Abstract
Background
The endocannabinoid system is involved in many physiological and pathological processes. Two receptors (cannabinoid receptor type 1 (CB1) and type 2 (CB2)) are known so far. Many unwanted psychotic side effects of inhibitors of this system can be addressed to the interaction with CB1. While CB1 is one of the most abundant neuroreceptors, CB2 is expressed in the brain only at very low levels. Thus, highly potent and selective compounds for CB2 are desired. N-aryl-((hetero)aromatic)-oxadiazolyl-propionamides represent a promising class of such selective ligands for the human CB2. Here, a library of various derivatives is studied for suitable routes for labelling with 18F. Such 18F-labelled compounds can then be employed as CB2-selective radiotracers for molecular imaging studies employing positron emission tomography (PET).
Results
By varying the N-arylamide substructure, we explored the binding pocket of the human CB2 receptor and identified 9-ethyl-9H-carbazole amide as the group with optimal size. Radioligand replacement experiments revealed that the modification of the (hetero)aromatic moiety in 3-position of the 1,2,4-oxadiazoles shows only moderate impact on affinity to CB2 but high impact on selectivity towards CB2 with respect to CB1. Further, we could show by autoradiography studies that the most promising compounds bind selectively on CB2 receptors in mouse spleen tissue. Molecular docking studies based on a novel three-dimensional structural model of the human CB2 receptor in its activated form indicate that the compounds bind with the N-arylamide substructure in the binding pocket. 18F labelling at the (hetero)aromatic moiety at the opposite site of the compounds via radiochemistry was carried out.
Conclusions
The synthesized CB2-selective compounds have high affinity towards CB2 and good selectivity against CB1. The introduction of labelling groups at the (hetero)aromatic moiety shows only moderate impact on CB2 affinity, indicating the introduction of potential labelling groups at this position as a promising approach to develop CB2-selective ligands suitable for molecular imaging with PET. The high affinity for human CB2 and selectivity against human CB1 of the herein presented compounds renders them as suitable candidates for molecular imaging studies.
Keywords: Cannabinoid receptors, Molecular modelling, Autoradiography, 18F labelling, Molecular imaging, PET, Neuroimaging
Background
The role of the endocannabinoid system in specific CNS disorders is related to the regulation of the temporal dynamics of neurotransmitter release by the retrograde cannabinoid signalling network [1]. Mediated by the G protein-coupled cannabinoid receptors [2], the release of endogenous or the administration of exogenous ligands affects both the long-term synaptic plasticity as well as the short-term regulation of synaptic transmission [1,3]. Two types of specific cannabinoid receptors have been cloned so far, termed cannabinoid receptor type 1 (CB1) and type 2 (CB2) [2,4]. The existence of additional cannabinoid-binding receptors has been suggested [5,6]. In contrast to classical neurotransmitters, endocannabinoids function as retrograde synaptic messengers which are released from postsynaptic neurons, diffuse across synapses, activate CB1 on presynaptic axons and eventually suppress neurotransmitter release [7]. In addition, endocannabinoids and their receptors control the decision about survival or death of neuronal cells [8], such that the pharmacological manipulation of this system might provide either neuroprotective or pro-apoptotic effects. A therapeutic role of cannabinoids has also been suggested for mood disorders [9], traumatic brain injury [10,11] and tumour treatment [12,13]. The development of CB2-selective anticancer agents is regarded to be advantageous in light of unwanted central effects exerted by binding of those agents to CB1 [14].
CB1 is abundantly expressed in the central nervous system and has been thoroughly investigated on cellular [15] and functional levels [16,17] in the brain. In contrast to this, only few and, to some extent, ambiguous data are available regarding CB2 expression and function in the brain. Initial reports stated that CB2 is mainly expressed peripherally in the cells and organs of the immune system such as the B lymphocyte-enriched marginal zone of the spleen or the cortex of lymph nodes [4,18]. However, more recent studies have proven structural and functional CB2 expression in primed glial (as reviewed in [5]) or neural progenitor cells [19]. Moreover, CB2 mRNA was detected in mouse [20] and rat cerebellum [21]. It could be shown in immunostaining studies that CB2 protein is abundant under basal conditions in neuronal and glial processes in the cerebellum and hippocampus of mouse and rat [22-24]. As reviewed recently by Atwood and Mackie [25], a growing number of reports substantiate a neuronal expression of CB2 through immunohistochemical data. Thus, it has been described that the activation of microglia is paralleled by an increase in CB2 immunoreactivity in the injured brain [26]. For instance, significantly increased CB2 expression levels were observed in severe Alzheimer's disease [27]. This indicates that CB2 expressed in the cerebellum might be involved in the pathogenesis of various neurodegenerative disorders and therefore might represent an interesting target for the diagnostics and therapy of such diseases [14,28]. Consequently, detailed investigation of the functional changes of both receptor subtypes CB1 and CB2 is needed to achieve a better understanding of the endocannabinoid system and the effects of potential cannabinoid therapeutics in the normal and diseased human brain [29].
Despite numerous in vitro approaches to directly identify CB2 in the brain, a non-invasive and quantitative analysis of these receptors in vivo has not been reported to date, probably due to the lack of ligands applicable for molecular imaging approaches such as positron emission tomography (PET). Recently, in a microPET study in a rat model, Evens et al. [30] observed specific binding of the type 2 cannabinoid receptor PET tracer 11C]NE40. In this model, hCB2 receptors were locally overexpressed in the brain after stereotactic injection of an adeno-associated viral vector (AAV2/7) encoding hCB2R with a point mutation (Asp80Asn) in the right striatum, yet the quantitative analysis of these receptors in native brain tissue remains a challenge.
Although CB2 has been already mentioned as a valuable biomarker of, e.g. neuroinflammation [30], the quantitative imaging of this target requires PET radioligands with superior affinity and specificity towards the low-abundance cerebral CB2 [31]. The 11C]-methoxy-labelled potent inverse CB2 agonist Sch225336 [32] showed only poor brain uptake in mice under baseline conditions. Various 18F-fluoroethoxy- [33] and 11C]-methoxy-labelled [34] 2-oxoquinoline derivatives were suggested as CB2 PET radioligands too, yet unfavourable brain-to-plasma ratios reflect the need for further improvement(s) regarding CB2 imaging in the healthy brain as recently reviewed [35].
Methods
Here, we extend the synthesis of various CB2-binding [36]N-aryl-((hetero)aromatic-oxadiazolyl-propionamides as potential PET imaging compounds. Guided by molecular docking studies employing a novel comparative model of the human CB2 receptor, the promising site for 18F labelling was selected. In vitro binding experiments using human CB2-transfected Chinese hamster ovary (CHO) cells were performed. The obtained affinity data are in good agreement with predicted binding strength and could be verified by autoradiographic studies on mouse spleen slices.
Results and discussion
Synthesis of N-aryl-((hetero)aromatic)-oxadiazolyl-propionamides
The substituted 1,2,4-oxadiazolyl-propionamides synthesized in this study are compiled in Scheme 1.
Scheme 1.
N-aryl-((hetero)aromatic)-oxadiazolyl-propionamides synthesized in this study.
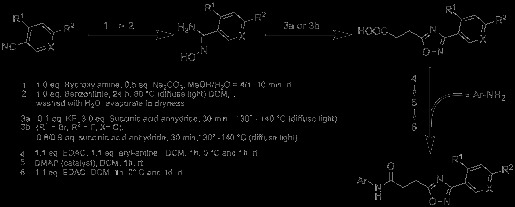
As depicted in Scheme 2, the two-step synthesis starts from a properly substituted nitrile and hydroxylamine yielding the hydroxy-amidin derivative. Following the route described by Cheng at al. [36], the products were dried after dilution with methylene chloride, and succinic acid anhydride was added to give the 3-phenyl-subsituted 1,2,4-oxadiazole acids. To support the formation of the oxadiazole acids, 0.1 eq. KF was added except for the compound with R1 = Br and R2 = F. In this case, the free 3-phenyl-substituted 1,2,4-oxadiazol-5-yl propanoic acid was protected, employing (diazomethyl)trimethylsilane. Subsequently, Br was substituted with CN by addition of 4.2 eq. KCN. Deprotection with LiOH yielded then the product. In a final step, the so obtained differently substituted 3-(hetero) aromatic-1,2,4-oxadiazol-5-yl-propanoic acids were coupled to various arylamides using standard (dimethylamino)-pyridine (DMAP)-catalysed carbodiimide chemistry. The obtained products were isolated either by semi-preparative high-performance liquid chromatography (HPLC) or crystallization (details can be found in the ‘Experimental’ section).
Scheme 2.
Synthesis of N-aryl-((hetero)aromatic)-oxadiazolyl-propionamides.
To suggest potential radiolabelling schemes of the compounds suitable for molecular imaging studies by PET, several strategies were evaluated, which are given in Scheme 3. For instance, 5e was synthesized by methylation of 4e with methyl iodide. This approach opens the way to introduce, for example, 11C or 18F by N-alkylation into the final compound. A further approach is the use of prosthetic groups, such as the introduction of fluorine-substituted alkyl chains via hydroxyl groups. In this study, 6k was synthesized from 6j by adding 1-fluoro-2-iodoethane. 18F can then be introduced into the molecule by using 18F-labelled alkylating agents. Another approach for the synthesis of 18F-labelled compounds is the replacement of fluorine with 18F via [2.2.2]cryptand chemistry, which is shown in this study as an example in the ‘Radiochemistry’ section.
Scheme 3.
Two strategies to introduce fluorine into the molecule, allowing labelling with18F.
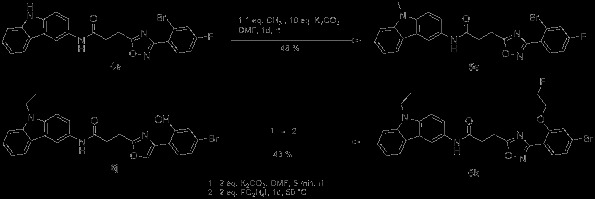
Figure 1 shows the single-crystal X-ray structure of compound 6f. As expected, the amide bond of this molecule (C5-N3 in Figure 1) is in trans configuration. Though several bonds in 6f are freely rotatable, the structure is planar. Thus, the dihedrals of the planes defined by the ring systems are 8.16(9)° between rings A and B and 11.2(1)° between rings B and C.
Figure 1.
X-ray crystal structure of 6f (50% thermal ellipsoids). The planar ring systems have been noted by A, B and C and are highlighted in grey.
In vitro binding studies
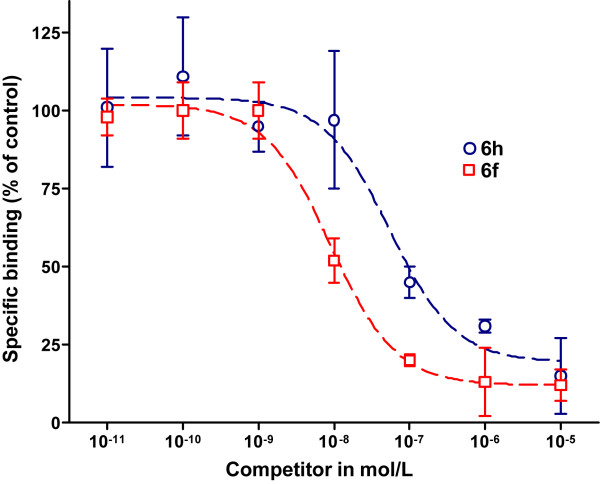
The specific binding of 3H]CP55,940 towards cell membranes obtained from CHO cells stably transfected with human CB1 (hCB1-CHO) or CB2 (hCB2-CHO) hCB-CHO accounted for 40% to 50% of total binding. Non-specific binding was related mainly to binding to the glass fibre (data not shown). For the binding of 3H]CP55,940 towards hCB1-CHO and hCB2-CHO cell membranes, KD values of 2.43 ± 1.89 nM (n = 3) and 1.48 ± 0.88 nM (n = 4) were determined in competitive binding experiments, which correspond with recently published data [37,38]. Kinetic analysis of the binding of 3H]CP55,940 towards hCB2-CHO cell membranes revealed the rate constants kon = 0.266 × 109 M−1 min−1 and koff = 0.196 min−1. The accordingly estimated KD value of 0.73 nM is consistent with the data obtained by saturation assay. The inhibition constants (Ki) for each of the human CB receptors are compiled together with reference compounds CP55,940, SR144528, and SR141716A in Table 1. In Figure 2, the inhibition curves of 6f and 6h on the hCB2-CHO cell homogenates obtained in the radioligand displacement experiments with 3H]CP55,940 are given as examples.
Table 1.
Affinities in nM and selectivity of the tested compounds towards hCB1 and hCB2 expressed in CHO cells
| Compound | hCB1 | hCB2 | Selectivitya |
|---|---|---|---|
|
1a |
>105 |
14.4 |
|
|
1b |
>105 |
>105 |
|
|
2a |
>105 |
722 |
|
|
2b |
>105 |
2,290 |
|
|
2c |
>105 |
>105 |
|
|
3b |
>105 |
>105 |
|
|
4e |
>105 |
>105 |
|
|
5e |
>105 |
250 |
|
|
6a |
>105 |
6.98 |
|
|
6b |
1,560 |
135 |
1.06 |
|
6c |
>105 |
1,920 |
|
|
6d |
7,470 |
12.5 |
2.78 |
|
6e |
>105 |
4.27 |
|
|
6f |
>105 |
5.89 |
|
|
6g |
397 |
2.38 |
2.22 |
|
6h |
>105 |
39.4 |
|
|
6i |
>105 |
>105 |
|
|
6j |
>105 |
5,710 |
|
|
6k |
>105 |
3,190 |
|
|
6l |
>105 |
223 |
|
|
6m |
>105 |
>105 |
|
|
7h |
6,010 |
>105 |
|
| CP55,940 |
2.43 |
1.27 |
0.28 |
| SR141716A |
1.26 |
n.d.b |
|
| SR144528 | n.d.b | 12.0 |
aAs calculated by pKi (CB2) – pKi (CB1) with pKi = -log (Ki); b., not determined.
Figure 2.
Radioligand displacement study of 6f and 6h against [3H]CP55,940 on membrane homogenates of hCB2-CHO cells.
The data on the hCB2 affinity and selectivity of the synthesized compounds are in good agreement with the results published by Cheng et al. [36] and are consistent with the therein reported potencies of similar compounds. For compound 6a (compound 44 in [36]), very high potency with an EC50 value of 0.85 nM was obtained by functional cAMP assays. Taken together with the high affinity measured for 6a (Ki = 6.98 nM), it is obvious that the efficacy of this compound is mainly caused by the strength of the direct physical interaction with CB2. Thus, the KD values can be seen as a direct measure of the activity of the compounds under investigation.
As the data in Table 1 show, the variation of the arylamide moiety has a significant impact on the affinity towards the cannabinoid receptors. The compounds with carbazolamides (Y4 in Scheme 1) show, on average, the highest affinity to hCB2. The 5-tert-butyl-isoxazol-3-yl derivative 1a shows high affinity too, while a quinoline substitution (Y2 in Scheme 1) results in less favourable properties. The approximately 100-fold lower hCB2 affinity of the 3-substituted quinoline 2a is in good agreement with published efficacy data, where a 6-substituted quinoline shows an approximately 50-fold lower [36] efficacy in comparison to 6a. The situation is completely different for CB1. Here, replacement of the carbazole group with a smaller moiety results in a strong loss of affinity towards the hCB1 receptor. For compounds 2c, 2b and 2a, binding on the hCB1 receptor could not be observed. Weak affinity towards hCB1 could be observed for 7h, which bears a benzyl-substituted indole at the left-hand side. Remarkably, the affinity towards hCB2 is lost in comparison to 6h despite the identical 2,4-diflourophenyl moieties at the 3-position of the oxadiazole ring. While the majority of the compounds bind to hCB2, those with an unsubstituted nitrogen in the ring system (aryles Y1, Y2, Y4a in Scheme 1) show no binding to hCB1 at all. In general, the affinity of all compounds towards hCB1 is much lower than that towards hCB2. This might indicate a different binding mode of 6h and 7h at hCB2.
