Abstract
Aims
To date, the therapeutic benefit of revascularization vs. medical therapy for stable individuals undergoing invasive coronary angiography (ICA) based upon coronary computed tomographic angiography (CCTA) findings has not been examined.
Methods and results
We examined 15 223 patients without known coronary artery disease (CAD) undergoing CCTA from eight sites and six countries who were followed for median 2.1 years (interquartile range 1.4–3.3 years) for an endpoint of all-cause mortality. Obstructive CAD by CCTA was defined as a ≥50% luminal diameter stenosis in a major coronary artery. Patients were categorized as having high-risk CAD vs. non-high-risk CAD, with the former including patients with at least obstructive two-vessel CAD with proximal left anterior descending artery involvement, three-vessel CAD, and left main CAD. Death occurred in 185 (1.2%) patients. Patients were categorized into two treatment groups: revascularization (n = 1103; 2.2% mortality) and medical therapy (n = 14 120, 1.1% mortality). To account for non-randomized referral to revascularization, we created a propensity score developed by logistic regression to identify variables that influenced the decision to refer to revascularization. Within this model (C index 0.92, χ2 = 1248, P < 0.0001), obstructive CAD was the most influential factor for referral, followed by an interaction of obstructive CAD with pre-test likelihood of CAD (P = 0.0344). Within CCTA CAD groups, rates of revascularization increased from 3.8% for non-high-risk CAD to 51.2% high-risk CAD. In multivariable models, when compared with medical therapy, revascularization was associated with a survival advantage for patients with high-risk CAD [hazards ratio (HR) 0.38, 95% confidence interval 0.18–0.83], with no difference in survival for patients with non-high-risk CAD (HR 3.24, 95% CI 0.76–13.89) (P-value for interaction = 0.03).
Conclusion
In an intermediate-term follow-up, coronary revascularization is associated with a survival benefit in patients with high-risk CAD by CCTA, with no apparent benefit of revascularization in patients with lesser forms of CAD.
Keywords: Computed tomography, Coronary revascularization, Medical therapy, Coronary artery disease
See page 3011 for the editorial comment on this article (doi:10.1093/eurheartj/ehs371)
Introduction
Numerous large-scale clinical studies have observed a distinct survival benefit for both percutaneous and surgical coronary revascularization when compared with medical therapy in stable patients with high-risk angiographic coronary artery disease (CAD) as demonstrated by invasive coronary angiography (ICA).1,2 Recently, coronary computed tomographic angiography (CCTA) has emerged as a novel non-invasive alternative for angiographic assessment of CAD extent and severity, with high diagnostic performance when compared with ICA, and prognostic value for prediction of adverse events based upon CCTA-identified CAD.3–5 Although the management of patients without obstructive CAD by CCTA is considered straightforward, little evidence is available regarding appropriate management of patients who are found to have high-grade coronary stenoses on CCTA. Whether CCTA findings can stratify individuals with CAD who may benefit from coronary revascularization vs. medical therapy remains unexplored. The goal of the present study was to determine the relative therapeutic impact of coronary revascularization vs. medical therapy on survival from a large international, multicentre, observational cohort of patients undergoing CCTA.
Methods
Patients
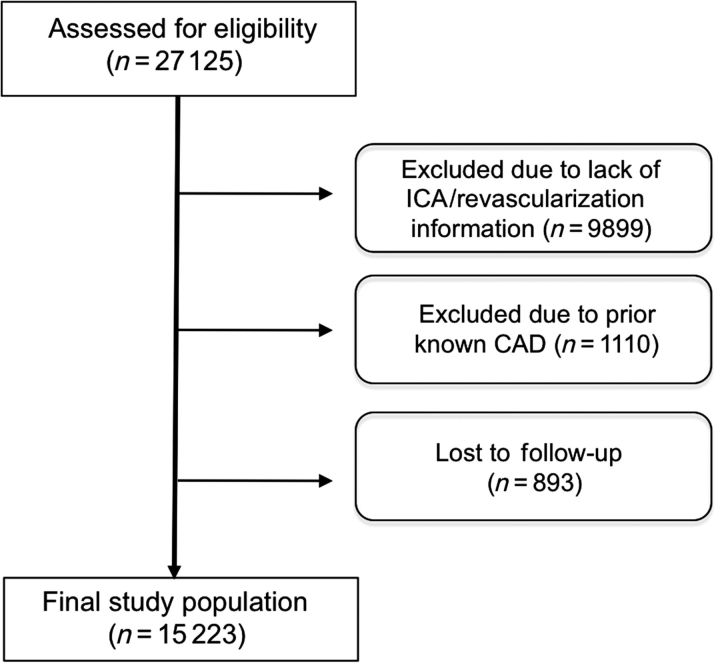
This study represents 15 223 stable patients without known CAD or suspected acute coronary syndrome from the CONFIRM Registry (Coronary CT Angiography EvaluatioN For Clinical Outcomes: An InteRnational Multicenter Registry), which has been previously described.6 CONFIRM enrolled consecutive adults ≥18 years of age between 2005 and 2009 who underwent ≥64-detector row CCTA for suspected CAD. Eight of 12 centres for which follow-up for coronary revascularization was available are represented in the present study (Cedars Sinai Medical Center, Los Angeles, CA; Harbor UCLA Medical Center, Los Angeles, CA; Tennessee Heart and Vascular Institute, Hendersonville, TN; Capital Cardiology Associates, Albany, NY; University of Munich, Munich, Germany; Ottawa Heart Institute, Ontario, Canada; Henry Ford Medical Center, Detroit, Michigan; Yonsei Medical Center, Seoul, Korea; University Hospital, Zurich, Switzerland; William Beaumont Hospital, Royal Oak, MI; Walter Reed Armey Medical Center, Washington DC; University Hospital of Parma, Parma, Italy). Institutional review board approval was obtained at each centre. Individuals with known CAD, as defined by prior myocardial infarction or prior coronary revascularization, were excluded from the present study analysis (Figure 1).
Figure 1.
Study flow chart.
Prior to the initiation of the scan, we prospectively collected information on the presence of categorical cardiac risk factors in each individual.7 Systemic arterial hypertension was defined as a documented history of high blood pressure or treatment with anti-hypertensive medications. Diabetes mellitus was defined by diagnosis of diabetes made previously by a physician and/or use of insulin or oral hypoglycemic agents. Dyslipidaemia was defined as known but untreated dyslipidaemia, or current treatment with lipid-lowering medications. A positive smoking history was defined as current smoking or cessation of smoking within 3 months of testing. Family history of coronary heart disease was determined by patient query. Symptom presentation was classified into one of four categories: typical chest pain/dyspnoea; atypical chest pain; non-cardiac pain or asymptomatic.
