Rheum Dis. 2006;65(Suppl. 3)):iii69–iii74. doi: 10.1136/ard.2006.058479. doi:10.1136/ard.2006.058479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Rawi MA, Watkins G, Mansel RE, Jiang WG. The effects of interleukin-7 on the lymphangiogenic properties of human endothelial cells. Int J Oncol. 2005;27:721–730. [PubMed] [Google Scholar]
- 15.Reiner A, Yekutieli D, Benjamini Y. Identifying differentially expressed genes using false discovery rate controlling procedures. Bioinformatics. 2003;19:368–375. doi: 10.1093/bioinformatics/btf877. doi:10.1093/bioinformatics/btf877. [DOI] [PubMed] [Google Scholar]
- 16.MacDougall ED, Kramer F, Polinsky P, Barnhart S, Askari B, Johansson F, Varon R, Rosenfeld ME, Oka K, Chan L, Schwartz SM, Bornfeldt KE. Aggressive very low-density lipoprotein (VLDL) and LDL lowering by gene transfer of the VLDL receptor combined with a low-fat diet regimen induces regression and reduces macrophage content in advanced atherosclerotic lesions in LDL receptor-deficient mice. Am J Pathol. 2006;168:2064–2073. doi: 10.2353/ajpath.2006.051009. doi:10.2353/ajpath.2006.051009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oka K, Pastore L, Kim IH, Merched A, Nomura S, Lee HJ, Merched-Sauvage M, Arden-Riley C, Lee B, Finegold M, Beaudet A, Chan L. Long-term stable correction of low-density lipoprotein receptor-deficient mice with a helper-dependent adenoviral vector expressing the very low-density lipoprotein receptor. Circulation. 2001;103:1274–1281. doi: 10.1161/01.cir.103.9.1274. [DOI] [PubMed] [Google Scholar]
- 18.Kim IH, Jozkowicz A, Piedra PA, Oka K, Chan L. Lifetime correction of genetic deficiency in mice with a single injection of helper-dependent adenoviral vector. Proc Natl Acad Sci U S A. 2001;98:13282–13287. doi: 10.1073/pnas.241506298. doi:10.1073/pnas.241506298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Terashima T, Kojima H, Fujimiya M, Matsumura K, Oi J, Hara M, Kashiwagi A, Kimura H, Yasuda H, Chan L. The fusion of bone-marrow-derived proinsulin-expressing cells with nerve cells underlies diabetic neuropathy. Proc Natl Acad Sci U S A. 2005;102:12525–12530. doi: 10.1073/pnas.0505717102. doi:10.1073/pnas.0505717102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rich BE. Autocrine expression of interleukin-7 rescues lymphoid expansion in interleukin-7-deficient mice. Immunology. 1997;92:374–380. doi: 10.1046/j.1365-2567.1997.00353.x. doi:10.1046/j.1365-2567.1997.00353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hack AA, Ly CT, Jiang F, Clendenin CJ, Sigrist KS, Wollmann RL, McNally EM. Gamma-sarcoglycan deficiency leads to muscle membrane defects and apoptosis independent of dystrophin. J Cell Biol. 1998;142:1279–1287. doi: 10.1083/jcb.142.5.1279. doi:10.1083/jcb.142.5.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. doi:10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 23.Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. doi:10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kitazawa H, Muegge K, Badolato R, Wang JM, Fogler WE, Ferris DK, Lee CK, Candeias S, Smith MR, Oppenheim JJ, Durum SK. IL-7 activates alpha4beta1 integrin in murine thymocytes. J Immunol. 1997;159:2259–2264. [PubMed] [Google Scholar]
- 25.Harvey EJ, Li N, Ramji DP. Critical role for casein kinase 2 and phosphoinositide-3-kinase in the interferon-gamma-induced expression of monocyte chemoattractant protein-1 and other key genes implicated in atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:806–812. doi: 10.1161/01.ATV.0000258867.79411.96. doi:10.1161/01.ATV.0000258867.79411.96. [DOI] [PubMed] [Google Scholar]
- 26.Ferrante A, Robinson BS, Singh H, Jersmann HP, Ferrante JV, Huang ZH, Trout NA, Pitt MJ, Rathjen DA, Easton CJ, Poulos A, Prager RH, Lee FS, Hii CS. A novel beta-oxa polyunsaturated fatty acid downregulates the activation of the IkappaB kinase/nuclear factor kappaB pathway, inhibits expression of endothelial cell adhesion molecules, and depresses inflammation. Circ Res. 2006;99:34–41. doi: 10.1161/01.RES.0000231292.66084.cd. doi:10.1161/01.RES.0000231292.66084.cd. [DOI] [PubMed] [Google Scholar]
- 27.Frey RS, Gao X, Javaid K, Siddiqui SS, Rahman A, Malik AB. Phosphatidylinositol 3-kinase gamma signaling through protein kinase Czeta induces NADPH oxidase-mediated oxidant generation and NF-kappaB activation in endothelial cells. J Biol Chem. 2006;281:16128–16138. doi: 10.1074/jbc.M508810200. doi:10.1074/jbc.M508810200. [DOI] [PubMed] [Google Scholar]
- 28.van Roon JA, Verweij MC, Wijk MW, Jacobs KM, Bijlsma JW, Lafeber FP. Increased intraarticular interleukin-7 in rheumatoid arthritis patients stimulates cell contact-dependent activation of CD4(+) T cells and macrophages. Arthritis Rheum. 2005;52:1700–1710. doi: 10.1002/art.21045. doi:10.1002/art.21045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.