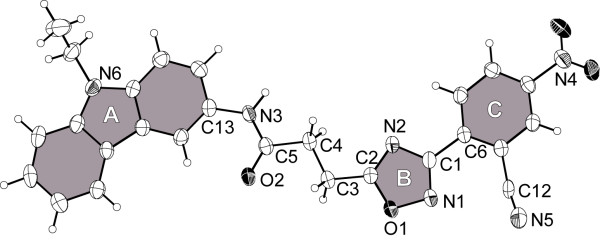
Comparing the compounds with the highest affinity (6g) and the best specificity for hCB2 (6e), the cause of this seems to be encoded in the functional group at para-position of the phenyl group. In concordance with published data [36], a fluorine atom at this position increases the affinity and efficacy towards hCB2. Depending on its electronegativity, a second substituent at ortho-position shows a significant impact on the affinity as shown in Figure 3. While the introduction of -Br (6e) or -CN (6a) leads to a higher affinity, the -OCH3 (6l) or -OCH2CH2F (6k) substituents decrease the affinity. Interestingly, replacement of the methoxy group with a hydroxyl moiety (6j) results in a further loss of affinity.
Figure 3.
Influence of substitution at ring C (cf. Figure1) on affinity towards hCB2. The pKi values were calculated according to pKi = −log (Ki). High pKi values represent high affinity.
Autoradiographic studies
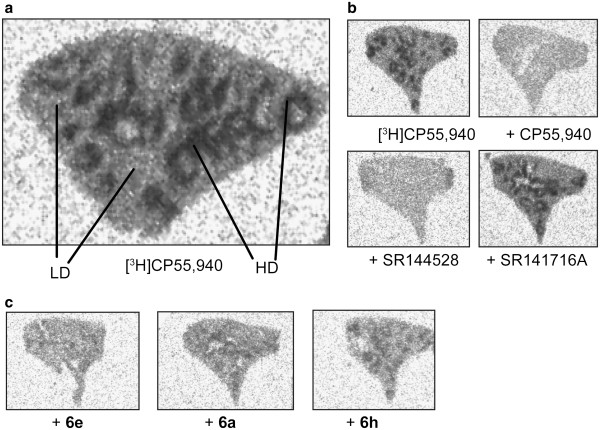
For further investigation of the CB2 affinity of the test compounds, a preliminary autoradiographic investigation of the displacement potential of reference and test compounds on mouse spleen tissue co-incubated with 3H]CP55,940 has been performed. Figure 4 shows representative autoradiograms of coronal mouse spleen sections incubated with 6 nM 3H]CP55,940 in the presence of 1 μM of test compounds 6a, 6e and 6h. For comparison, the autoradiograms for CP55,940, the CB1-selective antagonist SR141716A, and the CB2-selective antagonist SR144528 are shown in Figure 4b. As visible in Figure 4a, the distribution of 3H]CP55,940 was heterogeneous within the coronal spleen sections, showing a pattern similar to that reported previously [18,39]. Accordingly, high-density (HD) and low-density (LD) regions were assumed to reflect binding sites in the white or the red pulp, respectively.
Figure 4.
Autoradiogram of representative coronal sections of CD-1 mouse spleen tissue. (a) Incubated with 6 nM [3H]CP55,940. HD binding and LD binding represent the white and the red pulp, respectively (cf. text). (b) Incubation with 1 μM selective antagonists for CB2 (SR144528) and CB1 (SR141716A). (c) Compounds 6e, 6a and 6h compete at 1 μM with the binding of [3H]CP55,940 on CB2 in good agreement with the determined binding data: 6e >6a >6h.
To estimate the displacement efficacy of the reference and test compounds, the intensity of the radioligand binding in the HD regions was chosen to assess total binding of 3H]CP55,940. Specific binding of 3H]CPCP55,940 was calculated by subtracting the intensity of the homogenous binding of 3H]CP55,940 determined in the presence of 1 μM CP55,940 from the total binding values determined in the absence or presence of 1 μM compound. Accordingly, a high degree of specific binding of 3H]CP55,940 in mouse spleen has been identified, which accounts for approximately 90% of total 3H]CP55,940 binding. The density of specific binding sites of 6 nM 3H]CP55,940 in mouse spleen, estimated by converting the photostimulated luminescence per square millimetre values to femtomoles per milligram wet weight using 3H] standards, resembles, with 150 ± 25 fmol/mg wet weight, the values reported for the binding of 10 nM 3H]CP55,940 in rat spleen (59 to 108 fmol/mg wet weight) [18]. The assignment of the 3H]CP55,940 binding sites in mouse spleen to either CB1 or CB2 has been obtained by co-incubating the tissue with 3H]CP55,940 and either the CB1-selective SR141716A or the CB2-selective SR144,528 antagonist. As visible in the panels of Figure 4, co-incubation with 1 μM SR144528 displaced 78% of the specific binding of 6 nM 3H]CP55,940, while nearly no displacement has been detected in the presence of 1 μM SR141716A. In the latter, 94% of the specific CP55,940 binding remained. Thus, the basal binding of 3H]CP55,940 in mouse spleen was identified to reflect mainly CB2 binding, and the experimental conditions in this autoradiographic study are suitable to assess the CB2 binding potential of the test compounds.
At 1 μM, 6a, 6e, and 6h displaced 83%, 71%, and 66% of the specific binding of 6 nM 3H]CP55,940 respectively, and these data correlate to the rank order of CB2 affinity obtained on hCB2-CHO cells (Ki = 4.27, 6.98 and 39.4 nM, respectively). However, the displacement potential of the three compounds in mouse spleen is slightly lower than that calculated according to the Gaddum equation, based on which a fractional occupancy of 98%, 97% and 83%, respectively, of 3H]CP55,940 binding sites has been estimated (cf. ‘Experimental’ section). This difference between experimental and calculated data might reflect the previously reported non-significant variances between affinity values obtained on native tissue by in vitro autoradiography and in radioligand displacement studies using transfected cells [40]. Alternatively, as shown for the binding of WIN55,212-2 towards 3H]CP55,940-labelled mouse or human CB2, species differences might also explain the slightly distinct affinity of the studied compounds [41].
Molecular modelling
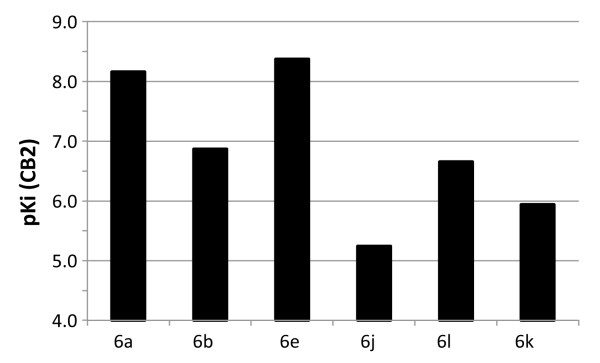
In order to gain more insight into the binding of the herein synthesized compounds towards hCB2, we performed molecular modelling studies. As no experimentally determined structures of cannabinoid receptors are available yet, we created a three-dimensional (3D) structure model of this G protein-coupled receptor (GPCR) by comparative modelling. Based on the results obtained by a sequence search with the BLAST server, the 3D structure of the human beta1-adrenergic receptor (bAR1) [PDB:2Y00] [42] was identified as the structure with the highest sequence similarity to the hCB2 sequence. However, during the course of this study, a GPCR structure with a bound agonist was published [43]. In comparison to the bAR1 structure [PDB:2Y00], which is crystalized with a bound inverse agonist, this novel structure is more opened and thus represents a better description of a GPCR in an active state. Thus, the X-ray structure of the human A2A adenosine receptor with UK-432097 ([PDB:3QAK]) [43] was chosen as the template for comparative modelling. The co-crystalized ligand of this structure was kept in place during the building process of the receptor model to ensure a state ready to host an agonist. Figure 5 shows the constructed 3D model of the human CB2 with the proposed binding pocket. In agreement with mutagenesis data compiled by Poso and Huffman [44], residues known to be involved in the binding of CP55,940 are accessible in our model.
Figure 5.
Model of the human cannabinoid receptor type 2. (a) Side view with the proposed transmembrane region shaded in grey. The protein is shown with the N-terminus at the top. (b) Top view from the extracellular space. The extracellular loops and the transmembrane helices are labelled to guide the reader. Only the residues forming the binding pocket are shown. The binding pocket identified by the Site Finder module of MOE (Chemical Computing Group Inc., Montreal, Canada) is shown as a surface with colour-coded features: H bonding (magenta), lipophilic (green), mild polar (blue).
The results of the docking of 6a, 6e and 6h are shown in Figure 6. The pose with the lowest predicted binding energy is shown in green. In the majority of the predicted poses, the molecules are bent and deviated significantly from a planar structure, which was observed for 6f in the single crystal. However, the bound conformation of a ligand is not necessarily identical to that of the free molecule in solution or in a packed crystal. Moreover, due to thermal effects, several differently populated conformations might exist. Typically, molecular docking procedures deliver multiple suggestions for the binding geometry of a ligand in a protein-ligand complex. Identifying the native pose out of these suggestions is often challenging, especially, if no experimentally determined reference structure is available. By taking into account the computed binding energy, it is possible to weight the suggested poses employing Boltzmann statistics.
Figure 6.
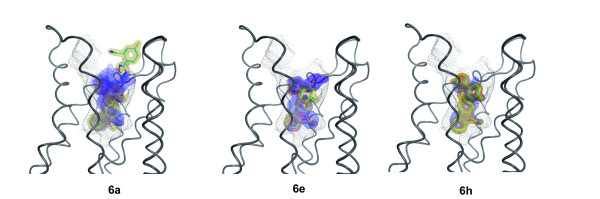
Predicted binding modes of 6e, 6a, 6h to human CB2 model after docking with GOLD. All compounds bind with their carbazole moiety (ring A, cf. Figure 1) in the binding pocket indicated by black dots. The figure was created with the software MOE (Chemical Computing Group Inc., Montreal, Canada) using the module PostDock [45]: The visibility of the surfaces is proportional to their binding energies based on Boltzman statistics. The surfaces are colour-coded with respect to the rmsd difference to the ligand with the lowest binding energy (from yellow to blue). As reference, the best-docked ligand is shown with cyan sticks.
In Figure 6 this is illustrated by encoding the energy in the visibility of the molecule according to the method implemented in the program PostDock [45]. While for 6h only one representative pose was predicted, for 6a and 6e, two and three lower populated binding modes were found, respectively. This is illustrated by the blue shades in Figure 6, which represent the less favourable poses of these compounds. Nevertheless, in all cases, the three compounds bind primarily with their arylamide moiety inside the binding pocket. A detailed analysis of the binding site revealed a hydrophobic microdomain at the bottom of the pocket. The comparison of 6a with 5e indicates that a substituent at the N atom of the ring system increases the affinity towards CB2. If such a substituent is missing like in 4e, no binding at hCB2 could be observed (data not shown). However, a replacement with a longer or bulkier moiety reduces the binding affinity too, as it is visible from the data obtained for 7h. As illustrated in Figure 7, the preferred binding of 7h is with the arylamide moiety outside the binding pocket, which, together with the determined binding data, substantiates a different binding mode of this compound. Thus, the 9-ethyl-9H-carbazole group seems to have an optimal size for fitting into the CB2 binding pocket.
Figure 7.
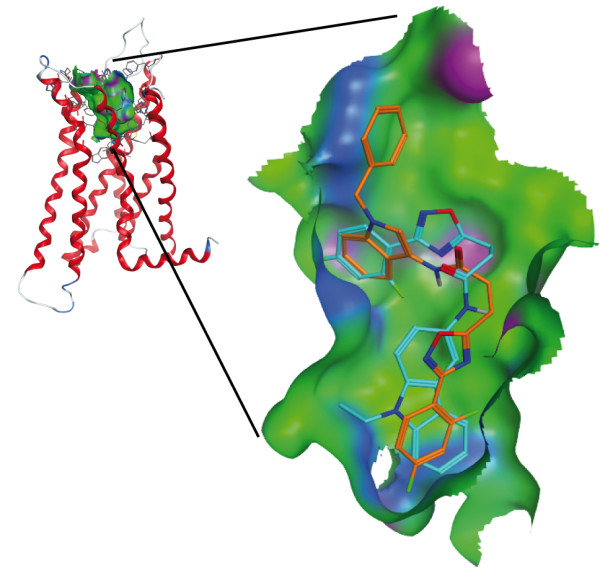
Comparison of the predicted binding modes of 7h and 6h to the human CB2 model. 6h (cyan) binds with the carbazole moiety in the binding pocket. For 7h (orange), the preferred binding is with ring A pointing outside of the cavity. This figure was created using MOE (Chemical Computing Group Inc., Montreal, Canada).
The low nanomolar affinity of 6a (Ki = 6.98 nM), 6e (Ki = 4.27 nM) and 6h (Ki = 39.4 nM) towards the human CB2 and the more than 1,000-fold selectivity over hCB1 (Ki > 1 μM) make these fluorine-substituted analogues the currently most promising candidates for further development of PET imaging agents within the series investigated. A proposed labelling at p-position of the phenyl group at the right-hand site seems to be favourable, e.g. the replacement of fluorine by 18F would allow applying these compounds for molecular imaging of CB2 receptors.
Radiochemistry
The labelling with 18F was performed exemplarily on 6g, yielding [18F]-6e, since 6e has been identified as highly selective for hCB2 with a high affinity of Ki = 4.27 nM (Scheme 4). 18F was introduced by microwave-assisted nucleophilic substitution of the 4-NO2 moiety employing [2.2.2]cryptand (Kryptofix®, VWR International GmbH, Darmstadt, Germany). The electron-withdrawing effect (−I effect) of the Br substituent in meta-position to the leaving group -NO2 enables the nucleophilic attack.
Scheme 4.
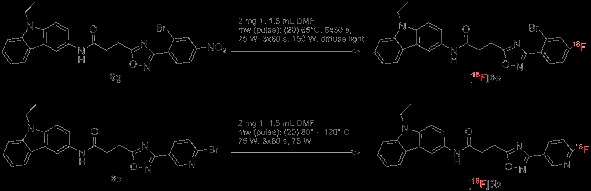
Radiochemistry.
However, this electron-withdrawing effect might not be strong enough as the reaction delivered only poor yields (3% radiochemical yield). An introduction of nitrogen into the aromatic ring should facilitate the nucleophilic substitution. Thus, the pyridine equivalent 6c was labelled with 18F under comparable conditions (Scheme 4). Though not supported by a further electron-withdrawing substituent, the radiochemical yield is significantly higher with 28%. Unfortunately, compounds with a pyridine moiety as ring C show only low affinity towards hCB2, as visible in Table 1 (compounds 1b, 2b, 2c, 3b, 6b, 6c).
Experimental
Chemistry
All reagents were commercially obtaineda and used without further purification unless otherwise stated. Anhydrous solvents were transferred via an oven-dried syringe or cannula. Flasks were oven-dried under vacuum and cooled under a constant stream of nitrogen. 1H, 13C, and 19F nuclear magnetic resonance (NMR) spectra were recorded on VARIAN GEMINI 2000 (200 MHz for 1H NMR, 50 MHz for 13C NMR, 188 MHz for 19F NMR; Palo Alto, CA, USA), VARIAN ‘MERCURY plus’ (300 MHz for 1H NMR, 75 MHz for 13C NMR, 228 MHz for 19F NMR) and VARIAN ‘MERCURY plus’ and BRUKER DRX-400 (400 MHz for 1H NMR, 100 MHz for 13C NMR, 367 MHz for 19F NMR; Billerica, MA, USA). The chemical shifts are reported relative to the residual solvent peak, which was used as an internal reference (chemical shifts in δ values, J in Hz). The following abbreviations were used to describe peak splitting patterns when appropriate: br = broad, s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, dd = doublet of doublet, ddd = triplet of doublet, pt = pseudo-triplet. High-resolution mass spectrometry (MS) was performed on a Bruker Daltonics APEX II FT-ICR, and low-resolved mass spectra (using electrospray ionization (ESI)) were recorded on a Bruker ESQUIRE. Reactions involving moisture-sensitive reactants were performed in oven-dried glassware under an atmosphere of nitrogen, reactants being added via a syringe. Flash column chromatography was performed on silica gel (VWR, 60 Å, 40 to 63 μm, VWR GmbH, Darmstadt, Germany) and analytical thin-layer chromatography (TLC) on pre-coated silica gel plates (Roth, 60 Å, F254, 0.25 mm, Carl Roth GmbH & Co. KG, Karlsruhe, Germany). HPLC was performed on silica gel (ReproSil-Pur 120 ODS3, C18, end-capped (endc.)) from JASCO (Jasco Labor- & Datentechnik GmbH, Groß-Umstadt, Germany) with a HPLC from Nordantec GmbH (Bremerhaven, Germany). All compounds were obtained with 95% purity.
