Abstract
Background:
Older adults who sustain hip fractures usually have multiple coexisting medical problems that may impact their treatment and outcomes. The geriatric fracture center (GFC) provides a model of care that standardizes treatment and optimizes outcomes. The purpose of this study is to determine whether GFC patients with a higher burden of comorbidity or specific comorbidities are at risk for worsened perioperative outcomes, such as increased time to surgery (TTS), postoperative complications, and longer length of hospital stay (LOS).
Method:
A total of 1077 patients aged 60 years and older who underwent surgery for a proximal femur fracture between April 15, 2005, and September 30, 2010, were evaluated. Comorbidities measured in the Charlson Comorbidity index were abstracted through chart review. Outcomes were TTS, postoperative complications, and LOS.
Results:
Most patients were white, with an average age of 85. One half lived in either a nursing home or an assisted living facility. The mean Charlson score was 3.06 and the nursing home residents had a significantly higher score compared to community dwellers (3.4 vs 2.8; P < .0001). Dementia was the most common comorbidity. There was no difference in the LOS or TTS based on Charlson score. The overall complication rate was 44% with delirium being the most common postoperative complication. Peripheral vascular disease, history of solid tumor, and peptic ulcer disease predicted delirium incidence. Charlson score predicted complication risk, with an odds ratio of 1.12 for each point increase.
Conclusion:
Frailty and comorbidity put this hip fracture population at high risk for adverse perioperative outcomes. This study shows that in the GFC model of care the comorbidity burden did not impact the TTS and LOS but did predict postoperative complication rate.
Keywords: hip fractures, dementia, comorbidity, model of care, perioperative outcomes
Osteoporosis and hip fractures are major public health concerns that are expected to become worse with the aging of the US population.1 More than 90% of hip fracture patients are older than 65 years and the fracture usually occurs in patients who have preexisting medical problems or comorbidities.2,3 It has long been recognized that comorbidity influences treatment and prognosis of an index condition.4,5
Mortality has been the most widely studied hip fracture outcome. Chronic obstructive pulmonary disease, heart failure, dementia, and malignancy have all been shown to be risk factors for increased mortality in the months following a hip fracture.6–8 Determining whether these conditions predict short-term perioperative outcomes such as time to surgery (TTS), postoperative complications, and length of hospital stay (LOS) in hip fractures may suggest a potential mechanism for the increased risk of poor outcomes following hospitalization. Delays in surgery have been associated with higher risk for complications,9 increased LOS,10 and increases in mortality in hip fracture patients.11 Postoperative complications, in turn, increase both short- and long-term mortality.8
The Geriatric Fracture Center (GFC) is a geriatric and orthopedic comanaged model of care that standardizes the care of hip fracture patients.12 The model has been reported to offer a reduced complication rate, TTS, and LOS. It is unclear whether patients in this model who have a higher burden of comorbidity or specific comorbidities are at higher risk for worsened perioperative outcomes, such as increased TTS, postoperative complications, and longer LOS. This study examines the relationship between comorbidity and these perioperative outcomes in a GFC model of care.
Methods
Description of the Model of Care
Patients in this study had surgical repair of a hip fracture at the GFC that operates in a 261-bed community-based teaching hospital. In the GFC care model, each patient is comanaged by orthopedic surgeons and geriatricians working together with shared responsibility for each patient. Patient risk is assessed preoperatively and documented as low, medium, high, or very high risk for adverse perioperative events. Medical comorbidities are identified and patients are optimized for surgery. Both the geriatrics and orthopedics teams see the patients daily throughout hospitalization. Standardized order sets are used at each stage of care and match the nursing care plan. In this program, 64% of patients have surgical repair within the first 24 hours and 95% within the first 48 hours of admission.12 The goal of surgery is stable fixation that allows for weight bearing as tolerated on the first postoperative day. Most patients are discharged on the third hospital day to a skilled nursing facility for rehabilitation.
Study Design
This is a retrospective cohort study. A dedicated research nurse, as part of a quality management program, collects baseline demographic and medical data and in-hospital outcomes through retrospective chart review. Interrater reliability testing is used to verify and maintain data integrity. The study was reviewed and approved by the University Research Subjects Review Board.
Patient Population
A total of 1077 patients 60 years of age or older, who were surgically treated for a proximal femur fracture between April 15, 2005, and September 30, 2010, were included in the study for analysis. Patients with a pathological fracture, a high-energy trauma, or a periprosthetic fracture were excluded.
