Abstract
The impact of chronic inflammatory conditions on immune function is substantial, and the simultaneous application of anti-inflammatory and immune modulating modalities has potential for reducing inflammation-induced immune suppression. Sorghum-based foods, teas, beers, and extracts are used in traditional medicine, placing an importance on obtaining an increased understanding of the biological effects of sorghum. This study examined selected anti-inflammatory and immune-modulating properties in vitro of Jobelyn™, containing the polyphenol-rich leaf sheaths from a West African variant of Sorghum bicolor (SBLS). Freshly isolated primary human polymorphonuclear (PMN) and mononuclear cell subsets were used to test selected cellular functions in the absence versus presence of aqueous and ethanol extracts of SBLS. Both aqueous and nonaqueous compounds contributed to reduced reactive oxygen species formation by inflammatory PMN cells, and reduced the migration of these cells in response to the inflammatory chemoattractant leukotriene B4. Distinct effects were seen on lymphocyte and monocyte subsets in cultures of peripheral blood mononuclear cells. The aqueous extract of SBLS triggered robust upregulation of the CD69 activation marker on CD3− CD56+ natural killer (NK) cells, whereas the ethanol extract of SBLS triggered similar upregulation of CD69 on CD3+ CD56+ NKT cells, CD3+ T lymphocytes, and monocytes. This was accompanied by many-fold increases in the chemokines RANTES/CCL5, Mip-1α/CCL3, and MIP-1β/CCL4. Both aqueous and nonaqueous compounds contribute to anti-inflammatory effects, combined with multiple effects on immune cell activation status. These observations may help suggest mechanisms of action that contribute to the traditional use of sorghum-based products, beverages, and extracts for immune support.
Key Words: CD69, leukotriene B4, migration, monocytes, natural killer cells, peripheral blood mononuclear cells, polymorphonuclear cells, ROS formation, T cells
Introduction
Chronic inflammation has a known negative impact on immune defense mechanisms,1 and is an underlying phenomenon for many health problems. Treatment modalities involving anti-inflammatory intervention are discussed for improved outcomes in antibacterial, antiviral, and anticancer treatments. The inflammatory response is essential to survival, but needs to be regulated; if unsuccessfully resolved, the chronic situation affects many aspects of immune, metabolic, hormonal, neurological, and cardiovascular health.2,3
The modulation of inflammatory status by bioactive consumables, particularly those present in common and traditional medicinal foods, is becoming an increasingly important factor in global preventive health management. Chronic inflammation is linked to metabolic diseases and obesity, and altered cell-mediated immune responses are affected by multiple mechanisms, due in part to a positive feedback loop between local inflammation in adipose tissue and dysregulated immune cell activation and production of pro-inflammatory mediators, such as leptin.4 As obesity continues to rise, representing malnutrition and metabolic disease, it is suggested that specific food components may exert specific immune regulatory effects.5 The geographical areas of the world with the biggest obesity problems overlap with the areas with the most people suffering from HIV, cancers, and cardiovascular disease. Therefore, at a global level, anti-inflammatory intervention, in conjunction with immune supportive modalities, may help reduce chronic inflammation and improve immune defense mechanisms.
Despite improved medical interventions, infectious diseases still take a toll. This involves acute bacterial, parasitic, and viral infections, as well as chronic infections, such as HIV, hepatitis, and many other chronic viral diseases. Increased bacterial resistance to antibiotic treatments has led to restrictions on the use of antibiotics, and support of immune function remains a fundamental factor.
Sorghum is a food grain that is grown across America, Africa, and Asia in areas that are too hot and dry to grow other grains and corn. Sorghum species are known to have a high content of antioxidants, including simple phenolic acids, as well as polyphenols, particularly 3-deoxyanthocyanidins, such as luteolinidin and apigenidin.6,7 The high content of these antioxidant compounds are present in sorghum-based beers and contribute to the inhibition of lipid peroxidation during mashing and boiling.8 The leaf sheath has a different chemical composition than the leaf blade.9 The sorghum leaf sheaths have a high concentration of dimeric 3-deoxy-anthocyanidins.10,11 Sorghum extracts have a strong chemoprotective potential and inhibit proliferation of gastrointestinal cancer cell lines, and these effects are partially independent of their antioxidant content.12 The anticancer properties of sorghum are due in part to the high content of 3-deoxyanthocyanidins.13
In addition to the high content of anti-inflammatory phenolic compounds, sorghum contains several groups of bioactive compounds with the capacity to induce pro-inflammatory immune responses. Water-soluble beta-glucans are found in sorghum14 that showed biologically active beta-glucans capable of initiating macrophage activation.
Sorghum seeds contain an antiviral peptide, shown to inhibit infection, replication, and spread of several viruses, including Herpes simplex and to a lesser extent the nonenveloped polio virus.15 It is not known whether some of the effect involves activation of immune defense mechanisms or are targeted directly at the virus envelope. It is also not known whether this or similar peptides are found in other parts of the plant.
Sorghum is used in traditional medicine in developing countries, including primary care of anemia,16,17 cancer, and a variety of infectious diseases, including viral diseases. An aqueous extract from sorghum leaf sheaths has hepatoprotective and hematopoietic effects,18 including the generation and hemoglobin-content of erythrocytes19; it is not known if this effect extends to other cell types produced in the bone marrow, such as, for example, natural killer (NK) cells. Sorghum is also used to help alleviate chronic pain, and an extract from the leaf base was shown to have central antinociceptive properties.20
In this study, we tested dried Sorghum bicolor leaf sheaths (SBLS) domesticated from a wild variety that is native to west Africa. This variety has been in use for centuries as traditional medicinal food by people of southwestern Nigeria. It differs from other varieties of S. bicolor in the intense dark brown pigmentation present. The botanical material is a proprietary blend of SBLS from the sub-Saharan belt in west Africa. SBLS is harvested, processed, packaged, and supplied under the trade name Jobelyn™ by Health Forever Products Inc. (Lagos, Nigeria) and Hains Herbal Products LLC (Gaithersburg, MD, USA). To better understand the contribution of various effects of S. bicolor on the immune system, as a mechanism of action for many of the traditional uses, we evaluated the biological effects of both aqueous and nonaqueous extracts from a west African SBLS extract in selected bioassays addressing effects on inflammatory cells and immune cell activation status.