Scan protocol and image reconstruction
Coronary computed tomographic angiography scans were performed on a variety of different scanner platforms (Lightspeed VCT, GE Healthcare, Milwaukee, WI; Somatom Definition CT, Siemens, Erlangen, Germany; Somatom Definition Flash CT, Siemens, Ehrlangen, Germany) in accordance to performance guidelines from the Society of Cardiovascular Computed Tomography (SCCT).8 Imaging of a test-bolus of contrast was performed at 2 mm superior to the takeoff of the left main coronary artery for precise timing of contrast injection. During the CCTA acquisition, 80–140 cc of iodinated contrast (Isovue 370, Bracco Diagnostics, Princeton, NJ; Omnipaque, GE Healthcare, Princeton, NJ; Visipaque, GE Healthcare, Princeton, NJ; or Imeron 350; Bracco Atlana Pharma, Konstanz, Germany) was injected followed by a 50 cc saline flush. Contrast timing was performed to optimize uniform contrast enhancement of the coronary arteries. The scan parameters were 64 × 0.625/0.750 mm collimation, tube voltage 100 or 120 mV, effective 400–650 mA. Dose reduction strategies—including ECG-gated tube current modulation, reduced tube voltage, and prospective axial triggering—were employed whenever feasible.
Helical or axial scan data were obtained with retrospective or prospective ECG gating, respectively. Images were reconstructed immediately after completion of the scan to identify motion-free coronary artery images. Optimal phase reconstruction was assessed by comparison of different phases, if available, and the phase with the least amount of coronary artery motion was chosen for analysis. Multiple phases were employed for image interpretation if motion-free images were different for different arteries. Coronary computed tomographic angiographys were evaluated by an array of post-processing imaging techniques, including axial, multiplanar reformat, maximum intensity projection, and short axis cross-sectional views. In all individuals, irrespective of image quality, every arterial segment was scored in an intent-to-diagnose fashion. If a coronary artery segment was uninterpretable despite these multiple techniques, the unevaluable segment was scored similar to the most proximal segment that was evaluable.
Non-invasive coronary artery analysis by CCTA
All scans were analysed by level III-equivalent cardiologists or radiologists with experience interpreting several thousand CCTA scans in direct accordance with the SCCT guidelines.9 Coronary computed tomographic angiography interpretation was uniform across all study sites, with coronary segments visually scored for the presence of coronary plaque using a 16-segment coronary artery model in an intent-to-diagnose fashion. In each coronary artery segment, coronary atherosclerosis was defined as tissue structures >1 mm2 that existed either within the coronary artery lumen or adjacent to the coronary artery lumen that could be discriminated from surrounding pericardial tissue, epicardial fat, or the vessel lumen itself. Coronary atherosclerotic lesions were quantified for stenosis by visual estimation. Luminal diameter stenosis severity was scored as none (0% luminal stenosis), mild (1–49% luminal stenosis), moderate (50–69% luminal stenosis), or severe (≥70% luminal stenosis). Percent obstruction of coronary artery lumen was based on a comparison of the luminal diameter of the segment exhibiting obstruction to the luminal diameter of the most normal appearing site immediately proximal to the plaque. In instances in which plaque was highly calcified, 2D oblique images were also visualized without maximal intensity projection (i.e. 0.625–0.75 mm isotropic voxel resolution) or multiplanar reformats with cross-sectional views to minimize partial volume averaging artefact of calcium.
For purposes of classification for per-vessel analyses, we considered four arterial territories: left main artery (LM); left anterior descending (LAD) artery; left circumflex (LCx) artery; and right coronary artery (RCA). Obstructive CAD in the diagonal branches, obtuse marginal branches, and posterolateral branches was considered as part of the LAD, LCx and RCA system, respectively. The posterior descending artery was considered as part of the RCA or LCx system, depending upon the coronary artery dominance. A ≥50% stenosis in the left main artery was considered obstructive in all models. Extent of obstructive CAD was defined by ≥50% stenosis in 0, 1, 2, or 3 coronary artery vessels.
Per-segment analysis was judged for individual coronary artery segments that included the LM artery; proximal, mid-, and distal LAD; first and second diagonal branch; proximal and distal LCx; first and second obtuse marginal branch; proximal, mid-, and distal right coronary artery; left and right posterolateral artery; and posterior descending artery. Coronary artery disease severity was calculated using clinical coronary artery plaque scores, as we have previously described.7,10 For assessment of the severity of CAD, a modified Duke CAD index, combining the location and degree of stenosis, was employed that we have previously demonstrated to provide incremental and linear gradations of prognostic risk of incident death in relations to extent and severity of CAD. Within the Duke CAD index, eight groups are present: Group 0 = No CAD; Group 1 = ≥1 segment with 1–49% stenosis; Group 2 = ≥2 segments with 1–49% stenosis AND at ≥1 proximal segment with any stenosis; Group 3 = ≥1 segment with 50–69% stenosis; Group 4 = ≥2 segments with 50–69% stenosis OR ≥1 segment with ≥70% stenosis; Group 5 = ≥3 segments with 50–69% stenosis OR ≥2 segments with ≥70% stenosis OR pLAD with ≥70% stenosis; Group 6 = ≥3 segments with ≥70% stenosis OR ≥2 segments with ≥70% stenosis AND pLAD with ≥70% stenosis); Group 7 = left main with ≥50% stenosis. Based upon these gradations and their associated prognosis, patients were also categorized into two separate groups that included non-high-risk CAD (Groups 0–4) and high-risk CAD (Groups 5–7).
Further, we examined the extent of CAD as determined by the number of major epicardial coronary vessels that possessed obstructive CAD (as defined by ≥50% stenosis), ranging from no CAD to three-vessel CAD. If a left main coronary stenosis of ≥50% was present, this was considered for the purposes of the study analysis equivalent to three-vessel CAD.