3-(3-(2-Bromo-4-fluorophenyl)-1,2,4-oxadiazol-5-yl)propanoic acid (8) and 2-bromo-4-fluorobenzamide (9)
To a mixture of deoxygenated hydroxylamine hydrochloride (517 mg, 7.4 mmol, 1.0 eq.) and sodium carbonate (394 mg, 3.7 mmol, 0.5 eq.), methanol/water (4:1, 35 mL) was added via a cannula, and the reaction mixture was stirred for 10 min. 2-Bromo-4-fluorobenzonitrile (1,487 mg, 7.4 mmol, 1.0 eq.) was added, and the reaction mixture was stirred under diffuse light for 18 h at 80°C. The reaction mixture was diluted with dichloromethane (DCM), washed with water and evaporated to dryness after concentration of the solvent in vacuo. To the deoxygenated crude intermediate succinic acid anhydride (638 mg, 6.4 mmol, 0.9 eq.) was added, and the reaction mixture was stirred under diffuse light for 30 min at 140°C. The crude material was purified by column chromatography on silica gel (eluting with petrol spirit or petrol ether/ethyl acetate/acetic acid 1:1:0.01) and by crystallization at room temperature (DCM/methanol/water) to give 8 (566 mg, 1.8 mmol, 24% yield) and 9 (559 mg, 2.6 mmol, 35% yield). 8: 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.84 (dd, J = 6 Hz, J = 9 Hz, 1H, CH), 7.47 (dd, J = 3 Hz, J = 8 Hz, 1H, CH), 7.14 (ddd, J = 3 Hz, J = 9 Hz, 1H, CH), 3.29 (t, J = 7 Hz, 2H, CH2), 3.01 (t, J = 7 Hz, 2H, CH2). 19F NMR (376 MHz, CDCl3): δ (ppm) = −107.89 (dd, J = 8 Hz, J = 14 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) = 177.7, 176.6, 167.2, 163.4 (d, J = 256 Hz), 133.3 (d, J = 9 Hz), 124.3 (d, J = 4 Hz), 122.8 (d, J = 10 Hz), 121.7 (d, J = 25 Hz), 114.9 (d, J = 22 Hz), 30.1, 21.7. High-resolution mass spectrometry (HRMS) (ESI, negative ion) m/z: 312.9, 314.9 [M-H]−. 9: 1H NMR (400 MHz, CD3OD): δ (ppm) = 7.51 (dd, J = 6 Hz, J = 8 Hz, 1H, CH), 7.47 (dd, J = 3 Hz, J = 9 Hz, 1H, CH), 7.19 (pt, J = 2 Hz, J = 8 Hz, 1H, CH). 19F NMR (376 MHz, CD3OD): δ (ppm) = −111.64 (dd, J = 8 Hz, J = 15 Hz). 13C NMR (101 MHz, CD3OD): δ (ppm) = 172.4, 164.2 (d, J = 252 Hz), 136.2 (d, J = 4 Hz), 131.5 (d, J = 9 Hz), 121.5 (d, J = 25 Hz), 121.0 (d, J = 10 Hz), 115.7 (d, J = 22 Hz). HRMS (ESI, positive ion) m/z: 217.96086 [M+H]+.
3-(3-(2-Bromo-4-nitrophenyl)-1,2,4-oxadiazol-5-yl)propanoic acid (10) and 2-bromo-4-nitrobenzamide (11)
The propanoic acid 10 was synthesized similar to the synthesis of 8 but with 2-bromo-4-nitrobenzonitrile as the reacting agent. The mixture was diluted with ethyl acetate, and potassium fluoride (2 mg, 34 μmol, 0.1 eq.) was added during the reaction with succinic acid anhydride. The reaction mixture was stirred under diffuse light for 30 min at 130°C. For the purification, petrol spirit or petrol ether/ethyl acetate/acetic acid (1:1:0.005) was used, resulting in 10 (24 mg, 70 μmol, 16% yield) and 11 (22 mg, 90 μmol, 20% yield). 10: 1H NMR (300 MHz, CDCl3/CD3OD 1:1): δ (ppm) = 8.56 (d, J = 2 Hz, 1H, CH), 8.29 (dd, J = 2 Hz, J = 9 Hz, 1H, CH), 7.89 (d, J = 9 Hz, 1H, CH). 13C NMR (75 MHz, CDCl3/CD3OD 1:1): δ (ppm) = 1,178.9, 173.0, 166.0, 148.5, 133.5, 132.2, 128.5, 122.0, 121.7, 29.4, 21.3. HRMS (ESI, negative ion) m/z: 339.95731 [M-H+]−, 680.92128 [2M-H+]−. 11: 1H NMR (300 MHz, CDCl3/CD3OD): δ (ppm) = 8.50 (d, J = 2 Hz, 1H, CH), 8.28 (dd, J = 2 Hz, J = 8 Hz, 1H, CH), 7.68 (d, J = 8 Hz, 1H, CH). 13C NMR (100 MHz, CDCl3/CD3OD): δ (ppm) = 171.4, 150.0, 145.7, 130.6, 129.1, 123.7, 120.7. HRMS (ESI, positive ion) m/z: 244.95569 [M+H]+.
3-(3-(2-Fluoro-4-nitrophenyl)-1,2,4-oxadiazol-5-yl)propanoic acid (12) and 2-fluoro-4-nitrobenzamide (13)
Compounds 12 and 13 were prepared using 2-fluoro-4-nitrobenzonitrile (prepared as in the synthesis for 10): 12: 807 mg, 2.9 mmol, 10% yield; 13: 93 mg, 0.5 mmol, 2% yield. 12: 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.26 (dd, J = 7 Hz, J = 8 Hz, 1H, CH), 8.06 to 8.18 (m, 2H, 2xCH), 3.32 (t, J = 7 Hz, 2H, CH2), 3.03 (t, J = 7 Hz, 2H, CH2). 19F NMR (282 MHz, CDCl3): δ (ppm) = −103.63 (dd, J = 7 Hz, J = 10 Hz). 13C NMR (75 MHz, CDCl3): δ (ppm) = 178.8, 176.0, 164.1 (d, J = 6 Hz), 160.5 (d, J = 263 Hz), 150.4 (d, J = 8 Hz), 131.8 (d, J = 3 Hz), 121.4 (d, J = 13 Hz), 119.5 (d, J = 4 Hz), 112.8 (d, J = 26 Hz), 30.7, 22.5. HRMS (ESI, negative ion) m/z: 280.03753 [M-H+]−, 316.01425 [M+Cl−]−, 561.08202 [2M-H+]−. 13: 1H NMR (400 MHz, CD3OD): δ (ppm) = 8.18 to 8.07 (m, 2H, 2xCH), 7.97 (dd, J = 7 Hz, J = 8 Hz, 2H, CH2). 19F NMR (376 MHz, CD3OD): δ (ppm) = −112.07 (t, J = 8 Hz). 13C NMR (101 MHz, CD3OD): δ (ppm) = 166.9, 160.8 (d, J = 254 Hz), 151.5 (d, J = 9 Hz), 132.8 (d, J = 3 Hz), 130.1 (d, J = 15 Hz), 120.5 (d, J = 4 Hz), 113.1 (d, J = 29 Hz). HRMS (ESI, positive ion) m/z: 185.03583 [M+H]+, 207.01776 [M+Na]+, 391.04643 [2M+Na]+.
3-(3-(2-Bromo-4-methoxyphenyl)-1,2,4-oxadiazol-5-yl)propanoic acid (14) and 2-bromo-4-methoxybenzamide (15)
Compounds 14 and 15 were prepared using 2-bromo-4-methoxybenzonitrile (prepared as in the synthesis of 10): 14: 2539 mg, 7.8 mmol, 33% yield; 15: impure. 14: 1H NMR (300 MHz, CD3OD): δ (ppm) = 7.74 (d, J = 9 Hz, 1H, CH), 7.31 (d, J = 2 Hz, 1H, CH), 7.04 (dd, J = 3 Hz, J = 9 Hz, 1H, CH), 3.86 (s, 3H, CH3), 3.24 (t, J = 7 Hz, 2H, CH2), 2.92 (t, J = 7 Hz, 2H, CH2). Impurity: δ (ppm) = 2.56 (s, 1.0H). 13C NMR (76 MHz, CD3OD): δ (ppm) = 180.2, 175.1, 168.8, 163.2, 133.9, 123.6, 121.5, 120.6, 114.4, 56.3, 31.1, 22.9. Impurity: δ (ppm) = 176.2, 29.9. HRMS (ESI, negative ion) m/z: 324.98282 [M-H+]−, 650.97272 [2M-H+]−. 15: 1H NMR (300 MHz, CD3OD): δ (ppm) = 7.44 (d, J = 9 Hz, 1H, CH), 7.19 (d, J = 3 Hz, 1H, CH), 7.00 (dd, J = 3 Hz, J = 9 Hz, 1H, CH), 3.83 (s, 3H, CH3). Impurity: δ (ppm) = 3.67 (s, 14.4H), 2.59 (s, 16H), 2.57 (s, 10H). HRMS (ESI, positive ion) m/z: 324.98282 [M-H+]−, 229.98094 [M+H]+.
3-(3-(2,4-Difluorophenyl)-1,2,4-oxadiazol-5-yl)propanoic acid (16)
Carboxylic acid 16 was prepared using 2,4-difluorobenzonitrile (prepared as in the synthesis for 10): 5684 mg, 22.4 mmol, 64% yield. 16: 1H NMR (300 MHz, CDCl3/CD3OD 1:1): δ (ppm) = 8.04 (dd, J = 8 Hz, J = 16 Hz, 1H, CH), 7.14 to 6.96 (m, 2H, 2xCH), 3.24 (t, J = 7 Hz, 2H, CH2), 2.92 (t, J = 7 Hz, 2H, CH2). 19F NMR (282 MHz, CDCl3/CD3OD 1:1): δ (ppm) = −104.91 to −105.25 (m, 1xF), −105.85 to −106.23 (m, 1xF). 13C NMR (75 MHz, CDCl3/CD3OD 1:1): δ (ppm) = 179.5, 174.2, 165.4 (d, J = 12 Hz, J = 254 Hz), 165.0 (d, J = 6 Hz), 161.9 (d, J = 12 Hz, J = 260 Hz), 132.8 (dd, J = 4 Hz, J = 10 Hz), 112.6 (dd, J = 4 Hz, J = 22 Hz), 112.1 (dd, J = 4 Hz, J = 13 Hz), 105.6 (pt, J = 26 Hz), 30.7, 22.5. HRMS (ESI, positive ion) m/z: 255.05735 [M+H]+, 277.03917 [M+Na]+, 531.09038 [2M+Na]+.
3-(3-(2-Cyano-4-fluorophenyl)-1,2,4-oxadiazol-5-yl)propanoic acid (17)
Lithium hydroxide monohydrate (50 mg, 1.200 mmol, 5.0 eq.) in water (1.2 mL) was added to 24 (66 mg, 241 μmol, 1.0 eq.) in methanol (9.5 mL) and stirred for 2.5 h at room temperature. Ethyl acetate (38 mL) and HCl solution (13 mL, 6 mol/L) were added, and the phases were mixed and separated. The aqueous HCl layer was washed with ethyl acetate, and the solvent was concentrated in vacuo. This material was used without further purification; 17: 58 mg, 221 μmol, 92% yield. 17: 1H NMR (300 MHz, CDCl3, 50°C): δ (ppm) = 8.51 (s broad, 1H, COOH), 8.15 (dd, J = 5 Hz, J = 9 Hz, 1H, CH), 7.53 (dd, J = 3 Hz, J = 8 Hz, 1H, CH), 7.42 (ddd, J = 3 Hz, J = 9 Hz, 1H, CH), 3.30 (t, J = 7 Hz, 2H, CH2), 3.01 (t, J = 7 Hz, 2H, CH2). 19F NMR (282 MHz, CDCl3, 50°C): δ (ppm) = −106.90 (dd, J = 8 Hz, J = 13 Hz). 13C NMR (75 MHz, CDCl3, 50°C): δ (ppm) = 178.9, 175.8, 165.6, 163.3 (d, J = 256 Hz), 132.3 (d, J = 9 Hz), 125.7 (d, J = 4 Hz), 121.8 (d, J = 25 Hz), 120.6 (d, J = 22 Hz), 116.0 (m), 113.3 (d, J = 9 Hz), 52.2, 30.0, 21.9. MS (ESI, positive ion) m/z: 284 [M+Na]+. MS (ESI, negative ion) m/z: 260 [M-H+]−.
3-(3-(2-Cyano-4-nitrophenyl)-1,2,4-oxadiazol-5-yl)propanoic acid (18)
The propanoic acid 18 was prepared using 26 (prepared as for the synthesis of 17): 119 mg, 412 μmol, 99% yield. 18: 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.74 (d, J = 2 Hz, 1H, CH), 8.58 (dd, J = 2 Hz, J = 9 Hz, 1H, CH), 8.38 (d, J = 9 Hz, 1H, CH), 3.27 (t, J = 7 Hz, hidden by solvent, CH2), 2.93 (t, J = 7 Hz, 2H, CH2). 13C NMR (76 MHz, CDCl3): δ (ppm) = 182.1, 174.9, 166.5, 150.4, 135.4, 132.7, 131.1, 129.1, 116.8, 113.4, 30.9, 23.0. MS (ESI, negative ion) m/z: 287 [M-H+]−, 575[2M-H+]−.
3-(3-(4-Fluoro-2-hydroxyphenyl)-1,2,4-oxadiazol-5-yl)propanoic acid (19)
To a mixture of deoxygenated tribromoborane (7,528 mg, 30.1 mmol, 8.0 eq.) in dry DCM (130 mL), a mixture of 20 (1,000 mg, 3.8 mmol, 1.0 eq.) in dry DCM (60 mL) was added via a cannula at −70°C. The reaction mixture was stirred for 1 h at −70°C and overnight at room temperature. The reaction mixture was neutralized with saturated aqueous NaHCO3 solution at 1°C, washed with ethyl acetate, and evaporated to dryness after concentration of the solvent in vacuo. The crude material was purified by column chromatography on silica gel (eluting with DCM/MeOH = 9:1 and DCM/MeOH = 1:1). Water was added and the product was additionally purified by washing with DCM to give 19 (188 mg, 746 μmol, 20%). 19: 1H NMR (400 MHz, CD3OD): δH (ppm) = 7.97 (pdd, J = 7 Hz, J = 9 Hz, 1H, CH), 6.79 to 6.71 (m, 2 H, 2xCH), 3.27 (t, J = 7 Hz, 2H, CH2), 2.94 (t, J = 7 Hz, 2H, CH2). 19F NMR (376 MHz, CD3OD): δF (ppm) = −109.06 to −109.20 (m). 13C NMR (101 MHz, CD3OD): δC (ppm) = 180.3, 175.1, 167.5, 166.9 (d, J = 250 Hz), 160.0 (d, J = 13 Hz), 131.6 (d, J = 11 Hz), 109.3 (d, J = 3 Hz), 108.5 (d, J = 23 Hz), 105.1 (d, J = 25 Hz), 31.0, 22.9. HR ESI -MS: C11H9FN2O4, m/z = 251.04731 [M-H+]−, 503.10122 [2M-H+]−.