Materials and Methods
Reagents, dyes, and monoclonal antibodies
The following buffers and reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA): Histopaque 1077 and 1119, phosphate-buffered saline (PBS), the RPMI-1640 culture medium, fetal calf serum, L-glutamine 200 mM, penicillin–streptomycin 100× solution, fibronectin, and bovine serum albumin. CD3-PerCP, CD56-PE, and CD69-FITC were obtained from BD Biosciences (San Jose, CA, USA). Sodium azide (NaN3) was obtained from LabChem Inc. (Pittsburgh, PA, USA). Leukotriene-B4 (LTB4) was obtained from Cayman Chemicals (Ann Arbor, MI, USA). The precursor dye DCF-DA was from Molecular Probes (Eugene, OR, USA).
S. bicolor source and handling
The botanical material was produced from SBLS from the sub-Saharan belt in west Africa. SBLS was harvested, processed, and supplied under the trade name Jobelyn™ by Health Forever Products Inc. To prepare sterile liquid fractions of the product suitable for addition to live cell cultures, 0.5 grams of the leaf sheath powder was added to 5 mL physiological saline for the aqueous extraction method, and to 5 mL 50% ethanol for the ethanol extraction method, in 15-mL vials. The vials were vortexed and placed on a rocker at room temperature for 1 h. Solids were removed by centrifugation at 900 g for 10 min, and the resulting liquid extracts further diluted in physiological saline. The liquid extracts were filtered through a 0.22 micron cellulose acetate filter to produce a sterile stock solution, from which serial dilutions were made in physiological saline. The serial dilutions added to live cell cultures were such that no more than 0.5% of ethanol was present in cell cultures. This dose of ethanol was considered to have no physiological effect in the assay, as verified by previous control tests.
Antioxidant capacity
The antioxidant capacity was evaluated by a panel of chemical oxygen radical absorbance capacity (ORAC) tests, each targeted at measuring the quenching of specific oxidative reactions. There are five predominant reactive species found in the body: peroxyl radicals, hydroxyl radicals, peroxynitrite, super oxide anion, and singlet oxygen. Total ORAC provides a measure of the total antioxidant power of a food/nutrition product against the five predominant reactive species. Testing was performed at Brunswick Laboratories (Southborough, MA, USA).
Cellular antioxidant protection
The cellular antioxidant protection assay using erythrocytes (CAP-e) was performed according to the method published by Honzel et al.,21 but using an accelerated and more sensitive microplate-based protocol. Briefly, a red blood cell suspension was prepared for the CAP-e assay by adding 0.1 mL packed red blood cells to 10 mL physiological saline (pH 7.4) in a V-bottom 96-well microplate. Six wells served as negative controls (no oxidative damage was induced) and six wells served as positive controls (maximum oxidative damage in the absence of any antioxidants). Gallic acid was used as a standard reference compound. Serial dilutions of either the aqueous extract or the ethanol extract were tested in duplicate. The level of ethanol in the cellular CAP-e assay was below 0.2%. Levels of ethanol below 2% do not affect the CAP-e assay, so the amount of ethanol in the test was considered negligible. The cells were incubated for 20 min to allow antioxidants to enter into the cells. Unabsorbed compounds were subsequently removed from the cells by two washes in physiological saline. Cell pellets were resuspended and lysed in water, and the precursor dye DCF-DA was added to the wells for 15 min. Oxidative damage was induced by AAPH for 1 h. The green fluorescence intensity, as a measurement of oxidative damage, was recorded at 488 nm using a Tecan SpectraFluor plate reader (Durham, NC, USA). The cellular antioxidant protection was calculated as the inhibition of oxidative damage reflected by the reduced fluorescence intensity in the wells, where the cells were pretreated with test products, compared to the baseline (negative controls) and maximum oxidative damage (positive controls).
Purification of polymorphonuclear and peripheral blood mononuclear cells
Healthy human volunteers between the ages of 18 and 65 years served as blood donors after written informed consent was obtained, as approved by the Sky Lakes Medical Center Institutional Review Board (FWA2603). Isolation of peripheral blood mononuclear cells (PBMC) and polymorphonuclear (PMN) cells was performed as previously described.22,23 The PMN cells were used for evaluation of anti-inflammatory activity in assays for production of reactive oxygen species (ROS) and migratory response to the inflammatory mediator LTB4. PBMC were used to establish lymphocyte cultures for the evaluation of phenotypic changes associated with immune modulation by the test product.
Evaluation of ROS formation in PMN cells
The PMN cells were incubated at 37°C, 5% CO2 for 90 min, either untreated or treated with serial dilutions of SBLS aqueous or ethanol extracts. A stock solution of the precursor dye DCF-DA was prepared by adding 0.18 mL dimethyl sulfoxide to a 50-μg aliquot of DCF-DA. A working solution was then prepared by adding 0.01 mL stock to 10 mL PBS. The PMN cells were washed twice in PBS to remove any unbound and unabsorbed compounds from the test product. The cells were resuspended in the DCF-DA working solution, and incubated for 1 h at 37°C to allow for the precursor dye to get absorbed into the PMN cells. All samples, except for the triplicate negative control samples, were then exposed to 167 mM H2O2 for 45 min to induce severe oxidative stress. Samples were washed twice in PBS to remove the peroxide, transferred to the cold RPMI 1640 medium, and stored on ice in the dark. The DCF-DA fluorescence intensity was immediately analyzed by flow cytometry. Data were collected in triplicate for controls and for each dose of the SBLS extracts. The mean fluorescence intensity of PMN cells was compared between untreated, H2O2-treated, and extract-pretreated cells. A reduction in mean fluorescence intensity in samples pretreated with test products before challenge with H2O2 indicated that the test product was able to reduce the ROS formation in PMN cells.
Migratory response to the inflammatory mediator LTB4
The PMN cell is a highly active and migratory cell type. The effect of exposure of PMN cells to the SBLS extracts on migration toward the inflammatory chemoattractant LTB4 was tested. The following experimental model was performed in quadruplicate for each control and treatment. Cells were incubated with serial dilutions of SBLS aqueous or ethanol extracts for 10 min. The top compartments of Millipore transwell (3.0-μm pore size) migration plates were coated with 50 μg/mL Fibronectin for 30 min. Meanwhile, RPMI 1640 with LTB4 (12 nM) was added to the bottom chamber wells of the transwell migration plate in a volume of 150 μL. Fibronectin was removed from the top wells by aspiration before plating of cells. Fifty microliters of cells (1×106/mL) was plated in the top chambers, and the top chamber plate was then lowered into the bottom plate and allowed to incubate for 4 h at 37°C. After the incubation, the top chambers were removed, and the relative number of cells that had migrated to the bottom chambers was evaluated by staining with CyQuant®. Fluorescence intensity was measured in a Tecan Spectrafluor fluorescence plate reader. Samples were assayed in quadruplicate and experiments repeated 3 times using PMN cells from different donors.