Statistical analysis and study design
SAS 9.2 (www.sas.com, Cary, NC) or SPSS 12.0 and 17.0 (www.SPSS.com, Chicago, IL) were used for all statistical analyses. Categorical variables are presented as frequencies and percentages. Continuous variables are presented as means ± 1 standard deviation or medians (interquartile range) when appropriate. Variables were compared with χ2 statistic for categorical variables and by Student's unpaired t-test or Wilcoxon non-parametric test where appropriate for continuous variables. We further examined individuals undergoing post-CCTA coronary revascularization vs. medical therapy alone. Given the observational nature of the present cohort study, we employed statistical measures to account for non-random allocation of individuals to medical therapy or coronary revascularization. Post-test coronary revascularization was defined as having occurred in the first 90 days following CCTA, and was selected based upon our prior published studies that have indicated that this timeframe is consistent with treatment based upon test findings (i.e. within a general episode of care) while revascularization occurring after this time occurs as a result of a worsening clinical state.11 Post-test medical therapy vs. revascularization was adjusted via a propensity score that accounted for the decision to refer an individual for revascularization and allocated patient assignment to treatment type in a manner similar to that for a randomized clinical trial, as we have previously described.12 In order to accomplish this, we performed a two-step process that entailed the development of the propensity score followed by execution of a multivariable Cox proportional hazards model that included the propensity score. The propensity score was developed using a logistic regression model that summarized predictors of referral of patients to revascularization vs. medical therapy. All potential factors known to influence this referral pattern were include a priori into the propensity score development with significant factors retained in the final model. All variables demonstrating significance (using P < 0.10 for interaction) were included in the final propensity score, which represented a summary measure for prediction of referral for revascularization.
Time to death from all causes was calculated using univariate Cox proportional hazards models to determine the relationship of medical therapy vs. coronary revascularization for time of freedom from death by all causes. We also performed a multivariable risk-adjusted Cox proportional hazards model. This approach controlled for the effect of baseline differences in the comparator cohorts as well as the impact of non-randomized treatment allocation on survival. A hazard ratio and 95% confidence interval was calculated from the Cox models. Model overfitting procedures were carefully considered. A two-tailed P-value of <0.05 was considered statistically significant.
Follow-up
The primary endpoint was time to death from all causes. Follow-up procedures were approved by all study centres' institutional review boards. Death status for non-US centres was gathered by clinical visits, telephone contacts, and questionnaires sent by mail; with verification of all reported events by hospital records or direct contact with a patient's attending physician. Death status for US centres was ascertained either by query of the Social Security Death Index or by scripted interview by experienced physician and/or nurse study investigators.
Results
Clinical characteristics of the study cohort
Baseline characteristics are listed in Table 1. Comparing with patients undergoing medical therapy, patients undergoing coronary revascularization were older, more likely to be male, more likely to possess CAD risk factors including hypertension, diabetes, dyslipidaemia, smoking, and family history of CAD. Patients undergoing revascularization also possessed greater rates of typical angina, obstructive CAD, and higher pre-test likelihood of CAD.
Table 1.
Baseline characteristics of the study groups
| Medical therapy | Revascularization | P-value | |
|---|---|---|---|
| n | 14 120 | 1103 | |
| Male sex, % (n) | 54 (7599) | 71 (783) | <0.0001 |
| Hypertension, % (n) | 47 (6570) | 59 (647) | <0.0001 |
| Diabetes mellitus, % (n) | 12 (1700) | 24 (261) | <0.0001 |
| Hypercholesterolaemia, % (n) | 54 (7533) | 67 (734) | <0.0001 |
| Smoking, % (n) | 16 (2239) | 22 (244) | <0.0001 |
| Family history of CAD, % (n) | 29 (4020) | 39 (421) | <0.0001 |
| Typicality | <0.0001 | ||
| Asymptomatic, % (n) | 31 (4116) | 24 (251) | |
| Non-cardiac angina, % (n) | 5 (659) | 5 (50) | |
| Atypical angina, % (n) | 36 (4774) | 29 (305) | |
| Typical angina or dyspnoea, % (n) | 29 (3862) | 42 (445) | |
| Age, mean (range) | 56 (18–92) | 63 (29–94) | <0.0001 |
| Extent of stenosis by CCTA | <0.0001 | ||
| Non-obstructive (<50%), % (n) | 87 (12 284) | 13 (139) | |
| Obstructive (≥50%), % (n) | 13 (1836) | 87 (964) | |
| Pre-test likelihood of CAD, median (range) | 0.46 (0.003–0.943) | 0.67 (0.003–0.943) | <0.0001a |
aDifferences tested using Wilcoxon test.
Clinical treatment and events
Individuals were followed for a median of 2.1 years (interquartile range 1.4–3.3 years). Among individuals undergoing revascularization (n = 1109), most (n = 1103) had the procedure within the first 90 days. More patients undergoing post-CCTA coronary revascularization did so by percutaneous coronary intervention (PCI) (n = 913; 82.8%) when compared with coronary artery bypass surgery (CABG) (n = 228; 20.7%), with a small minority undergoing both PCI and CABG (n = 38, 3.5%). Coronary revascularization rates differed in relation to the extent and severity of CAD, with 3.8% (534 of 14 111), and 51.2% (569 of 1.112) of revascularization occurring in patients with non-high-risk CAD and high-risk CAD, respectively (P < 0.01).
During follow-up, a total of 185 deaths occurred (1.22% of the entire population), with the majority of deaths occurring in the first year (n = 85), followed to a lesser degree during the second year (n = 53) and more than 2 years (n = 44) after testing. As stratified by treatment method, death occurred in 1.14% (161 of 14 120) of those treated with medical therapy and 2.18% (24 of 1103) of those treated with coronary revascularization
Propensity score
From the logistic regression analysis, the most significant predictors of referral to coronary revascularization were presence of obstructive CAD and pre-test probability of CAD as calculated by the method of Diamond and Forrester13 Table 2. Family history of CAD was significantly associated with referral to revascularization and was thus included in the propensity score. At a 0.10 significance level for interactions, the interaction between pre-test probability of CAD and obstructive CAD was significant (P = 0.0344) and was also included in the final propensity score model (C index 0.91, χ2 = 1248, P < 0.0001).
Table 2.