3-(3-(4-Fluoro-2-methoxyphenyl)-1,2,4-oxadiazol-5-yl)propanoic acid (20)
Carboxylic acid 20 was prepared using 4-fluoro-2-methoxybenzonitrile (prepared as in the synthesis for 8): 1,250 mg, 4.7 mmol, 15% yield. 20: 1H NMR (400 MHz, CD3OD): δ (ppm) = 7.99 (dd, J = 7 Hz, J = 9 Hz, 1H, CH), 6.97 (dd, J = 2 Hz, J = 11 Hz, 1H, CH), 6.82 (ddd, J = 2 Hz, J = 8 Hz, J = 11 Hz, 1H, CH), 3.93 (s, 3H, CH3), 3.21 (t, J = 7 Hz, 2H, CH2), 2.82 (t, J = 7 Hz, 2H, CH2). Impurity: δ (ppm) = 3.16 (q, J = 7 Hz, 6.5H), 2.96 (s, 4.6H), 1.28 (t, J = 7 Hz, 10.2H). 19F NMR (376 MHz, CD3OD): δ (ppm) = −108.48 (td, J = 7 Hz, J = 11 Hz, J = 15 Hz). 13C NMR (101 MHz, CD3OD): δ (ppm) = 180.3, 177.0 (broad), 167.1, 166.9 (d, J = 250 Hz), 161.3 (d, J = 11 Hz), 133.8 (d, J = 11 Hz), 113.2 (d, J = 3 Hz), 108.3 (d, J = 22 Hz), 101.1 (d, J = 27 Hz), 56.7, 33.4 (broad), 22.1 (broad). Impurity: δ (ppm) = 177.6 (broad), 47.5, 23.8, 9.1. HRMS (ESI, negative ion) m/z: 265.06292 [M-H+]−, 531.13261 [2M-H+]−.
3-(3-(6-Bromopyridin-3-yl)-1,2,4-oxadiazol-5-yl)propanoic acid (21)
Compound 21 was prepared using 6-bromonicotinonitrile (prepared as in the synthesis for 8): 164 mg, 550 μmol, 10% yield. 21: 1H NMR (300 MHz, CDCl3/CD3OD 1:1, 30°C): δ (ppm) = 8.95 (dd, J = 1 Hz, J = 2 Hz, CH), 8.22 (dd, J = 2 Hz, J = 8 Hz, 1H, CH), 7.68 (dd, J = 1 Hz, J = 8 Hz, 1H, CH), 3.24 (t, J = 7 Hz, 2H, CH2), 2.92 (t, J = 7 Hz, 2H, CH2). 13C NMR (76 MHz, CDCl3/CD3OD 1:1, 30°C): δ (ppm) = 180.6, 174.1, 166.1, 149.2, 145.0, 137.9, 129.3, 123.3, 30.6, 22.5. HRMS (ESI, positive ion) m/z: 297.98160 [M+H]+, 319.96382 [M+Na]+, 616.93871 [2M+Na]+.
3-(3-(6-Fluoropyridin-3-yl)-1,2,4-oxadiazol-5-yl)propanoic acid (22)
Compound 22 was prepared using 6-fluoronicotinonitrile (prepared as in synthesis for 10): 197 mg, 830 μmol, 5% yield. 22: 1H NMR (400 MHz, CDCl3/CD3OD 1:1): δ (ppm) = 8.87 to 8.83 (m, 1 H, CH), 8.48 (ddd, J = 2 Hz, J = 7 Hz, J = 8 Hz, 1H, CH), 7.15 (ddd, J = 0.4 Hz, J = 3 Hz, J = 9 Hz, 1H, CH), 3.24 (t, J = 7 Hz, 2H, CH2), 2.93 (t, J = 7 Hz, 2H, CH2). 19F NMR (376 MHz, CDCl3/CD3OD 1:1): δ (ppm) = −65.88 (d, J = 6 Hz). 13C NMR (101 MHz, CDCl3/CD3OD 1:1): δ (ppm) = 180.5, 174.2, 166.0, 165.6 (d, J = 244 Hz), 147.4 (d, J = 15 Hz), 141.2 (d, J = 9 Hz), 122.2 (d, J = 5 Hz), 110.9 (d, J = 37 Hz), 30.6, 22.5. HRMS (ESI, negative ion) m/z: 236.04779 [M-H+]−, 272.02442 [M-Cl−]−, 473.10191 [2M-H+]−.
Methyl 3-(3-(2-bromo-4-fluorophenyl)-1,2,4-oxadiazol-5-yl)propanoate (23)
(Diazomethyl)trimethylsilane (2 M in hexane, 1.3 mL, 2.6 mmol, 1.5 eq.) was added dropwise to deoxygenated 8 (547 mg, 1.7 mmol, 1.0 eq.) in dry toluene/methanol (9:1, 20 mL) at 0°C, and the reaction mixture was stirred for 30 min at room temperature. The crude product was purified by column chromatography on silica gel (eluting with petrol spirit or petrol ether/ethyl acetate 2:1) after concentration of the solvent in vacuo to give 23 (560 mg, 1.7 mmol, 98% yield). 23: 1H NMR (400 MHz, CDCl3): δ (ppm) = 7.84 (dd, J = 6 Hz, J = 9 Hz, 1H, CH), 7.47 (dd, J = 3 Hz, J = 8 Hz, 1H, CH), 7.14 (ddd, J = 3 Hz, J = 9 Hz, 1H, CH), 3.73 (s, 3H, CH3), 3.29 (t, J = 7 Hz, 2H, CH2), 2.94 (t, J = 7 Hz, 2H, CH2). 19F NMR (376 MHz, CDCl3): δ (ppm) = −108.04 (dd, J = 8 Hz, J = 14 Hz). 13C NMR (101 MHz, CDCl3): δ (ppm) = 178.0, 171.6, 167.2, 163.3 (d, J = 256 Hz), 133.3 (d, J = 9 Hz), 124.5 (d, J = 4 Hz), 122.7 (d, J = 10 Hz), 121.6 (d, J = 25 Hz), 114.8 (d, J = 21 Hz), 52.1, 30.2, 22.0. MS (ESI, positive ion) m/z: 351 [M+Na]+.
Methyl 3-(3-(2-cyano-4-fluorophenyl)-1,2,4-oxadiazol-5-yl)propanoate (24)
Copper(I) chloride (12 mg, 122 μmol, 0.4 eq.) was added to deoxygenated 10 (100 mg, 304 μmol, 1.0 eq.) in dry dimethylacetamide (2.2 mL). Potassium cyanide (79 mg, 1.215 mmol, 4.0 eq.) was added after 15 min, and the reaction mixture was stirred for 14 h at 130°C. Saturated ammonium chloride (2.2 mL), ethyl acetate (8.7 mL) and water (7.6 mL) were added, and the phases were mixed and separated. The aqueous layer was washed with ethyl acetate. The crude product was purified by column chromatography on silica gel (eluting with petrol spirit or petrol ether/ethyl acetate 2:1) after concentration of the solvent in vacuo to give 24 (23 mg, 83 μmol, 27% yield). 24: 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.15 (dd, J = 5 Hz, J = 9 Hz, 1H, CH), 7.53 (dd, J = 3 Hz, J = 8 Hz, 1H, CH), 7.42 (ddd, J = 3 Hz, J = 9 Hz, 1H, CH), 3.73 (s, 3H, CH3), 3.03 (t, J = 7 Hz, 2H, CH2), 2.96 (t, J = 7 Hz, 2H, CH2). 19F NMR (282 MHz, CDCl3): δ (ppm) = −106.85 (ddd, J = 5 Hz, J = 8 Hz). 13C NMR (100 MHz, CDCl3): δ (ppm) = 179.1, 171.6, 165.5, 163.2 (d, J = 256 Hz), 132.3 (d, J = 9 Hz), 125.6 (d, J = 4 Hz), 121.8 (d, J = 25 Hz), 120.6 (d, J = 21 Hz), 116.2 (d, J = 3 Hz), 113.0 (d, J = 10 Hz), 52.2, 30.1, 22.0. MS (ESI, positive ion) m/z: 276 [M+H]+, 298 [M+Na]+.
Methyl 3-(3-(2-bromo-4-nitrophenyl)-1,2,4-oxadiazol-5-yl)propanoate (25)
Compound 25 was prepared using 10 (prepared as in the synthesis of 23): 230 mg, 646 μmol, 98% yield. 25: 1H NMR (400 MHz, CDCl3): δ (ppm) = 8.58 (d, J = 2 Hz, 1H, CH), 8.25 (dd, J = 2 Hz, J = 9 Hz, 1H, CH), 8.06 (d, J = 9 Hz, 1H, CH), 3.73 (s, 3H, CH3), 3.32 (t, J = 7 Hz, 2H, CH2), 2.96 (t, J = 7 Hz, 2H, CH2). 13C NMR (101 MHz, CDCl3): δ (ppm) = 178.7, 171.5, 166.5, 148.9, 133.9, 132.7, 129.2, 122.7, 122.1, 52.2, 30.1, 22.0. MS (ESI, positive ion) m/z: 378 [M+Na]+.
Methyl 3-(3-(2-cyano-4-nitrophenyl)-1,2,4-oxadiazol-5-yl)propanoate (26)
The carboxylic acid 26 was prepared using 10 (prepared as for the synthesis of 24): 83 mg, 275 μmol, 43% yield. 26: 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.68 (d, J = 2 Hz, 1H, CH), 8.54 (dd, J = 2 Hz, J = 9 Hz, 1H, CH), 8.39 (d, J = 9 Hz, 1H, CH), 3.73 (s, 3H, CH3), 3.34 (t, J = 7 Hz, 2H, CH2), 2.98 (t, J = 7 Hz, 2H, CH2). 13C NMR (75 MHz, CDCl3): δ (ppm) = 179.9, 171.5, 164.9, 148.7, 134.3, 131.3, 129.7, 127.4, 115.4, 112.7, 52.2, 30.0, 22.0. MS (ESI, positive ion) m/z: 303 [M+H]+, 325 [M+Na]+.
General amide coupling method A
The coupling reaction was initiated by dropwise addition of N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide (EDAC; 50 mg, 322 μmol, 1.1 eq.) in dry DCM (2.5 mL) to a mixture of 1.0 eq. of the deoxygenated propanoic acid derivate and 1.1 eq. amine in dry DCM (2.5 mL) at 0°C. The reaction mixture was stirred for 1 h at 0°C followed by stirring for 1 h at room temperature. A tip of spatula of 4-DMAP was added at 0°C, and the reaction mixture was stirred for 1 h at room temperature. EDAC (1.1 eq.) in dry DCM (2.5 mL) was added at 0°C, and the reaction mixture was again stirred for 14 h at room temperature followed by evaporation to dryness after concentration of the solvent in vacuo.
3-(3-(6-Bromopyridin-3-yl)-1,2,4-oxadiazol-5-yl)-N-(5-tert-butylisoxazol-3-yl)propanamide (1a)
Compound 1a was prepared using 14 and 5-tert-butylisoxazol-3-amine according to method A. The crude material was purified by column chromatography on silica gel (elution with petrol spirit/ethyl acetate 2:1) and by HPLC (RP18, ReproSil-Pur 120 ODS3, endc., methanol/water = 85:15) and by crystallization at room temperature (methanol), giving 1a (19 mg, 44 μmol, 27% yield). 1a: 1H NMR (400 MHz, CDCl3): δ (ppm) = 10.20 (s broad, 1H, NH), 9.01 (d, J = 2 Hz, 1H, CH), 8.16 (dd, J = 2 Hz, J = 8 Hz, 1H, CH), 7.59 (d, J = 8 Hz, 1H, CH), 6.69 (s, 1H, CH), 3.39 (t, J = 7 Hz, 2H, CH2), 3.11 (t, J = 7 Hz, 2H, CH2), 1.31 (s, 9H, 3×CH3). 13C NMR (76 MHz, CDCl3): δ (ppm) = 182.2, 179.5, 168.9, 165.8, 158.0, 149.1, 145.0, 137.0, 128.5, 122.5, 93.6, 33.2, 32.5, 28.7, 22.1. HRMS (ESI, positive ion): C17H18BrN5O3, m/z = 420.06635 [M+H]+, 839.12559 [2M+H]+.
3-(3-(6-Fluoropyridin-3-yl)-1,2,4-oxadiazol-5-yl)-N-(5-tert-butylisoxazol-3-yl)propanamide (1b)
Compound 1b was synthesized similar to 1a with 22 (30 mg, 127 μmol, 1.0 eq.) as carboxylic acid component. Before evaporating, water (5.0 mL) was added, and the reaction mixture was washed with ethyl acetate. The crude material was purified as described for 1a, giving 1b (14 mg, 38 μmol, 30% yield). 1b: 1H NMR (300 MHz, CD3OD): δH (ppm) = 8.85 (d, 1H, CH), 8.52 (ddd, J = 2 Hz, J = 8 Hz, J = 9 Hz, 1H, CH), 7.26 to 7.19 (m, 1H, CH), 6.54 (s, 1H, CH), 3.34 (t, J = 7 Hz, 2H, CH2), 3.06 (t, J = 7 Hz, 2H, CH2), 1.32 (s, 9H, 3×CH3). 19F NMR (282 MHz, CD3OD): δF (ppm) = −67.33 (d, J = 7 Hz). HR ESI +MS: C17H18FN5O2, m/z = 360.14680 [M+H]+, 382.12896 [M+Na]+, 719.28664 [2M+H]+, 741.26859 [2M+Na]+.
3-(3-(2-Cyano-4-fluorophenyl)-1,2,4-oxadiazol-5-yl)-N-(quinolin-3-yl)propanamide (2a)
Compound 2a was prepared following general amide coupling method A with 17 and quinoline-3-amine. The crude material was purified by column chromatography on silica gel (elution with petrol spirit/ethyl acetate 1:1, ethyl acetate and DCM/methanol 1:1) and by crystallization at room temperature (acetonitrile), giving pure 2a (25 mg, 64 μmol, 51% yield). 2a: 1H NMR (300 MHz, CDCl3/CD3OD = 1:1, 50°C): δH (ppm) = 8.83 (d, J = 3 Hz, 1H, CH), 8.65 (d, J = 2 Hz, 1H, CH), 8.14 (dd, J = 5 Hz, J = 9 Hz, 1H, CH), 7.94 (pd, J = 8 Hz, 1H, CH), 7.77 (d, J = 1 Hz, J = 8 Hz, 1H, CH), 7.64 to 7.42 (m, 4H, CH), 3.41 (t, J = 7 Hz, 2H, CH2), 3.11 (t, J = 7 Hz, 2H, CH2). 19F NMR (282 MHz, CDCl3, 50°C): δF (ppm) = −107.89 (td, J = 5 Hz, J = 8 Hz, J = 16 Hz). APT NMR (75 MHz, CDCl3/CD3OD = 1:1, 50°C): δC (ppm) = 180.6, 171.2, 166.3, 164.1, (d, J = 255 Hz), 145.1, 144.9, 133.1, 133.0 (d, J = 9 Hz), 129.12, 129.08, 128.6, 128.3, 127.9, 127.6, 126.3 (J = 4 Hz), 125.2, 122.4 (d, J = 26 Hz), 121.5 (d, J = 22 Hz), 116.8 (d, J = 3 Hz), 113.4 (d, J = 9 Hz), 32.9, 22.5. HR ESI +MS: C21H14FN5O2, m/z = 388.12051 [M+H]+, 410.10243 [M+Na]+, 775.23385 [2M+H]+, 797.21557 [2M+Na]+.