PBMC cultures for immunostaining and flow cytometry
To evaluate the activation status of NK cells, T lymphocytes, and monocytes, multiparameter flow cytometry was performed to allow electronic gating on CD3− CD56+ NK cells, CD3+ T cells, and high forward/side scatter monocytes. These electronic gates were then followed by evaluation of the expression level (mean fluorescence intensity) of the CD69 activation marker on each cell type. Freshly isolated PBMC were plated in sterile U-bottom 96-well culture plates (Nunc, Roskilde, Denmark) and exposed to serial dilutions of SBLS aqueous or ethanol extracts for 18 h. Cells were transferred to V-bottom 96-well plates (Nunc), washed, and resuspended in PBS buffer containing 1% bovine serum albumin and 0.02% NaN3; monoclonal antibodies were added and incubated in the dark at room temperature for 10 min. The monoclonal antibodies were CD3-PerCP (red), CD56-PE (orange), and CD69-FITC (green). The amounts used for each monoclonal antibody was previously titrated to provide optimal staining. The cells were washed and resuspended in 0.05 mL PBS buffer containing 0.02% NaN3 and transferred into 5-mL Falcon tubes each containing 0.4 mL of 1% formalin in PBS. Samples were acquired by flow cytometry using a FACSCalibur cytometer (Becton-Dickinson, San Jose, CA, USA). Analysis was performed using the FlowJo (Tree Star Inc., Ashland, OR, USA) software.
Cytokine analysis
PBMC treated with the ethanol extract of SBLS were prepared as indicated above and plated in a flat bottom 96-well plate (Costar Corning, NY, USA). All reagents were prepared and the assay was executed according to the Human Cytokine Magnetic 25-Plex Panel assay guidelines (Life Technologies, Carlsbad, CA, USA). A standard curve was created using three different concentrations of standards. Culture supernatants were tested in duplicate as follows: two samples from untreated cell cultures as controls, two samples from cell cultures exposed to 111 mg/L of the ethanol extract, and two samples from cell cultures exposed to 37 mg/L of the ethanol extract. A blank was also used to control for background fluorescence. Results were read using a LuminexMagPix instrument (Applied Biosystems, Foster City, CA, USA). Data were analyzed using Microsoft Excel.
Statistical analysis
Statistical analysis was performed using Student's t-test. Levels of statistical significance between data sets were significant if the P value was less than .05, highly significant if P<.01, and a very high level of significance if P<.001.
Results
Anti-inflammatory effects and antiviral immune support
Antioxidant capacity and cellular protection
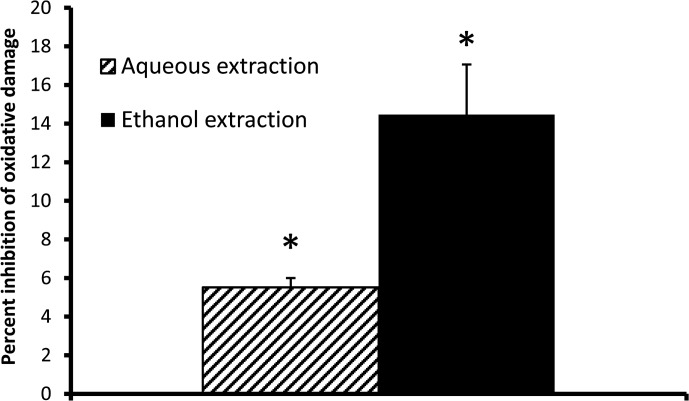
The west African SBLS was tested for antioxidant capacity in a panel of chemical antioxidant assays and also tested in the CAP-e assay. The importance of testing in both chemical and cellular assays stems from the observation that many compounds with chemical antioxidant capacity are not always able to enter and protect living cells. SBLS was found to contain high amounts of anthocyanidins, including the two 3-deoxyanthocyanidins apigenidin and luteolinidin, and a total content per dry weight of 3-deoxyanthocyanidins exceeding 40,000 μg/g, equaling over 4% of the dry weight (Table 1). SBLS was shown to have a very high chemical antioxidant capacity of 37, 622 μM Trolox equivalents/gram (Table 2), where the strongest capacities for quenching free radicals were seen for hydroxyl-free radicals and superoxide anion. In parallel, aqueous and ethanol extracts of SBLS were compared in the CAP-e bioassay, where both provided significant protection of cellular damage at a dose of 270 mg/L (Fig. 1). At lower doses, the antioxidant protection in the CAP-e assay was below the level of detection.
Table 1.
Content and Properties of Flavones in the West African Sorghum bicolor Leaf Sheaths
| μg/g | Rings | Phenolic class | Solubility in water | Solubility in ethanol | |
|---|---|---|---|---|---|
| Naringenin | 130 | 3 | Flavanone | Almost insoluble | Yes |
| Apigeninidin | 39,900 | 3 | 3-deoxyanthocyanidin | ||
| Luteolinidin | 450 | 3 | 3-deoxyanthocyanidin | ||
| Apigenin | 6910 | 3 | Flavone | ||
| Luteolin | 570 | 3 | Flavone | Almost insoluble | Yes |
Table 2.
Antioxidant Capacity of the West African Sorghum bicolor Leaf Sheaths
| (μmol TE/g) | |
|---|---|
| Antioxidant power against peroxyl-free radicals | 3549 |
| Antioxidant power against hydroxyl-free radicals | 18,387 |
| Antioxidant power against peroxynitrite | 269 |
| Antioxidant power against superoxide anion | 11,417 |
| Antioxidant power against singlet oxygen | 4000 |
| Total oxygen radical scavenging capacity (ORAC) | 37,622 |
FIG. 1.