Logistic regression model predicting referral to revascularization
| Variable | β coefficient (95% CI) | χ2 | P-value |
|---|---|---|---|
| Intercept | −3.48 (−3.68 to −3.28) | 1177.43 | <0.0001 |
| Family history of CAD | 0.1848 (0.03–0.34) | 5.6449 | 0.0175 |
| DF pre-test probablility | 0.3749 (0.18–0.57) | 14.7235 | 0.0001 |
| Obstructive CAD | – | 1110.59 | <0.0001 |
| Normal | 1 | Reference | Reference |
| Non-obstructive | −0.3437 (−0.57 to −0.12) | 8.9508 | 0.0028 |
| Obstructive CAD | 2.8367 (2.64–3.03) | 792.3851 | <0.0001 |
| DF probability vs. Obstructive CAD | – | 6.7387 | 0.0344 |
| Normal vs. DF_probability | 1 | Reference | Reference |
| Non-obstructive vs. DF_probability | −0.2872 (−0.51 to −0.06) | 6.2515 | 0.0124 |
| Obstructive CAD vs. DF_probability | −0.1778 (−0.38 to 0.02) | 3.1141 | 0.0776 |
Survival analysis
The final Cox regression model for prediction of all-cause mortality included the following variables: age (linear and non-linear); male gender; hypertension; dyslipidaemia; diabetes; angina typicality; smoking; treatment type (revascularization vs. medical therapy); CAD severity categorized as non-high-risk CAD vs. high-risk CAD; the interaction of treatment and CAD severity; and the propensity score predicting referral to revascularization Table 3. When checking the proportional hazards assumptions for the Cox model, a significant time effect was observed to interact with CAD severity. To account for this, two additional terms were added to the final Cox regression model; the first was a two-way interaction between time and CAD severity, the second was a three-way interaction between time, CAD severity, and treatment. The final hazard ratios estimate the effect of treatment (revascularization vs. medical therapy) at different levels of CAD severity, and are given at the median follow-up time of 2.1 years.
Table 3.
Cox proportional hazards model predicting death by any cause
| Effect | Univariate |
Multivariate |
||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P-value | Hazard ratio | 95% CI | P-value | |
| Male sex | 0.9 | 0.66–1.22 | 0.502 | 1.17 | 0.85–1.61 | 0.3326 |
| Hypertension | 2.31 | 1.66–3.22 | <0.0001 | 1.8 | 1.28–2.54 | 0.0007 |
| Dyslipidaemia | 0.67 | 0.50–0.91 | 0.0104 | 0.67 | 0.49–0.91 | 0.01 |
| Diabetes | 1.77 | 1.25–2.50 | 0.0013 | 1.83 | 1.29–2.60 | 0.0007 |
| Age, linear | 1.09 | 1.07–1.11 | <0.0001 | 0.87 | 0.81–0.97 | 0.002 |
| Age vs. age, non-linear | 1.001 | 1.001–1.001 | <0.0001 | 1.002 | 1.001–1.002 | <0.0001 |
| Typicality of chest pain | ||||||
| Asymptomatic | 1 | Reference | Reference | 1 | Reference | Reference |
| Non-cardiac | 1.61 | 0.88–2.95 | 0.1215 | 1.31 | 0.71–2.42 | 0.3834 |
| Atypical | 0.46 | 0.29–0.71 | 0.0006 | 0.47 | 0.30–0.74 | 0.0012 |
| Typical/dyspnoea | 1.21 | 0.84–1.72 | 0.3029 | 1.1 | 0.77–1.59 | 0.5943 |
| Smoker | 1.72 | 1.22–2.41 | 0.0018 | 2.2 | 1.56–3.12 | <0.0001 |
| Treatment (revasc vs. medical Tx) | 0.69 | 0.42–1.12 | 0.1308 | N/A | N/A | 0.4484 |
| High-risk vs. low-risk CAD | 1.49 | 0.95–2.34 | 0.0836 | N/A | N/A | 0.5821 |
| Revasc vs. high-risk CAD | N/A | N/A | 0.0899 | N/A | N/A | 0.0161 |
| High risk vs. time | N/A | N/A | 0.3652 | N/A | N/A | 0.4477 |
| High risk vs. time vs. early revasc | 0.1179 | 0.03 | ||||
| Revasc vs. medical Tx (high-risk CAD at 2.1 year follow-up) | 0.52 | 0.30–0.89 | 0.38 | 0.18–0.83 | ||
| Revasc vs. medical Tx (low-risk CAD at 2.1 year follow-up) | 0.87 | 0.46–1.64 | 3.24 | 0.76–13.9 | ||
| Propensity | 51.6 | 21.9–121.4 | <0.0001 | 4.84 | 1.41–16.6 | 0.012 |
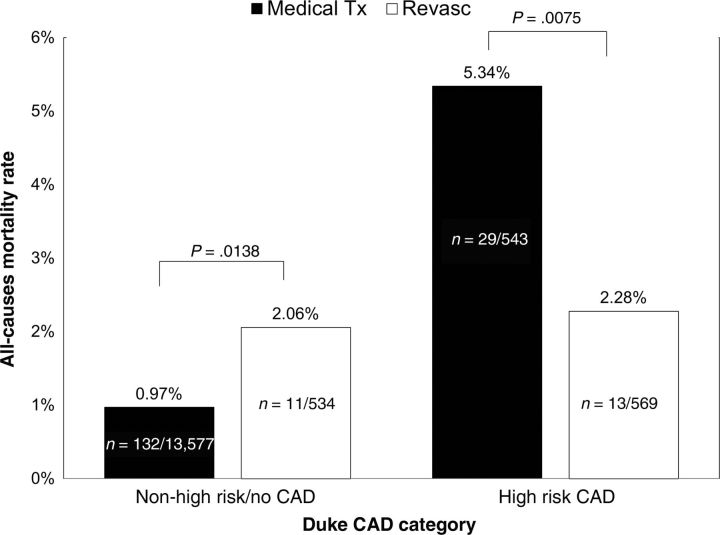
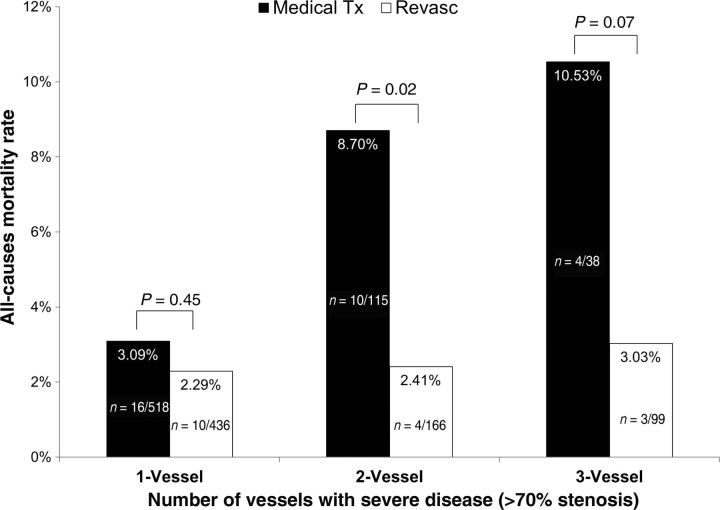
The univariate relationship between CAD severity and death with respect to revascularization vs. medical therapy can be observed in Figures 2 and 3. Figure 2 depicts the overall mortality of high-risk and non-high-risk CAD patients in the revascularization and medical therapy groups. A survival benefit with revascularization was seen for patients with high-risk CAD, whereas patients with less severe CAD demonstrated lower mortality rates when treated with medical therapy alone. Figure 3 illustrates the mortality rate with the respective treatments by the extent of CAD as defined by number of major epicardial vessels with obstructive CAD. In patients with single-vessel CAD, no significant difference in mortality was observed between those undergoing and not undergoing revascularization. However, an observational survival benefit for those undergoing revascularization was observed in patients with two-vessel disease, with a trend towards benefit noted for patients with three-vessel disease.