3-(3-(6-Fluoropyridin-3-yl)-1,2,4-oxadiazol-5-yl)-N-(quinolin-3-yl)propanamide (2b)
The synthesis of 2b followed the procedure described for 2a with 21 as reactant. The crude material was purified under the same conditions as 2a, resulting in 20 mg of 2b (55 μmol, 44% yield). 2b: 1 H NMR (400 MHz, CDCl3/CD3OD = 1:1): δH (ppm) = 8.86 to 8.79 (m, 2H, 2xCH), 8.68 (d, J = 2 Hz, 1H, CH), 8.46 (ddd, J = 2 Hz, J = 8 Hz, J = 8 Hz, 1H, CH), 7.93 (d, J = 8 Hz, 1H, CH), 7.77 (dd, J = 2 Hz, J = 8 Hz, 1H, CH), 7.65 to 7.58 (m, 1H, CH), 7.55 to 7.48 (m, 1H, CH), 7.12 (dd, J = 3 Hz, J = 9 Hz, 1H, CH), 3.34 (t, J = 7 Hz, 2H, CH2), 3.11 (t, J = 7 Hz, 2H, CH2). 19F NMR (376 MHz, CDCl3/CD3OD = 1:1): δF (ppm) = −66.00 (d, J = 7 Hz). 13C NMR (101 MHz, CDCl3/CD3OD = 1:1): δC (ppm) = 180.7, 171.3, 166.1, 165.6 (d, J = 244 Hz), 147.5 (d, J = 15 Hz), 145.0, 144.6, 141.2 (d, J = 9 Hz), 133.2, 129.19, 129.16, 128.44, 128.41, 128.1, 125.1, 122.3 (d, J = 5 Hz), 110.9 (d, J = 37 Hz), 32.9, 22.5. HR ESI +MS: C19H14FN5O2, m/z = 364.12066 [M+H]+, 386.10258 [M+Na]+, 727.23464 [2M+H]+, 749.21527 [2M+Na]+.
3-(3-(6-Bromopyridin-3-yl)-1,2,4-oxadiazol-5-yl)-N-(quinolin-3-yl)propanamide (2c)
Compound 2c was synthesized by method A, by coupling 21 (50 mg, 168 μmol, 1.0 eq.) to quinolin-3-amine. Before evaporating to dryness, water (5.0 mL) was added to the reaction mixture and subsequently washed with ethyl acetate. The crude material was purified as described for 2a, giving 2c (33 mg, 77 μmol, 46% yield). 2c: 1H NMR (400 MHz, CDCl3/CD3OD = 1:1): δH (ppm) = 8.94 (dd, J = 1 Hz, J = 2 Hz, 1H, CH), 8.78 (d, J = 2 Hz, 1H, CH), 8.70 (d, J = 2 Hz, 1H, CH), 8.18 (dd, J = 2 Hz, J = 8 Hz, 1H, CH), 7.93 (d, J = 8 Hz, 1H, CH), 7.76 (d, J = 1 Hz, J = 8 Hz, 1H, CH), 7.63 (dd, J = 1 Hz, J = 8 Hz, 1H, CH), 7.63 to 7.56 (m, 1H, CH), 7.53 to 7.47 (m, 1H, CH), 3.38 (t, J = 7 Hz, 2H, CH2), 3.10 (t, J = 7 Hz, 2H, CH2). 13C NMR (101 MHz, CDCl3/CD3OD = 1:1): δC (ppm) = 180.5, 170.8, 166.0, 149.1, 144.9, 144.7, 144.3, 137.7, 132.9, 129.1, 129.0, 128.9, 128.3, 128.2, 127.8, 124.8, 123.1, 32.6, 22.4. HR ESI +MS: C19H14BrN5O2, m/z = 424.04075 [M+H]+, 446.02267 [M+Na]+, 847.07404 [2M+H]+, 869.05547 [2M+Na]+.
3-(3-(6-Fluoropyridin-3-yl)-1,2,4-oxadiazol-5-yl)-N-(1-ethyl-1H-indol-5-yl)-propanamide (3b)
Compound 3b was synthesized by coupling deoxygenated 21 (50 mg, 211 μmol, 1.0 eq.) to 1-ethyl-1H-indol-5-amine (37 mg, 232 μmol, 1.1 eq.) via method A. The crude material was purified by column chromatography on silica gel (elution with petrol spirit/ethyl acetate 1:1) and by crystallization at room temperature (DCM/petrol spirit), giving 3b (61 mg, 162 μmol, 77% yield). 3b: 1H NMR (300 MHz, CDCl3): δH (ppm) = 8.91 (d, J = 2 Hz, 1H, CH), 8.41 (dt, J = 2 Hz, J = 8 Hz, 1H, CH), 7.76 (s, 1H, CH), 7.61 (s broad, 1H, NH), 7.24 (d, J = 10 Hz, 1H, CH), 7.21 (d, J = 2 Hz, 1H, CH), 7.10 (d, J = 3 Hz, 1H, CH), 7.02 (dd, J = 3 Hz, J = 9 Hz, 1H, CH), 6.41 (d, J = 3 Hz, 1H, CH), 4.12 (q, J = 7 Hz, 2H, CH2), 3.38 (t, J = 7 Hz, 2H, CH2), 2.97 (t, J = 7 Hz, 2H, CH2), 1.43 (t, J = 7 Hz, 3H, CH3). 19F NMR (282 MHz, CDCl3): δF (ppm) = −64.21 (d, J = 5 Hz). 13C NMR (76 MHz, CDCl3): δC (ppm) = 179.8, 168.4, 165.6, 165.0 (d, J = 244 Hz), 147.4 (d, J = 16 Hz), 140.2 (d, J = 9 Hz), 133.5, 129.7, 128.8, 128.1, 121.5 (d, J = 5 Hz), 115.9, 113.2, 110.1 (d, J = 38 Hz), 109.5, 41.2, 33.0, 22.5, 15.6. HR ESI +MS: C20H18FN5O2, m/z = 380.15202 [M+H]+, 402.13403 [M+Na]+, 759.29638 [2M+H]+, 781.27720 [2M+Na]+.
3-(3-(2-Bromo-4-fluorophenyl)-1,2,4-oxadiazol-5-yl)-N-(9H-carbazol-3-yl)propanamide (4e)
Compound 4e was prepared using 8 and 9H-carbazol-3-amine via coupling method A. The crude product was purified by column chromatography on silica gel (elution with petrol spirit/ethyl acetate 1:1, ethyl acetate and DCM/methanol 1:1) and by HPLC (RP18, ReproSil-Pur 120 ODS3, endc., methanol/water = 85:15) and by crystallization at room temperature (chloroform), giving 60 mg of 4e (125 μmol, 64% yield). 4e: 1H NMR (300 MHz, CD3OD): δ (ppm) = 8.26 (d, J = 2 Hz, 1H, CH), 7.96 (d, J = 8 Hz, 1H, CH), 7.82 (dd, J = 6 Hz, J = 9 Hz, 1H, CH), 7.55 (dd, J = 3 Hz, J = 9 Hz, 1H, CH), 7.49 to 7.29 (m, 4H, 4×CH), 7.21 (td, J = 2 Hz, J = 8 Hz, 1H, CH), 7.11 (t, J = 7 Hz, 1H, CH), 3.38 (t, J = 7 Hz, 2H, CH2), 3.04 (t, J = 7 Hz, 2H, CH2). 19F NMR (282 MHz, CD3OD): δ (ppm) = −110.02 (td, J = 6 Hz, J = 8 Hz). 13C NMR (76 MHz, CD3OD): δ (ppm) = 180.8, 171.4, 168.5, 164.8 (d, J = 254 Hz), 142.1, 138.7, 134.6 (d, J = 9 Hz), 131.1, 126.8, 126.1 (d, J = 4 Hz), 124.3, 124.2, 123.8 (d, J = 10 Hz), 122.4 (d, J = 25 Hz), 121.0, 120.7, 119.7, 116.0 (d, J = 22 Hz), 113.6, 111.8, 111.6, 33.4, 23.2. HR ESI +MS: C23H16BrFN4O2, m/z = 501.03343 [M+Na]+, 979.07777 [2M+Na]+.
3-(3-(2-Bromo-4-fluorophenyl)-1,2,4-oxadiazol-5-yl)-N-(9-methyl-9H-carbazol-3-yl)propanamide (5e)
Compound 5e was synthesized by adding iodomethane (8 mg, 56 μmol, 1.3 eq.) in dry dimethylformamide (0.5 mL) dropwise to deoxygenated 4e (21 mg, 43 μmol, 1.0 eq.) and potassium carbonate (59 mg, 430 μmol, 10.0 eq.). The reaction mixture was stirred for 20 h at room temperature followed by evaporation to dryness after concentration of the solvent in vacuo. The crude product was purified by hot filtration in methanol and by HPLC (RP18, ReproSil-Pur 120 ODS3, endc., acetonitrile/water = 85:15) with subsequent crystallization in the fridge (methanol) to give 5e (8 mg, 17 μmol, 39% yield). 5e: 1H NMR (300 MHz, CDCl3/CD3OD 1:1): δ (ppm) = 8.30 (d, J = 2 Hz, 1H, CH), 8.00 (d, J = 8 Hz, 1H, CH), 7.81 (d, J = 9 Hz, 1H, CH), 7.57 to 7.35 (m, hidden by solvent, 4×CH), 7.33 (d, J = 9 Hz, 1H, CH), 7.20 to 7.11 (m, 2 H, 2×CH), 3.81 (s, 3H, CH3), 3.40 (t, J = 7 Hz, 2H, CH2), 3.03 (t, J = 7 Hz, 2H, CH2). 19F NMR (282 MHz, CDCl3/CD3OD 1:1): δ (ppm) = −108.76 (td, J = 6 Hz, J = 8 Hz). HR ESI +MS: C23H16BrFN4O2, m/z = 515.04893 [M+Na]+, 1,007.10847 [2M+Na]+.
3-(3-(2-Cyano-4-nitrophenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6a)
For the synthesis of 6a, 17 was coupled to 9-ethyl-9H-carbazol-3-amine as described in method A. After synthesis, water (5.0 mL) was added, and the reaction mixture was washed with ethyl acetate and evaporated to dryness after concentration of the solvent in vacuo. The crude product was purified by column chromatography on silica gel (elution with petrol spirit/ethyl acetate (2:1) + five drops of triethylamine and DCM/methanol (1:1) + five drops of triethylamine) and by crystallization at room temperature (DCM/methanol), giving pure 6a (27 mg, 60 μmol, 50% yield). 6a: 1H NMR (300 MHz, CDCl3/CD3OD = 1:1, 50°C): δH (ppm) = 8.28 (d, J = 2 Hz, 1H, CH), 8.13 (dd, J = 5 Hz, J = 9 Hz, 1H, CH), 7.99 (d, J = 8 Hz, 1H, CH), 7.54 (dd, J = 2 Hz, J = 8 Hz, 1H, CH), 7.52 to 7.37 (m, 3H, 3×CH), 7.35 (d, J = 8 Hz, 1H, CH), 7.31 (d, J = 9 Hz, 1H, CH), 7.13 (pt, J = 7 Hz, 1H, CH), 4.31 (q, J = 7 Hz, 2H, CH2), 3.40 (t, J = 7 Hz, 2H, CH2), 3.04 (t, J = 7 Hz, 2H, CH2), 1.36 (t, J = 7 Hz, 3H, CH3). 19F NMR (282 MHz, CDCl3/CD3OD = 1:1, 50°C): δF (ppm) = −107.74 (ps). 13C NMR (75 MHz, CDCl3/CD3OD = 1:1, 50°C): δC (ppm) = 179.6, 169.2, 165.1, 162.9 (d, J = 255 Hz), 140.1, 136.9, 131.9 (d, J = 9 Hz), 129.5, 125.32, 125.26 (d, J = 4 Hz), 122.5, 122.4, 121.3 (d, J = 26 Hz), 120.3 (d, J = 22 Hz), 119.9, 119.2, 118.2, 115.8, 112.4, 112.2, 108.1, 107.9, 37.0, 32.0, 21.9, 12.9. HR ESI +MS: C26H20FN5O2, m/z = 496.13901 [M+Na]+, 969.28932 [2M+Na]+.
3-(3-(6-Fluoropyridin-3-yl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6b)
Compound 6b was synthesized as 6a by coupling deoxygenated 22 with 9-ethyl-9H-carbazol-3-amine following method A. The crude material was purified by column chromatography on silica gel (elution with petrol spirit/ethyl acetate 1:1 and DCM/methanol 1:1) and by crystallization at room temperature (DCM/methanol), giving 6b (42 mg, 98 μmol, 47% yield). 6b: 1H NMR (400 MHz, CDCl3/CD3OD = 1:1): δH (ppm) = 8.83 (d, J = 2 Hz, 1H, CH), 8.47 to 8.38 (m, 1H, CH), 8.28 (d, J = 2 Hz, 1H, CH), 7.98 (d, J = 8 Hz, 1H, CH), 7.47 (dd, J = 2 Hz, J = 9 Hz, 1H, CH), 7.44 to 7.33 (m, 2H, 2×CH), 7.30 (d, J = 9 Hz, 1H, CH), 7.13 (t, J = 7 Hz, 1H, CH), 7.07 (dd, J = 2 Hz, J = 8 Hz, 1H, CH), 4.30 (q, J = 7 Hz, 2H, CH2), 3.36 (t, J = 7 Hz, 2 H, CH2), 3.03 (t, J = 7 Hz, 2H, CH2), 1.34 (t, J = 7 Hz, 3H, CH3). 19F NMR (282 MHz, CDCl3/CD3OD = 1:1): δF (ppm) = −65.79 (d, J = 7 Hz). 13C NMR (101 MHz, CDCl3/CD3OD = 1:1): δC (ppm) = 180.7, 170.3, 165.9, 165.5 (d, J = 244 Hz), 147.4 (d, J = 15 Hz), 141.1 (d, J = 9 Hz), 141.0, 137.8, 130.5, 126.4, 123.5, 123.3, 122.2 (d, J = 5 Hz), 120.9, 120.1, 119.2, 113.3, 110.8 (J = 37 Hz), 109.1, 109.0, 38.0, 32.8, 22.8, 14.0. HR ESI +MS: C24H20FN5O2, m/z = 452.14902 [M+Na]+, 859.32705 [2M+H]+, 881.30861 [2M+Na]+.
3-(3-(6-Bromopyridin-3-yl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6c)
Compound 6c was prepared as described for 6b with 21 as carboxylic acid reactant. The crude material was purified as described for 6b, giving 6c (42 mg, 86 μmol, 51% yield).6c: 1H NMR (300 MHz, CDCl3/CD3OD = 1:1): δH (ppm) = 8.93 (dd, J = 1 Hz, J = 2 Hz, 1H, CH), 8.28 (d, J = 2 Hz, 1H, CH), 8.16 (dd, J = 2 Hz, J = 8 Hz, 1H, CH), 7.97 (d, J = 8 Hz, 1H, CH), 7.60 (dd, J = 1 Hz, J = 8 Hz, 1H, CH), 7.47 (dd, J = 2 Hz, J = 9 Hz, 1H, CH), 7.44 to 7.33 (m, 2H, 2×CH), 7.30 (d, J = 9 Hz, 1H, CH), 7.17 to 7.09 (m, 1H, CH), 4.30 (q, J = 7 Hz, 2H, CH2), 3.36 (t, J = 7 Hz, 2H, CH2), 3.03 (t, J = 7 Hz, 2H, CH2), 1.35 (t, J = 7 Hz, 3H, CH3). 13C NMR (76 MHz, CDCl3/CD3OD = 1:1): δC (ppm) = 180.8, 170.2, 166.0, 149.1, 144.9, 141.0 137.77, 137.75, 130.5, 129.2, 126.4, 123.4, 123.3, 120.9, 120.0, 119.2, 113.3, 109.1, 109.0, 38.0, 32.8, 22.8, 14.0. HR ESI +MS: C24H20BrN5O2, m/z = 490.08770 [M+H]+, 512.06951 [M+Na]+, 979.16767 [2M+H]+, 1,001.14935 [2M+Na]+.