Variables
Baseline variables obtained for all patients include age, race, sex, residence prior to admission, and preexisting comorbidities as defined by the Charlson protocol. The Charlson Comorbidity index (CCI) is a widely used and validated score that has been shown to predict 1 year and inpatient mortality in hospitalized patients,13,14 as well as the functional status of patients 1 year after cardiac surgery15 and survival of critically ill patients.16 A CCI score is calculated for each patient based on the presence and severity of the diseases that are included in the index.5
Outcome variables were TTS, postoperative complications, and LOS. Time to surgery was defined as the time from admission registration to the time the patient entered the operating room. Length of hospital stay was defined as the number of days in which the patient was in the hospital based on the midnight census method. In-hospital complications other than mortality have been previously described in more detail and include delirium, renal insufficiency, hypoxia, pneumonia, new congestive heart failure, myocardial infarction, cerebrovascular disease, deep vein thrombosis, pulmonary embolism, another fracture, implant dislocation, periprosthetic fracture, fracture fixation failure, reoperation, surgical site infection, urinary tract infection, new arrhythmia, hemorrhagic cerebrovascular accident, intracranial bleed, gastrointestinal bleed, and retroperitoneal bleed.12
Statistical Analyses
Statistical analyses were conducted using SPSS software for Windows. This study assessed the impact of the individual Charlson score as well as comorbid diseases on in-hospital perioperative outcomes in 1,077 patients who were comanaged by orthopedic surgeons and geriatric hospitalists in a GFC. Descriptive statistics characterized the overall CCI according to patient characteristic. The prevalence of individual comorbidities was tallied.
The association of baseline characteristics with TTS and with LOS was calculated via Student t test and analysis of variance (ANOVA) where appropriate. The relationship between the overall CCI and the outcomes of TTS and LOS was determined using linear regression.
Unadjusted and adjusted risk of experiencing any complication according to CCI was determined by logistic regression. The risk of developing specific complications was determined according to the prevalence of specific comorbidities at baseline. We used an a priori cutoff of outcomes with an incidence of 10% or more so as (a) to have enough power to look at independent predictors and (b) to identify risk factors for common complications. Delirium and renal insufficiency were the only 2 complications with an incidence of 10% or more. Comorbidities with prevalence over 10% were evaluated as predictors for these outcomes. Odds ratios (ORs) for each of the comorbidities were calculated using logistic regressions.
Results
Demographics
Ninety-six percent of patients were white and 76.9% were female, with an average age of 85 years (standard deviation [SD] 8.4). Almost one-half (48.9%) resided in the community prior to admission, 36% were admitted from nursing homes, and 14% were admitted from assisted living facilities. The mean CCI was 3.06 (SD 2.1). The score did not differ between the different age groups or between white patients versus other races. Men had a higher Charlson score that trended toward significance (3.3 vs 3.0, P = .09). Nursing home patients had a significantly higher Charlson score compared to community dwellers (3.4 vs 2.8, P < .001; Table 1).
Table 1.
Demographic Data and Charlson Comorbidity Index (CCI) of Patients Admitted to the Geriatric Fracture Center.a
| Characteristic | Distribution | CCI, mean (SD) | P Valueb |
|---|---|---|---|
| Age, mean (SD) | 85.2 (8.4) | 3.06 (2.1) | |
| Age group categories | |||
| <80, n (%) | 256 (23.8) | 3.00 (2.2) | |
| 80-84, n (%) | 198 (18.9) | 3.19 (2.2) | |
| 85-89, n (%) | 288 (26.7) | 3.23 (2.2) | |
| >89, n (%) | 335 (31.1) | 2.95 (2.1) | .31 |
| Gender | |||
| Male, n (%) | 249 (23.1) | 3.28 (2.3) | |
| Female, n (%) | 828 (76.9) | 3.00 (2.1) | .09 |
| Race | |||
| White, n (%) | 1030 (95.6) | 3.08 (2.1) | |
| Other, n (%) | 47 (0.04) | 2.74 (2.4) | .15 |
| Residence prior to admission | |||
| Community dwelling, n (%) | 547 (48.9) | 2.82 (2.1) | |
| Nursing home, n (%) | 384 (35.7) | 3.40 (2.2) | |
| Assisted living, n (%) | 146 (13.6) | 3.09 (1.9) | .0001 |
Abbreviations: ANOVA, analysis of variance; SD, standard deviation.
a N = 1077.
b Calculated using Student t test for dichotomous variables and ANOVA for more than 2 variables.