Figure 2.
Observed death rates for patients with obstructive coronary artery disease undergoing medical therapy (medical Tx) or revascularization (revasc) by high-risk vs. non-high-risk coronary artery disease.
Figure 3.
Observed death rates for patients undergoing medical therapy (medical Tx) or revascularization (revasc) by obstructive coronary stenosis for one-, two-, or three-vessels.
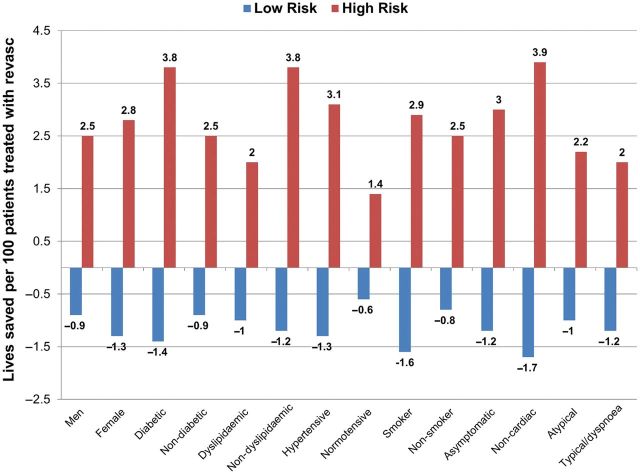
Univariate Cox proportional hazards models of survival in relationship to revascularization or medical therapy can be seen in Table 3. In multivariable Cox proportional hazards models, when compared with medical therapy, revascularization was associated with a survival advantage for patients with high-risk CAD (hazards ratio 0.38, 95% confidence interval 0.18–0.83), with no difference in survival for patients with non-high-risk CAD (HR 3.24, 95% CI 0.76–13.89) at the median follow-up period (P-value for interaction of revascularization and high-risk CAD and time = 0.03). Using the final Cox model, we also evaluated subgroups to determine whether any particular cohort benefited more from coronary revascularization Figure 4. Improvement in survival was evident for all subgroups when high-risk CAD was treated with revascularization with no specific subgroup benefiting substantially more than another.
Figure 4.
Predicted all-cause mortality rates in patients treated with medical therapy vs. coronary revascularization as stratified by non-high-risk coronary artery disease, and high-risk coronary artery disease. Exploratory analyses that do not reach statistical significance.
Discussion
The present results of this large multinational registry of patients without known CAD undergoing CCTA provide evidence that stable patients with high-risk CAD—inclusive of those with two-vessel obstructive CAD with proximal left anterior descending artery involvement, three-vessel obstructive CAD, and obstructive left main CAD—experience a survival benefit from undergoing revascularization when compared with standard medical therapy alone. In contradistinction, those with lesser forms of CAD—which represent the predominant majority of individuals undergoing CCTA—do not experience any difference in survival by undergoing revascularization. These results were evident in observed rates of mortality following revascularization as well as after adjustment for the assignment of individuals to revascularization vs. medical therapy. On the basis of the final Cox models, the benefits for revascularization in the high-risk CAD subset are suggestive of generalizability across a myriad of patient subgroups.
Our group has previously reported on the prognostic value of CCTA-identified CAD extent and severity, and has observed increased risk of death and major adverse cardiac events14 for CCTA measures of high-risk CAD, obstructive CAD, and even for milder forms of non-obstructive CAD.7,15–17 The prognostic value of CCTA-identified CAD is incremental to traditional measures of CAD risk assessment—including being additive to measures of left-ventricular function, myocardial perfusion, coronary artery calcium scores, and clinical evaluation—and generalizable across an array of patient subgroups including those without and with known CAD, diabetics, women, and asymptomatic patients.17–22 In this article, we sought to further determine whether there exists a threshold of angiographic CAD above which revascularization might hold a relative therapeutic advantage over medical therapy alone. Given the large-scale international multisite nature of the CONFIRM registry, these results extend prior studies by examining the potential therapeutic benefit of CCTA-guided decision making. Future studies examining whether CCTA can improve appropriate referral to cardiac catheterization with intended coronary revascularization now appear warranted.
Recently, large-scale registries and randomized controlled trials (RCT) have suggested that ischaemia-guided revascularization may be superior to angiographically guided revascularization for reduction of events.12,23,24 In particular, the recent FAME, or Fractional Flow Reserve vs. Angiography for Multivessel Evaluation, trial demonstrated a higher survival for patients undergoing revascularization based upon lesion-specific assessment of coronary artery stenosis haemodynamic significance.25 Similarly, a prior large-scale registry from our own group has revealed that revascularization based upon a function of % ischaemic myocardium as judged by non-invasive myocardial perfusion testing may identify individuals who may benefit from coronary revascularization over medical therapy alone.12 The results of the present study do not conflict with these prior landmark studies, and whether ischaemia-guided revascularization is superior to angiographically guided revascularization by CCTA is beyond the scope of the study. Rather, given the recent designation of CCTA by American College of Cardiology Appropriate Use Criteria as an appropriate alternative to functional stress for symptomatic individuals with suspected CAD, these data may be useful for practitioners to identify thresholds at which cardiac catheterization with intended revascularization after CCTA may be considered reasonable or not.26
On its surface, the results of the present study may appear incongruous with recent RCTs that have challenged the notion that the addition of coronary revascularization to optimal medical therapy (OMT) is superior to OMT alone in stable patients with angiographically obstructive CAD.27,28 Particularly germane to the present discussion are the results of the COURAGE (Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation) trial, which evaluated 2287 patients with angiographic obstructive CAD who were assigned to revascularization plus OMT vs. OMT alone. At a 4.6-year follow-up, no differences were noted between groups for MACE events or angina-related quality of life. And indeed, in the present study, for the vast majority of patients with CCTA-identified CAD—including those with angiographically obstructive CAD—no survival benefit was observed for those undergoing coronary revascularization vs. medical therapy alone. However, for a small minority of patients undergoing CCTA who were identified as having high-risk CAD (7.3%; 1112 of 15 223), a survival benefit from revascularization was observed. The propitious outcomes following revascularization in patients with angiographically high-risk CAD may be explained by differences in the CONFIRM and COURAGE cohorts. By design, the COURAGE trial systematically excluded patients with obstructive left main CAD and may have been affected by unobserved negative selection biases for patients with more high-risk forms of angiographic CAD.29 In contrast, the present study assessed the outcomes of patients across the entire range of CAD severity—including those with high-risk CAD—without pre-defined selection criteria. Further, the present study design was an observational one, with no mandated post-test treatments and thus, medical regimens and medical compliance are unknown. In this regard, the patients in our registry are assumed to have achieved usual rather than OMT, which may have served to heighten the outcome disparities between the revascularization and medical therapy groups. In this real world comparative effectiveness study, whether the outcomes of patients with high-risk CAD taking an OMT regimen could be improved by revascularization cannot be properly tested. Given the observational nature of this study, all of its results should be considered hypothesis-generating rather than hypothesis-proving; future randomized trials further exploring the present findings should be performed to corroborate the present findings.