3-(3-(2-Fluoro-4-nitrophenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6d)
Compound 6d was synthesized as 6a using 12. The crude product was purified by crystallization in the fridge (DCM), resulting in 69 mg of pure compound (145 μmol, 81% yield). 6d: 1H NMR (400 MHz, CD3CN, 70°C): δ (ppm) = 8.39 (s broad, 1H, NH), 8.34 to 8.24 (m, 2H, 2×CH), 8.18 to 8.10 (m, 2H, 2xCH), 8.07 (d, J = 8 Hz, 1H, CH), 7.57 to 7.43 (m, 4H, 4xCH), 7.20 (t, J = 7 Hz, 1H, CH), 4.41 (q, J = 7 Hz, 2H, CH2), 3.40 (t, J = 7 Hz, 2H, CH2), 3.02 (t, J = 7 Hz, 2H, CH2), 1.39 (t, J = 7 Hz, 3H, CH3). 19F NMR (376 MHz, CD3CN, 90°C): δ (ppm) = −106.52 (s, broad). HR ESI +MS: C25H20FN5O4, m/z = 496.13901 [M+Na]+, 969.28932 [2M+Na]+.
3-(3-(2-Bromo-4-fluorophenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6e)
Compound 6e was prepared as described for 6a using 8 as carboxylic acid. The crude product was purified by column chromatography on silica gel (elution with DCM/methanol 100:1) and by crystallization at room temperature (DCM/methanol) to give 6e (112 mg, 221 μmol, 76% yield) 6e: 1H NMR (300 MHz, CDCl3): δ (ppm) = 8.26 (d, J = 2 Hz, 1H, CH), 8.02 (d, J = 8 Hz, 1H, CH), 7.83 (dd, J = 6 Hz, J = 9 Hz, 1H, CH), 7.69 (s broad, 1H, NH), 7.54 to 7.42 (m, 3H, 3xCH), 7.38 (d, J = 8 Hz, 1H, CH), 7.31 (d, J = 9 Hz, 1H, CH), 7.20 (pt, J = 8 Hz, 1H, CH), 7.12 (ddd, J = 2 Hz, J = 9 Hz, 1H, CH), 4.32 (q, J = 7 Hz, 2H, CH2), 3.45 (t, J = 7 Hz, 2H, CH2), 3.02 (t, J = 7 Hz, 2H, CH2), 1.40 (t, J = 7 Hz, 3H, CH3). 19F NMR (282 MHz, CDCl3): δ (ppm) = −107.95 (dd, J = 8 Hz, J = 14 Hz). 13C NMR (101 MHz, CDCl3): δ (ppm) = 178.6, 168.4, 167.2, 163.3 (d, J = 255 Hz), 140.4, 137.3, 133.3 (d, J = 9 Hz), 129.2, 125.9, 124.5 (d, J = 4 Hz), 123.0, 122.8 (d, J = 10 Hz), 122.7, 121.6 (d, J = 25 Hz), 120.6, 119.5, 118.8, 114.9 (d, J = 21 Hz), 113.0, 108.6, 108.5, 37.6, 33.1, 22.4, 13.78. HR ESI +MS: C25H20BrFN4O2, m/z = 507.08249 [M+H]+, 529.06444 [M+Na]+, 1013.15637 [2M+H]+, 1035.13966 [2M+Na]+.
3-(3-(2-Cyano-4-nitrophenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6f)
Compound 6f was synthesized as 6a using 18. The crude product was purified by column chromatography on silica gel (elution with petrol spirit/ethyl acetate (2:1) + five drops of triethylamine and DCM/methanol (1:1) + five drops of triethylamine) and by crystallization at room temperature (DCM/methanol and acetonitrile), resulting in 6f (25 mg, 51 μmol, 25%). 6f: 1H NMR (300 MHz, CD3CN, 70°C): δH (ppm) = 8.71 (d, J = 2 Hz, 1H, CH), 8.54 (dd, J = 2 Hz, J = 9 Hz, 1H, CH), 8.38 (d, J = 9 Hz, 1H, CH), 8.32 (ps, 1H, CH), 8.07 (pd, J = 8 Hz, 1H, CH), 7.54 (dd, J = 2 Hz, J = 9 Hz, 1H), 7.51 to 7.43 (m, 3H, 3×CH), 7.20 (pt, J = 7 Hz, 1H, CH), 4.41 (q, J = 7 Hz, 2H, CH2), 3.43 (t, J = 7 Hz, 2H, CH2), 3.04 (t, J = 7 Hz, 2H, CH2), 1.39 (t, J = 7 Hz, 3H CH3). HR ESI +MS: C26H20N6O4, m/z = 503.14351 [M+Na]+, 983.29765 [2M+Na]+.
3-(3-(2-Bromo-4-nitrophenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6g)
Compound 6g was prepared as 6a but with 10 as the reactant. The crude product was purified as described for 6e, yielding 6g (106 mg, 198 μmol, 68%). 6g: 1H NMR (300 MHz, CDCl3, 50°C): δH (ppm) = 8.57 (d, J = 2 Hz, 1H, CH), 8.36 to 8.14 (m, 2H, 2×CH), 8.03 (pd, J = 8 Hz, 2H, 2×CH), 7.62 to 7.13 (m, hidden by solvent), 4.35 (q, J = 7 Hz, 2H, CH2), 3.49 (ps broad, 2H, CH2), 3.04 (ps broad, 2H, CH2), 1.43 (t, J = 7 Hz, 3H, CH3). HR ESI +MS: C25H20BrN5O4, m/z = 556.05940 [M+Na]+, 1,067.14721 [2M+H]+, 1,089.12816 [2M+Na]+.
3-(3-(2,4-Difluorophenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6h)
Compound 6h was synthesized similar to the procedure described for 6a using 16 as carboxylic acid. The crude product was purified by column chromatography on silica gel (elution with petrol spirit/ethyl acetate 2:1, petrol spirit/ethyl acetate 2:1 and DCM/methanol 1:1) and by crystallization at room temperature (DCM/methanol 1:1), yielding 6h (39 mg, 88 μmol, 49%). 6h: 1H NMR (400 MHz, CDCl3/CD3OD = 1:1): δH (ppm) = 8.29 (d, J = 2 Hz, 1H, CH), 8.04 (pdd, J = 8 Hz, J = 16 Hz, 1H, CH), 8.00 (d, J = 8 Hz, 1H, CH), 7.49 (dd, J = 2 Hz, J = 9 Hz, 1H, CH), 7.46 to 7.36 (m, 2H, 2xCH), 7.33 (d, J = 8 Hz, 1H, CH), 7.14 (pt, J = 6 Hz, 1H, CH), 7.06 to 6.97 (m, 2H, 2xCH), 4.33 (q, J = 7 Hz, 2H, CH2), 3.38 (t, J = 7 Hz, 2H, CH2), 3.03 (t, J = 7 Hz, 2H, CH2), 1.37 (t, J = 7 Hz, 3H, CH3). 19F NMR (376 MHz, CDCl3/CD3OD = 1:1): δF (ppm) = −105.08 (pdd, J = 7 Hz, J = 14 Hz, 1xF), −105.89 to −106.04 (m, 1×F). 13C NMR (101 MHz, CDCl3/CD3OD = 1:1): δC (ppm) = 179.8, 170.4, 165.4 (dd, J = 12 Hz, J = 254 Hz), 165.0 (d, J = 6 Hz), 161.9 (dd, J = 12 Hz, J = 260 Hz), 141.1, 137.9, 132.7 (dd, J = 4 Hz, J = 10 Hz), 130.5, 126.4, 123.5, 123.4, 120.9, 120.2, 119.2, 113.4, 112.6 (dd, J = 4 Hz, J = 22 Hz), 112.1 (dd, J = 4 Hz, J = 13 Hz), 109.2, 109.0, 105.6 (t, J = 26 Hz), 38.0, 32.9, 22.8, 14.0. HR ESI +MS: C25H20F2N4O2, m/z = 447.16279 [M+H]+, 469.14455 [M+Na]+, 893.31771 [2M+H]+, 915.29946 [M+Na]+.
3-(3-(4-Amino-2-fluorophenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6i)
Tin(II) chloride (40 mg, 211 μmol, 5.0 eq.) was added to deoxygenated 6d (20 mg, 42 μmol, 1.0 eq.) in ethanol (1.0 mL), and the reaction mixture was stirred for 30 min at reflux. Saturated sodium carbonate solution (2.0 mL) was added at 0°C. The precipitate was filtered up, washed with water, diluted in chloroform/methanol (1:1), filtered and evaporated to dryness after concentration of the solvent in vacuo. The crude material was purified by column chromatography on silica gel (elution with chloroform/methanol 100:1) and by crystallization in the fridge (acetonitrile) to give 6i (12 mg, 26 μmol, 62% yield) 6i: 1H NMR (300 MHz, CD3CN, 65°C): δ (ppm) = 8.38 (s broad, 1H, NH), 8.32 (s, 1H, CH), 8.07 (d, J = 8 Hz, 1H, CH), 7.74 (t, J = 8 Hz, 1H, CH), 7.60 to 7.36 (m, 4H, 4×CH), 7.19 (t, J = 7 Hz, 1H, CH), 6.61 to 6.41 (m, 2H, 2×CH), 4.71 (s broad, 2 H, NH2), 4.39 (q, J = 7 Hz, 2H, CH2), 3.31 (t, J = 7 Hz, 2H, CH2), 2.96 (t, J = 7 Hz, 2H, CH2), 1.38 (t, J = 7 Hz, 3H, CH3). 19 F NMR (282 MHz, CD3CN, 65°C): δ (ppm) = −110.20 (dd, J = 9 Hz, J = 13 Hz). HRMS (ESI, positive ion) m/z: 466.16522 [M+Na]+, 909.34029 [2M+Na]+.
3-(3-(4-Fluoro-2-hydroxyphenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6j)
Compound 6j was prepared using 19 as described in the synthesis of 6a. The crude material was purified as described for 6h, giving 38 mg of 6j (85 μmol, 23% yield). 6j: 1H NMR (300 MHz, CD3CN): δ (ppm) = 8.55 (s, broad, 1H, NH), 8.35 (d, J = 1 Hz, 1H, CH), 8.06 (pd, J = 6 Hz, 1H, CH), 8.00 (dd, J = 5 Hz, J = 7 Hz, 1H, CH), 7.55 to 7.42 (m, 4H, 4xCH), 7.18 (td, J = 1 Hz, J = 5 Hz, 1H, CH), 6.84 to 6.74 (m, 2H, 2xCH), 4.38 (q, J = 5 Hz, 2H, CH2), 3.37 (t, J = 5 Hz, 2H, CH2), 3.02 (t, J = 5 Hz, 2H, CH2), 1.35 (t, J = 5 Hz, 3H, CH3). 19F NMR (282 MHz, CD3CN): δ (ppm) = −108.31 to −108.44 (m). HR ESI +MS: C25H21FN4O3, m/z = 445.16724 [M+H]+, 467.14897 [M+Na]+, 889.32803 [2M+H]+, 911.30937 [2M+Na]+.
3-(3-(4-Fluoro-2-(2-fluoroethoxy)phenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)-propanamide (6k)
Potassium carbonate (14 mg, 101 μmol, 2.0 eq.) was added to deoxygenated 6j (22 mg, 50 μmol, 1.0 eq.) in dry dimethylformamide (0.5 mL), and the reaction mixture was stirred for 5 min. To the reaction mixture, 1-fluoro-2-iodoethane (18 mg, 101 μmol, 2.0 eq.) was added, and the reaction mixture was stirred for 20 h at 50°C. The reaction mixture was added to ice water, washed with ethyl acetate and evaporated to dryness after concentration of the solvent in vacuo. The crude material was purified by column chromatography on silica gel (elution with petrol spirit or petrol ether/ethyl acetate 1:1) and by crystallization at room temperature (DCM/methanol) to give 6k (11 mg, 22 μmol, 43% yield). 6k: 1H NMR (300 MHz, CDCl3/CD3OD 1:1): δ (ppm) = 8.52 (s, broad, 1H, NH), 8.36 (d, J = 2 Hz, 1H, CH), 8.06 (pd, J = 8 Hz, 1H, CH), 7.94 (dd, J = 7 Hz, J = 9 Hz, 1H, CH), 7.58 to 7.39 (m, 4H, 4×CH), 7.18 (pt, J = 7 Hz, 1H, CH), 6.93 (dd, J = 2 Hz, J = 11 Hz, 1H, CH), 6.86 (dt, J = 2 Hz, J = 8 Hz, 1H, CH), 4.88 to 4.78/4.71 to 4.61 (dm, J = 48 Hz, 1 H/1H, CH2), (m, 1H, CH2), 4.47 to 4.32/4.31 to 4.22 (m, 2H, 1H/1H, 2×CH2), 3.31 (t, J = 7 Hz, 2H, CH2), 2.98 (t, J = 7 Hz, 2H, CH2), 1.35 (t, J = 7 Hz, 3H, CH3). 19F NMR (282 MHz, CDCl3/CD3OD 1:1): δ (ppm) = −108.79 to −108.96 (m, 1F), −223.78 to −224.42 (m, 1F). HR ESI +MS: C27H24F2N4O3, m/z = 513.17095 [M+Na]+, 1,003.35233 [2M+Na]+.
3-(3-(4-Fluoro-2-methoxyphenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)-propanamide (6 l)
Compound 6l was synthesized via coupling method A as described for 6a with 20 as carboxylic acid. The crude material was purified following the procedure for 6e, yielding 6l (76 mg, 166 μmol, 57%). 6l: 1H NMR (400 MHz, CDCl3/CD3OD = 1:1): δH (ppm) = 8.29 (d, J = 2 Hz, 1H, CH), 8.02 to 7.91 (m, 2H, 2×CH), 7.48 (dd, J = 2 Hz, J = 8 Hz, 1H, CH), 7.43 to 7.33 (m, 2H, 2×CH), 7.30 (d, J = 9 Hz, 1H, CH), 7.13 (pt, J = 7 Hz, 1H, CH), 6.79 (dd, J = 2 Hz, J = 11 Hz, 1H, CH), 6.72 (dd, J = 2 Hz, J = 8 Hz, 1H, CH), 4.29 (q, J = 7 Hz, 2 H, CH2), 3.88 (s, 3H, CH3), 3.35 (t, J = 7 Hz, 2H, CH2), 3.01 (t, J = 7 Hz, 2H, CH2), 1.34 (t, J = 7 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3/CD3OD = 1:1): δC (ppm) = 178.7, 170.5, 166.6, 166.2 (d, J = 251 Hz), 160.4 (d, J = 11 Hz), 141.1 137.8, 133.3 (d, J = 11 Hz), 130.5, 126.4, 123.5, 123.4, 120.9, 120.1, 119.2, 113.4, 112.3 (d, J = 3 Hz), 109.2, 109.0, 108.0 (d, J = 22 Hz), 100.5 (d, J = 26 Hz), 56.4, 38.0, 33.0, 22.8, 14.0. HR ESI +MS: C26H23FN4O3, m/z = 459.18247 [M+H]+, 917.35797 [2M+H]+.
3-(3-(2-Bromo-4-methoxyphenyl)-1,2,4-oxadiazol-5-yl)-N-(9-ethyl-9H-carbazol-3-yl)propanamide (6m)
Compound 6m was synthesized as 6a with 14 as carboxylic acid reactant. The crude material was purified by column chromatography on silica gel (elution with DCM/methanol 100:1 and DCM/methanol 100:2) and by crystallization at room temperature (DCM/methanol), yielding 6m (83 mg, 159 μmol, 54%). 6m: 1H NMR (400 MHz, CDCl3/CD3OD = 1:1): δH (ppm) = 8.29 (s, 1H, CH), 8.00 (d, J = 8 Hz, 1H, CH), 7.72 (d, J = 8 Hz, 1H, CH), 7.55 to 7.27 (m, hidden by solvent, 4×CH), 7.22 (d, J = 2 Hz, 1H, CH), 7.15 (t, J = 7 Hz, 1H, CH), 6.92 (dd, J = 2 Hz, J = 9 Hz, 1H, CH), 4.32 (q, J = 7 Hz, 2H, CH2), 3.80 (s, 3H, CH3), 3.38 (t, J = 7 Hz, 2H, CH2), 3.02 (t, J = 7 Hz, 2H, CH2), 1.36 (t, J = 7 Hz, 3H, CH3). 13C NMR (101 MHz, CDCl3/CD3OD = 1:1): δC (ppm) = 179.4, 170.4, 168.2, 162.4, 141.0, 137.8, 133.3, 130.5, 126.4, 123.4, 123.3, 123.2, 120.9, 120.7, 120.13, 120.09, 119.2, 113.9, 113.4, 109.1, 109.0, 56.0, 38.0, 32.9, 22.8, 14.0. HR ESI +MS: C26H23BrN4O3, m/z = 519.10249 [M+H]+, 541.08500 [M+Na]+, 1,037.19973 [2M+H]+, 1,059.18045 [2M+Na]+.