Cellular antioxidant protection from oxidative damage. Aqueous and ethanol extractions of the Sorghum bicolor leaf sheaths (SBLS) were compared in the cellular antioxidant protection using erythrocytes (CAP-e) assay at a dose of 270 mg/L. The level of ethanol in the cellular CAP-e assay was below 0.2%. Levels of ethanol below 2% do not affect the CAP-e assay, so the amount of ethanol in the test was considered negligible. The ethanol extract provided better protection than the aqueous extract, but both fractions were able to provide significant protection of intracellular oxidative damage when comparing levels of cellular damage between cultures with and without SBLS extract (*P<.05).
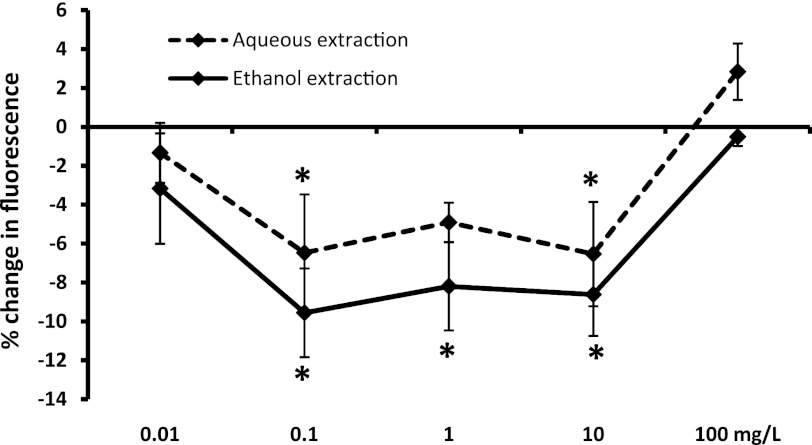
ROS formation by PMN cells
PMN cells are capable of rapid formation of ROS in response to inflammatory stimuli. The produced ROS serves both as antimicrobial defense mechanisms and also accelerates an inflammatory response. When ROS production was induced in PMN cells previously treated with the ethanol extract of SBLS at doses ranging from 0.1 to 10 mg/L, significant reduction in ROS formation was seen when compared to the level of ROS induction in untreated cells (P<.05; Fig. 2). The inhibitory effect by the aqueous extract was slightly milder, and only the doses 0.1 and 10 mg/L reached statistical significance.
FIG. 2.
Formation of reactive oxygen species (ROS) in polymorphonuclear (PMN) cells. Freshly isolated primary human PMN cells were either untreated or pretreated with serial dilutions of SBLS aqueous or ethanol extracts, then cultured under conditions of oxidative stress to provoke intracellular ROS formation. All test conditions were performed in quadruplicate. The data are shown as mean±SD and represent one of three separate experiments using cells from three different donors (*P<.05).
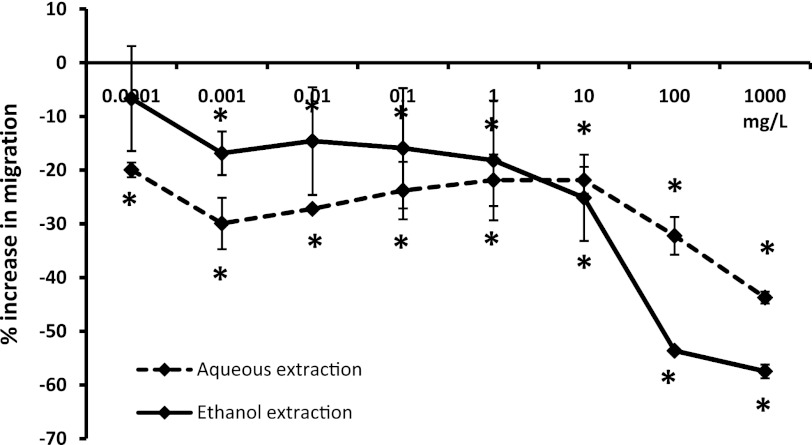
Effect on migratory behavior of PMN cells toward the inflammatory chemoattractant LTB4
The PMN cell population contributes 50%–70% of circulating white blood cells. The cells are capable of rapidly entering an activated state where they migrate toward chemoattractants of either microbial or inflammatory nature. The effect of SBLS on the migration of PMN cells in response to the inflammatory chemoattractant LTB4 was evaluated, where both the aqueous and ethanol extracts were compared over a dose range of 0.1 μg/L–1 g/L. A strong inhibition of PMN migration toward LTB4 was seen at the dose range of 100–1000 mg/L, but even at lower doses of SBLS, a significant reduction in PMN migration was seen, remaining significantly lower than untreated cells at 0.001 mg/L (P<.05; Fig. 3).
FIG. 3.
Migration of PMN cells toward the inflammatory chemoattractant leukotriene B4 (LTB4). Freshly isolated primary human PMN cells were either untreated or pretreated with serial dilutions of SBLS aqueous or ethanol extracts, then added to the top chambers of trans-well migration plates. LTB4 was added to the bottom chambers, except in negative control wells. All test conditions were performed in quadruplicate. The data are shown as mean±SD and represent one of three separate experiments using cells from three different donors (*P<.05).
Effect on activation of NK cells, NKT cells, T cells, and monocytes using the cell surface marker CD69
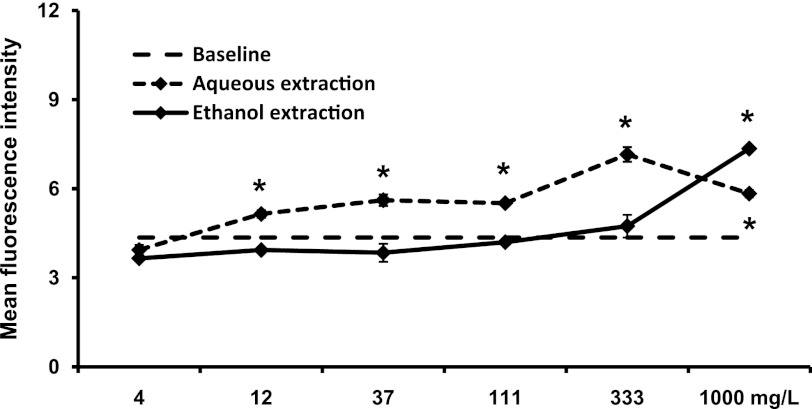
The NK cells in blood circulation are characterized as CD3− CD56+ lymphocytes, and are predominantly negative for the activation marker CD69. Given appropriate test stimuli, CD69 expression will increase and correlates to increased cytotoxic capacity.24 The treatment of PBMC cultures with aqueous and ethanol extracts of SBLS showed that the aqueous extract induced a strong increase in CD69 expression on CD3− CD56+ NK cells (at 100–1000 mg/L: P<.05; Fig. 4). In contrast, a significant effect on NK cells by the ethanol extract was only seen at the highest dose used (1000 mg/L).