Charlson Score and Individual Comorbidities
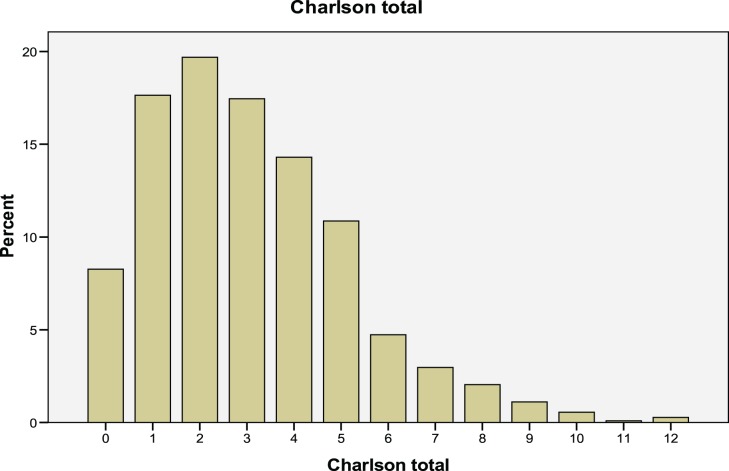
The frequencies of preexisting comorbidities are shown in Table 2. Dementia was the most common comorbid condition affecting 47.8% of the population. Other comorbid diseases affecting more than 20% of the population were peptic ulcer (35%), peripheral vascular disease (30%), any solid tumor (22%), congestive heart failure (21%), pulmonary disease (21%), and diabetes (20.3%). More than 10% of the population had a history of cerebrovascular disease (15%) and myocardial infarctions (13%). The distribution of Charlson scores are shown in Figure 1; 25.9% had a score of 0 or 1; 19.7% had a score of 2; 31.8% had a score of 3 or 4; and 22.7% had a score of 5 or more.
Table 2.
Time to Surgery (TTS) and Length of Stay (LOS) According to Demographic Data and Comorbidities of the Patients Admitted to the Geriatric Fracture Center.a
| Characteristic | Distribution N (%) | TTS (hours), mean (SD) | P Valueb | LOS (days), mean (SD) | P Valueb |
|---|---|---|---|---|---|
| Overall, mean (SD) | 21.35 (13.7) | 4.19 (1.9) | |||
| Age group categories | |||||
| <80 | 256 (23.8) | 22.9 (13.9) | 4.4 (1.9) | ||
| 80-85 | 198 (18.4) | 20.6 (14.5) | 4.2 (1.9) | ||
| 85-90 | 288 (26.7) | 21.1 (13.2) | 4.1 (1.5) | ||
| >90 | 335 (31.1) | 20.5 (13.3) | .16 | 4.2 (2.1) | .35 |
| Gender | |||||
| Male | 249 (23.1) | 23.2 (16.1) | 4.5 (2.2) | ||
| Female | 828 (76.9) | 20.6 (12.8) | .53 | 4.1 (1.7) | .53 |
| Race | |||||
| White | 1030 (95.6) | 21.1 (13.5) | 4.2 (1.8) | ||
| Other | 47 (4.4) | 24.2 (13.7) | .38 | 4.4 (2.7) | .39 |
| Residence prior to admission | |||||
| Community dwelling | 547 (50.7) | 21.4 (13.9) | 4.3 (1.9) | ||
| Nursing home | 384 (35.7) | 21.1 (12.4) | 3.9 (1.7) | ||
| Assisted living | 146 (13.6) | 21.4 (15.7) | .94 | 4.3 (2.1) | .003 |
| Comorbidities | |||||
| Dementia | 515 (47.8) | 21.1 (14.4) | .95 | 4.2 (1.8) | .95 |
| Ulcer disease | 377(35.0) | 21.1 (14.4) | .70 | 4.1 (1.8) | .70 |
| Peripheral vascular disease | 324 (30.1) | 19.9 (11.4) | .62 | 4.1 (1.7) | .63 |
| Any solid tumor | 236 (21.9) | 19.9 (12.7) | .52 | 4.0 (1.8) | .52 |
| Congestive heart failure | 231 (21.4) | 20.8 (11.8) | .51 | 4.2 (1.6) | .52 |
| Pulmonary disease | 231 (21.4) | 22.1 (15.1) | .51 | 4.4 (2.3) | .52 |
| Diabetes (incl with end organ damage) | 219 (20.3) | 20.6 (11.9) | .50 | 4.2 (1.9) | .51 |
| Cerebrovascular disease | 157 (14.6) | 22.8 (15.6) | .45 | 4.2 (2.1) | .46 |
| Myocardial infarction | 137 (12.7) | 21.4 (13.0) | .44 | 4.3 (2.2) | .44 |
| Moderate to severe renal disease | 98 (9.1) | 22.3 (14.9) | .43 | 4.5 (2.5) | .42 |
| Connective tissue disease | 44 (4.1) | 22.9 (9.9) | .38 | 4.1 (1.4) | .39 |
| Hemiplegia | 37 (3.4) | 21.1 (10.2) | .38 | 4.7 (3.6) | .39 |
| Metastatic solid tumor | 30 (2.8) | 19.3 (9.5) | .37 | 3.9 (1.3) | .38 |
| Leukemia | 12 (1.1) | 18.1 (6.6) | .38 | 4.1 (1.6) | .38 |
| Lymphoma | 12 (1.1) | 18.1 (6.6) | .38 | 4.1 (1.6) | .38 |
| Mild liver disease | 10 (0.9) | 18.0 (5.4) | .37 | 4.5 (4.1) | .38 |
| Moderate to severe liver diseasec | 2 (0.2) | ||||
| AIDSc | 0 (0) | ||||
Abbreviations: ANOVA, analysis of variance; SD, standard deviation.