The present results harmonize with prior large-scale registries that have demonstrated a similar hazards reduction for death in patients with angiographically high-risk CAD treated with revascularization. Mark et al.2 examined 9263 patients referred for invasive angiography during a 7-year period. In this cohort, a growing benefit for revascularization was noted for patients with increasingly severe CAD, with an almost 40% lower rate of cardiovascular death in patients with three-vessel obstructive CAD undergoing coronary artery bypass grafting in favour of medical therapy. Similarly, the Coronary Artery Surgery Study examined 11 508 patients for sudden death, comparing those treated with medical therapy vs. coronary revascularization by surgical methods.1 In this cohort with high-risk CAD, a 25% lower rate of sudden death was observed in patients undergoing revascularization. Both of these studies identified the most favourable outcomes of patients with high-risk CAD to be related to surgical, as opposed to percutaneous revascularization. Importantly, these investigations were performed in an era that preceded contemporary stent technologies. In the current study, we did not specifically examine the mortality differences associated with revascularization by percutaneous methods vs. CABG because to do so would have compromised statistical power and because angiographic data was not available for review. However, given the recent large-scale RCTs that have demonstrated a similar survival advantage among patients treated with surgical vs. percutaneous revascularization methods, we believe that combination of these methods into a single revascularization category is both contemporary in its scope as well as scientifically justified.30–32
This study is not without limitations. While the patient cohorts represent those from a diversity of clinical practice sites and countries, all study results are subject to the limitations of observational data. In particular, unobserved confounders and selection and/or referral biases may have affected our study results. It is notable to mention, however, that we performed careful statistical modelling to account for patterns of referral to coronary revascularization vs. medical therapy, in a manner that simulated an RCT design.12 Further, we aimed to examine those patients who underwent revascularization as a response to CCTA findings and chose a 90-day window after CCTA performance to define early post-test revascularization. This window of time was chosen as lengthier periods of time after testing generally represent clinical worsening that exceeds the warranty of an episode of care for which the original test was ordered.11,33,34
Notably, in this prospective multicentre observational cohort study, post-CCTA treatment regimens were not mandated. Our study results may have differed, particularly in the high-risk CAD subset, if OMT had been mandated; future randomized studies addressing this issue may be warranted. Further, it should be noted that we examined baseline characteristics—including CAD risk factors—as binary variables, rather than as continuous ones. Thus, the duration of CAD risk factor presence and the severity of the risk remain unknown. Finally, we examined all-cause mortality as a primary endpoint, given its unparalleled clinical importance and freedom from ascertainment bias. Nevertheless, revascularization is not expected to affect rates of non-cardiac deaths and the relative therapeutic impact of revascularization vs. medical therapy specifically on cardiac death alone or on sudden cardiac death remains unknown.
Conclusion
In an intermediate-term follow-up of 15 223 patients without known CAD undergoing CCTA from an international multicentre observational cohort study, coronary revascularization in patients with high-risk CAD was associated with significantly lower rates of all-cause mortality when compared with medical therapy alone. In contrast, no apparent benefit of revascularization was realized for patients with lesser forms of CAD. These observational findings may be potentially useful as hypothesis-generating data upon which future randomized trials can be considered.
Funding
No funding has been provided for the CONFIRM registry to date.
Conflict of interest: J.M. received research support, serves on the speaker's bureau, and is a consultant to GE Healthcare.
References
- 1.Holmes DR, Jr, Davis KB, Mock MB, Fisher LD, Gersh BJ, Killip T, III, Pettinger M. The effect of medical and surgical treatment on subsequent sudden cardiac death in patients with coronary artery disease: a report from the coronary artery surgery study. Circulation. 1986;73:1254–1263. doi: 10.1161/01.cir.73.6.1254. doi:10.1161/01.CIR.73.6.1254. [DOI] [PubMed] [Google Scholar]
- 2.Mark DB, Nelson CL, Califf RM, Harrell FE, Jr, Lee KL, Jones RH, Fortin DF, Stack RS, Glower DD, Smith LR, DeLong ER, Smith PK, Reves JG, Jollis JG, Tcheng JE, Muhlbaier LH, Lowe JE, Phillips HR, Pryor DB. Continuing evolution of therapy for coronary artery disease. Initial results from the era of coronary angioplasty. Circulation. 1994;89:2015–2025. doi: 10.1161/01.cir.89.5.2015. doi:10.1161/01.CIR.89.5.2015. [DOI] [PubMed] [Google Scholar]
- 3.Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724–1732. doi: 10.1016/j.jacc.2008.07.031. doi:10.1016/j.jacc.2008.07.031. [DOI] [PubMed] [Google Scholar]
- 4.Miller JM, Rochitte CE, Dewey M, Arbab-Zadeh A, Niinuma H, Gottlieb I, Paul N, Clouse ME, Shapiro EP, Hoe J, Lardo AC, Bush DE, de Roos A, Cox C, Brinker J, Lima JA. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med. 2008;359:2324–2336. doi: 10.1056/NEJMoa0806576. doi:10.1056/NEJMoa0806576. [DOI] [PubMed] [Google Scholar]