FIG. 4.
Expression of the CD69 activation marker on CD3− CD56+ natural killer (NK) cells. Peripheral blood mononuclear cells (PBMC) were cultured for 18 h in the absence (baseline) or presence of either an aqueous extraction or an ethanol extraction from SBLS. The aqueous extract triggered a significant, dose-dependent increase in expression of the CD69 activation marker on NK cells. For data points where CD69 expression on treated cell cultures was significantly higher than on untreated cells (baseline, 4.36±0.43), this is indicated (*P<.05). Conditions were assayed in triplicate, and the results shown are mean±SD values from a representative of three separate experiments using PBMC from three different donors.
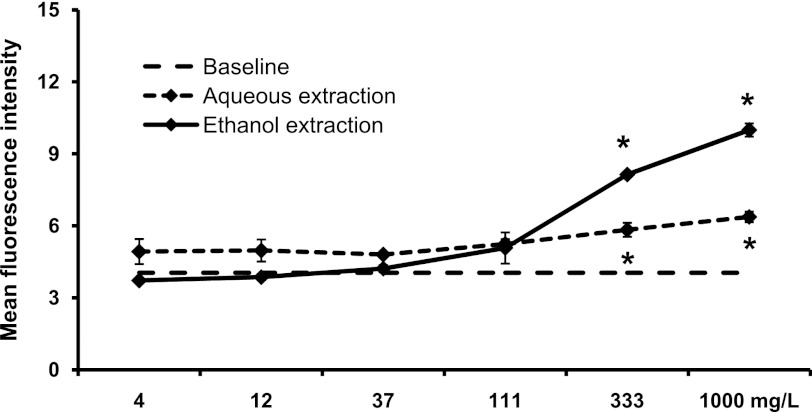
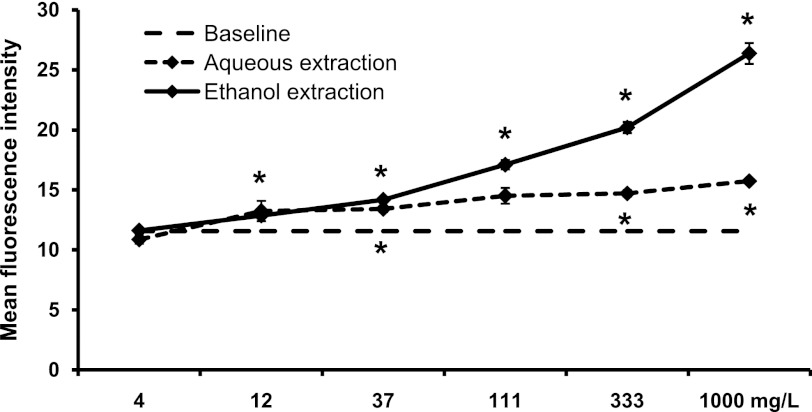
In contrast, the ethanol extract provoked a strong increase in CD69 expression on CD3+ CD56+ NKT cells (at 333–1000 mg/L: P<.05), whereas the aqueous extract only induced a milder effect at the same doses (Fig. 5). This parallels what was seen for CD3+ T cells (Fig. 6). Thus, different compounds in SBLS are responsible for the activation of NK versus NKT and T cells.
FIG. 5.
Expression of the CD69 activation marker on CD3+ CD56+ natural killer T (NKT) cells. PBMC were cultured for 18 h in the absence (baseline) or presence of either an aqueous extraction or an ethanol extraction from SBLS. The aqueous extract triggered a mild, but significant increase in expression of the CD69 activation marker on NKT cells. The ethanol fraction triggered a robust, dose-dependent increase in the CD69 expression on NKT cells. For data points where CD69 expression on treated cell cultures was significantly higher than on untreated cells (baseline, 4.05±0.17), this is indicated (*P<.05). Conditions were assayed in triplicate, and the results shown are mean±SD values from a representative of three separate experiments using cells from three different donors.
FIG. 6.
Expression of the CD69 activation marker on CD3+ T cells. PBMC were cultured for 18 h in the absence (baseline, 1.34±0.01) or presence of either an aqueous extraction or an ethanol extraction from SBLS. The ethanol extract triggered a significant, dose-dependent increase in expression of the CD69 activation marker on T cells. Conditions were assayed in triplicate, and the results shown are mean±SD values from a representative of three separate experiments using cells from three different donors.
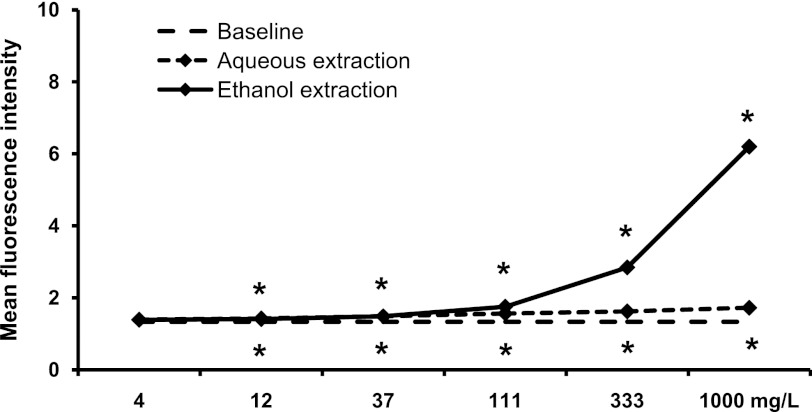
In addition, the expression level of CD69 on the monocyte population, as distinguished by its forward/side scatter properties during flow cytometry analysis, was affected by both the aqueous and ethanol extracts from SBLS (Fig. 7). CD69 is constitutively expressed on human monocytes; however, treatment with SBLS extracts led to significant increases in expression (P<.05). The ethanol extract produced a more robust, dose-dependent response than the aqueous extract.
FIG. 7.
Expression of the CD69 activation marker on monocytes. PBMC were cultured for 18 h in the absence (baseline, 11.56±0.30) or presence of either an aqueous extraction or an ethanol extraction from SBLS. The aqueous extract had a minor, but significant effect on monocytes. In contrast, the ethanol extract triggered a significant, dose-dependent increase in expression of the CD69 activation marker on monocytes. Conditions were assayed in triplicate, and the results shown are mean±SD values from a representative of three separate experiments using cells from three different donors.