a N = 1077.
b Calculated using Student t test for dichotomous variables and ANOVA for more than 2 variables.
c Unable to calculate due to low prevalence.
Figure 1.
Frequency distribution of the Charlson Comorbidity index.
Perioperative Outcomes
The mean TTS was 21.35 hours (SD 13.7) and mean LOS was 4.19 days (SD 1.9). There was no difference in the TTS and LOS among the different age groups. There was also no difference in the TTS among community dwellers, nursing home, or assisted living residents. Nursing home residents had a shorter LOS compared to community dwellers (3.9 vs 4.3, P = .003). This was likely a result of simpler discharge planning for nursing home residents who are discharged to their previous facility. The most common postoperative complications were delirium (32%) and renal insufficiency (16%). Other less frequent postoperative complications included hypoxia (9.1%), postoperative pneumonia (2.8%), and either new or exacerbation of congestive heart failure (2.0%). In-hospital mortality was 2.5%.
Charlson Comorbidity Score and Individual Comorbidities as a Predictor of Perioperative Outcomes
The overall complication rate was 44%. There was no significant linear relationship between patients’ total CCI and their TTS (r = .041) or LOS (r = .011). However, the CCI did predict postoperative complication rate with an unadjusted OR of 1.12 (95% confidence interval [CI] 1.06 -1.19) and an OR of 1.12 (95% CI 1.06-1.19) per additional point, after adjusting for age, gender, and place of residence. Thirty-four percent of patients in the lowest quartile of the Charlson score (CCI 0-1) had at least 1 complication compared to 52% of patients in the highest quartile (CCI >5). The proportions were 46% and 48%, respectively, in the second quartile (CCI = 2) and the third quartile (CCI of 3-4).
The associations between comorbid diseases and the 2 most common postoperative complications are listed in Table 3. Peptic ulcer disease, peripheral vascular disease, and any tumor were the only comorbid illnesses that were significantly associated with delirium. Peptic ulcer disease and any solid tumor were significantly associated with renal insufficiency. There was a trend toward significance for cerebrovascular disease to be associated with both delirium and renal insufficiency.
Table 3.
| Delirium (n = 347) | Renal Insufficiency (n = 174) | |
|---|---|---|
| Peripheral vascular disease (n = 324) | 1.57 (1.19-2.06) | 1.14 (0.81-1.63) |
| Any solid tumor (n = 236) | 1.46 (1.08-1.98) | 1.69 (1.17-2.44) |
| Cerebrovascular disease (n = 157) | 1.37 (0.96-2.0) | 1.52 (0.99-2.33) |
| Peptic ulcer disease (n = 377) | 1.32 (1.01-1.72) | 1.41 (1.01-1.96) |
| Dementia (n = 515) | 1.20 (0.92-1.55) | 1.2 (0.89-1.72) |
| Congestive heart failure (n = 231) | 1.19 (0.87-1.62) | 1.09 (0.74-1.61) |
| Pulmonary disease (n = 231) | 1.09 (0.80-1.49) | 1.01 (0.68-1.50) |
| Diabetes and diabetes with end organ damage (n = 219) | 1.05 (0.76-1.44) | 0.95 (0.63-1.43) |
| Myocardial infarction (n = 137) | 1.01 (0.73-1.58) | 1.14 (0.70-1.84) |
Abbreviations: ANOVA, analysis of variance; CI, confidence interval; OR, odds ratio; SD, standard deviation.
a OR (95% CI).
b Only comorbidities with a frequency of greater than 10% and postoperative complications with a frequency of greater than 10% were included in the analyses.