3-(3-(2,4-Difluorophenyl)-1,2,4-oxadiazol-5-yl)-N-(1-benzyl-1H-indol-3-yl)-propanamide (7h)
Compound 7h was prepared by coupling of 16 with 1-benzyl-1H-indol-3-amine hydrochloride according to coupling method A. The crude product was purified by column chromatography on silica gel (elution with petrol spirit/ethyl acetate 2:1 and ethyl acetate), yielding 7h (71 mg, 155 μmol, 87%). For affinity data, the product (20 mg) was further purified by HPLC (RP18, ReproSil-Pur 120 ODS3, endc., acetonitrile/0.057 M triethylammonium acetate = 65:35) and by crystallization at room temperature (ethanol/water) to an aspic. The aspic was dried in a speed vacuum to give 14.7 mg of pure solid product. 7h: 1H NMR (400 MHz, CDCl3): δH (ppm) = 8.07 to 7.98 (m, 1H, CH), 7.92 (s broad, 1H, NH), 7.78 (s, 1H, CH), 7.51 (d, J = 8 Hz, 1H, CH), 7.36 to 7.22 (m, hidden by solvent, 4×CH), 7.19 (pt, J = 7 Hz, 1H, CH), 7.16 to 7.10 (m, 2H, 2×CH), 7.07 (pt, J = 7 Hz, 1H, CH), 7.02 to 6.92 (m, 2H, 2×CH), 5.24 (s, 2H, CH2), 3.42 (t, J = 7 Hz, 2H, CH2), 3.06 (t, J = 7 Hz, 2H, CH2). 19F NMR (376 MHz, CDCl3): δF (ppm) = −104.01 to −104.26 (m, 1xF), −104.97 to −105.20 (m, 1xF). 13C NMR (101 MHz, CDCl3): δC (ppm) = 178.8, 167.7, 164.7 (dd, J = 12 Hz, J = 254 Hz), 164.6 (d, J = 6 Hz), 161.3 (dd, J = 12 Hz, J = 260 Hz), 137.4, 134.1, 132.1 (dd, J = 4 Hz, J = 10 Hz), 128.9, 127.8, 127.0, 122.6, 121.1, 119.9, 119.3, 116.8, 114.1, 112.1 (dd, J = 4 Hz, J = 22 Hz), 111.6 (dd, J = 4 Hz, J = 13 Hz), 110.1, 105.3 (t, J = 25 Hz), 50.3, 32.6, 22.6. HR ESI +MS: C26H20F2N4O2, m/z = 481.14447 [M+Na]+, 939.29960 [2M+Na]+.
X-ray structural analysis
The single-crystal X-ray diffraction data of compound 6f were collected on a diffractometer IPDS-2T (Stoe & Cie GmbH, Darmstadt, Germany) at 180 K using Mo-Kα radiation (l = 71.073 pm). The data reduction was performed using the STOE X-AREA software package (2006, Stoe & Cie, Darmstadt, Germany). The structure was solved by direct methods using the program SHELXS-97 and refined using SHELXL-97 [46]. All non-H atoms were refined anisotropically, and hydrogen (H) atoms were added geometrically using a riding model. Graphical visualization of the structure was performed using the program Diamond 3.2e (DIAMOND 3.2e, K. Brandenburg, 2010, Crystal Impact GbR, Bonn, Germany). The crystal structure has been deposited at the Cambridge Crystallographic Data Centre and allocated the deposition number CCDC 841516.
Cell culture
CHO cell lines stably transfected with human CB1 (hCB1-CHO; obtained from Euroscreen, Gosselies, Belgium) or human CB2 (hCB2-CHO; obtained from Paul L. Prather, Department of Pharmacology and Toxicology, College of Medicine, University of Arkansas for Medical Sciences, USA) were cultured in Ham's F12 medium or Dulbecco's modified Eagle's medium (DMEM) with 10% fetal calf serum, 100 IU/mL penicillin, 100 μg/mL streptomycin and 250 or 400 μg/mL of the selection antibiotic Geneticin (G418), respectively. The cells were cultured in a humidified atmosphere with 5% CO2 at 37°C. Confluent cell layers of 175-cm2 flasks were scraped; cells were harvested by centrifugation (800 rpm, 3 min), counted by trypan blue staining, suspended in 200 μL of 50 mM tris(hydroxymethyl)aminomethane (TRIS; pH 7.4) at 4°C and stored at −32°C until the binding experiment.
Determination of CB receptor affinity
Competitive binding experiments were performed with crude membrane homogenates obtained from hCB1-CHO or hCB2-CHO cells as described above. Increasing concentrations of test compounds (0.01 nM to 10 μM) diluted from 10 mM stock solutions in DMSO were incubated with 30 to 50 μg of membrane protein (equivalent to 0.5 to 1 × 106 hCB1-CHO or hCB2-CHO cells) and 0.5 nM [3H]CP55,940 (6,438 GBq/mmol; PerkinElmer Life and Analytical Sciences, Rodgau, Germany) in a final volume of 1 mL in a binding buffer (50 mM TRIS (pH 7.4) at 21°C, 0.1% bovine serum albumin (BSA), 5 mM MgCl2, 1 mM EDTA) for 90 min at room temperature. Non-specific binding of [3H]CP55,940 was determined in the presence of 10 μM CP55,940. Incubations were terminated by rapid filtration through a GF-B glass fibre filter pre-incubated for 90 min at room temperature in freshly prepared 0.5% polyvinylpyrrolidone + 0.1% Tween 20 solution using a 48-well cell harvester (Brandel, Gaithersburg, MD, USA).
Saturation binding of CP55,940 towards hCB1-CHO or hCB2-CHO cell membranes was analysed in homologous competition experiments performed with increasing concentrations of CP55,940 (0.01 nM to 10 μM) and one concentration of [3H]CP55,940 (0.5 nM). Kinetic analysis of the binding of CP55,940 towards hCB2-CHO cell membranes was performed in one association experiment using three concentrations of [3H]CP55,940 (0.207, 0.458 and 0.873 nM) and incubation times of up to 120 min.
Binding data were analysed using GraphPad Prism version 2.01 (GraphPad Software, Inc., San Diego, CA, USA) by non-linear regression to provide estimates of the half maximal inhibitory concentration (IC50) values and the rate of association. KD values of 3H]CP55,940 from homologous radioligand displacement experiments were estimated according to Jeffries et al. [47] by KD = IC50 – [radioligand] and from kinetic experiments according to Hulme and Trevethick [48]. The corresponding inhibition constants (Ki) of test compounds were derived from the IC50 values using the Cheng-Prusoff equation [49]. All binding experiments were performed in triplicates, and data are given as mean values from independent experiments.
Animals
Animal experiments were performed under procedures approved by the State of Saxony Animal Care and Use Committee and conducted in accordance with the German Law for the Protection of Animals. Female CD-1 mice (10 to 12 weeks old, 20 to 25 g), obtained from the Medizinisch-Experimentelles Zentrum der Universität Leipzig (Leipzig, Germany), were used for the experiments.
Autoradiographic binding study
The animals were anesthetized and sacrificed, and their spleens were rapidly removed and frozen in isopentane at −35°C. The spleens were brought to −15°C in a cryostat (Microm International GmbH, Walldorf, Germany), and a series of 12-μm-thick coronal sections obtained from the spleens of three animals were collected in parallel on microscopic glass slides. The sections were dried at room temperature and stored at −35°C until they were processed for receptor binding autoradiography.
For autoradiographic experiments, the slides were brought to room temperature, dried in a stream of cold air, pre-incubated in a binding buffer (50 mM TRIS (pH 7.4) at 21°C, 5% BSA) for 15 min at room temperature and dried again in a stream of cold air. To account for total binding, samples were incubated for 2 h at room temperature with 6 nM [3H]CP55,940 (5,328 GBq/mmol, PerkinElmer Life and Analytical Sciences, Rodgau, Germany). Sections were incubated in the presence of 1 μM CP55,940 (non-specific binding), SR144528 (CB2-selective inverse agonist), SR141716A (CB1-selective antagonist), or a test compound in binding buffer. Samples were washed twice for 30 min at 4°C in 50 mM TRIS (pH 7.4) at 4°C, containing 1% BSA, dipped briefly in ice-cold deionized water (5 s), dried in a stream of cold air and exposed along with [3H] standards (Amersham/GE Healthcare, Piscataway, NJ, USA) for 14 days to [3H]-sensitive screen plates (Fuji Photo Film, Co. Ltd., Tokyo, Japan).
The image plates were analysed using a BAS-1800II system bioimaging analyser (Fuji Photo Film, Co. Ltd., Tokyo, Japan). Scan data were visualized and processed by computer-assisted microdensitometry (Aida version 2.31, Raytest Isotopenmessgeräte GmbH, Straubenhardt, Germany). Irregular regions of interest (ROIs) were drawn on selected areas of the spleen labelled as red pulp (low-density binding) and white pulp (high-density binding) according to Lynn and Herkenham [18] and Massi et al. [39]. The intensity of the radioligand binding was assessed by measuring the background-corrected optical density in each ROI expressed as photostimulated luminescence per square millimetre The theoretical fractional occupancy (f) of 3H]CP55,940 and the reference and test compounds at CB2 was calculated using the Gaddum equation: fA = [A] / ([A] + KDA(1 + [B]/KD,B)) [50].
Receptor modelling
The receptor model was created employing the ‘Homology Modeling’ module of the program Molecular Operation Environment (MOE, version 2010.11, Chemical Computing Group Inc. Montreal, Canada). For this purpose, the sequence of the human CB2 [Swiss-Prot:P34972] [4] was subjected to a PDB search on the protein structure database implemented in MOE 2010.11 with the Z cut-off set to 2.0 while leaving the other parameters at their default values. The search suggested the human adenosine receptor A2a (hAA2R) as the template for the comparative modelling procedure. Since the recently published structure of the agonist-bound hAA2 receptor [43] is not included in the MOE protein database, the hAA2R structure [PDB:3EML] [51] was chosen as reference for the sequence alignment. The alignment of the human CB2 sequence with that of hAA2R is given as Additional files 1, 2, and 3. After downloading the 3D structure of [PDB:3QAK] [43] from the RCSB database, the structure was superimposed on the crystal structure 3eml. After removal of all lipid molecules, ions and water molecules, the residues between 209 and 222, which belong to lysozyme T4, were removed from the structure. In order to improve the modelling accuracy, the co-crystalized agonist UK-432097 of [PDB:3QAK] remained in the system during the modelling procedure. Out of ten independently generated models employing the default parameters of the Homology Modeling module, a consensus model was computed. To remove clashes and optimize the overall geometry of the protein, the system was subjected to an energy minimization employing the implemented AMBER89 force field using the default values. The quality of the model was verified by running the Protein Report module of MOE. After removal of the template ligand UK-432097, the apo receptor was energy-minimized to convergence, giving the final hCB2 model structure.
Docking studies
The small compounds were built in MOE with subsequent geometry optimization based on the MMFF force field as incorporated in the program package with a distance-dependent dielectric constant. The residues of the proposed binding pocket were defined based on the Site Finder module of MOE. The docking site included residue Y1905.39 (numbering scheme according to Ballesteros and Weinstein [52]), which is known to be involved in ligand binding (cf.[53] and references cited therein). For the molecular docking studies, the program GOLD [54] (version 5) with parameters set to their default values was employed. All single bonds of the small ligands were allowed to rotate freely during the docking simulation. The side chains of the residues composing the binding pocket were treated as flexible, and the atoms of the protein backbone were kept at fixed positions. This approach is considered to represent the main characteristics of an induced fit [55]. The predicted poses were clustered by the GOLD software with a cut-off of 1.0 Å (heavy atoms only), giving representative geometries of the small molecules. For each of the compounds, all final poses were subjected to a further analysis with the program PostDock [45].
Radiochemistry
The radiolabelling procedure was initiated by addition of K[18F]/K2.2.2 and K2CO3 to 2 mg of precursor in dry N,N-dimethylformamide under diffuse light and argon atmosphere (6g: 293 MBq [18F]fluoride, K2.2.2 (7.4 μmol/GBq) and K2CO3 (3.2 μmol/GBq); 6c: 303.2 MBq [18F]fluoride, K2.2.2 (7.4 μmol/GBq) and K2CO3 (3.2 μmol/GBq)). The reaction mixtures were subjected to microwave radiation in closed vessels employing a Discover microwave synthesizer (CEM GmbH, Kamp-Lintfort, Germany) with the following scheme: heating to 60°C (30 s, 45 to 75 W with subsequent cooling to 20°C followed by three radiation cycles (100°C (60 s, 150 W); 20°C). Subsequently, 5 eq. SnCl2 was added and the reaction mixture was subjected to three heating cycles (120°C (60 s, 150 W); 20°C). The yields of the labelled compounds were determined by radio-TLC (EE/petrol ether = 2:1).
Conclusions
Various N-aryl-3-((hetero)aromatic-oxadiazolyl-propionamides have been synthesized. Among those, 9-ethyl-9H-carbazole-substituted compounds showed the highest affinity towards CB2 and good selectivity against CB1 receptors. The study revealed strong relationships between the structure of the CB2 ligands and their CB2 affinity and selectivity. The data of this study indicate that a modification of the compounds at the amide moiety shows a stronger impact on binding and specificity of the compounds toward CB2 than variation at the (hetero)aromatic ring located at the 3-position of the oxadiazoles. Thus, introduction of functional groups for subsequent 18F labelling at this moiety is a promising strategy for the creation of CB2-selective radioligands suitable for (neuro)imaging by PET. Such imaging studies are currently in process at our laboratory.
Endnotes
aVendors of chemicals (details can be obtained from the authors upon request): ABCR GmbH (Karlsruhe, Germany), Activate Scientific GmbH (Prien, Germany), Alfa Aesar Johnson Matthey Forschungschemikalien GmbH (Karlsruhe, Germany), Apollo Scientific LTD (Cheshire, UK), AppliChem GmbH (Darmstadt, Germany), Carl Roth GmbH & Co. KG (Karlsruhe, Germany), Fisher Scientific GmbH (Schwerte, Germany), Green Chempharm, Inc. (Bardonia, NY, USA), Hande Sciences (Suzhou, China), Oakwood Products Inc. (West Columbia, SC, USA), Princeton BioMolecular Research (Munich, Germany), Sigma-Aldrich Chemie GmbH (Munich, Germany), VWR International GmbH (Darmstadt, Germany).
Competing interests
The authors declare that they have no competing interests.
Disclaimer
Some of the syntheses described here have partially been disclosed in the patent DE 10 2010 063 974 A1.
Supplementary Material
Contains crystal data and structure refinement for compound X-ray structural data of 6f. Crystal data: C26H20N6O4, Mm = 480.48 g mol–1, triclinic, space group (no. 2), a = 766.4(1) pm, b = 1236.3(1) pm, c = 1278.1(2) pm, α = 109.04(1)°, β = 100.34(1)°, γ = 91.31(1)°, V = 1,121.8(2)·× 106 pm3, Z = 2, rcalc = 1.423 g cm–3, μ(Mo-Kα) = 0.10 mm–1, crystal size 0.32 × 0.08 × 0.07 mm3, Qmax = 50°, 6,766 reflections collected, 3,761 unique, Rint = 0.046, 2,382 observed reflections, 330 parameters, R1 = 0.043 [I > 2s(I)], wR2 = 0.082 (all data), maximum and minimum residual electron density 0.18 and –0.14 × 10–6 Å–3, respectively.
Contains the alignment of the sequence of hCB2 with hAA2R.