Effect on cytokine/chemokine expression
The changes in cytokine levels were tested using a human 25-plex Luminex panel. Fifteen of the 25 cytokines and chemokines showed an increase in cell cultures treated with an ethanol extract of SBLS (111 mg/L). The changes in mean fluorescence intensity as well as the calculated levels (ng/mL) are shown for these cytokines in Table 3. The most robust increases were seen for IL-6, MCP-1, MIP-1α, and MIP-1β.
Table 3.
Changes in Cytokine Levels After Exposure of Peripheral Blood Mononuclear Cells to SBLS Ethanol Extract
| Target | MFI (untreated)a | MFI (SBLS)b | Fold changec | Concentration (ng/mL)d | |
|---|---|---|---|---|---|
| IL-1β | 1.00 | 9.00 | 9 | <12.07 | |
| IL-1Rα | 2.50 | 21.00 | 8 | 72.58 | |
| IL-6 | 6.50 | 1129.50 | 174 | 430.68 | |
| IL-7 | 0.75 | 4.75 | 6 | 16.73 | |
| CXCL8 | IL-8 | 660.25 | 8511.50 | 13 | >388.9 |
| CSIF | IL-10 | 0.50 | 7.50 | 15 | <30.25 |
| IL-12 | 2.50 | 11.50 | 5 | 13.08 | |
| IL-13 | 0.50 | 2.50 | 5 | <8.78 | |
| IL-15 | 1.00 | 8.50 | 9 | <11.61 | |
| CCL2 | MCP1 | 13.00 | 1890.25 | 145 | >592.51 |
| CCL3 | MIP-1α | 0.50 | 120.50 | 241 | 62.97 |
| CCL4 | MIP-1β | 2.50 | 418.75 | 168 | 127.27 |
| CCL5 | RANTES | 156.00 | 337.25 | 2 | 94.84 |
| IFN-α | 1.00 | 12.00 | 12 | 33.38 | |
| CXCL10 | IP10 | 1.00 | 5.50 | 6 | <1.62 |
No change: IL-2, IL-2R, IL-4, IL-5, IL-17, eotaxin/CCL11, GM-CSF, IFN-γ, MIG/CXCL9, TNF-α.
Cytokine levels in untreated cell cultures.
SBLS ethanol extract (111 mg/L).
Calculated from net mean fluorescence intensity (MFI) data.
Calculated from the standard curves for the Luminex array. Concentrations reflect treatment with SBLS.
SBLS, Sorghum bicolor leaf sheaths.
Discussion
The unique properties of the west African SBLS reported here go beyond a simple content of antioxidant polyphenols and water-soluble proinflammatory glucans. The complexity is illustrated by the presence of antioxidants and anti-inflammatory compounds in both the aqueous and ethanol-based extracts, as well as the presence of immune modulating compounds with selectively different biological effects in the aqueous versus ethanol-based extracts. For example, select ethanol soluble sorghum bran fractions have been demonstrated to inhibit the pro-inflammatory cytokines IL-1β and TNF-α in vitro.25 In addition, the antioxidant protection capacity of the leaf sheaths from SBLS is many-fold higher than that reported for cereal grains and vegetables (Table 2).26 At least some of the antioxidants are able to enter into and protect live cells from oxidative damage, as shown by the CAP-e bioassay. Beyond the simple antioxidant cellular bioavailability, anti-inflammatory action was seen at much lower doses than the cellular antioxidant protection measured in the CAP-e assay, indicating complex cellular signaling by both aqueous and nonaqueous compounds in SBLS. Previous work21 has elucidated the relative advantages and disadvantages of differing assays that could be used to characterize the antioxidant properties of SBLS. ORAC is a natural assay for determining the antioxidant potential of SBLS at the basic chemistry level. The cell-based antioxidant protection in an erythrocyte model (CAP-e) assay is better suited for capturing antioxidant protection of SBLS within a cellular environment, but without eliciting complex signaling and inflammatory pathways. The ROS formation in PMN cells assay may be better suited to studying such signaling and inflammatory pathways.27,28 Antioxidant assays that employ malignant or transformed cell lines (e.g., hepatocarcinoma) may not serve as a good model for such an evaluation due to the complex nature of genomic instability present in such cell lines. Data generated in assays using cells of such complexity and dysregulation does not allow for conclusive interpretation pertaining to a test product's antioxidant capacity, as cellular signaling and life/death decisions are additional factors affecting the total free radical levels.
The data presented here show three different anti-inflammatory activities: Reduced production of free radicals by PMN cells, reduced migratory responsiveness toward the inflammatory chemoattractant LTB4 and induction of several anti-inflammatory cytokines. The previously published proinflammatory effects of water-soluble beta-glucans in sorghum,14 are therefore only one part of the overall biological effects, and we suggest that other compounds are responsible for the anti-inflammatory effect reported here. Antiviral effects (traditional medicine) linked to the use of hot teas made from sorghum, may be explained in part by the NK cell–activating properties associated with the aqueous extract.29,30
The CD56 cell surface molecule is also called the neural cell adhesion molecule, and is expressed on almost all circulating NK cells in the blood, and only on a minor subset of CD3+ T cells. The CD3+ CD56+ T cell phenotype is associated with increased MHC-unrestricted antitumor activity, and the adoptive transfer of such cells as part of immunotherapy for treatment of solid tumors is currently subject for discussion.31 The cell type has also been reported to play a role in recognition of bacterial super-antigens and attenuated vaccines.32 The activation of human CD3+ CD56+ T cells by the SBLS ethanol extract suggests that SBLS contains compounds capable of supporting immune defense reactions toward transformed cells, as well as possibly support certain types of vaccine responses. Since this was tested on PBMC cultures, we cannot conclude whether this was a direct effect on T cells, or whether SBLS acted on, for example, monocytes, and that the activation was cytokine mediated.
Human monocytes constitutively express CD69 cell surface molecules. The present study indicates that SBLS upregulates CD69 expression. Monocytes differentiate and become active through several known pathways, depending on the environmental signals present. For example, it has been shown that exposure to certain cytokines can shift differentiation of monocytes from dendritic cells to activated macrophages.33 Similarly, it may be possible that elevated CD69 expression may influence differentiation and activation of monocytes. Additional data also support this hypothesis.34 Further research is warranted to investigate the relationship between CD69 expression and monocyte activation.