Discussion
The population described in this study are elderly individuals with a high burden of comorbidity, a high prevalence of dementia, and one-half residing in nursing homes and assisted living facilities. The mean patient age in this study is the oldest among the 21 studies reported in a literature review comparing different models of hip fracture care.17 Thus, it is not surprising that the population has a high burden of comorbid illness with a mean CCI of 3.04. This score has been associated with a 1 year mortality rate of 19% in this model of care.18 Nursing home patients had a significantly higher score, and men had a trend toward a higher score when compared to women (P = .09). Nursing home patients are in general sicker than community dwellers and the higher burden of comorbidities in men compared to women has been previously described.19
Two published reports of the model of care in this study have shown lower TTS, LOS, and complication rate when compared to usual or standard care.12,20 This study shows that the comorbidity burden of the patients did not affect either their TTS or their LOS. It is likely that the GFC model mitigates the effects of chronic comorbidity by expediting TTS and using standardized practices that decrease the LOS.
The Charlson score predicted a higher complication rate; for each point increase in the Charlson score there was a 12% greater likelihood of having a complication. In addition, the proportion with at least 1 complication increased significantly among patients above the lowest quartile of comorbidity; approximately one-third of patients with a Charlson score less than 2 experienced a postoperative complication, whereas half of the patients with a score of 2 or more had a complication.
With regard to specific comorbid diseases, we had expected that patients with dementia would be more likely to develop delirium, as has been shown among hip fracture patients in usual care,21–23 but this was not the case. This may be reflective of the standardized protocol-driven care to reduce the risk of delirium, including minimizing tethers and medications associated with causing delirium, and treating pain more aggressively. It should also be noted that, since this is a retrospective study, ascertainment rates are based on chart review and may have underestimated subtle changes that occurred.
In fact, only peptic ulcer disease, peripheral vascular disease and solid tumors were significantly associated with delirium. In addition, both peptic ulcer disease and solid tumors predicted renal insufficiency. There was a trend toward significance for cerebrovascular disease to predict both delirium and renal insufficiency. Since the operational definition of peptic ulcer disease includes the use of proton pump inhibitors,5 this diagnosis may be serving as a surrogate for previous hospitalizations, frequent interaction with the health care system overall, or polypharmacy. None of these was measured directly, but each could potentially increase the risk of development of delirium. Peripheral vascular disease and cerebrovascular disease have been shown to be predictors of delirium in nonhip fracture postoperative patients.24 It has been proposed that both these comorbidities are associated with atherosclerotic burden and, as such, may increase the risk of cerebral embolization during surgery.24 A possible mechanism for the association of solid tumors with delirium and renal insufficiency is suggested by a multivariate analysis in patients undergoing colorectal cancer surgery which showed that hypotension and low albumin were associated with postoperative delirium.25 Both of these conditions may result in postoperative renal insufficiency due to kidney hypoperfusion. Further investigation of these comorbidities as risk factors for complications should be done prospectively to confirm these findings.
Patients in this study were treated using the GFC model of care in a community hospital. In this model, orthopedic surgeons and geriatricians manage the patient together from admission until discharge and standardized treatment plans are followed. In this GFC model, the burden of comorbid illness did not affect either the TTS or the LOS. Patients with a greater burden of chronic disease were more likely to have at least 1 postoperative complication, even in this optimized approach to care.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The authors received support from NIA grant T32 020493-05, the AO Research Foundation, and Highland Hospital in the preparation of this manuscript. None of these sources of support had any role in the development of this article.