Contains the 3D coordinates of the modelled human cannabinoid receptor 2.
Contributor Information
Thomas Rühl, Email: t.ruehl@hzdr.de.
Winnie Deuther-Conrad, Email: w.deuther-conrad@hzdr.de.
Steffen Fischer, Email: s.fischer@hzdr.de.
Robert Günther, Email: r.guenther@hzdr.de.
Lothar Hennig, Email: hennig@organik.chemie.uni-leipzig.de.
Harald Krautscheid, Email: krautscheid@rz.uni-leipzig.de.
Peter Brust, Email: p.brust@hzdr.de.
Acknowledgements
This work has been supported by the DFG (project BR 1360/12-1). The authors wish to thank Bernhard Wünsch from Westfälische Wilhelms-Universität Münster for the fruitful discussions. The authors are grateful to Claudia Birkenmeyer, Ramona Oehme and Aniko Kretschmar from Universität Leipzig for the mass spectrometry and the HRMS data.
References
- Fowler CJ, Rojo ML, Rodriguez-Gaztelumendi A. Modulation of the endocannabinoid system: neuroprotection or neurotoxicity? Exp Neurol. 2010;224(1):37–47. doi: 10.1016/j.expneurol.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346(6284):561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Alger B. Retrograde signaling in the regulation of synaptic transmission: focus on endocannabinoids. Prog Neurobiol. 2002;68(4):247–286. doi: 10.1016/s0301-0082(02)00080-1. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Stella N. Cannabinoid signaling in glial cells. Glia. 2004;48(4):267–277. doi: 10.1002/glia.20084. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII Classification of cannabinoid receptors Pharmacol Rev. 2002;54(2):161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science. 2002;296(5568):678–682. doi: 10.1126/science.1063545. [DOI] [PubMed] [Google Scholar]
- Guzmán M, Sánchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol Ther. 2002;95(2):175–184. doi: 10.1016/s0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- Witkin JM, Tzavara ET, Davis RJ, Li X, Nomikos GG. A therapeutic role for cannabinoid CB1 receptor antagonists in major depressive disorders. Trends Pharmacol Sci. 2005;26(12):609–617. doi: 10.1016/j.tips.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Shohami E, Novikov M, Bass R. Long-term effect of HU-211, a novel non-competitive NMDA antagonist, on motor and memory functions after closed head injury in the rat. Brain Res. 1995;674(1):55–62. doi: 10.1016/0006-8993(94)01433-i. [DOI] [PubMed] [Google Scholar]
- Maas A, Murray G, Henney H, Kassem N, Legrand V, Mangelus M, Muizelaar J, Stocchetti N, Knoller N. Efficacy and safety of dexanabinol in severe traumatic brain injury: results of a phase III randomised, placebo-controlled, clinical trial. Lancet Neurol. 2006;5(1):38–45. doi: 10.1016/S1474-4422(05)70253-2. [DOI] [PubMed] [Google Scholar]
- Alexander A, Smith PF, Rosengren RJ. Cannabinoids in the treatment of cancer. Cancer Lett. 2009;285(1):6–12. doi: 10.1016/j.canlet.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Oesch S, Gertsch J. Cannabinoid receptor ligands as potential anticancer agents - high hopes for new therapies? J Pharm Pharmacol. 2009;61(7):839–853. doi: 10.1211/jpp/61.07.0002. [DOI] [PubMed] [Google Scholar]
- Pertwee RG, Thomas A. In: The cannabinoid receptors. Reggio PH, editor. Humana, New York; 2010. Therapeutic applications for agents that act at CB1 and CB2 receptors; pp. 361–392. [Google Scholar]
- Mackie K. Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol. 2006;46:101–122. doi: 10.1146/annurev.pharmtox.46.120604.141254. [DOI] [PubMed] [Google Scholar]
- Turu G, Hunyady L. Signal transduction of the CB1 cannabinoid receptor. J Mol Endocrinol. 2010;44(2):75–85. doi: 10.1677/JME-08-0190. [DOI] [PubMed] [Google Scholar]
- Ludányi A, Hu SS-J, Yamazaki M, Tanimura A, Piomelli D, Watanabe M, Kano M, Sakimura K, Maglóczky Z, Mackie K, Freund TF, Katona I. Complementary synaptic distribution of enzymes responsible for synthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in the human hippocampus. Neuroscience. 2011;174:50–63. doi: 10.1016/j.neuroscience.2010.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn AB, Herkenham M. Localization of cannabinoid receptors and nonsaturable high-density cannabinoid binding-sites in peripheral-tissues of the rat - implications for receptor-mediated immune modulation by cannabinoids. J Pharmacol Exp Ther. 1994;268(3):1612–1623. [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Egia A, Mechoulam R, Guzmán M, Galve-Roperh I. Non-psychoactive CB2 cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006;20(13):2405–2407. doi: 10.1096/fj.06-6164fje. [DOI] [PubMed] [Google Scholar]
- Skaper S, Buriani A, DalToso R, Petrelli L, Romanello S, Facci L, Leon A. The ALIAmide palmitoylethanolamide and cannabinoids, but not anandamide, are protective in a delayed postglutamate paradigm of excitotoxic death in cerebellar granule neurons. P Natl Acad Sci USA. 1996;93(9):3984–3989. doi: 10.1073/pnas.93.9.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Sickle MD, Duncan M, Kingsley PJ, Mouihate A, Urbani P, Mackie K, Stella N, Makriyannis A, Piomelli D, Davison JS, Marnett LJ, Di Marzo V, Pittman QJ, Patel KD, Sharkey KA. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310(5746):329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- Gong J-P, Onaivi ES, Ishiguro H, Liu Q-R, Tagliaferro PA, Brusco A, Uhl GR. Cannabinoid CB2 receptors: immunohistochemical localization in rat brain. Brain Res. 2006;1071(1):10–23. doi: 10.1016/j.brainres.2005.11.035. [DOI] [PubMed] [Google Scholar]
- Onaivi ES, Ishiguro H, Gong J-P, Patel S, Meozzi PA, Myers L, Perchuk A, Mora Z, Tagliaferro PA, Gardner E, Brusco A, Akinshola BE, Hope B, Lujilde J, Inada T, Iwasaki S, Macharia D, Teasenfitz L, Arinami T, Uhl GR. Brain neuronal CB2 cannabinoid receptors in drug abuse and depression: from mice to human subjects. PLoS One. 2008;3(2):e1640. doi: 10.1371/journal.pone.0001640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez J, Bermúdez-Silva FJ, Mackie K, Ledent C, Zimmer A, Cravatt BF, de Fonseca FR. Immunohistochemical description of the endogenous cannabinoid system in the rat cerebellum and functionally related nuclei. J Comp Neurol. 2008;509(4):400–421. doi: 10.1002/cne.21774. [DOI] [PubMed] [Google Scholar]
- Atwood BK, Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160(3):467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton JC, Wright JL, McPartland JM, Tyndall JDA. Cannabinoid CB1 and CB2 receptor ligand specificity and the development of CB2-selective agonists. Curr Med Chem. 2008;15(14):1428–1443. doi: 10.2174/092986708784567716. [DOI] [PubMed] [Google Scholar]
- Halleskog C, Mulder J, Dahlström J, Mackie K, Hortobágyi T, Tanila H, Kumar Puli L, Färber K, Harkany T, Schulte G. WNT signaling in activated microglia is proinflammatory. Glia. 2011;59(1):119–131. doi: 10.1002/glia.21081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotter EL, Abood ME, Glass M. The endocannabinoid system as a target for the treatment of neurodegenerative disease. Br J Pharmacol. 2010;160(3):480–498. doi: 10.1111/j.1476-5381.2010.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham ES, Ashton JC, Glass M. Cannabinoid receptors: a brief history and "what's hot". Front Biosci. 2009;14:944–957. doi: 10.2741/3288. [DOI] [PubMed] [Google Scholar]
- Evens N, Vandeputte C, Coolen C, Janssen P, Sciot R, Baekelandt V, Verbruggen AM, Debyser Z, Van Laere K, Bormans GM. Preclinical evaluation of [11C]NE40, a type 2 cannabinoid receptor PET tracer. Nuclear Med Biol. 2011;39(3):389–399. doi: 10.1016/j.nucmedbio.2011.09.005. [DOI] [PubMed] [Google Scholar]
- Roche M, Finn DP. Brain CB2 receptors: implications for neuropsychiatric disorders. Pharmaceuticals. 2010;3(8):2517–2553. doi: 10.3390/ph3082517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavey BJ, Kozlowski JA, Hipkin RW, Gonsiorek W, Lundell DJ, Piwinski JJ, Narula S, Lunn CA. Triaryl bis-sulfones as a new class of cannabinoid CB2 receptor inhibitors: identification of a lead and initial SAR studies. Bioorg Med Chem Lett. 2005;15(3):783–786. doi: 10.1016/j.bmcl.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Evens N, Muccioli GG, Houbrechts N, Lambert DM, Verbruggen AM, Van Laere K, Bormans GM. Synthesis and biological evaluation of carbon-11- and fluorine-18-labeled 2-oxoquinoline derivatives for type 2 cannabinoid receptor positron emission tomography imaging. Nucl Med Biol. 2009;36(4):455–465. doi: 10.1016/j.nucmedbio.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Gao M, Wang M, Miller KD, Hutchins GD, Zheng Q-H. Synthesis and in vitro biological evaluation of carbon-11-labeled quinoline derivatives as new candidate PET radioligands for cannabinoid CB2 receptor imaging. Bioorg Med Chem. 2010;18(6):2099–2106. doi: 10.1016/j.bmc.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Evens N, Bormans GM. Non-invasive imaging of the type 2 cannabinoid receptor, focus on positron emission tomography. Curr Top Med Chem. 2010;10(15):1527–1543. doi: 10.2174/156802610793176819. [DOI] [PubMed] [Google Scholar]
- Cheng Y, Albrecht BK, Brown J, Buchanan JL, Buckner WH, DiMauro EF, Emkey R, Fremeau RT, Harmange J-C, Hoffman BJ, Huang L, Huang M, Lee JH, Lin F-F, Martin MW, Nguyen HQ, Patel VF, Tomlinson SA, White RD, Xia X, Hitchcock SA. Discovery and optimization of a novel series of N-arylamide oxadiazoles as potent, highly selective and orally bioavailable cannabinoid receptor 2 (CB2) agonists. J Med Chem. 2008;51(16):5019–5034. doi: 10.1021/jm800463f. [DOI] [PubMed] [Google Scholar]
- Shoemaker JL, Joseph BK, Ruckle MB, Mayeux PR, Prather PL. The endocannabinoid noladin ether acts as a full agonist at human CB2 cannabinoid receptors. J Pharmacol Exp Ther. 2005;314(2):868–875. doi: 10.1124/jpet.105.085282. [DOI] [PubMed] [Google Scholar]
- Gullapalli S, Amrutkar D, Gupta S, Kandadi MR, Kumar H, Gandhi M, Karande V, Narayanan S. Characterization of active and inactive states of CB1 receptor and the differential binding state modulation by cannabinoid agonists, antagonists and inverse agonists. Neuropharmacology. 2010;58(8):1215–1219. doi: 10.1016/j.neuropharm.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Massi P, Patrini G, Rubino T, Fuzio D, Parolaro D. Changes in rat spleen cannabinoid receptors after chronic CP-55,940: an autoradiographic study. Pharmacol Biochem Behav. 1997;58(1):73–78. doi: 10.1016/s0091-3057(96)00379-6. [DOI] [PubMed] [Google Scholar]
- McPartland JM, Glass M, Pertwee RG. Meta-analysis of cannabinoid ligand binding affinity and receptor distribution: interspecies differences. Br J Pharmacol. 2007;152(5):583–593. doi: 10.1038/sj.bjp.0707399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shire D, Calandra B, Rinaldi-Carmona M, Oustric D, Pessegue B, Bonnin-Cabanne O, LeFur G, Caput D, Ferrara P. Molecular cloning, expression and function of the murine CB2 peripheral cannabinoid receptor. Biochim Biophys Acta. 1996;1307(2):132–136. doi: 10.1016/0167-4781(96)00047-4. [DOI] [PubMed] [Google Scholar]
- Warne T, Moukhametzianov R, Baker JG, Nehmé R, Edwards PC, Leslie AGW, Schertler GFX, Tate CG. The structural basis for agonist and partial agonist action on a β(1)-adrenergic receptor. Nature. 2011;469(7329):241–244. doi: 10.1038/nature09746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu F, Wu H, Katritch V, Han GW, Jacobson KA, Gao Z-G, Cherezov V, Stevens RC. Structure of an agonist-bound human A2A adenosine receptor. Science. 2011;332(6027):322–327. doi: 10.1126/science.1202793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poso A, Huffman JW. Targeting the cannabinoid CB2 receptor: modelling and structural determinants of CB2 selective ligands. Br J Pharmacol. 2008;153(2):335–346. doi: 10.1038/sj.bjp.0707567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley EA, Deslongchamps G. PostDock: a novel visualization tool for the analysis of molecular docking. Comput Visual Sci. 2006;12(1):1–7. [Google Scholar]
- Sheldrick GM. A short history of SHELX. Acta Crystallogr, A, Found Crystallogr. 2008;64(Pt 1):112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- Jeffries WB, Waugh D, Abel PW. Analysis of data from “cold saturation” radioligand binding experiments. Methods Mol Biol. 1997;73:331–342. doi: 10.1385/0-89603-399-6:331. [DOI] [PubMed] [Google Scholar]
- Hulme EC, Trevethick MA. Ligand binding assays at equilibrium: validation and interpretation. Br J Pharmacol. 2010;161(6):1219–1237. doi: 10.1111/j.1476-5381.2009.00604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Foreman JC, Johansen T. Textbook of receptor pharmacology. 2. CRC, Boca Raton; 2003. [Google Scholar]
- Jaakola V-P, Griffith MT, Hanson MA, Cherezov V, Chien EYT, Lane JR, Ijzerman AP, Stevens RC. The 2.6 Ǻngström crystal structure of a human A2A adenosine receptor bound to an antagonist. Science. 2008;322:1211–1217. doi: 10.1126/science.1164772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros JA, Weinstein H. In: Receptor molecular biology. Methods in neurosciences, vol 25. Sealfon SC, editor. Elsevier, San Diego; 1995. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors; pp. 366–428. [Google Scholar]
- Abood ME. Molecular biology of cannabinoid receptors. Handb Exp Pharmacol. 2005;168:81–115. doi: 10.1007/3-540-26573-2_3. [DOI] [PubMed] [Google Scholar]
- Verdonk ML, Cole JC, Hartshorn MJ, Murray CW, Taylor RD. Improved protein–ligand docking using GOLD. Proteins. 2003;52(4):609–623. doi: 10.1002/prot.10465. [DOI] [PubMed] [Google Scholar]
- Gunasekaran K, Nussinov R. How different are structurally flexible and rigid binding sites? Sequence and structural features discriminating proteins that do and do not undergo conformational change upon ligand binding. J Mol Biol. 2007;365(1):257–273. doi: 10.1016/j.jmb.2006.09.062. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Contains crystal data and structure refinement for compound X-ray structural data of 6f. Crystal data: C26H20N6O4, Mm = 480.48 g mol–1, triclinic, space group (no. 2), a = 766.4(1) pm, b = 1236.3(1) pm, c = 1278.1(2) pm, α = 109.04(1)°, β = 100.34(1)°, γ = 91.31(1)°, V = 1,121.8(2)·× 106 pm3, Z = 2, rcalc = 1.423 g cm–3, μ(Mo-Kα) = 0.10 mm–1, crystal size 0.32 × 0.08 × 0.07 mm3, Qmax = 50°, 6,766 reflections collected, 3,761 unique, Rint = 0.046, 2,382 observed reflections, 330 parameters, R1 = 0.043 [I > 2s(I)], wR2 = 0.082 (all data), maximum and minimum residual electron density 0.18 and –0.14 × 10–6 Å–3, respectively.
Contains the alignment of the sequence of hCB2 with hAA2R.
Contains the 3D coordinates of the modelled human cannabinoid receptor 2.