Several cytokines showed increased expression in this assay, including some that showed a multifold increase in expression. Particularly noteworthy was the increase in RANTES/CCL5, MIP-1α/CCL3, and MIP-1β/CCL4, since these chemokines have previously been implicated in suppression of HIV activity.35 Other effects included many-fold increases in both pro- and anti-inflammatory cytokines. The increased secretion of the proinflammatory cytokine IL-6 was one of the most robust changes with a 174-fold increase compared to untreated cells. Other affected proinflammatory cytokines, included IL-1β and IL-8. The anti-inflammatory cytokines, IL-10 and IL-13, were upregulated 5–15-fold in culture supernatants treated with the SBLS ethanol extract. Interferon-alpha (IFN-α) production was increased 12-fold, and since this cytokine has a dual action as both a pro- and anti-inflammatory regulating factor, this suggests that a sequence of events orchestrate the immune actions in response to SBLS.
Thus, both the cellular activation events seen among multiple cell types and the secreted cytokines point to a complex effect of SBLS. Our current hypothesis suggests that SBLS contains compounds that directly activate monocytes, both to express CD69 and to secrete a complex profile of cytokines that, in turn, help activate T cells. Further work is needed to differentiate which cytokines are contributed directly from an effect of SBLS onto monocytes, NK cells, and T cells, and which effects require a concerted interaction between these cell types. The complex and highly selective effects of water-based and ethanol-based extracts suggests multiple, potentially synergistic, active compounds. The work presented here reflects in vitro testing on human cell subsets, and therefore cannot be directly translated to conclude whether ingestion of SBLS may trigger similar immune modulating effects in vivo. The data suggest that there may be value in evaluating anti-inflammatory and immune modulating effects in clinical studies, which may also help explain the traditional use of sorghum-based products and extracts thereof in traditional medicine across the world. Given the dichotomy of inflammation as a two-edged sword, and the complex action of SBLS on cytokine profiles, it is possible that consumption of SBLS may support pro-inflammatory acute immune defense reactions, while also assisting the resolution of an inflammatory insult. Such complexity needs to be addressed in clinical studies in vivo, using specific models to address acute immune responses to a controlled challenge, versus chronic viral infections and inflammatory conditions.
Acknowledgments
The study was performed at NIS Labs, an independent contract research laboratory specializing in natural products research, and sponsored by Health Forever Products Inc., Lagos, Nigeria.
Author Disclosure Statement
G.S.J., K.F.B., and J.L.B. are employees of NIS Labs, an independent contract lab. B.O. is an employee at Dover Sciences. A.O. and O.O. are employees of Health Forever Products Inc.
References
- 1.Kanterman J. Sade-Feldman M. Baniyash M. New insights into chronic inflammation-induced immunosuppression. Semin Cancer Biol. 2012;22:307–318. doi: 10.1016/j.semcancer.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Elenkov IJ. Iezzoni DG. Daly A. Harris AG. Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12:255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- 3.Elenkov IJ. Neurohormonal-cytokine interactions: Implications for inflammation, common human diseases and well-being. Neurochem Intl. 2008;52:40–51. doi: 10.1016/j.neuint.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 4.de Heredia FP. Gómez-Martínez S. Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71:332–338. doi: 10.1017/S0029665112000092. [DOI] [PubMed] [Google Scholar]
- 5.Dall'Asta M. Derlindati E. Ardigò D. Zavaroni I. Brighenti F. Del Rio DM. Macrophage polarization: the answer to the diet/inflammation conundrum? Nutr Metab Cardiovasc Dis. 2012;22:387–392. doi: 10.1016/j.numecd.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 6.Dykes L. Rooney LW. Sorghum and millet phenols and antioxidants. Review. J Cereal Sci. 2006;44:236–251. [Google Scholar]
- 7.Devi PS. Kumar MS. Das SM. Evaluation of antiproliferative activity of red sorghum bran anthocyanin on a human breast cancer cell line (mcf-7) Int J Breast Cancer. 2011;2011:891481. doi: 10.4061/2011/891481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bröhan M. Jerkovic V. Collin S. Potentiality of red sorghum for producing stilbenoid-enriched beers with high antioxidant activity. J Agric Food Chem. 2011;59:4088–4094. doi: 10.1021/jf1047755. [DOI] [PubMed] [Google Scholar]
- 9.Mueller-Harvey I. Reed JD. Identification of phenolic compounds and their relationships to in-vitro digestibility of sorghum leaves from bird-resistant and non-bird-resistant varieties. J Sci Food Agric. 1992;60:179–196. [Google Scholar]
- 10.Kayode APP. Nout MJR. Linnemann AR. Joseph D. Hounhouigan JD. Berghofer E. Siebenhandl-Ehn S. Uncommonly high levels of 3-deoxyanthocyanidins and antioxidant capacity in the leaf sheaths of dye sorghum. J Agric Food Chem. 2011;59:1178–1184. doi: 10.1021/jf103963t. [DOI] [PubMed] [Google Scholar]
- 11.Geera B. Ojwang LO. Awika JM. New highly stable dimeric 3-deoxyanthocyanidin pigments from Sorghum bicolor leaf sheath. J Food Sci. 2012;77:C566–C572. doi: 10.1111/j.1750-3841.2012.02668.x. [DOI] [PubMed] [Google Scholar]
- 12.Yang L. Browning JD. Awika JM. Sorghum 3-deoxyanthocyanins possess strong phase II enzyme inducer activity and cancer cell growth inhibition properties. J Agric Food Chem. 2009;57:1797–1804. doi: 10.1021/jf8035066. [DOI] [PubMed] [Google Scholar]