- 5.Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, Nieman K, van Werkhoven JM, Pundziute G, Weustink AC, de Vos AM, Pugliese F, Rensing B, Jukema JW, Bax JJ, Prokop M, Doevendans PA, Hunink MG, Krestin GP, de Feyter PJ. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135–2144. doi: 10.1016/j.jacc.2008.08.058. doi:10.1016/j.jacc.2008.08.058. [DOI] [PubMed] [Google Scholar]
- 6.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah MH, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan KM, Chow B, Delago A, Hadamitzky M, Hausleiter J, Karlsberg RP, Kaufmann P, Maffei E, Nasir K, Pencina MJ, Raff GL, Shaw LJ, Villines TC. Rationale and design of the CONFIRM (COronary CT Angiography EvaluatioN For Clinical Outcomes: An International Multicenter) registry. J Cardiovasc Comput Tomogr. 2011;5:84–92. doi: 10.1016/j.jcct.2011.01.007. doi:10.1016/j.jcct.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, Lippolis NJ, Berman DS, Callister TQ. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–1170. doi: 10.1016/j.jacc.2007.03.067. doi:10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 8.Abbara S, Arbab-Zadeh A, Callister TQ, Desai MY, Mamuya W, Thomson L, Weigold WG. SCCT guidelines for performance of coronary computed tomographic angiography: a report of the Society of Cardiovascular Computed Tomography Guidelines Committee. J Cardiovasc Comput Tomogr. 2009;3:190–204. doi: 10.1016/j.jcct.2009.03.004. doi:10.1016/j.jcct.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 9.Raff GL, Abidov A, Achenbach S, Berman DS, Boxt LM, Budoff MJ, Cheng V, DeFrance T, Hellinger JC, Karlsberg RP. SCCT guidelines for the interpretation and reporting of coronary computed tomographic angiography. J Cardiovasc Comput Tomogr. 2009;3:122–136. doi: 10.1016/j.jcct.2009.01.001. doi:10.1016/j.jcct.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Lin FY, Devereux RB, Roman MJ, Meng J, Jow VM, Jacobs A, Weinsaft JW, Shaw LJ, Berman DS, Gilmore A, Callister TQ, Min JK. Assessment of the thoracic aorta by multidetector computed tomography: age- and sex-specific reference values in adults without evident cardiovascular disease. J Cardiovasc Comput Tomogr. 2008;2:298–308. doi: 10.1016/j.jcct.2008.08.002. doi:10.1016/j.jcct.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 11.Staniloff HM, Forrester JS, Berman DS, Swan HJ. Prediction of death, myocardial infarction, and worsening chest pain using thallium scintigraphy and exercise electrocardiography. J Nucl Med. 1986;27:1842–1848. doi: [PubMed] [Google Scholar]
- 12.Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short-term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2907. doi: 10.1161/01.CIR.0000072790.23090.41. doi:10.1161/01.CIR.0000072790.23090.41. [DOI] [PubMed] [Google Scholar]
- 13.Diamond GA, Forrester JS. Analysis of probability as an aid in the clinical diagnosis of coronary-artery disease. N Engl J Med. 1979;300:1350–1358. doi: 10.1056/NEJM197906143002402. doi:10.1056/NEJM197906143002402. [DOI] [PubMed] [Google Scholar]
- 14.Dorfman R, Li W, Sun L, Lin F, Wang Y, Sandford A, Pare PD, McKay K, Kayserova H, Piskackova T, Macek M, Czerska K, Sands D, Tiddens H, Margarit S, Repetto G, Sontag MK, Accurso FJ, Blackman S, Cutting GR, Tsui LC, Corey M, Durie P, Zielenski J, Strug LJ. Modifier gene study of meconium ileus in cystic fibrosis: statistical considerations and gene mapping results. Hum Genet. 2009;126:763–778. doi: 10.1007/s00439-009-0724-8. doi: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin FY, Shaw LJ, Dunning AM, Labounty TM, Choi JH, Weinsaft JW, Koduru S, Gomez MJ, Delago AJ, Callister TQ, Berman DS, Min JK. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2-center study of 2,583 patients undergoing 64-detector row coronary computed tomographic angiography. J Am Coll Cardiol. 2011;58:510–519. doi: 10.1016/j.jacc.2010.11.078. doi:10.1016/j.jacc.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 16.Min JK, Dunning A, Lin FY, Achenbach S, Al-Mallah M, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan K, Chow BJ, Delago A, Hadamitzky M, Hausleiter J, Kaufmann P, Maffei E, Raff G, Shaw LJ, Villines T, Berman DS. Age- and sex-related differences in all-cause mortality risk based on coronary computed tomography angiography findings results from the international multicenter CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter Registry) of 23,854 patients without known coronary artery disease. J Am Coll Cardiol. 2011;58:849–860. doi: 10.1016/j.jacc.2011.02.074. doi:10.1016/j.jacc.2011.02.074. [DOI] [PubMed] [Google Scholar]
- 17.Chow BJ, Small G, Yam Y, Chen L, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, Cademartiri F, Callister TQ, Chang HJ, Cheng V, Chinnaiyan KM, Delago A, Dunning A, Hadamitzky M, Hausleiter J, Kaufmann P, Lin F, Maffei E, Raff GL, Shaw LJ, Villines TC, Min JK. Incremental prognostic value of cardiac computed tomography in coronary artery disease using confirm: coronary computed tomography angiography evaluation for clinical outcomes: an international multicenter registry. Circ Cardiovasc Imaging. 2011;4:463–472. doi: 10.1161/CIRCIMAGING.111.964155. doi:10.1161/CIRCIMAGING.111.964155. [DOI] [PubMed] [Google Scholar]
- 18.Min JK, Lin FY, Dunning AM, Delago A, Egan J, Shaw LJ, Berman DS, Callister TQ. Incremental prognostic significance of left ventricular dysfunction to coronary artery disease detection by 64-detector row coronary computed tomographic angiography for the prediction of all-cause mortality: results from a two-centre study of 5330 patients. Eur Heart J. 2010;31:1212–1219. doi: 10.1093/eurheartj/ehq020. doi:10.1093/eurheartj/ehq020. [DOI] [PubMed] [Google Scholar]