References
- 1. Dennison E, Mohamed MA, Cooper C. Epidemiology of osteoporosis. Rheum Dis Clin North Am. 2006;32(4):617–629 [DOI] [PubMed] [Google Scholar]
- 2. Burge R, Bess DH, Daniel Solomon, John Wong, Alison King, Anna Tosteson. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–475 [DOI] [PubMed] [Google Scholar]
- 3. Zuckerman JD. Hip fracture. N Engl J Med. 1996;334(23):1519–1525 [DOI] [PubMed] [Google Scholar]
- 4. Yancik R, Ershler W, Satariano W, Hazzard W, Cohen HJ, Ferrucci L. Report of the national institute on aging task force on comorbidity. J Gerontol A Biol Sci Med Sci. 2007;62(3):275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383 [DOI] [PubMed] [Google Scholar]
- 6. de Luise C, Brimacombe M, Pedersen L, Sørensen HT. Comorbidity and mortality following hip fracture: a population-based cohort study. Aging Clin Exp Res. 2008;20(5):412–418 [DOI] [PubMed] [Google Scholar]
- 7. Penrod JD, Litke A, Hawkes WG, et al. The association of race, gender, and comorbidity with mortality and function after hip fracture. J Gerontol A Biol Sci Med Sci. 2008;63(8):867–872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roche JJ, Wenn RT, Sahota O, Moran CG. Effect of comorbidities and postoperative complications on mortality after hip fracture in elderly people: prospective observational cohort study. BMJ. 2005;331(7529):1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lefaivre KA, Macadam SA, Davidson DJ, Gandhi R, Chan H, Broekhuyse HM. Length of stay, mortality, morbidity and delay to surgery in hip fractures. J Bone Joint Surg Br. 2009;91(7):922–927 [DOI] [PubMed] [Google Scholar]
- 10. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436–1442 [DOI] [PubMed] [Google Scholar]
- 11. Mendelson D, Friedman SM, Nicholas JA. Evidenced Based Orthopedics. 1st Edition Bhandari M, ed. Chicester, West Sussex: Blackwell Publishing Ltd.; 2012:93–99 [Google Scholar]
- 12. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349–1356 [DOI] [PubMed] [Google Scholar]
- 13. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619 [DOI] [PubMed] [Google Scholar]
- 14. D'Hoore W, Sicotte C, Tilquin C. Risk adjustment in outcome assessment: the Charlson comorbidity index. Methods Inf Med. 1993;32(5):382–387 [PubMed] [Google Scholar]
- 15. Jaeger AA, Hlatky MA, Paul SM, Gortner SR. Functional capacity after cardiac surgery in elderly patients. J Am Coll Cardiol. 1994;24(1):104–108 [DOI] [PubMed] [Google Scholar]
- 16. Poses RM, McClish DK, Smith WR, Bekes C, Scott WE. Prediction of survival of critically ill patients by admission comorbidity. J Clin Epidemiol. 1996;49(7):743–747 [DOI] [PubMed] [Google Scholar]
- 17. Kammerlander C, Roth T, Friedman SM, et al. Ortho-geriatric service–a literature review comparing different models. Osteoporos Int. 2010;21(suppl 4):S637–S646 [DOI] [PubMed] [Google Scholar]
- 18. Schnell S. The 1-year mortality of patients treated in a hip fracture program for elders. Geriatric Orthopaedic Surg Rehabil. 2011;1(1):6–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sterling RS. Gender and race/ethnicity differences in hip fracture incidence, morbidity, mortality, and function. Clin Orthop Relat Res. 2011;469(7):1913–1918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Friedman SM, Mendelson DA, Bingham KW, Kates SL. Impact of a comanaged Geriatric Fracture Center on short-term hip fracture outcomes. Arch Intern Med. 2009;169(18):1712–1717 [DOI] [PubMed] [Google Scholar]
- 21. Lee HB, Mears SC, Rosenberg PB, Leoutsakos JM, Gottschalk A, Sieber FE. Predisposing factors for postoperative delirium after hip fracture repair in individuals with and without dementia. J Am Geriatr Soc. 2011;59(12):2306–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Juliebo V, Bjøro K, Krogseth M, Skovlund E, Ranhoff AH, Wyller TB. Risk factors for preoperative and postoperative delirium in elderly patients with hip fracture. J Am Geriatr Soc. 2009;57(8):1354–1361 [DOI] [PubMed] [Google Scholar]
- 23. Marcantonio ER, Flacker JM, Michaels M, Resnick NM. Delirium is independently associated with poor functional recovery after hip fracture. J Am Geriatr Soc. 2000;48(6):618–624 [DOI] [PubMed] [Google Scholar]
- 24. Bucerius J, Gummert JF, Borger MA, et al. Predictors of delirium after cardiac surgery delirium: effect of beating-heart (off-pump) surgery. J Thorac Cardiovasc Surg. 2004;127(1):57–64 [DOI] [PubMed] [Google Scholar]
- 25. Tei M, Ikeda M, Haraguchi N, et al. Risk factors for postoperative delirium in elderly patients with colorectal cancer. Surg Endosc. 2010;24(9):2135–2139 [DOI] [PubMed] [Google Scholar]