- 13.Shih C-H. Siu S-O. Ng R. Wong E. Chiu LCM. Chu IK. Lo C. Quantitative analysis of anticancer 3-deoxyanthocyanidins in infected sorghum seedlings. J Agric Food Chem. 2007;55:254–259. doi: 10.1021/jf062516t. [DOI] [PubMed] [Google Scholar]
- 14.Ramesh HP. Tharanathan RN. Non-cellulosic mixed linkage beta-D-glucan in sorghum, Sorghum bicolor (L.) Moench—localization and biological activity studies. Indian J Exp Biol. 2000;38:155–159. [PubMed] [Google Scholar]
- 15.Camargo Filho I. Cortez DA. Ueda-Nakamura T. Nakamura CV. Dias Filho BP. Antiviral activity and mode of action of a peptide isolated from Sorghum bicolor. Phytomedicine. 2008;15:202–208. doi: 10.1016/j.phymed.2007.07.059. [DOI] [PubMed] [Google Scholar]
- 16.Erah Patrick O. AsonyeCanice C. Okhamaf Augustine O. Response of Trypanosoma brucei brucei–induced anaemia to a commercial herbal preparation. Afr J Biotechnol. 2003;2:307–311. [Google Scholar]
- 17.Oladiji AT. Jacob TO. Yakubu MT. Anti-anaemic potentials of aqueous extract of Sorghum bicolor (L.) Moench stem bark in rats. J Ethnopharmacol. 2007;111:651–656. doi: 10.1016/j.jep.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 18.Akande IS. Oseni AA. Biobaku OA. Effects of aqueous extract of Sorghum bicolor on hepatic, histological and haematological indices in rats. J Cell Animal Biol. 2010;4:137–142. [Google Scholar]
- 19.Ogwumike OO. Hemopoietic effect of aqueous extract of the leaf sheath of Sorghum bicolor in albino rats. Afr J Biomed Res. 2002;5:69–71. [Google Scholar]
- 20.Nwinyi FC. Kwanashie HO. Evaluation of aqueous methanolic extract of Sorghum bicolor leaf base for antinociceptive and anti-inflammatory activities. Afr J Biotechnol. 2009;8:4642–4649. [Google Scholar]
- 21.Honzel D. Carter SG. Redman KA. Schauss AG. Endres JR. Jensen GS. Comparison of chemical and cell-based antioxidant methods for evaluation of foods and natural products: Generating multifaceted data by parallel testing using erythrocytes and polymorphonuclear cells. J Agric Food Chem. 2008;56:8319–8325. doi: 10.1021/jf800401d. [DOI] [PubMed] [Google Scholar]
- 22.Jensen GS. Patterson KM. Yoon I. Yeast culture has anti-inflammatory effects and specifically activates NK cells. Comp Immunol Microbiol Infect Dis. 2008;31:487–500. doi: 10.1016/j.cimid.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 23.Jensen GS. Benson KF. Carter SG. Endres JR. GanedenBC30 cell wall and metabolites: anti-inflammatory and immune modulating effects in vitro. BMC Immunol. 2010;24:11. doi: 10.1186/1471-2172-11-15. 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Clausen J. Vergeiner B. Enk M. Petzer AL. Gastl G. Gunsilius E. Functional significance of the activation-associated receptors CD25 and CD69 on human NK-cells and NK-like T-cells. Immunobiology. 2003;207:85–93. doi: 10.1078/0171-2985-00219. [DOI] [PubMed] [Google Scholar]
- 25.Burdette A. Garner PL. Mayer EP. Hargrove JL. Hartle DK. Greenspan P. Anti-inflammatory activity of select sorghum (Sorghum bicolor) brans. J Med Food. 2010;13:879–887. doi: 10.1089/jmf.2009.0147. [DOI] [PubMed] [Google Scholar]
- 26.USDA Database for the Oxygen Radical Absorbance Capacity (ORAC) of Selected Foods. U.S. Department of Agriculture; 2010. Release 2. [Google Scholar]
- 27.Kang J. Li Z. Wu T. Jensen GS. Schauss AG. Wu X. Antioxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.) Food Chem. 2010;122:610–617. [Google Scholar]
- 28.Kang J. Thakali KM. Xie C. Kondo M. Tong Y. Ou B. Jensen GS. Medina MB. Schauss AG. Wu X. Bioactivities of açaí (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012;133:671–677. [Google Scholar]
- 29.Park JH. Darvin P. Lim EJ. Joung YH. Hong DY. Park EU. Park SH. Choi SK. Moon ES. Cho BW. Park KD. Lee HK. Kim MJ. Park DS. Chung IM. Yang YM. Hwanggeumchal sorghum induces cell cycle arrest, and suppresses tumor growth and metastasis through jak2/stat pathways in breast cancer xenografts. PLoS One. 2012;7:e40531. doi: 10.1371/journal.pone.0040531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu L. Huang Z. Qin P. Yao Y. Meng X. Zou J. Zhu K. Ren G. Chemical characterization of a procyanidin-rich extract from sorghum bran and its effect on oxidative stress and tumor inhibition in vivo. J Agric Food Chem. 2011;59:8609–8615. doi: 10.1021/jf2015528. [DOI] [PubMed] [Google Scholar]
- 31.Mesiano G. Todorovic M. Gammaitoni L. Leuci V. Giraudo Diego L. Carnevale-Schianca F. Fagioli F. Piacibello W. Aglietta M. Sangiolo D. Cytokine-induced killer (CIK) cells as feasible and effective adoptive immunotherapy for the treatment of solid tumors. Expert Opin Biol Ther. 2012;12:673–684. doi: 10.1517/14712598.2012.675323. [DOI] [PubMed] [Google Scholar]
- 32.Saikh KU. Dyas B. Kissner T. Ulrich RG. CD56+-T-cell responses to bacterial superantigens and immune recognition of attenuated vaccines. Clin Diagn Lab Immunol. 2003;10:1065–1073. doi: 10.1128/CDLI.10.6.1065-1073.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delneste Y. Charbonnier P. Herbault N. Magistrelli G. Caron G. Bonnefoy JY. Jeannin P. Interferon-gamma switches monocyte differentiation from dendritic cells to macrophages. Blood. 2003;101:143–150. doi: 10.1182/blood-2002-04-1164. [DOI] [PubMed] [Google Scholar]
- 34.De Maria R. Cifone MG. Trotta R. Rippo MR. Festuccia C. Santoni A. Testi R. Triggering of human monocyte activation through CD69, a member of the natural killer cell gene complex family of signal transducing receptors. J Exp Med. 1994;180:1999–2004. doi: 10.1084/jem.180.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cocchi F. DeVico AL. Garzino-Demo A. Arya SK. Gallo RC. Lusso P. Identification of RANTES, MIP-1 alpha, and MIP-1 beta as the major HIV-suppressive factors produced by CD8+ T cells. Science. 1995;270:1811–1815. doi: 10.1126/science.270.5243.1811. [DOI] [PubMed] [Google Scholar]