- 19.van Werkhoven JM, Schuijf JD, Gaemperli O, Jukema JW, Kroft LJ, Boersma E, Pazhenkottil A, Valenta I, Pundziute G, de Roos A, van der Wall EE, Kaufmann PA, Bax JJ. Incremental prognostic value of multi-slice computed tomography coronary angiography over coronary artery calcium scoring in patients with suspected coronary artery disease. Eur Heart J. 2009;30:2622–2629. doi: 10.1093/eurheartj/ehp272. doi:10.1093/eurheartj/ehp272. [DOI] [PubMed] [Google Scholar]
- 20.van Werkhoven JM, Schuijf JD, Gaemperli O, Jukema JW, Boersma E, Wijns W, Stolzmann P, Alkadhi H, Valenta I, Stokkel MP, Kroft LJ, de Roos A, Pundziute G, Scholte A, van der Wall EE, Kaufmann PA, Bax JJ. Prognostic value of multislice computed tomography and gated single-photon emission computed tomography in patients with suspected coronary artery disease. J Am Coll Cardiol. 2009;53:623–632. doi: 10.1016/j.jacc.2008.10.043. doi:10.1016/j.jacc.2008.10.043. [DOI] [PubMed] [Google Scholar]
- 21.Choi EK, Choi SI, Rivera JJ, Nasir K, Chang SA, Chun EJ, Kim HK, Choi DJ, Blumenthal RS, Chang HJ. Coronary computed tomography angiography as a screening tool for the detection of occult coronary artery disease in asymptomatic individuals. J Am Coll Cardiol. 2008;52:357–365. doi: 10.1016/j.jacc.2008.02.086. doi:10.1016/j.jacc.2008.02.086. [DOI] [PubMed] [Google Scholar]
- 22.Hadamitzky M, Hein F, Meyer T, Bischoff B, Martinoff S, Schomig A, Hausleiter J. Prognostic value of coronary computed tomographic angiography in diabetic patients without known coronary artery disease. Diabetes Care. 2010;33:1358–1363. doi: 10.2337/dc09-2104. doi:10.2337/dc09-2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaw LJ, Berman DS, Maron DJ, Mancini GB, Hayes SW, Hartigan PM, Weintraub WS, O'Rourke RA, Dada M, Spertus JA, Chaitman BR, Friedman J, Slomka P, Heller GV, Germano G, Gosselin G, Berger P, Kostuk WJ, Schwartz RG, Knudtson M, Veledar E, Bates ER, McCallister B, Teo KK, Boden WE. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation. 2008;117:1283–1291. doi: 10.1161/CIRCULATIONAHA.107.743963. doi:10.1161/CIRCULATIONAHA.107.743963. [DOI] [PubMed] [Google Scholar]
- 24.Hachamovitch R, Rozanski A, Shaw LJ, Stone GW, Thomson LE, Friedman JD, Hayes SW, Cohen I, Germano G, Berman DS. Impact of ischaemia and scar on the therapeutic benefit derived from myocardial revascularization vs. medical therapy among patients undergoing stress-rest myocardial perfusion scintigraphy. Eur Heart J. 2011;32:1012–1024. doi: 10.1093/eurheartj/ehq500. doi:10.1093/eurheartj/ehq500. [DOI] [PubMed] [Google Scholar]
- 25.Tonino PA, De Bruyne B, Pijls NH, Siebert U, Ikeno F, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, Fearon WF. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N Engl J Med. 2009;360:213–224. doi: 10.1056/NEJMoa0807611. doi:10.1056/NEJMoa0807611. [DOI] [PubMed] [Google Scholar]
- 26.Taylor AJ, Cerqueira M, Hodgson JM, Mark D, Min J, O'Gara P, Rubin GD, Kramer CM, Berman D, Brown A, Chaudhry FA, Cury RC, Desai MY, Einstein AJ, Gomes AS, Harrington R, Hoffmann U, Khare R, Lesser J, McGann C, Rosenberg A, Schwartz R, Shelton M, Smetana GW, Smith SC., Jr ACCF/SCCT/ACR/AHA/ASE/ASNC/NASCI/SCAI/SCMR 2010 appropriate use criteria for cardiac computed tomography. A report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the Society of Cardiovascular Computed Tomography, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the American Society of Nuclear Cardiology, the North American Society for Cardiovascular Imaging, the Society for Cardiovascular Angiography and Interventions, and the Society for Cardiovascular Magnetic Resonance. J Am Coll Cardiol. 2010;56:1864–1894. doi: 10.1016/j.jacc.2010.07.005. doi:10.1016/j.jacc.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS. Optimal medical therapy with or without pci for stable coronary disease. N Engl J Med. 2007;356:1503–1516. doi: 10.1056/NEJMoa070829. doi:10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 28.Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, Goldberg SH, Hlatky MA, Jones TL, Molitch ME, Nesto RW, Sako EY, Sobel BE. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. doi: 10.1056/NEJMoa0805796. doi:10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kereiakes DJ, Teirstein PS, Sarembock IJ, Holmes DR, Jr, Krucoff MW, O'Neill WW, Waksman R, Williams DO, Popma JJ, Buchbinder M, Mehran R, Meredith IT, Moses JW, Stone GW. The truth and consequences of the courage trial. J Am Coll Cardiol. 2007;50:1598–1603. doi: 10.1016/j.jacc.2007.07.063. doi:10.1016/j.jacc.2007.07.063. [DOI] [PubMed] [Google Scholar]
- 30.Park SJ, Kim YH, Park DW, Yun SC, Ahn JM, Song HG, Lee JY, Kim WJ, Kang SJ, Lee SW, Lee CW, Park SW, Chung CH, Lee JW, Lim DS, Rha SW, Lee SG, Gwon HC, Kim HS, Chae IH, Jang Y, Jeong MH, Tahk SJ, Seung KB. Randomized trial of stents versus bypass surgery for left main coronary artery disease. N Engl J Med. 2011;364:1718–1727. doi: 10.1056/NEJMoa1100452. doi:10.1056/NEJMoa1100452. [DOI] [PubMed] [Google Scholar]
- 31.Seung KB, Park DW, Kim YH, Lee SW, Lee CW, Hong MK, Park SW, Yun SC, Gwon HC, Jeong MH, Jang Y, Kim HS, Kim PJ, Seong IW, Park HS, Ahn T, Chae IH, Tahk SJ, Chung WS, Park SJ. Stents versus coronary-artery bypass grafting for left main coronary artery disease. N Engl J Med. 2008;358:1781–1792. doi: 10.1056/NEJMoa0801441. doi:10.1056/NEJMoa0801441. [DOI] [PubMed] [Google Scholar]
- 32.Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW. Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. doi: 10.1056/NEJMoa0804626. doi:10.1056/NEJMoa0804626. [DOI] [PubMed] [Google Scholar]
- 33.Shaw LJ, Peterson ED, Shaw LK, Kesler KL, DeLong ER, Harrell FE, Jr, Muhlbaier LH, Mark DB. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation. 1998;98:1622–1630. doi: 10.1161/01.cir.98.16.1622. doi:10.1161/01.CIR.98.16.1622. [DOI] [PubMed] [Google Scholar]
- 34.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA. 2003;290:1624–1632. doi: 10.1001/jama.290.12.1624. doi:10.1001/jama.290.12.1624. [DOI] [PubMed] [Google Scholar]